Abstract
Both standard and sustained-release injectable formulations of buprenorphine (Bup and BupSR, respectively) are used as preemptive analgesics, potentially affecting gas anesthetic requirements. This study tested the effects of Bup and BupSR on isoflurane requirements and confirmed that buprenorphine could reduce isoflurane requirements during a laparotomy in mice. We hypothesized that both Bup and BupSR would significantly decrease the required minimum alveolar concentration (MAC) of isoflurane. C57BL/6 mice received either isotonic crystalloid fluid (control), Bup (0.1 mg/kg), or BupSR (1.2 mg/kg) subcutaneously 10 min prior to the induction of anesthesia. Each anesthetized mouse was tested at 2 isoflurane concentrations. A 300-g noxious stimulus was applied at each isoflurane concentration, alternating between hindfeet. In addition, a subset of mice underwent terminal laparotomy or 60 min of anesthesia after injection with Bup, BupSR, or saline to ensure an appropriate surgical plane of anesthesia. Mice were maintained at the lowest isoflurane concentration that resulted in 100% of mice at a surgical plane from the aforementioned MAC experiments (control, 2.0%; Bup and BupSR, 1.7%). Analysis showed that both Bup and BupSR significantly decreased isoflurane requirements by 25.5% and 14.4%, respectively. The isoflurane MAC for the control injection was 1.80% ± 0.09%; whereas Bup and BupSR decreased MAC to 1.34% ± 0.08% and 1.54% ± 0.09%, respectively. Sex was not a significantly different between the injection groups during MAC determination. All of the mice that underwent surgery achieved a surgical plane of anesthesia on the prescribed regimen and recovered normally after discontinuation of isoflurane. Lastly, heart and respiratory rates did not differ between mice that underwent surgery and those that were anesthetized only. Bup and BupSR are MAC-sparing in male and female C57BL/6 mice and can be used for effective multimodal anesthesia.
Abbreviations: Bup, standard-formulation buprenorphine; BupSR, sustained-release buprenorphine; MAC, minimum alveolar concentration
Isoflurane is a commonly used volatile anesthetic for rodent procedures. Acting at the level of the spinal cord and brainstem, isoflurane inhibits sensory processing, nociceptive signaling, and motor response to noxious stimulation.3,18,39 The anesthetic strengths of isoflurane include a steep dose-dependent curve and a rapid response to a change in dosing. However, isoflurane can cause hypotension, hypothermia and respiratory suppression in rodents.9,18,20,23 Most significantly, isoflurane does not provide analgesia, leaving a potential for pain and distress after routine procedures.18,50
Opioids bind to specific opioid receptors within the central nervous system and peripheral tissues to inhibit neurotransmitter release creating sedative and analgesic effects and are used preemptively to ensure continuous analgesic coverage in the postoperative period and to prevent postsurgical hypersensitivity.18,30,39 Buprenorphine, a partial μ agonist and partial κ antagonist analgesic, is commonly used in mice and has been shown to provide 3 to 6 h of antinociception in mice, depending on the dose, when injected subcutaneously.8,19,22,31 One concern with Bup is that the short duration of action requires frequent handling of mice, potentially disrupting normal behavior and adding stress for the animal.1,23 The emergence of the polymer-coated buprenorphine sustained-release (BupSR) has provided extended analgesic coverage in mice. However, great variability has been reported across pharmacokinetic and pharmacodynamics testing, with some studies reporting the drug to be effective for up to 12 h,4,8 24 h29,31 or 48 h,26 all of which are significantly longer than the duration of Bup. Regardless of formulation, buprenorphine administered preemptively potentially affects isoflurane gas requirements intraoperatively in mice. Opioids, when used as part of multimodal anesthesia, have consistently proven to exhibit minimum alveolar concentration (MAC) sparing effects in animals and humans due to both analgesic and sedative effects during gas anesthetic events.10,11,24,41,54 The MAC of an inhaled anesthetic is the concentration that produces immobility in response to a noxious stimulus in 50% of subjects.14,15 Despite the common use of Bup and BupSR in mice, buprenorphine has not been evaluated as a MAC sparing drug. Considering that Bup, BupSR and isoflurane can lead to respiratory suppression and poor respiratory drive, having accurate dosing information for the use of these drugs will improve their safety in mice.
The purpose of this study was to determine the MAC sparing effects of clinically relevant doses of Bup and BupSR on male and female mice anesthetized with isoflurane, as well as monitoring the effects of these drugs on the heart rate (HR) and respiratory rate (RR) of the anesthetized mouse. We hypothesized that both would significantly decrease MAC requirements. In addition, secondary experiments were performed to confirm that the Bup and BupSR isoflurane doses were safe and adequate during experimental laparotomies. Our study aimed to provide laboratory personnel and research scientists with clinically relevant isoflurane concentrations for use in mice, following prelaparotomy Bup and BupSR administration.
Materials and Methods
Mice.
Young adult (age, 8 to 16 wk) male and female C57BL/6J mice (Mus musculus; n = 51; Jackson Laboratories, Bar Harbor, ME) were used in this study. Mice were housed under a 12:12-h light:dark cycle in same-sex groups of no more than 5 animals per cage in static polycarbonate microisolation cages (Max 75, Alternative Design, Siloam Springs, AR) containing disposable bedding (0.12-in. Bed-O-Cobs, The Andersons, Maumee, OH) and with cotton squares (Ancare, Bellmore, NY) for environmental enrichment. Mice were fed standard pelleted laboratory rodent chow (no. 5001, LabDiet, St Louis, MO) without restriction and received municipal water supplied by bottle. Sentinel mice were tested routinely in our facility and were found to be free from fur mites, pinworms, and contagious pathogens, including mouse hepatitis virus, mouse parvoviruses, rotavirus, ectromelia virus, Sendai virus, pneumonia virus of mice, Theiler murine encephalomyelitis virus, reovirus, Mycoplasma pulmonis, lymphocytic choriomeningitis virus, mouse adenovirus, and polyomavirus. Procedures were approved by the University of Pennsylvania's IACUC. Mice were allowed at least 1 wk to acclimate to the AAALAC-accredited housing facility and cage environment prior to the start of the study. Each mouse in this study underwent no more than 3 anesthetic events with at least a 7-d washout period between anesthetic events.
This project consisted of 2 separate sets of experiments. Experiment 1 evaluated isoflurane MAC after administration of Bup or BupSR; experiment 2 confirmed that the opioid-adjusted isoflurane doses produced a surgical plane of anesthesia for laparotomy. In both experiments, the initial drug administration, induction of anesthesia, and animal preparation were identical.
Preanesthetic dosing, anesthetic induction, preparation of the mice, and noxious stimulation.
Mice were weighed on a digital scale (model KD-160, Tanita, Arlington Heights, IL) prior to dosing. Experimental groups and isoflurane doses were assigned randomly (JM) prior to each experiment. Mice received either isotonic crystalloid fluid (0.1 mL SC; 0.9% sodium chloride or Lactated Ringer Solution, Hospira, Lake Forest, IL), Bup (0.1 mg/kg SC; Buprenex, C III, Patterson Vet Generics; 0.3 mg/mL diluted to 0.02 mg/mL), or BupSR (1.2 mg/kg SC; Buprenorphine SR Lab ZooPharm, Windsor, CO). All injections were administered into the interscapular region of each mouse. At 10 min after injection, anesthesia was induced at 4.0% isoflurane and 100% O2 in an anesthetic induction chamber until mice lost their righting reflex. Mice were transferred to a nose cone to receive 2.0% isoflurane and 0.6 L/min O2. Application of eye lube (Akorn, Lake Forest, IL), placement of a rectal thermometer (ThermoWorks, American Fork, UT), and instrumentation of ECG leads on the mouse's forepaws (ECGenie, Mouse Specifics, Framingham, MA) were performed. The mice received 2.0% isoflurane for approximately 2 min while being instrumented. After instrumentation, the isoflurane concentration was adjusted to the first experimental concentration (described later). Mice were maintained on a circulating water blanket (Stryker T/Pump, Kalamazoo, MI) after induction and throughout the procedure. Temperature was not used as a dependent variable due to the importance of maintaining body temperature on other physiologic parameters.5
HR, RR (manually measured by thoracic excursions), body temperature monitored by a rectal probe (9 mm; RET3, ThermoWorks, connected to a thermometer [TW2-193, MicroTherma, ThermoWorks]), and plane of anesthesia were recorded every 5 min. HR was monitored by electrocardiography (ECGenie and eMouse 11 Analysis Software, Mouse Specifics). The isoflurane concentration at the fresh gas outlet was measured in real time by using an anesthetic gas monitor (Poet IQ2, Anesthetic Gas Monitor Criticare Systems, Waukesha, WI). Because isoflurane concentrations were collected from the inspiration gas stream, our isoflurane concentrations were only an estimate of the true alveolar isoflurane concentration. A surgical plane of anesthesia was defined as the loss of hindlimb withdrawal at a 300-g noxious stimulus (Touch Test, North Coast Medical, Gilroy, CA). The device is a handheld filament that delivers 300 g when depressed manually and that bends when this force is reached, limiting the amount force delivered. This amount of stimulation reliably and consistently replicated the response to a firm toe pinch, without causing lameness or pain in the mouse after the procedure.16,28 The stimulus was delivered 4 times, alternating between hindfeet, with at least 30 s between tests of each foot. When a brisk positive response to the noxious stimulus occurred on the first test, no further stimuli were performed to prevent testing from significantly altering the animal's plane of anesthesia. A positive response was defined as any movement by the mouse in response to the noxious stimulus. Response to each individual stimulus was scored as either positive or negative.45
Experiment 1: MAC determination.
To determine isoflurane MAC for each drug, 61 anesthetic trials (male mice, 34; female mice, 27) were performed at 2 randomized and increasing isoflurane concentrations. The concentrations were always increased to prevent the effects of neural inertia from keeping the mice at a deeper plane of anesthesia than on initial exposure.21,48 Each mouse was anesthetized with isoflurane for 20 min before the initial noxious stimulus test, to allow the injected agents to take full effect; a 10-min isoflurane equilibration period was implemented between concentrations.7,47,52 After the 300-g noxious stimulus test at the initial concentration, isoflurane was set to an increased concentration (increases ranged from 0.1% to 0.3%) for an additional 10 min, and the depth of anesthesia was tested at the second concentration. Isoflurane concentrations evaluated ranged from 1.5% to 2.1% for control mice and 1.0% to 1.9% for the mice receiving a buprenorphine injection, in 0.1% increments. Because of these ranges, the experimenter was not blinded to the injection that the mouse received. On completion of the experiment, mice were returned to their home cage for recovery and were monitored until normal grooming behaviors and ambulation were noted.
Experiment 1: Statistical analysis.
All statistical analyses for experiment 1 were performed by using SigmaPlot 12.3 (Systat Software, San Jose, CA). In addition, normal distribution of the data was confirmed before the use of parametric statistics. The statistical analysis of the effects of Bup and BupSR injections on isoflurane MAC was performed by both bracketing analysis and quantal analysis. For the bracketing analysis, an estimate of the isoflurane concentration at which the mouse transitioned from a positive response to the noxious stimulus to a negative response was determined for each mouse by averaging the isoflurane concentration between the positive and negative responses. When a mouse had both positive and negative responses at the same isoflurane concentration (occurred in 10% of trials), this concentration was used as the estimate of the transitioning isoflurane concentration. Two-way ANOVA was performed to analyze the effects of sex and drug on isoflurane MAC. When significant differences were detected, Tukey posthoc analysis was performed. Significance was set at a P value less than 0.05. Quantal analysis of MAC was performed by graphing the percentage of mice at a surgical plane of anesthesia for each isoflurane concentration tested for each of the 3 injections. Isoflurane MAC was determined as the concentration at which 50% of the mice were at a surgical plane of anesthesia. When a mouse had both a positive and negative response to the noxious stimulus at the same isoflurane concentration, the animal was considered to not be at a surgical plane of anesthesia. This decision is based on current veterinary recommendations, thus assuring that any positive response is interpreted as a nonsurgical plane of anesthesia.2,50
Experiment 2: Use of Bup and BupSR under surgical conditions.
Mice were randomly assigned to receive either terminal laparotomy or a sham laparotomy anesthetic event for 60 min. The time to complete the laparotomy took less than 45 min from induction of surgery. Four or 5 mice (2 or 3 male and female mice) were used in each group for each of the 3 injections (control, Bup, BupSR). A total of 25 laparotomy or sham procedures were performed between the 3 groups. The isoflurane concentration used for the control mice was 2.0%, which was the lowest concentration at which 100% of the mice were at a surgical plane of anesthesia. This concentration was then conservatively decreased by 15% (to 1.7%) for both Bup and BupSR, in light of the decrease in MAC values in experiment 1. Cardiorespiratory parameters (HR and RR) were monitored for each trial and recorded every 5 min.
For laparotomy, mice were shaved from xiphoid to pubis, and the abdomen was aseptically scrubbed with 3 alternating rounds of diluted chlorhexidine and alcohol; 0.25 mg of lidocaine (Xylocaine 2%, APP Pharmaceuticals, Lake Zurich, IL; diluted 1:4 with sterile water for a final concentration of 0.5%) was given subcutaneously as a line block at 5 min prior to first incision. Prior to the incision, the mice were confirmed to be at a surgical plane of anesthesia according to a negative response to the 300-g noxious stimulus.
The laparotomy procedure was designed to provide extensive manipulation of the abdominal viscera. A 2.0-cm midline incision was made, and the cranial mesenteric artery was identified and exposed for 5 min. The left kidney was then identified and exposed for 5 min. A 2-layer closure was performed, with the body wall sutured with 4-0 monofilament suture in a simple interrupted fashion. The skin was sutured with 3-0 monofilament suture in a simple continuous fashion. Isoflurane was discontinued, and return to a positive hindlimb withdrawal indicated a successful anesthetic event and laparotomy. The mouse was then deeply anesthetized with isoflurane until a deep surgical plane of anesthesia (no response to firm toe pinch on both hindlimbs) was reached, and the mouse was euthanized by cervical dislocation.
Experiment 2: Statistical analysis.
Two-way ANOVA was performed on the time to reach a surgical plane of anesthesia, with the main effects of injection and sex. In light of the results from experiment 1, we analyzed the effect of sex on the HR and RR responses in the mice. We found that the effect of sex on HR and RR was not significant and, because we did not assume the sphericity of the data, we performed 3-way ANOVA with Greenhouse–Geisser correction, with main effects of drug, procedure, and time. Bonferroni posthoc analysis was used for multiple comparisons. The data were analyzed over the first 40 min, as this period was the time required to complete the laparotomy. Statistics in this section was performed by using SPSS (version 17, SPSS).
Results
Experiment 1: MAC determination.
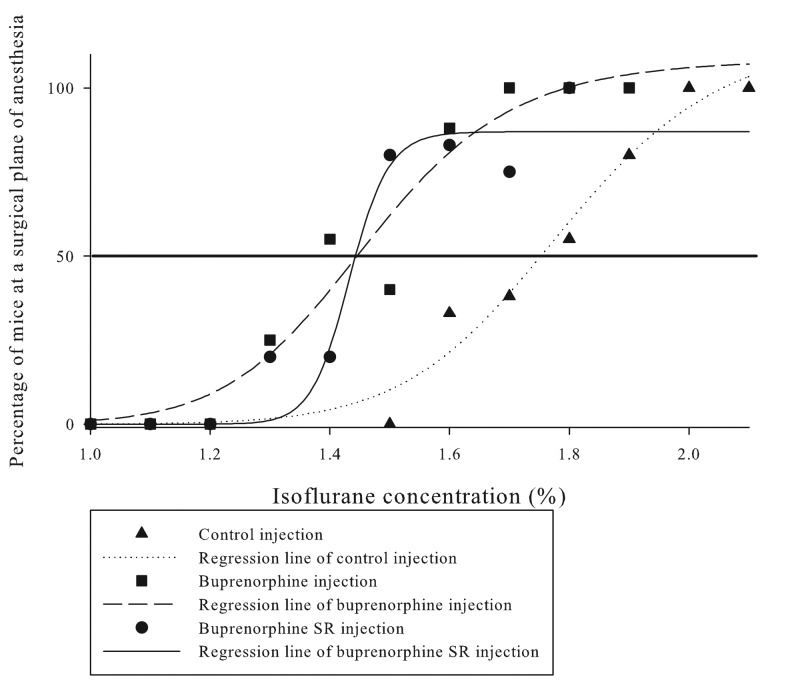
Experiment 1 directly tested the effects of Bup and BupSR on isoflurane requirements in male and female C57BL/6J mice. Sex did not significantly affect isoflurane MAC (P = 0.53). Bracketing data analysis demonstrated a statistically significant (P < 0.001) decreases in isoflurane requirements of 25.5% after Bup administration and 14.4% after BupSR (Table 1). Post hoc analysis of the bracketing technique showed that all 3 groups were statistically different from each other in regard to isoflurane MAC. Figure 1 highlights the results of the quantal analysis, which demonstrated an 18% reduction in isoflurane MAC after both drugs.
Table 1.
Isoflurane MAC (%) in C57BL/6 mice premedicated with standard buprenorphine, buprenorphine SR, or an isotonic crystalloid fluid (control) 10 min prior to isoflurane anesthesia induction
| Bracketing analysis (mean ± 1 SD) | Quantal analysis | |
| Control (n = 18) | 1.80% ± 0.09% | 1.75% |
| Standard buprenorphine (n = 11) | 1.34% ± 0.08% | 1.44% |
| Buprenorphine SR (n = 11) | 1.54% ± 0.09% | 1.44% |
The effect of drug injection type differed significantly (P < 0.001) between groups, but sex did not have a significant effect (P = 0.53) between groups. Post hoc analysis revealed that all 3 injections had significantly different effects on isoflurane MAC.
Figure 1.
Quantal analysis of Bup, BupSR, and control groups (data pooled for male and female mice). The horizontal black line bisecting the graph is the 50% line, which indicates the isoflurane concentration at which 50% of the mice were at a surgical plane or MAC. Results show a noticeable left shift of the mice that received Bup or BupSR. Overall, we noted an estimated 20% decrease in isoflurane concentration with both Bup and BupSR.
Experiment 2: Use of Bup and BupSR under laparotomy conditions.
All mice in experiment 2 achieved a surgical plane of anesthesia that was adequate for laparotomy and were allowed to recover to the point of leaving the surgical plane of anesthesia after discontinuation of isoflurane anesthesia. The time required to achieve a surgical plane did not differ significantly between sexes or injected agents, although variability was greater for mice that received either Bup or BupSR (time [mean ± 1 SD] to reach surgical plane [pooled for male and female mice]: control, 9.8 ± 0.9 min; Bup, 14.6 ± 9.0 min; BupSR, 16.9 ± 11.0 min).
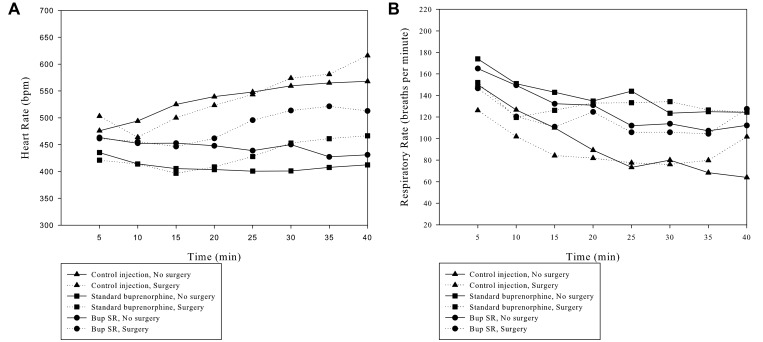
Figure 2 reports the HR and RR of the mice during the anesthetic events. As in experiment 1, sex did not significantly affect HR (P = 0.796) or RR (P = 0.312). In addition, HR did not differ with regard to preanesthetic drug type (P = 0.215) or procedure type (P = 0.208) but did show a significant effect due to time (P = 0.005). In addition, Greenhouse–Geisser analysis showed significant differences in RR with regard to drug (P < 0.001), procedure type (P = 0.046), and time (P < 0.001).
Figure 2.
A) Effect of each anesthetic combination on heart rate over time. B) Effect of each anesthetic combination on respiratory rate over time. T = 0 is the initiation of anesthesia. The data with standard deviations are included in Supplemental Table 1.
Discussion
Preemptive analgesia in mice is an example of multimodal anesthesia, which is used to ensure continuous analgesic coverage in the transition to the postoperative period, reduction of anesthetic requirements and prevention of postsurgical hypersensitivity.30,51 The sedation and potential respiratory suppression commonly associated with opiates necessitate the importance of understanding their effect on isoflurane requirements during anesthesia. Consistent with other opiates or anesthetic combinations in other species,10,11,27,40 we demonstrated decreases in isoflurane requirements after the administration of Bup (25.5% decrease in isoflurane MAC) or BupSR (14.4% decrease; Table 1). In addition, we demonstrated that these lower isoflurane concentrations after opiate administration are sufficient to maintain a surgical plane of anesthesia in a laparotomy procedure.
In the current investigation, we used 2 methods of analysis to determine isoflurane MAC—bracketing analysis and quantal analysis. Bracketing attempts to identify the individual isoflurane concentration at which an animal transitions to a surgical plane of anesthesia, whereas quantal analysis uses the population's results at different isoflurane percentages to generate a dose–response curve from which MAC is determined.45 Typically, as was the case in the current study, the results of the 2 analyses are similar.41,45 The differences in the current study are likely representative of the small percentage of mice that had both a positive and negative response to the noxious stimulus at the same isoflurane concentration. For the bracketing analysis, this point was considered to be an excellent estimate for the transition isoflurane concentration, whereas in the quantal analysis, this event would result in the animal being classified as not at a surgical plane of anesthesia. This decision was based on current recommendations for researchers to delay initiating laparotomy surgery whenever a mouse has a positive withdrawal reflex to a noxious stimulus.2,50
The bracketing analysis demonstrated significant effects of both Bup and BupSR on isoflurane MAC and significant differences in the effects of Bup and BupSR on isoflurane requirements, when compared with the control injection. The differences in MAC reduction noted between the 2 buprenorphine preparations may be attributable to the slower release of drug from BupSR. Sustained-release formulations generally do not have linear absorption rates immediately after subcutaneous administration, leading to variability in the onset of drug action.8,49 Previous studies have shown pharmacokinetic variability after the administration of BupSR in several species, including mice.25,36,37,49,56,57
In our current study, the variability in the time required to achieve therapeutic serum concentrations is demonstrated by the high variability required to reach a surgical plane of anesthesia in experiment 2 after either Bup or BupSR injection when compared with the variability in the control mice. To date, no work has examined the pharmacokinetics of Bup or BupSR absorption during this very early time frame after drug administration,8,30 so it is difficult to assess when the opiates achieve serum concentrations sufficiently high to provide both analgesic effects (which occur at lower concentrations) and sedative effects, to decrease the isoflurane anesthesia requirements. In addition, differences between animals in response to the drugs—as well as the potential effect of the microbiome on drug activity—may account for some of this variability. The initial analysis in experiment 1 included a time point at 20 min after injection. Because of the results in experiment 2, this time point was removed from the analysis, and only time points that were at least 30 min after injection were analyzed. Even with this modification, the fact that no pharmacokinetic analyses were performed in experiment 1 is a limitation of the study because we cannot truly confirm the serum concentrations of the drugs at the time of the analysis. Ultimately, this variability in buprenorphine activity early in the procedure necessitates close monitoring of the anesthetic plane in each mouse until steady serum concentrations in the therapeutic range are achieved.
The current study reports an isoflurane MAC for control mice of 1.80% ± 0.09% (Table 1), which is similar to that of a study7 that likewise tested the response to a noxious stimulus on a hindlimb. The anatomic site (tail, forelimb, hindlimb) and nature (compression, surgical incision, intubation) of stimulation is critical in determining the resulting MAC values. The tail is less sensitive than hindlimbs, subsequently requiring less anesthetic to eliminate a response to stimulation.2,15,17,53,56 Consideration of these factors—along with the age, strain, and health status of the mice—are critical when comparing MAC values between studies.46
We confirmed the MAC-sparing effects of the 2 opiates by testing them under surgical conditions. We performed laparotomies in mice at 1.7% isoflurane after opioid administration and at 2.0% isoflurane after a control injection. The 1.7% isoflurane concentration safely achieved and maintained a surgical plane of anesthesia for a laparotomy in mice, although more painful procedures may require higher isoflurane concentrations after Bup or BupSR administration. Given the steep dose–response curve for inhalants,3 small increases in the isoflurane dose delivered will safely deepen the plane of anesthesia without bringing the mouse to an unsafe plane of anesthesia. In the current study, we did not assess the upper limits of a safe isoflurane administration range, but this experiment could be incorporated into future studies. In addition, researchers should understand that many other factors affect the buprenorphine and isoflurane requirements of anesthetized mice in biomedical research, including the procedures performed, the strain and health of the mice, and the experience of the surgeon. An additional benefit of the decreased isoflurane MAC after buprenorphine administration is the decreased exposure of personnel to waste anesthetic gases.42
To test the effects of Bup or BupSR combined with isoflurane under surgical conditions, we administered a subcutaneous local anesthetic line block prior to the laparotomy incision. Local anesthetics, such as mepivacaine and bupivacaine, are known to be MAC-sparing when administered as locoregional anesthesia for oral or veterinary dental procedures.43,44 In addition, lidocaine can have systemic anesthetic effects, having been shown to decrease the induction time and prolong the inhibition of the hindlimb withdrawal reflex when administered intraperitoneally with ketamine and xylazine in CD1 mice.13 The lidocaine administered in experiment 2 may have enhanced the depth of anesthesia in response to the Bup or BupSR with isoflurane; however we believe the effect was negligible, because the absorption of lidocaine after subcutaneous administration in our current study would be slower than after the intraperitoneal delivery in the cited study.23
In experiment 2, we monitored HR and RR of all groups (control, Bup, and BupSR) during laparotomy and sham procedures (Figure 2 A and B). Researchers need to know normal responses in HR and RR to accurately monitor and treat potential anesthetic complications before they become untreatable. We hypothesized that the procedure performed (either a laparotomy or sham) would have a profound effect on HR and RR, given that the mice received the lowest isoflurane–opiate dose likely to maintain a surgical plane of anesthesia. However, the procedure had relatively little effect on these dependent variables compared with the effect of the anesthetic protocol. The effect of the procedure achieved statistical significance (P = 0.046) for RR but not HR (P = 0.235). Alternatively, the anesthetic protocol had a more profound effect on both HR and RR (P < 0.001 for both). This response is consistent with 2 previous studies.16,35 In one of these studies,16 RR during isoflurane anesthesia changed approximately 70% of the time after a noxious stimulus, whereas the other study35 reported a small but significant change in RR in response to surgical stimulation. Neither of these studies reported meaningful changes in HR in response to surgical stimulation. One explanation for these reported responses could be global autonomic reflex blunting, which has been reported in humans and animals.12,57 However, this effect is unlikely, because the isoflurane concentrations that we documented were just above the calculated MAC values, at which we would still expect a robust response from the autonomic nervous system. Regardless, the isoflurane concentrations administered in the current study—both with and without Bup or BupSR—appear to have been adequate to suppress large changes in HR and RR in response to skin incision and manipulation of the abdominal viscera. This finding is relevant to researchers in that they will be unable to refine their assessment of deeply anesthetized animals according to the mouse's HR and RR response to a noxious stimulus.
Isoflurane had a significant effect on RR. The control mice, which received the highest concentration of isoflurane, had a significantly lower RR than did either of the other 2 groups of mice; this finding is consistent with previous studies.6 This result is surprising, given that the other 2 groups of mice received either Bup or BupSR. Although Bup has less respiratory-suppressive effects than pure μ-agonists, its use is still associated with dose-dependent respiratory effects.32,55 A decrease in RR is not specifically indicative of respiratory depression, which is defined as the inability to respond to abnormal respiratory parameters such as hypercarbia or hypoxia.33 In the current study, we used 100% oxygen as the carrier gas, in an attempt to remove hypoxia as a variable in the evaluation of RR. Further work using pulse oximetry and blood gas analysis and monitoring minute ventilation and tidal volume is necessary to confirm the presence of respiratory depression.
This study did not use the traditional ‘up-down’ method for bracketing MAC determination, in light of the risk of neural inertia in preventing accurate assessment of the transition from unconsciousness to consciousness.21,48 Neural inertia describes the resistance of the brain to transition between states of consciousness and unconsciousness. This state means that the response of a mouse to a given isoflurane concentration is significantly influenced by whether the mouse was conscious or unconscious before being exposed to the new concentration.21,34,38
Our study demonstrated that preemptive Bup and BupSR reduced isoflurane requirements by approximately 20% in both male and female C57BL/6 mice. Furthermore, we demonstrated that preemptive administration of either Bup or BupSR was effective at lowering the isoflurane requirements in surgical conditions. Additionally, there was marked variability in the time required for the drugs to significantly affect the isoflurane requirements in these mice. In conclusion, the addition of preemptive analgesics provides a MAC-sparing effect during procedures, allowing time for the drugs to reach a therapeutic analgesic effect before the animal is likely to experience any pain. Taken collectively, these results will assist the research community in improving the wellbeing of the mice undergoing anesthesia, and highlight the benefits of multimodal administration of analgesia.
Supplementary Material
Cardiopulmonary parameters (mean ± 1 SD) in mice during isoflurane anesthesia after pretreatment with or without subcutaneous buprenorphine
Acknowledgment
We thank the Office of the Vice Provost of Research at the University of Pennsylvania for partially funding this project and the salary of Philip C LaTourette.
References
- 1.Balcombe JP, Barnard ND, Sandusky C. 2004. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43:42–51. [PubMed] [Google Scholar]
- 2.Buitrago S, Martin TE, Tetens-Woodring J, Belicha-Villanueva A, Wilding GE. 2008. Safety and efficacy of various combinations of injectable anesthetics in BALB/c mice. J Am Assoc Lab Anim Sci 47:11–17. [PMC free article] [PubMed] [Google Scholar]
- 3.Campagna JA, Miller KW, Forman SA. 2003. Mechanisms of actions of inhaled anesthetics. N Engl J Med 348:2110–2124. 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
- 4.Carbone ET, Lindstrom KE, Diep S, Carbone L. 2012. Duration of action of sustained-release buprenorphine in 2 strains of mice. J Am Assoc Lab Anim Sci 51:815–819. [PMC free article] [PubMed] [Google Scholar]
- 5.Caro AC, Hankenson FC, Marx JO. 2013. Comparison of novel thermoregulatory devices used during anesthesia of C57BL/6 mice and the corelation of body temperature and physiologic parameters. J Am Assoc Lab Anim Sci 52:577–583. [PMC free article] [PubMed] [Google Scholar]
- 6.Cesarovic N, Jirkof P, Rettich A, Nicholls F, Arras M. 2012. Combining sevoflurane anesthesia with fentanyl-midazolam or s-ketamine in laboratory mice. J Am Assoc Lab Anim Sci 51:209–218. [PMC free article] [PubMed] [Google Scholar]
- 7.Cesarovic N, Nicholls F, Rettich A, Kronen P, Hassig M, Jirkof P, Arras M. 2010. Isoflurane and sevoflurane provide equally effective anaesthesia in laboratory mice. Lab Anim 44:329–336. 10.1258/la.2010.009085. [DOI] [PubMed] [Google Scholar]
- 8.Clark TS, Clark DD, Hoyt RF., Jr 2014. Pharmacokinetic comparison of sustained-release and standard buprenorphine in mice. J Am Assoc Lab Anim Sci 53:387–391. [PMC free article] [PubMed] [Google Scholar]
- 9.Constantinides C, Mean R, Janssen BJ. 2011. Effects of isoflurane anesthesia on the cardiovascular function of the C57BL/6 mouse. ILAR J 52:e21–e31. [PMC free article] [PubMed] [Google Scholar]
- 10.Criado AB, Gómez de Segura IA, Tendillo FJ, Marsico F. 2000. Reduction of isoflurane MAC with buprenorphine and morphine in rats. Lab Anim 34:252–259. 10.1258/002367700780384717. [DOI] [PubMed] [Google Scholar]
- 11.Criado AB, Gómez e Segura IA. 2003. Reduction of isoflurane MAC by fentanyl or remifentanil in rats. Vet Anaesth Analg 30:250–256. 10.1046/j.1467-2995.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- 12.Daniel M, Weiskopf RB, Noorani M, Eger EI., 2nd 1998. Fentanyl augments the blockade of the sympathetic response to incision (MAC-BAR) produced by desflurane and isoflurane: desflurane and isoflurane MAC-BAR without and with fentanyl. Anesthesiology 88:43–49. 10.1097/00000542-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Dholakia U, Clark-Price SC, Keating SCJ, Stern AW. 2017. Anesthetic effects and body weight changes associated with ketamine-xylazine-lidocaine administered to CD1 mice. PLoS One 12:1–11. 10.1371/journal.pone.0184911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eger EI., 2nd 2001. Age, minimum alveolar anesthetic concentration, and minimum alveolar anesthetic concentration-awake. Anesth Analg 93:947–953. 10.1097/00000539-200110000-00029. [DOI] [PubMed] [Google Scholar]
- 15.Eger EI, 2nd, Saidman LJ, Brandstater B. 1965. Minimum alveolar anesthetic concentration: a standard of anesthetic potency. Anesthesiology 26:756–763. 10.1097/00000542-196511000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Erickson RL, Terzi MC, Jaber SM, Hankenson FC, McKinstry-Wu A, Kelz MB, Marx JO. 2016. Intraperitoneal continuous-rate infusion for the maintenance of anesthesia in laboratory mice (Mus musculus). J Am Assoc Lab Anim Sci 55:548–557. [PMC free article] [PubMed] [Google Scholar]
- 17.Figueiró MR, Soares JH, Ascoli FO, Werre S, Gómez de Segura IÁ. 2016. Isoflurane MAC determination in dogs using three intensities of constant-current electrical stimulation. Vet Anaesth Analg 43:464–471. 10.1111/vaa.12341. [DOI] [PubMed] [Google Scholar]
- 18.Fish RE, Danneman PJ, Brown M, Karas AZ, editors. 2008. Anesthesia and analgesia in laboratory animals. Burlington (MA): Elsevier. [Google Scholar]
- 19.Foley PL, Liang H, Crichlow AR. 2011. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci 50:198–204. [PMC free article] [PubMed] [Google Scholar]
- 20.Fox JG, Anderson LC, Otto GM, Pritchett-Corning KR, Whary MT, editors. 2015. Laboratory animal medicine, 3rd ed Boston (MA): Elsevier; 10.1016/B978-0-12-409527-4.00001-8 [DOI] [Google Scholar]
- 21.Friedman EB, Sun Y, Moore JT, Hung HT, Meng QC, Perera P, Joiner WJ, Thomas SA, Eckenhoff RG, Sehgal A, Kelz MB. 2010. A conserved behavioral state barrier impedes transitions between anesthetic-induced unconsciousness and wakefulness: evidence for neural inertia. PLoS One 5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gades NM, Danneman PJ, Wixson SK, Tolley EA. 2000. The magnitude and duration of the analgesic effect of morphine, butorphanol, and buprenorphine in rats and mice. Contemp Top Lab Anim Sci 39:8–13. [PubMed] [Google Scholar]
- 23.Gargiulo S, Greco A, Gramanzini M, Esposito S, Affuso A, Brunetti A, Vesce G. 2012. Mice anesthesia, analgesia, and care, part I: anesthetic considerations in preclinical research. ILAR J 53:E55–E69. 10.1093/ilar.53.1.55. [DOI] [PubMed] [Google Scholar]
- 24.Grasso SC, Ko JC, Weil AB, Hess JA, Paranjape V, Payton M. 2018. Effects of transdermal fentanyl solution application and subsequent naloxone hydrochloride administration on minimum alveolar concentration of isoflurane in dogs. J Am Vet Med Assoc 253:431–436. 10.2460/javma.253.4.431. [DOI] [PubMed] [Google Scholar]
- 25.Guzman DS, Knych HK, Olsen GH, Paul-Murphy JR. 2017. Pharmacokinetics of a sustained release formulation of buprenorphine after intramuscular and subcutaneous administration to American kestrels (Falco sparverius). J Avian Med Surg 31:102–107. 10.1647/2015-155. [DOI] [PubMed] [Google Scholar]
- 26.Healy JR, Tonkin JL, Kamarec SR, Saludes MA, Ibrahim SY, Matsumoto RR, Wimsatt JH. 2014. Evaluation of an improved sustained-release buprenorphine formulation for use in mice. Am J Vet Res 75:619–625. 10.2460/ajvr.75.7.619. [DOI] [PubMed] [Google Scholar]
- 27.Ilkiw JE, Pascoe PJ, Tripp LD. 2002. Effects of morphine, butorphanol, buprenorphine, and U50488H on the minimum alveolar concentration of isoflurane in cats. Am J Vet Res 63:1198–1202. 10.2460/ajvr.2002.63.1198. [DOI] [PubMed] [Google Scholar]
- 28.Jaber SM, Hankenson FC, Heng K, McKinstry-Wu A, Kelz MB, Marx JO. 2014. Dose regimens, variability, and complications associated with using repeat-bolus dosing to extend a surgical plane of anesthesia in laboratory mice. J Am Assoc Lab Anim Sci 53:684–691. [PMC free article] [PubMed] [Google Scholar]
- 29.Jirkof P, Tourvieille A, Cinelli P, Arras M. 2015. Buprenorphine for pain relief in mice: repeated injections vs sustained-release depot formulation. Lab Anim 49:177–187. 10.1177/0023677214562849. [DOI] [PubMed] [Google Scholar]
- 30.Katz J, Cohen L, Schmid R, Chan VW, Wowk A. 2003. Postoperative morphine use and hyperalgesia are reduced by preoperative but not intraoperative epidural analgesia: implications for preemptive analgesia and the prevention of central sensitization. Anesthesiology 98:1449–1460. 10.1097/00000542-200306000-00023. [DOI] [PubMed] [Google Scholar]
- 31.Kendall LV, Wegenast DJ, Smith BJ, Dorsey KM, Kang S, Lee NY, Hess AM. 2016. Efficacy of sustained-release buprenorphine in an experimental laparotomy model in female mice. J Am Assoc Lab Anim Sci 55:66–73. [PMC free article] [PubMed] [Google Scholar]
- 32.Khanna IK, Pillarisetti S. 2015. Buprenorphine—an attractive opioid with underutilized potential in treatment of chronic pain. J Pain Res 8:859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko S, Goldstein DH, VanDenKerkhof EG. 2003. Definitions of "respiratory depression" with intrathecal morphine postoperative analgesia: a review of the literature. Can J Anaesth 50:679–688. [DOI] [PubMed] [Google Scholar]
- 34.Kuizenga MH, Colin PJ, Reyntjens KMEM, Touw DJ, Nalbat H, Knotnerus FH, Vereecke HEM, Struys MMRF. 2018. Test of neural inertia in humans during general anaesthesia. Br J Anaesth 120:525–536. 10.1016/j.bja.2017.11.072. [DOI] [PubMed] [Google Scholar]
- 35.Lipiski M, Arras M, Jirkof P, Cesarovic N. 2017. Premedication with fentanyl-midazolam improves sevoflurane anesthesia for surgical intervention in laboratory mice. Exp Biol Med (Maywood) 242:1287–1298. 10.1177/1535370217707730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nunamaker EA, Halliday LC, Moody DE, Fang WB, Lindeblad M, Fortman JD. 2013. Pharmacokinetics of 2 formulations of buprenorphine in macaques (Macaca mulatta and Macaca fascicularis). J Am Assoc Lab Anim Sci 52:48–56. [PMC free article] [PubMed] [Google Scholar]
- 37.Nunamaker EA, Stolarik DF, Ma J, Wilsey AS, Jenkins GJ, Medina CL. 2014. Clinical efficacy of sustained-release buprenorphine with meloxicam for postoperative analgesia in beagle dogs undergoing ovariohysterectomy. J Am Assoc Lab Anim Sci 53:494–501. [PMC free article] [PubMed] [Google Scholar]
- 38.Paul M, Fisher DM. 2001. Are estimates of MAC reliable? Anesthesiology 95:1362–1370. 10.1097/00000542-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 39.Plumb DC. 2015. Plumb's veterinary drug handbook, 8th ed, Ames (IA): Wiley–Blackwell. [Google Scholar]
- 40.Queiroz-Williams P, Doherty TJ, da Cunha AF, Leonardi C. 2016. Effects of ketamine and lidocaine in combination on the sevoflurane minimum alveolar concentration in alpacas. Can J Vet Res 80:141–145. [PMC free article] [PubMed] [Google Scholar]
- 41.Sebel PS, Glass PS, Fletcher JE, Murphy MR, Gallagher C, Quill T. 1992. Reduction of the MAC of desflurane with fentanyl. Anesthesiology 76:52–59. 10.1097/00000542-199201000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Smith JC, Bolon B. 2002. Atmospheric waste isoflurane concentrations using conventional equipment and rat anesthesia protocols. Contemp Top Lab Anim Sci 41:10–17. [PubMed] [Google Scholar]
- 43.Snyder CJ, Snyder LB. 2013. Effect of mepivacaine in an infraorbital nerve block on minimum alveolar concentration of isoflurane in clinically normal anesthetized dogs undergoing a modified form of dental dolorimetry. J Am Vet Med Assoc 242:199–204. 10.2460/javma.242.2.199. [DOI] [PubMed] [Google Scholar]
- 44.Snyder LB, Snyder CJ, Hetzel S. 2016. Effects of buprenorphine added to bupivacaine infraorbital nerve blocks on isoflurane minimum alveolar concentration using a model for acute dental/oral surgical pain in dogs. J Vet Dent 33:90–96. 10.1177/0898756416657232. [DOI] [PubMed] [Google Scholar]
- 45.Sonner JM. 2002. Issues in the design and interpretation of minimum alveolar anesthetic concentration (MAC) studies. Anesth Analg 95:609–614 [table of contents.]. [DOI] [PubMed] [Google Scholar]
- 46.Sonner JM, Gong D, Li J, Eger EI, 2nd, Laster MJ. 1999. Mouse strain modestly influences minimum alveolar anesthetic concentration and convulsivity of inhaled compounds. Anesth Analg 89:1030–1034. [DOI] [PubMed] [Google Scholar]
- 47.Tao F, Skinner J, Yang Y, Johns RA. 2010. Effect of PSD-95/SAP90 and/or PSD-93/chapsyn-110 deficiency on the minimum alveolar anesthetic concentration of halothane in mice. Anesthesiology 112:1444–1451. 10.1097/ALN.0b013e3181dcd3dc. [DOI] [PubMed] [Google Scholar]
- 48.Tarnal V, Vlisides PE, Mashour GA. 2016. The neurobiology of anesthetic emergence. J Neurosurg Anesthesiol 28:250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thiede AJ, Garcia KD, Stolarik DF, Ma J, Jenkins GJ, Nunamaker EA. 2014. Pharmacokinetics of sustained-release and transdermal buprenorphine in Göttingen minipigs (Sus scrofa domestica). J Am Assoc Lab Anim Sci 53:692–699. [PMC free article] [PubMed] [Google Scholar]
- 50.Tranquilli WJ, Thurmon JC, Grimm KA, Lumb WV. 2007. Lumb & Jones’ veterinary anesthesia and analgesia, 4th ed Ames (IA): Blackwell. [Google Scholar]
- 51.Troncy E, Junot S, Keroack S, Sammut V, Pibarot P, Genevois JP, Cuvelliez S. 2002. Results of preemptive epidural administration of morphine with or without bupivacaine in dogs and cats undergoing surgery: 265 cases (1997–1999). J Am Vet Med Assoc 221:666–672. 10.2460/javma.2002.221.666. [DOI] [PubMed] [Google Scholar]
- 52.Tsukamoto A, Iimuro M, Sato R, Yamazaki J, Inomata T. 2015. Effect of midazolam and butorphanol premedication on inhalant isoflurane anesthesia in mice. Exp Anim 64:139–145. 10.1538/expanim.14-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valverde A, Morey TE, Hernandez J, Davies W. 2003. Validation of several types of noxious stimuli for use in determining the minimum alveolar concentration for inhalation anesthetics in dogs and rabbits. Am J Vet Res 64:957–962. 10.2460/ajvr.2003.64.957. [DOI] [PubMed] [Google Scholar]
- 54.Williamson AJ, Soares JHN, Pavlisko ND, McAlister Council-Troche R, Henao-Guerrero N. 2017. Isoflurane minimum alveolar concentration sparing effects of fentanyl in the dog. Vet Anaesth Analg 44:738–745. 10.1016/j.vaa.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 55.Yassen A, Olofsen E, Kan J, Dahan A, Danhof M. 2007. Pharmacokinetic-pharmacodynamic modeling of the effectiveness and safety of buprenorphine and fentanyl in rats. Pharm Res 25:183–193. 10.1007/s11095-007-9440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zbinden AM, Maggiorini M, Petersen-Felix S, Lauber R, Thomson DA, Minder CE. 1994. Anesthetic depth defined using multiple noxious stimuli during isoflurane/oxygen anesthesia. I. Motor reactions. Anesthesiology 80:253–260. 10.1097/00000542-199402000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Zbinden AM, Petersen-Felix S, Thomson DA. 1994. Anesthetic depth defined using multiple noxious stimuli during isoflurane/oxygen anesthesia. II. Hemodynamic responses. Anesthesiology 80:261–267. 10.1097/00000542-199402000-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cardiopulmonary parameters (mean ± 1 SD) in mice during isoflurane anesthesia after pretreatment with or without subcutaneous buprenorphine