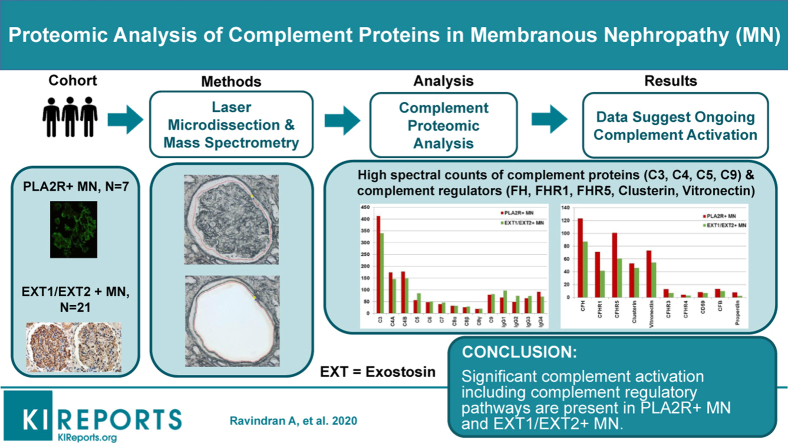
Abstract
Introduction
Membranous nephropathy (MN) is the most common cause of nephrotic syndrome in Caucasian adults. Phospholipase A2 receptor (PLA2R)– and exostosin 1 (EXT1)/exostosin 2 (EXT2)–associated MN represent the most common primary and secondary forms of MN. The complement profile using a proteomics approach has not been studied in these 2 common forms of MN.
Methods
We used laser microdissection and mass spectrometry (MS/MS) to dissect glomeruli and identify glomerular complement proteins in PLA2R-associated (n = 7), EXT1/EXT2-associated MN (n = 21), and 11 control cases (time 0 transplant biopsies).
Results
MS/MS identified high total spectral counts for PLA2R and EXT1/EXT2 in corresponding cases of PLA2R- and EXT1/EXT2-positive MN. Both PLA2R- and EXT1/EXT2-associated MN had high spectral counts of complement proteins C3, C4, C5, C6, C7, C8, and C9. Complement protein C1 was present in low spectral counts in EXT1/EXT2-associated MN. Regulators of complement activation that were detected in MN included higher spectral counts of FH, FHR-1, FHR-5, clusterin, vitronectin and lower spectral counts of FHR-3, FHR-4, and CD59. Low spectral counts of FB and properdin, key components of the alternative pathway, also were detected. IgG4 and IgG1 were the most abundant IgG subclasses in PLA2R- and EXT1/EXT2-associated MN. Lower spectral counts for C3, C4, and C5 were detected in control cases when compared with MN.
Conclusion
Significant complement activation is present in MN as evidenced by large spectral counts of complement proteins from C3- and C4-based pathways, including regulatory proteins of complement pathways. These data suggest that anticomplement drugs may be effective in treatment for MN.
Keywords: complement, laser microdissection, mass spectrometry, membranous nephropathy
Graphical abstract
See Commentary on Page 572
MN is the most common cause of nephrotic syndrome in Caucasian adults. It is characterized by deposition of immune complexes and complement proteins along the glomerular basement membrane, and depending on its underlying etiology, is classified as either primary or secondary MN.1, 2, 3 Target antigens for primary MN, which account for 75% to 80% of cases, are PLA2R (60%–70%), thrombospondin type-1 domain-containing 7A (1%–5%), and the recently described neural epidermal growth factor-like 1 protein (∼5%–10%).2, 3, 4, 5, 6, 7 Secondary MN, which accounts for 20% to 25% of MN, is associated with autoimmune diseases, malignancies, infections, and drugs.1 EXT1 and EXT2 have been recently identified as putative antigens in 30% to 40% of secondary autoimmune MN.8 Thus, PLA2R-associated and EXT1/EXT2-associated MN are the 2 largest subgroups of MN.
Although complement deposition is characteristic of MN, a comprehensive and thorough description of complement proteins in PLA2R- and EXT1/EXT2-associated MN has not been described. Routine renal biopsy evaluation is typically limited to C1q and C3c staining, which provides only limited information regarding the nature of the involvement of complement in MN and offers only limited understanding of the role of complement in the disease process. A detailed analysis of the contribution of complement in MN has the potential to inform our understanding of the role played by glomerular deposition of complement proteins and their cleavage products in both causing and amplifying injury to glomerular endothelial cells and podocytes.9, 10, 11
In this study, we sought to address this knowledge gap by identifying which complement proteins are deposited in MN, as this insight may establish whether specific complement pathways are triggered in this disease. This knowledge is relevant as numerous anticomplement drugs are being developed, and their targeted use requires a detailed understanding of specific disease processes. The aim of this study, therefore, was to complete a proteomic analysis of PLA2R- and EXT1/EXT2-associated MN using microdissection and MS/MS to define the complement proteins involved in this disease.12,13
Methods
Patient Selection
We selected a cohort of 7 cases of PLA2R-positive MN and 21 cases of EXT1/EXT2-positive cases on kidney biopsy for MS studies. Controls included 11 cases of day 0 (time 0) donor kidney biopsies before transplantation. The clinical features, biopsy findings, and MS data on detection of PLA2R and EXT1/EXT2 in MN were reported previously.8 We have subsequently performed proteomic analysis of the complement pathways in these cases. These biopsies were received in the Renal Pathology Laboratory, Department of Laboratory Medicine and Pathology, Mayo Clinic, for diagnosis and interpretation between January 2015 and May 2018. Light microscopy, immunofluorescence microscopy, including PLA2R studies, and electron microscopy were performed in each case of MN. The study was approved by the Mayo Clinic Institutional Review Board.
Statistical analyses were performed using JMP software, version 14 (SAS Institute Inc., Cary, NC). Continuous variables are reported as average (±SD and range). Parametric tests (t-test) or nonparametric tests (Wilcoxon rank-sum test) were used for statistical comparisons of the complement profile of PLA2R-associated MN versus EXT1/EXT2-associated MN, as appropriate for the test variable. Further, for comparisons within the same group (i.e., IgG1 versus IgG4 spectra in EXT1/EXT2-associated MN cohort and in PLA2R-associated MN cohort), a paired t-test, or Wilcoxon signed-rank test was used as appropriate for the test variable. Statistical significance (P value) was based on a 2-sided significance level of 0.05.
Protein Identification by Laser Capture Microdissection, Trypsin Digestion, Nano LC-Orbitrap MS/MS
For each case, 10-μm-thick formalin-fixed paraffin-embedded tissue sections were obtained and mounted on a special PEN membrane laser microdissection slide and using a Zeiss (Oberkochen, Germany) Palm Microbeam microscope, the glomeruli were microdissected to reach approximately 250–500,000 μM2 per case. Resulting formalin-fixed paraffin-embedded tissue fragments were digested with trypsin and collected for MS/MS analysis. The trypsin-digested peptides were identified by nano-flow liquid chromatography electrospray tandem MS/MS using a Thermo Scientific Q-Exactive Mass Spectrometer (Thermo Fisher Scientific, Bremen, Germany) coupled to a Thermo Ultimate 3000 RSLCnano HPLC system. All MS/MS samples were analyzed using Mascot and X! Tandem set up to search a Swissprot human database. Scaffold (version 4.8.3; Proteome Software Inc., Portland, OR) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted at greater than 95.0% probability by the Scaffold Local false discovery rate algorithm with protein identifications requiring a 2-peptide minimum and a 95% probability using Protein Prophet.14
Results
Detection of PLA2R and EXT1/EXT2 in PLA2R-Associated and EXT1/EXT2-Associated MN by MS/MS
High spectral counts of PLA2R and EXT1/EXT2 were detected in PLA2R- and EXT1/EXT2-associated MN, respectively, with average PLA2R total spectra counts of 86 (SD ± 28, median 89, range 45–134) and average EXT1 and EXT2 total spectral counts of 65 (SD ± 35, median 71, range 11–155) and 83 (SD ± 38, median 83, range 19–160). PLAR2 spectral counts in controls and EXT1/EXT2-associated MN averaged only 7 (SD ± 5, median 8, range 0–19). Both EXT1 and EXT2 were absent in controls and PLA2R-associated MN. These data have previously been reported.8
MS/MS Detection of Complement and Complement Regulatory Proteins in MN PLA2R-Associated MN
Complement Proteins
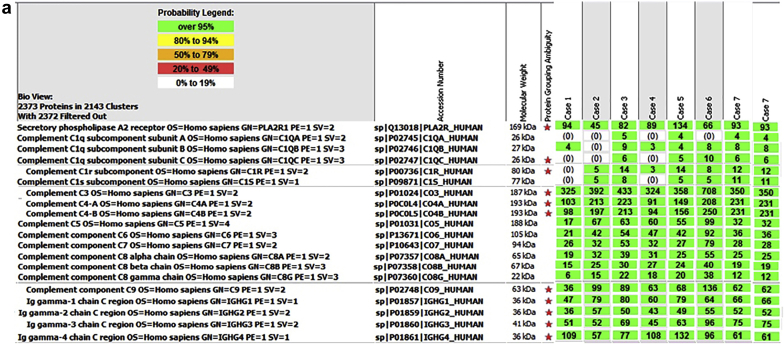
Complement component 3 (C3) was the most abundant complement protein detected in PLA2R-associated MN, with average total spectral counts of 413 (SD ± 136; range 324–708). Other abundant complement proteins included C4 (C4B average 177, SD ± 63, range 94–250; C4A 174, SD ± 59, range 91–231), C5 (average 56, SD ± 26, range 17–99), and C6–9 (C6 average 48, SD ± 22; C7 average 40, SD ± 20; C8α average 32, SD ± 12; and C9 average 79, SD ± 32) (Figure 1). C1 was absent or minimal, with average spectral counts of C1q subunit A, B, and C of 2 (SD ± 2), 5 (SD ± 3) and 4 (SD ± 4), respectively. Low spectral counts of C1r (8, SD ± 6) and C1s (5, SD ± 4) also were detected.
Figure 1.
(a) Proteomic identification of complement proteins in phospholipase A2 receptor (PLA2R)–associated membranous nephropathy (MN). Complement proteins identified in 7 cases of PLA2R-associated MN is shown. Numbers in green boxes represent spectral counts of tandem mass spectrometry (MS/MS) matches to a respective protein. Protein grouping ambiguity (red star) indicates shared amino acid sequences for certain proteins. (b) Proteomic identification of complement proteins in exostosin (EXT)1/EXT2–associated MN. Complement proteins identified in 21 cases of EXT1/EXT2-associated MN are shown. Numbers in green boxes represent spectral counts of MS/MS matches to a respective protein. Protein grouping ambiguity (red star) indicates shared amino acid sequences for certain proteins.
Complement-Regulating Proteins
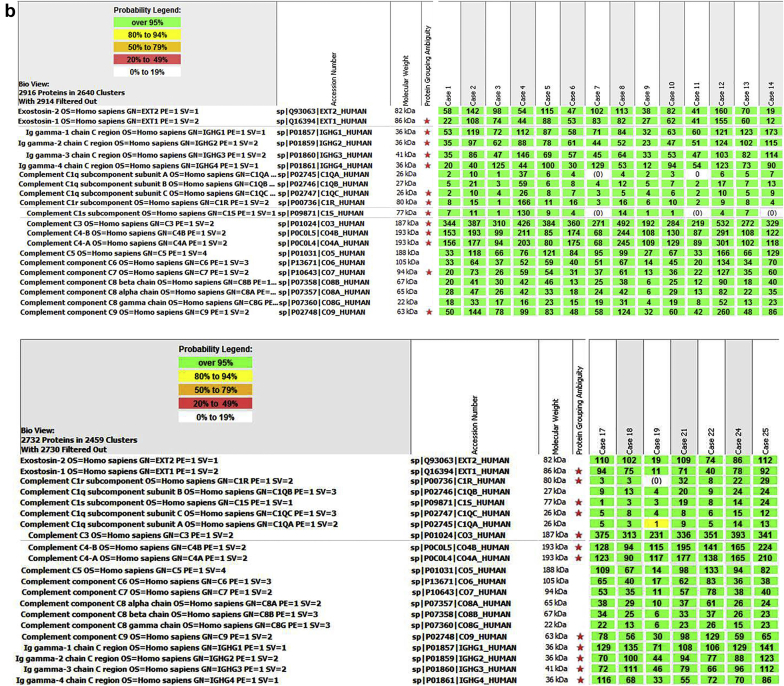
High spectral counts of complement factor H (FH; average 123, SD ± 40, range 69–189), FHR-5 (average 101, SD ± 46, range 57–197), FHR-1 (average 71, SD ± 28, range 39–119), and FHR-2 (average 38, SD ± 17, range 19–68) were detected (Figure 2). Clusterin (average 53, SD ± 12), vitronectin (average 73, SD ± 29) and CD59 (average 8, SD ± 2) were also detected. Low spectral counts of properdin (average 8, SD ± 4) and FB (average 13, SD ± 4) also were present.
Figure 2.
(a) Proteomic identification of complement-regulating proteins in in phospholipase A2 receptor (PLA2R)–associated membranous nephropathy (MN). Complement-regulating proteins identified in 7 cases of PLA2R-associated MN is shown. Numbers in green boxes represent spectral counts of tandem mass spectrometry (MS/MS) matches to a respective protein. (b) Proteomic identification of complement-regulating proteins in exostosin (EXT)1/EXT2–associated MN. Complement-regulating proteins identified in 21 cases of EXT1/EXT2-associated MN are shown. Numbers in green boxes represent spectral counts of MS/MS matches to a respective protein.
Igs
IgG4 was the most abundant Ig (average 91, SD ± 28, range 57–132, P = 0.22, compared with IgG1), followed by IgG1 (average 68, SD ± 12, range 47–80), IgG3 (average 64, SD ± 18, range 45–96), and IgG2 (average 49, SD ± 7, range 36–57).
EXT1/EXT2-Associated MN
Complement Proteins
C3 was the most abundant complement protein with average total spectral counts of 340 (SD ± 83; range 192–532), followed by C4 (C4B average 149, SD ± 59, range 68–291; C4A 146, SD ± 59, range 68–301) and C5 (average 85, SD ± 38, range 14–166) (Figure 1). C6–9 were also detected (C6 average 51, SD ± 26; C7 average 46, SD ± 26; C8α average 32, SD ± 17; and C9 average 82, SD ± 51). Low spectral counts of C1 proteins were present with average spectral counts of C1q subunits A, B, and C of 7 (SD ± 8), 13 (SD ± 13), and 8 (SD ± 5), respectively; very low spectral counts of C1r (average 18, SD ± 35) and C1s (average 12, SD ± 28) were present.
Complement-Regulating Proteins
High spectral counts of FH (average 87, SD ± 23, range 49–130), FHR-5 (average 61, SD ± 22, range 8–108), FHR-1 (average 42, SD ± 21, range 12–77), and FHR-2 (average 26, SD ± 11, range 11–52) were found (Figure 2). Clusterin (average 46, SD ± 26), vitronectin (average 55, SD ± 29), and CD59 (average 7, SD ± 2) also were detected.
Igs
All 4 subclasses of IgG were detected: IgG1 was the most abundant Ig (average 97, SD ± 36, range 32–173, P = 0.006 compared with IgG4), followed by IgG2 (average 75, SD ± 29, range 23–124), IgG3 (average 74, SD ± 30, range 33–146), and IgG4 (average 71, SD ± 35, range 12–129, P = 0.006 compared with IgG1).
Statistical Comparison of MS/MS Spectral Counts Complement Proteins and Complement-Regulating Proteins Among PLA2R Cohort Versus EXT Cohort
On comparing the MS/MS spectral counts of PLA2R-MN cohort (n = 7) versus EXT1/EXT2-associated MN cohort (n = 21), statistical significance was observed in certain complement proteins including C1qA (average PLA2R-MN: 2, SD ± 2, and average EXT1/EXT2-associated MN: 7, SD ± 8, P = 0.03), and complement-regulating proteins including CFH (average PLA2R-MN: 123, SD ± 40, and average EXT1/EXT2-associated MN: 87, SD ± 23, P = 0.03), CFHR1 (average PLA2R-MN: 71, SD ± 28, and average EXT1/EXT2-associated MN: 42, SD ± 21, P = 0.02), CFHR5 (average PLA2R-MN: 101, SD ± 46, and average EXT1/EXT2-associated MN: 61, SD ± 22, P = 0.008), properdin (average PLA2R-MN: 8, SD ± 4, and average EXT1/EXT2-associated MN: 3, SD ± 2, P = 0.005) and vitronectin (average PLA2R-MN: 73, SD ± 29, and average EXT-MN: 55, SD ± 29, P = 0.04).
Control Cases
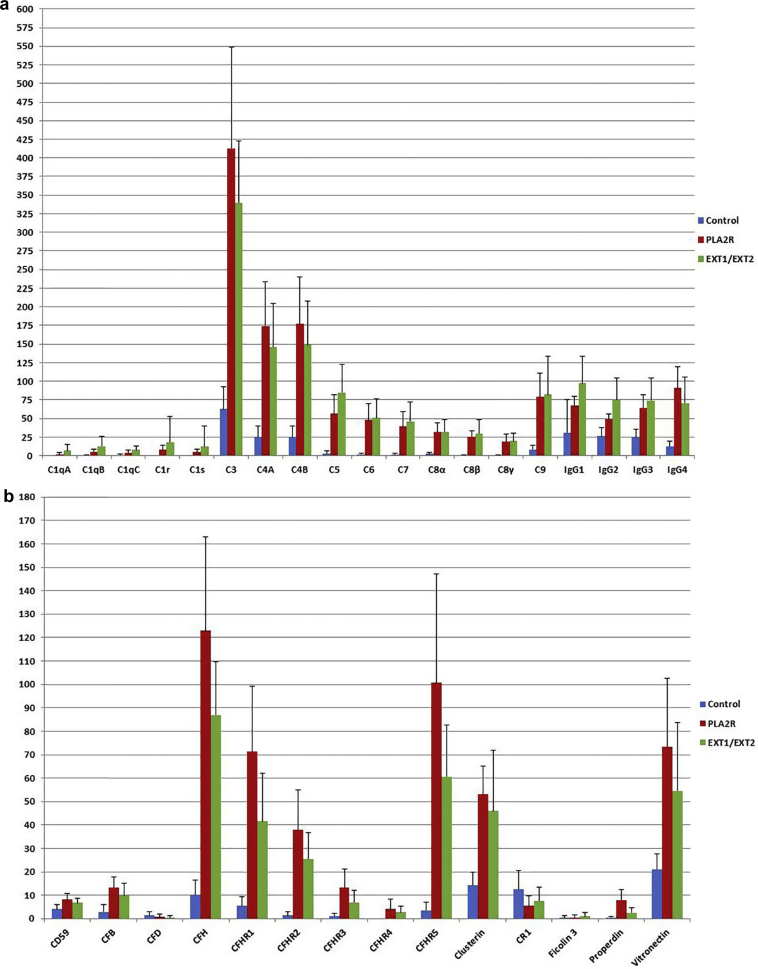
Control cases included 11 protocol (time 0) biopsies before transplantation. The average spectral counts of complement proteins for C3, C4-B, C4-A, and C5 were 63 (SD ± 30), 25 (SD ± 14), 25 (SD ± 15), and 3 (SD ± 3), respectively. The average spectral counts of complement-regulating proteins were as follows: FH (average 10, SD ± 6), FHR-5 (average 4, SD ± 3), FHR-1 (average 6, SD ± 4), vitronectin (average 21, SD ± 6), and clusterin (average 14, SD ± 6). The average IgG spectral counts were as follows: IgG1 (average 31, SD ± 44), IgG2 (average 26, SD ± 12), IgG3 (average 25, SD ± 11), and IgG4 (average 13, SD ± 7).
Discussion
MN, the most common cause of nephrotic syndrome in Caucasian adults, describes a histopathologic pattern of injury characterized by subepithelial glomerular deposition of immune complexes. These deposits activate complement to trigger glomerular basement membrane remodeling, podocyte damage with loss of slit diaphragms, and endothelial cell injury. To the best of our knowledge, the extent of complement involvement in MN has not been studied at the proteomic level. Instead, most data have come from immunostaining of MN kidney biopsies with antibodies to complement proteins like C3, C4, and C1q.
In this study, we used laser capture microdissection and MS/MS to identify and quantitate the spectrum of complement proteins in MN and to compare the proteomic profile of these proteins in PLA2R-associated and EXT1/EXT2-associated MN. We identified 1500 to 2000 glomerular proteins per case and found that complement proteins ranked among the list of proteins with the highest spectral counts, even exceeding the spectral counts of the putative antigens, PLA2R and EXT1/EXT2 and their corresponding IgG. This distribution may reflect the primacy of complement in driving the MN disease process once immune complexes appear and trigger complement activity. The finding of large amounts of complement products also may explain why the proteinuria (clinical remission) often lags behind the immunologic remission (i.e., disappearance of anti-PLA2R antibodies), because the complement proteins likely persist in the glomerular capillaries and cause continuing capillary wall injury.15,16 Another point to be considered is that at different stages of MN, the extent of complement activation and regulation may vary in an individual case, and hence the variability in the spectral counts in individual MN cases.
Complement can be activated via the classical, lectin, and alternative pathways, all of which converge on C3. Indeed, our studies show that C3 is the single most abundant complement protein in MN (Figure 2). C4 is the second most abundant complement protein. The identification of C4 indicates activation of the classical and/or lectin pathways. Large spectral counts of C5 and C9, along with moderate spectral counts of C6, C7, and C8, also were present indicating activation of the terminal pathway of complement. Interestingly, both PLA2R-associated MN and EXT1/EXT2-associated MN showed similar spectral counts with no statistical significance for C3, C4, C5, C6, C7, C8, and C9 (Table 1, Figure 3). Small spectral counts for C1q, C1r, and C1s were present in EXT1/EXT2-associated MN, which appeared higher than in PLA2R-associated MN, with statistical significance observed in spectral counts of C1qA only (average EXT1/EXT2-associated MN: 7, SD ± 8, versus average PLA2R-MN: 2, SD ± 2, P = 0.03). Control cases also showed moderate spectral counts of C3 and C4 that likely indicates modest activation of the complement pathways due to ischemia/reperfusion injury.
Table 1.
Tandem mass spectroscopy spectral counts of complement proteins, complement-regulating proteins, and Igs in membranous nephropathy
| Complement | Controls, average (±SD) n = 11 |
PLA2R, average (±SD) n = 7 |
EXT1/EXT2, average (±SD) n = 21 |
P value |
|---|---|---|---|---|
| C1qA | ND | 2 (±2) | 7 (±8) | 0.03 |
| C1qB | ND | 5 (±3) | 13 (±13) | 0.06 |
| C1qC | ND | 4 (±4) | 8 (±5) | 0.12 |
| C1r | ND | 8 (±6) | 18 (±35) | 0.59 |
| C1s | ND | 5 (±4) | 12 (±28) | 0.65 |
| C3 | 63 (±30) | 413 (±136) | 340 (±83) | 0.19 |
| C4A | 25 (±15) | 174 (±59) | 146 (±59) | 0.21 |
| C4B | 25 (±14) | 177 (±63) | 149 (±59) | 0.27 |
| C5 | 3 (±3) | 56 (±26) | 85 (±38) | 0.051 |
| C6 | 2 (±2) | 48 (±22) | 51 (±26) | 0.96 |
| C7 | 2 (±1) | 40 (±20) | 46 (±26) | 0.44 |
| C8α | 2 (±2) | 32 (±12) | 32 (±17) | 0.85 |
| C8β | ND | 26 (±8) | 30 (±18) | 0.59 |
| C8γ | ND | 19 (±10) | 20 (±10) | 0.63 |
| C9 | 9 (±5) | 79 (±32) | 82 (±51) | 0.59 |
| IgG1 | 31 (±44) | 68 (±12) | 97 (±36) | 0.06 |
| IgG2 | 26 (±12) | 49 (±7) | 75 (±29) | 0.03 |
| IgG3 | 25 (±11) | 64 (±18) | 74 (±30) | 0.49 |
| IgG4 | 13 (±7) | 91 (±28) | 71 (±35) | 0.13 |
| CD59 | 4 (±2) | 8 (±2) | 7 (±2) | 0.09 |
| CFB | 3 (±3) | 13 (±4) | 10 (±5) | 0.13 |
| CFD | ND | ND | ND | Not applicable |
| CFH | 10 (±6) | 123 (±40) | 87 (±23) | 0.03 |
| CFHR1 | 6 (±4) | 71 (±28) | 42 (±21) | 0.02 |
| CFHR2 | ND | 38 (±17) | 26 (±11) | 0.09 |
| CFHR3 | ND | 13 (±8) | 7 (±5) | 0.08 |
| CFHR4 | ND | 4 (±5) | 3 (±3) | 0.60 |
| CFHR5 | 4 (±3) | 101 (±46) | 61 (±22) | 0.008 |
| Clusterin | 14 (±6) | 53 (±12) | 46 (±26) | 0.07 |
| CR1 | 13 (±8) | 5 (±4) | 8 (±6) | 0.47 |
| Ficolin 3 | ND | ND | ND | Not applicable |
| Properdin | ND | 8 (±4) | 3 (±2) | 0.005 |
| Vitronectin | 21 (±6) | 73 (±29) | 55 (±29) | 0.04 |
EXT, exostosin; ND, none detected (spectral count of 1 or not detected); PLA2R, phospholipase A2 receptor.
Statistical significance (P value) is performed comparing PLA2R versus EXT1/EXT2-associated membranous nephropathy. The significant P values are highlighted in bold.
Figure 3.
(a) Histogram comparing the complement proteins and Igs in controls, phospholipase A2 receptor (PLA2R)–associated membranous nephropathy (MN) and exostosin (EXT)1/EXT2–associated MN. The average spectral counts (± SD, indicated by 1-sided vertical error bars) are shown on the y-axis and the complement proteins and Igs on the x-axis. (b) Histogram comparing the complement-regulating proteins in controls, PLA2R-associated MN and EXT1/EXT2-associated MN. The average spectral counts (± SD, indicated by 1-sided vertical error bars) are shown on the y-axis and the complement-regulating proteins on the x-axis.
All subtypes of IgG were detected in MN. In PLA2R-associated MN, IgG4 was dominant, although IgG1, IgG2, and IgG3 also were seen. This is in keeping with other recent studies that show similar findings.17,18 On the other hand, in EXT1/EXT2-associated MN, IgG1 was the dominant IgG subtype, although IgG2, IgG3, and IgG4 also were present. Thus, the antibody in PLA2R-associated membranous nephropathy is predominantly IgG4 and in EXT1/EXT2-associated MN it is IgG1. In vivo, immune complexes generated by either of these IgG subtypes activate complement. With IgG1-based immune complexes, the effect is potent, whereas with IgG4-based immune complexes, it is less so; however, IgG4 also fixes and activates complement after interaction with antigen.19, 20, 21, 22 It is likely that the classical pathway is initially triggered, with generation of the C3 convertase C4b2a. Cleavage of C3 by C4b2a leads to the generation of C3bBb, the C3 convertase of the alternative pathway, creating a powerful amplification loop and consistent with the large spectral counts of both C3 and C4 that were identified.
Binding of C3b to either C4b2a or C3bBb creates C4b2aC3b and C3bBbC3b, C5 convertases that trigger the terminal pathway by cleaving C5 into C5a, a powerful anaphylatoxin, and C5b, the first in a series of complement proteins required for the formation of membrane attack complex. In both PLAR2- and EXT1/EXT2-associated MN, terminal pathway activity is significant and likely an important contributor to disease.
MS/MS also showed large spectral counts of the complement-regulating proteins FH, FHR-1, FHR-2, and FHR-5, which are fluid-phase as opposed to cell-surface bound regulators (Figure 2). The MS/MS spectral counts were abundant in the PLA2R-MN compared with EXT1/EXT2-associated MN with respect to the following complement-regulating proteins: CFH (P = 0.03), CFHR1 (P = 0.02), CFHR5 (P = 0.008), properdin (P = 0.005), and vitronectin (P = 0.04), perhaps indicating a higher complement regulatory activity in PLA2R-associated MN. FH controls complement in the glomerular microenvironment and is especially important for preventing complement activity in the glycomatrix that overlies endothelial pores. FHR-1, -2, and -5 are structurally similar to FH but lack its regulatory N-terminal domain. Although their precise role in complement regulation remains to be determined, current data suggest that FHR-1, FHR-2, and FHR-5 are competitive inhibitors of FH. It is interesting to note that high spectral counts for vitronectin and clusterin and low spectral counts for CD59 were found that inhibit MAC formation or blunt its damaging effects.23 Interestingly, MS/MS did not detect mannan-binding lectin serine protease 1 or 2 (MASP-1 or MASP-2) of the lectin pathway that are responsible for cleaving C4 and C2 to form the C3 convertase.
Finally, multiple new anticomplement therapeutics are in clinical trials, and others are in the developmental pipeline. These new therapies include drugs targeting unique proteins at different levels of the classical, lectin, and alternative complement pathways, as well as complement proteins essential for mounting any type of complement response such as C3. Our data suggest that in MN, the entire complement cascade is active, with both the classical/lectin and alternative pathways driving and contributing to activation of the terminal pathway, but the preferential mode of activation will vary according to the pathogenic process (i.e., PLA2R- or EXT1/EXT2-driven processes). In the future, targeted complement pathway blockade therapies are likely to be designed that treat specific forms of MN (e.g., PLA2R- versus EXT1/EXT2-associated MN).
To summarize, large spectral counts of complement proteins and complement regulatory proteins are present in MN. The main complement proteins detected were C3, C4, C5, C6, C7, C8, and C9. The main complement-regulating proteins detected were FH, FHR5, FHR1, FHR2, FHR3, clusterin, vitronectin, and CFB. Taken together, the findings indicate on-going activation of the complement cascade and also the regulatory pathways of complement in MN.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We thank the Mayo Clinic Genome Facility-Proteomics Core (a shared resource of the Mayo Clinic Cancer Center [NCI P30 CA15083], Department of Laboratory Medicine and Pathology and the Pathology Research Core, Mayo Clinic). PR is a recipient of European Research Council ERC-2012-ADG_20120314 grant 322947 and 7th Framework Programme of the European Community contract 2012-305608 (European Consortium for High-Throughput Research in Rare Kidney Diseases), and the National Research Agency grant MNaims (ANR-17- CE17-0012-01). Research is supported in part by the US National Institutes of Health grant R01 DK110023 to RJS.
References
- 1.Couser W.G. Primary membranous nephropathy. Clin J Am Soc Nephrol. 2017;12:983–997. doi: 10.2215/CJN.11761116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ronco P., Debiec H. Pathogenesis of membranous nephropathy: recent advances and future challenges. Nat Rev Nephrol. 2012;8:203–213. doi: 10.1038/nrneph.2012.35. [DOI] [PubMed] [Google Scholar]
- 3.Ronco P., Debiec H. Pathophysiological advances in membranous nephropathy: time for a shift in patient's care. Lancet. 2015;385:1983–1992. doi: 10.1016/S0140-6736(15)60731-0. [DOI] [PubMed] [Google Scholar]
- 4.Beck L.H., Jr., Bonegio R.G., Lambeau G. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomas N.M., Beck L.H., Jr., Meyer-Schwesinger C. Thrombospondin type-1 domain-containing 7a in idiopathic membranous nephropathy. N Engl J Med. 2014;371:2277–2287. doi: 10.1056/NEJMoa1409354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomas N.M., Hoxha E., Reinicke A.T. Autoantibodies against thrombospondin type 1 domain-containing 7A induce membranous nephropathy. J Clin Invest. 2016;126:2519–2532. doi: 10.1172/JCI85265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sethi S., Debiec H., Madden B. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 2020;97:163–174. doi: 10.1016/j.kint.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Sethi S., Madden B.J., Debiec H. Exostosin 1/exostosin 2–associated membranous nephropathy. J Am Soc Nephrol. 2019;30:1123–1136. doi: 10.1681/ASN.2018080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salant D.J. Unmet challenges in membranous nephropathy. Curr Opin Nephrol Hypertens. 2019;28:70–76. doi: 10.1097/MNH.0000000000000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bomback A.S., Markowitz G.S., Appel G.B. Complement-mediated glomerular diseases: a tale of 3 pathways. Kidney Int Rep. 2016;1:148–155. doi: 10.1016/j.ekir.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma H., Sandor D.G., Beck L.H. The role of complement in membranous nephropathy. Semin Nephrol. 2013;33:531–542. doi: 10.1016/j.semnephrol.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sethi S., Gamez J.D., Vrana J.A. Glomeruli of Dense Deposit Disease contain components of the alternative and terminal complement pathway. Kidney Int. 2009;75:952–960. doi: 10.1038/ki.2008.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sethi S., Fervenza F.C., Zhang Y. C3 glomerulonephritis:clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int. 2012;82:465–473. doi: 10.1038/ki.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nesvizhskii A., Keller A., Kolker E. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 15.De Vriese A.S., Glassock R.J., Nath K.A. A Proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol. 2017;28:421–430. doi: 10.1681/ASN.2016070776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck L.H., Fervenza F.C., Beck D.M. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol. 2011;22:1543–1550. doi: 10.1681/ASN.2010111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Na W., Yi K., Song Y.S. Dissecting the relationships of IgG subclasses and complements in membranous lupus nephritis and idiopathic membranous nephropathy. PLoS One. 2017;12 doi: 10.1371/journal.pone.0174501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song Y.S., Min K.-W., Kim J.H. Differential diagnosis of lupus and primary membranous nephropathies by IgG subclass analysis. Clin J Am Soc Nephrol. 2012;7:1947–1955. doi: 10.2215/CJN.04800511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garred P., Michaelsen T., Aase A. The IgG subclass pattern of complement activation depends on epitope density and antibody and complement concentration. Scand J Immunol. 1989;30:379–382. doi: 10.1111/j.1365-3083.1989.tb01225.x. [DOI] [PubMed] [Google Scholar]
- 20.Lucisano Valim Y.M., Lachmann P.J. The effect of antibody isotype and antigenic epitope density on the complement-fixing activity of immune complexes:a systematic study using chimaeric anti-NIP antibodies with human Fc regions. Clin Exp Immunol. 1991;84:1–8. doi: 10.1111/j.1365-2249.1991.tb08115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell M.W., Reinholdt J., Kilian M. Anti-inflammatory activity of human IgA antibodies and their Fabα fragments:inhibition of IgG-mediated complement activation. Eur J Immunol. 1989;19:2243–2249. doi: 10.1002/eji.1830191210. [DOI] [PubMed] [Google Scholar]
- 22.van der Zee J.S., van Swieten P., Aalberse R.C. Inhibition of complement activation by IgG4 antibodies. Clin Exp Immunol. 1986;64:415–422. [PMC free article] [PubMed] [Google Scholar]
- 23.Rastaldi M.P., Candiano G., Musante L. Glomerular clusterin is associated with PKC-α/β regulation and good outcome of membranous glomerulonephritis in humans. Kidney Int. 2006;70:477–485. doi: 10.1038/sj.ki.5001563. [DOI] [PubMed] [Google Scholar]