Abstract
The long-distance translocation of nutrients and mobile molecules between different terminals is necessary for plant growth and development. Plasmodesmata-mediated symplastic trafficking plays an important role in accomplishing this task. To facilitate intercellular transport, plants have evolved diverse plasmodesmata with distinct internal architecture at different cell–cell interfaces along the trafficking route. Correspondingly, different underlying mechanisms for regulating plasmodesmal structures have been gradually revealed. In this review, we highlight recent studies on various plasmodesmal architectures, as well as relevant regulators of their de novo formation and transition, responsible for phloem loading, transport, and unloading specifically. We also discuss the interesting but unaddressed questions relating to, and potential studies on, the adaptation of functional plasmodesmal structures.
Keywords: Callose, phloem translocation, plasmodesmata, symplastic trafficking, unloading
A review of recent studies on plasmodesmal architecture and relevant regulators of their formation and transition involved in phloem translocation.
Introduction
Phloem-mediated translocation of photoassimilates, such as sucrose, is one of the most critical processes for plant growth and development. It includes three steps: loading, transport, and unloading. The supply end that produces and exports assimilates is termed the ‘source’, while the consumption end is referred to as the ‘sink’. From source to sink, both symplastic and apoplastic trafficking are involved, and their contributions vary in different organs/tissues and species (Comtet et al., 2017; Milne et al., 2018). In this review, we will focus on symplastic trafficking in higher plants.
Plant cells, unlike animal cells, are isolated to some extent by their surrounding cell wall. To facilitate intercellular communication, plants have formed specialized plasma membrane-lined tubes, termed plasmodesmata (PD), that connect neighboring cells to form a continuous multicellular cytoplasm known as the symplast (Faulkner, 2018). The symplast allows both local and long-distance molecular transport through the phloem (Ayre et al., 2003). To date, many molecular species are known to traffic through the PD and are translocated by the phloem, including nutrients, proteins, RNAs and hormones, indicating that they may function in expanded domains (Turgeon and Wolf, 2009; Ham and Lucas, 2017). Furthermore, symplastic trafficking is crucial and contributes to plant development and stress responses by transporting mobile signaling molecules, such as ABA and salicylic acid (Wang et al., 2013; Yadav et al., 2014; Lim et al., 2016; Otero et al., 2016; Li et al., 2018; Tylewicz et al., 2018).
Typical PD consist of the outer plasma membrane and appressed endoplasmic reticulum (ER), termed the desmotubule, along with the supporting cell wall, creating the foundations for diverse plasmodesmal structures (Tilsner et al., 2016) (Fig. 1). PD are classified as simple and branched based on their morphologies. The simple form can be transformed into the branched form during sink–source transition in leaves, accompanied by reduced conductivity for macromolecules (Oparka et al., 1999). Recently, a separate classification that differentiates types I and II PD has been developed, which is dependent on the status of the cytoplasmic sleeve between the plasma membrane and desmotubule (Nicolas et al., 2017). Type I PD lack visible cytoplasmic sleeve and internal tethers, but are surprisingly more permeable to molecules than type II PD, which possess a visible sleeve (Yan et al., 2019).
Fig. 1.
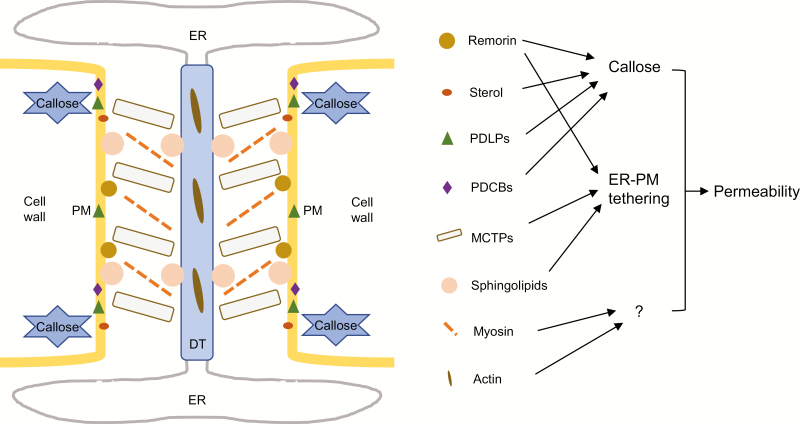
A schematic diagram showing the major localized plasmodesmal components that are involved in permeability control. These components are localized at the plasmodesma (left panel, except choline) and modulate plasmodesmal permeability by different mechanisms (right panel). Remorin, sterol, PDLPs, and PDCBs can induce callose biosynthesis and thus restrict plasmodesmal aperture. Remorin, MCTPs and sphingolipids affect the ER–plasma membrane connection and control the cytoplasmic sleeve space. Myosin and actin were previously reported, without a detailed working model, to alter the plasmodesmal permeability. DT, desmotubule; ER, endoplasmic reticulum; MCTP, multiple C2 domains and transmembrane region protein; PDCB, PD CALLOSE-BINDING PROTEIN; PDLP, PD-LOCALIZED PROTEIN; PM, plasma membrane.
Callose, an extracellular polysaccharide deposited around the PD (Fig. 1), is a well-known regulator of plasmodesmal aperture and the permeability of the corresponding cell-to-cell interface (Zavaliev et al., 2011; De Storme and Geelen, 2014; Cui and Lee, 2016). Although other factors, including reactive oxygen species (Benitez-Alfonso and Jackson, 2009), PD-localized proteins (Simpson et al., 2009; Lee et al., 2011), and sterol (Grison et al., 2015) (Fig. 1), have been reported to control plasmodesmal permeability, their downstream terminal targets are callose. A previous study found that salicylic acid signaling triggered plasmodesmal closure by altering lipid organization in a manner dependent on the protein remorin. Moreover, callose levels increased on both the apical/basal and lateral walls of salicylic acid-treated root cells (Huang et al., 2019), implying combined modulation of plasmodesmal gating. Unlike these cases, in phloem unloading modulator (plm) mutants, a reduction in plant complex sphingolipids with saturated very-long-chain fatty acids (VLCFAs) led to defective plasmodesmal ultrastructure maintenance without altering callose accumulation (Yan et al., 2019), a case that will be described in detail below. Another example of callose-independent dynamic control of plasmodesmal transport by light and the circadian clock has been recently revealed; however, the direct regulation mechanism was not uncovered (Brunkard and Zambryski, 2019).
Additionally, more novel models of plasmodesmal permeability modulation have been proposed. Mechanosensitive control indicates that the pressure forces resulting from changes in the environment disturb the equilibrium position of the ER–desmotubule complex, as well as inducing plasmodesmal closure and transport blocking (Park et al., 2019). Along with previous work on plasmodesmal ultrastructures (Nicolas et al., 2017; Brault et al., 2019), the selective role of tethers connecting ER to plasma membrane in plasmodesmal trafficking is highlighted in Fig. 1. INCREASED SIZE EXCLUSION LIMIT (ISE)1 and ISE2 are two chloroplastic DEVH-BOX RNA helicases that were found to positively control secondary complex PD formation in embryos and adult leaves via an elusive mechanism (Kobayashi et al., 2007; Burch-Smith and Zambryski, 2010), establishing a link between chloroplast and plasmodesmal function. Moreover, the cytoskeleton (Hsieh et al., 2017; Diao et al., 2018), myosin (Radford and White, 2011) (Fig. 1), and cell wall formation (Knox and Benitez-Alfonso, 2014) were all thought to potentially influence plasmodesmal permeability. However, direct evidence indicating alterations of plasmodesmal internal organization is still lacking.
In this review, we summarize the diverse forms of PD and architecture regulation at various cell-to-cell interfaces that contribute to phloem loading, transport, and unloading processes, as well as the relevant techniques for studying plasmodesmal ultrastructure. Additionally, we discuss unaddressed questions and potential approaches for future investigations on the regulatory mechanisms of plasmodesmal structures and functions.
Regulation of the conductivity of pore-plasmodesmata units that connect companion cells and sieve elements modulates phloem loading
The phloem consists of conducting cells, namely sieve elements (SEs), and their surrounding companion cells (CCs) and parenchyma (Knoblauch and Oparka, 2012; Heo et al., 2014). After asymmetric division from the same precursor cell as the CC, an SE undergoes a maturation process, similar to programmed cell death with enucleation and the loss of some organelles (Furuta et al., 2014). Eventually, only partial cellular components are retained, including plastids, mitochondria, P-proteins, and the smooth ER structure (Oparka and Turgeon, 1999). Therefore, mature SEs must rely on CCs to survive by the aid of remodeled pore-PD units (PPUs) that connect SEs and CCs to form a complex system (Lee and Frank, 2018) (Fig. 2). Each PPU consists of hundreds of branched plasmodesmal strands on the CC side and a wide pore on the SE side. This asymmetric shape satisfies the high-volume load from the CC to the SE side (Oparka and Turgeon, 1999). Two pathways are thought to be responsible for trafficking via PD: the cytoplasm and the ER/desmotubule. SEs, however, have no integral cytoplasm in which the desmotubule connecting the residual ER and the normal CC ER may provide a potential channel for flow (Blackman et al., 1998; Oparka and Cruz, 2000; Heo et al., 2017).
Fig. 2.
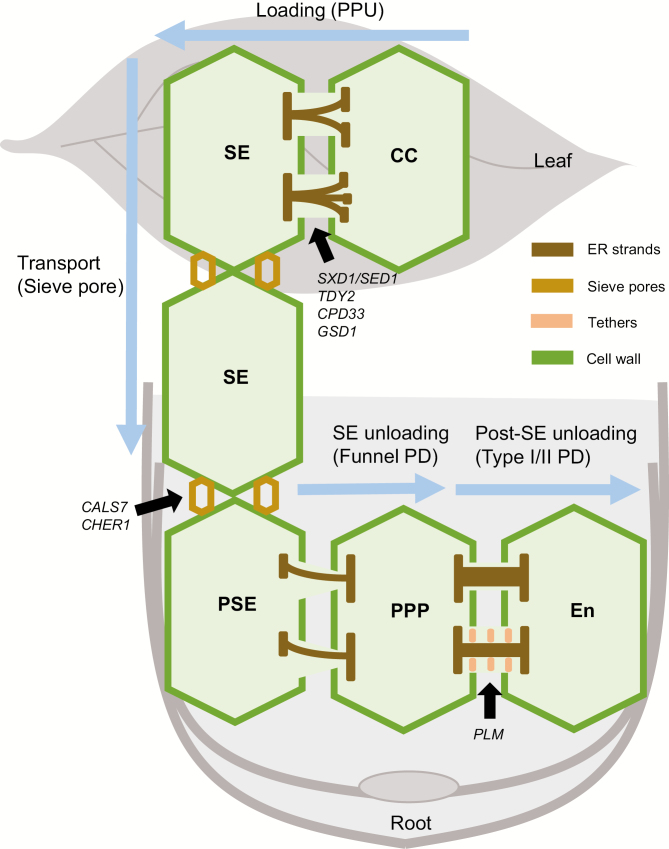
Diverse plasmodesmal forms and regulators responsible for phloem translocation. In source tissues, mobile molecules that are present in CCs diffuse through PPUs into SEs, followed by long-distance transport in SE cells that are connected by sieve pores within sieve plates. At the region of differentiated PSE, those molecules are batch-unloaded into PPP via funnel-shaped PD, and subsequently moved further into the endodermis. The post-SE unloading process through the PPP–endodermis interface can be modulated by the dynamic proportion of type I and type II PD. The key genes that play regulatory roles are listed. CC, companion cell; En, endodermis; ER, endoplasmic reticulum; PPP, phloem pole pericycle; PPU, pore-PD unit; SE, sieve element.
Another notable property of PPUs is their size exclusion limit, which is up to 70 kDa (Oparka and Turgeon, 1999) and is likely required for the loading of larger macromolecules. Thus, the reduction of plasmodesmal permeability would result in compromised phloem loading. For example, in maize, three mutants were reported to show defects in the symplastic transport of photoassimilates, including sucrose export defective1 (sxd1/sed1), tie-dyed2 (tdy2), and Carbohydrate partitioning defective33 (Cpd33). The sxd1 mutation resulted in a tocopherol deficiency and overproduced callose at the bundle sheath–vascular parenchyma interface that led to abnormal plasmodesmal morphology and prevented symplastic transport in leaf blades (Russin et al., 1996; Botha et al., 2000). Because tocopherol is an antioxidant that protects chloroplasts from photooxidative damage, callose deposition may be the consequence of enhanced oxidative stress in the source leaves of the sxd1 mutant (Hofius et al., 2004). TIE-DYED2 (TDY2), a callose synthase gene, is highly expressed in the vascular tissues of developing leaves. Loss-of-function does not affect either callose accumulation or the plasmodesmal structure and frequency but does affect sucrose movement between CCs and SEs, as well as vascular differentiation (Baker and Braun, 2008; Slewinski et al., 2012). Like TDY2, the absence of CPD33, which encodes multiple C2 domains and transmembrane region proteins (MCTPs), resulted in a reduced number of PD at the CC–SE interface, with consequently weakened sucrose export (Tran et al., 2019). In rice, overexpression of GRAIN SETTING DEFECT1 (GSD1), which encodes a putative remorin protein with specific expression in CCs, inhibited plasmodesmal permeability and the export and translocation of soluble sugars by increasing callose deposition (Gui et al., 2014).
Although the unique structures of PPUs have been discovered over the years, how these PPUs are formed during plant developmental phases has not been well studied. As phloem loading does not usually occur in sink tissues, it would be interesting to isolate the PD in the same leaf undergoing a sink–source transition and perform morphological, transcriptomic, and proteomic analyses. However, optimization of the purification method is required.
Sieve-plate pores deformed from plasmodesmata facilitate long-distance transport
As conducting cells, SEs are the most highly specialized cells in the phloem. Individual SEs are connected one by one to establish a long semi-hollow tube, with sieve pores formed in the sieve plates facilitating mass flow inside (Fig. 2). Sieve pores are differentiated from the original PD during SE maturation (Kalmbach and Helariutta, 2019). At the beginning of the transformation, callose is deposited around the edges and replaces cellulose in the primary cell wall (Esau and Thorsch, 1985). When a sufficient amount of callose has filled the whole area of the future pore, the degradation of callose commences until a larger pore of up to 1 µm is formed; callose is retained only around the pore (Esau and Cheadle, 1959; Esau and Thorsch, 1985; Mullendore et al., 2010).
Due to its essential contributions, callose has been found to be involved in the modulation of the sieve pore diameter. In mutants of callose synthase 7 (cals7), a gene with specific expression patterns in SEs, significant depletion of callose deposition was observed at the sieve plate, which led to reduced density, smaller sieve pores, and impaired root growth (Barratt et al., 2011; Xie et al., 2011). With the loss of its central components after maturation, the sieve pore is no longer a real or complete plasmodesma, and regulation of plasmodesmal permeability, depending on the membrane microdomain, is not applicable. However, callose still serves as a dynamic regulator. For instance, in response to local phloem damage, callose is instantly deposited extracellularly around the pores and constricts the sieve pore corridor. The Ca2+ concentration is the stimulus for this process (Amsbury et al., 2017). Additionally, overproduction of callose in the phloem by cals3m, a gain-of-function mutation of CALS3, inversely blocks SE transport (Vatén et al., 2011).
SE differentiation and enucleation are governed by the NAC DOMAIN CONTAINING PROTEIN (NAC) 45/86-DEPENDENT EXONUCLEASE-DOMAIN PROTEINS (NENs) pathway. The double mutant nac45/86 exhibits the formation of sieve pores but defective phloem transport and unloading (Furuta et al., 2014), suggesting the potential indirect control of sieve pore development by SE enucleation. The identification of CHOLINE TRANSPORTER-LIKE 1 (cher1) mutants provided evidence for the link between sieve pore biogenesis and lipid homeostasis, as well as the presence of PD-localized proteins (Kraner et al., 2017b). These cher1 mutants have fewer, smaller, and structurally abnormal sieve pores in their roots, resulting in impaired phloem translocation (Dettmer et al., 2014). Another study found that plasmodesmal maturation from simple to branched PD was suppressed in cher1 leaves, leading to the absence of complex PD (Kraner et al., 2017a). Thus, these two structural reconstructions may be similar to some extent and may involve common regulatory factors. However, which lipid components are responsible for the sieve pore and plasmodesmal defects has yet to be determined, as the supply of choline did not complement the cher1 mutant, and the difference in global lipid composition was not dramatic (Kraner et al., 2017b). Thus, it is highly possible that other proteins or local lipid species, apart from choline, are potential cargos of CHER1. Comparative proteomic profiling of PD in Arabidopsis wild-type Col-0 and cher1 leaves identified plasmodesmal structure-associated proteins that were already known but also some that were newly discovered (Kraner et al., 2017b). These promising candidates include C2 calcium/lipid-binding plant phosphoribosyl transferase and maternal effect embryo arrest protein (MEE9), which were both localized in the PD (Kraner et al., 2017b).
Specific funnel-plasmodesmata mediate sieve element unloading with unknown regulation mechanism
The SE unloading domain in root tips was first revealed by green fluorescent protein (GFP) fusion proteins expressed under the CC-specific AtSUC2 promoter. Unlike free GFP, all soluble GFP-fusion variants were unloaded but were restricted to a narrow zone of cells adjacent to the mature protophloem (Stadler et al., 2005). This region, which is responsible for protophloem sieve element (PSE) unloading, was ~300 µm long starting from the first differentiating protophloem cell (Ross-Elliott et al., 2017). The adjacent cells that connect PSE, called phloem pole pericycle (PPP), act as the principle contributors to SE unloading. This role was further confirmed by the blocked PSE–PPP movement after plasmodesmal closure as a result of specific induction of callose accumulation around the SE–PPP interface using the inducible cals3m system. Beyond this unloading zone toward the mature part of the root, callose was strongly accumulated at the SE cell walls, which reduced external flow by blocking the corresponding PD (Ross-Elliott et al., 2017).
Interestingly, it was found that molecular unloading does not occur at a constant rate but in distinct pulses, termed ‘batch unloading’ (Ross-Elliott et al., 2017), suggesting unusual properties of the PD in this interface. Indeed, a novel architecture, termed ‘funnel PD’, was observed solely at the PSE–PPP interface (Ross-Elliott et al., 2017). Asymmetric funnel-shaped PD exhibit larger apertures toward the PSE entrance but narrow toward the PPP side, enabling bulk flow for unloading with low pressure (Fig. 2). Additionally, the relative distribution and total number of PD between the PSE–PPP interface are both higher than that at the PSE–CC and PSE–metaphloem interfaces in the unloading zone. However, ~10% of normal simple PD were found at the PSE–PPP junction (Ross-Elliott et al., 2017). Due to the small proportion, their contributions to SE unloading may be minor.
Until recently, the formation and regulation of funnel-PD development were elusive in the absence of identification or characterization of relevant mutants. It may be worth investigating the genes that affect plasmodesmal complexity, such as CHER1, which controls the formation of complex PD. Due to the universal role of callose in plasmodesmal gate control, the absence of CALS may functionally impair funnel PD. Among the 12 CALS members in Arabidopsis, CALS7 (Xie et al., 2011) and CALS8 (Yan et al., 2019) exhibit specific expression patterns in SEs and PPP, respectively, and may be excellent candidates.
Sphingolipids maintain the plasmodesmal ultrastructure specifically for post-sieve element unloading
The PPP acts as a repository for unloaded macromolecules and is a potential filter in root tip cells for molecules that are subsequently transported beyond the PPP to their destination. Trafficking from PPP to its neighboring cell layer, the endodermis, is termed ‘post-SE unloading’ (Patrick, 1997; Yan et al., 2019) (Fig. 2). While the SE–PPP interface is designed to promote SE unloading, the PPP–endodermis interface is the inverse bottleneck for the post-SE unloading process. All simple, branched, type I and type II PD, including a few intermediates with no clear spokes and partial detachment between the ER and plasma membrane, were observed at the PPP–endodermis interface (Yan et al., 2019). Unlike the equal percentages of type I and type II, simple PD are more numerous than branched PD, which is consistent with the observation that simple PD are more permeable. Interestingly, our previous study found that the plm mutant lacked the typical type II PD. This absence of type II PD, however, is specific to the PPP–endodermis interface, but not the SE–PPP interface, in the roots (Yan et al., 2019), which indicates a unique functional property of PLM for post-SE unloading. This information, therefore, reinforces the distinguishable difference between the SE and post-phloem transport.
Consistent with the near loss of the sleeve, no significant differences were detected between the width of simple and branched PD in the plm mutant. Additionally, the ratio of simple and branched PD was similar to that in the wild-type (Yan et al., 2019), implying potential independent mechanisms for the modulation of these two morphological transformations.
Type I PD were enriched in young tissues with newly divided cell walls, while type II PD were more numerous in relatively older tissues. Unexpectedly, the extreme narrow sleeve of type I PD enabled also small molecule diffusion and macromolecule trafficking (Nicolas et al., 2017). More surprisingly, the predominant presentation of type I PD at the PPP–endodermis interface increased its conductivity, leading to enhanced post-phloem unloading and earlier root elongation (Yan et al., 2019). These findings challenge the previous model, which assumes that the ER–plasma membrane gap is the space for the transport stream (Epel, 1994; Kragler et al., 1998), suggesting the need to reconsider the accessible route through the PD. A supporting case is the observation of fluorescent probe trafficking between cells via the desmotubule in tobacco and Torenia (Cantrill et al., 1999). Recently, MCTPs were found to act as ER–plasma membrane tethers of the PD (Brault et al., 2019) (Fig. 1), which may regulate the ER–plasma membrane contacts within the PD. This work uncovers part of the mysterious internal structure of the PD and directs us to reconsider the role of these tethers in internal molecular transport. Specifically, is this role positive, negative, or both? Future investigations on the diverse selective and non-selective trafficking routes within the plasmodesmal channel and plasmodesmal structural modification would allow us to understand the evolution and adaptation of the PD in changing developmental contexts.
Characterization of the PLM gene revealed a mechanism of specific membrane components for maintaining the plasmodesmal structure and function with no effect on plasmodesmal density and callose deposition. PLM encodes a novel protein involved in the biosynthesis pathways of VLCFA-containing sphingolipids, especially ceramides and glycosyl inositol phosphoryl ceramides (GIPCs) (Yan et al., 2019). Based on the enrichment of GIPCs in plasmodesmal microdomains (Grison et al., 2015) (Fig. 1), we speculate that this deficiency may influence the maintenance of the plasmodesmal architecture to some extent. However, a link between ceramides and PD has been introduced. Their interaction may be either direct (i.e. regulation of plasmodesmal functions by ceramides) or indirect (i.e. ceramides act as the backbone for the GIPCs or as signal molecules for plasmodesmal modification). It has been demonstrated that inhibition of ceramide synthesis affects the endomembrane ultrastructure and that VLCFA-sphingolipids are involved in the molecular secretory pathway (Markham et al., 2011). Therefore, various loss-of-function mutants of sphingolipid biosynthesis genes are excellent tools for further clarifying the relationship between plant sphingolipids and plasmodesmal structural organization.
Updated approaches for observation of plasmodesmal structure
As nanoscale channels (diameter of 20–50 nm) embedded in the cell walls between two adjacent cells, PD are difficult to observe by conventional light microscopy. Therefore, plasmodesmal research is highly dependent on technical developments. Electron microscopy (EM) was first developed in 1931 and became a common technique in the 1950s. Due to the shorter wavelength of electrons than photons, EM provides images at much higher resolution, up to 1 nm. This advancement enhanced the study of plasmodesmal ultrastructure and laid the foundation for current models of plasmodesmal architecture (Roberts, 2018). However, one problem of this method is that the chemical fixation or embedding procedure may cause structural artifacts due to poor sample penetration and slow cellular matrix crosslinking (Gilkey and Staehelin, 1986). With the development of cryo-EM, which uses rapid low-temperature fixation and high-pressure freezing, the cellular architecture was preserved, and the native plasmodesmal structure was revealed (McIntosh et al., 2005).
However, as conventional EM is two-dimensional, information on the whole plasmodesmal internal architecture is still missing. Recently, a new electron tomography method has provided excellent images displaying the three-dimensional organization of PD. Technological advancements include the acquisition of tilt series of longitudinal views ranging from −70° to +70° at high magnifications and subsequent tomogram reconstruction, combination, and image segmentation (Nicolas et al., 2017). By this method, two types of PD were clearly presented with evident differences (described above).
Additionally, field emission scanning electron microscopy (FESEM) can be used to image PD while they are exposed on the sample surface. Thus, cryo-FESEM, which uses similar pre-preparation methods as cryo-EM, has been exploited to reveal the plasmodesmal details within the cell wall (Vesk et al., 2000). An example of this feat is the remarkably clear images of sieve plates and sieve pores acquired from a range of species obtained by Faulkner et al. (2008) and Mullendore et al. (2010).
Meanwhile, new advances in fluorescence imaging using confocal laser scanning microscopy have been widely used for fluorescent molecules and structures. However, due to the diffraction limit of light (~200 nm) and insufficient labeling markers for the plasmodesmal microdomain and its components, the capacity for resolving fine details of plasmodesmal ultrastructure with high resolution is unsatisfactory. Improvements in super-resolution imaging surpassed the diffraction barrier and bridged the gap between fluorescence and electron microscopy (Huang et al., 2009; Lippincott-Schwartz and Patterson, 2009; Huang, 2010). Three-dimensional structured illumination microscopy has been used to examine the PD by imaging antibody-labeled callose and the VIRAL MOVEMENT PROTEIN (MP) of the tobacco mosaic virus fused to GFP, which interacts with the PD (Fitzgibbon et al., 2010). The simple PD in epidermal cells and central cavities of the complex PD were previously revealed at 100 nm resolution (Roberts and Oparka, 2003; Faulkner et al., 2008). Other than imaging of the molecular complex, observational technology extends to the single molecule level. Internal reflection fluorescence microscopy, stimulated emission depletion, photoactivation localization microscopy, and stochastic optical reconstruction microscopy are examples that may have the potential to observe the PD at the single molecule level (Klar and Hell, 1999; Komis et al., 2015; Schubert, 2017; Paes et al., 2018). However, optimization of the procedures may be necessary when utilized for plasmodesmal imaging, as the plasmodesmal ultrastructure has not yet been revealed by those methods.
Perspectives
Various plasmodesmal structural forms have been found at different cell–cell interfaces responsible for phloem translocation, including PPUs for phloem loading, large sieve pores for long-distance transport, and funnel PD for unloading. This diverse set of plasmodesmal architectures strongly implies the dynamic function and adaptive ability of PD spatially and temporally. However, several questions remain. Specifically, how do those various forms of PD develop? And, how are the transitions between various architectures regulated? Moreover, other tissues/organs (e.g. fruits) and species (e.g. crops and trees) than Arabidopsis roots have not been investigated, which is likely due to the difficult and time-consuming techniques. It is not known yet if the principal mediation role of PPP for phloem unloading is universal. Due to the developmental variation of vascular tissues, we speculate that more novel plasmodesmal architectures facilitating the adaptation to complex contexts will be discovered. Comparison among plant species would also be helpful for understanding the coevolution between the PD and plants.
The diverse and specific plasmodesmal structures indicate the potential diverse underlying regulatory mechanisms. Concerning the plasmodesmal pore gating system, callose deposition dominated for a long time, until the revelation of the regulation by lipid homeostasis within the plasmodesmal microdomain. Indeed, plasmodesmal structure and permeability are modulated by common factors, such as callose, and also by other unique components. For example, the impact on type I and type II plasmodesmal architectural transition by the plm mutation was discovered at the PPP–endodermis interface, but not the SE–PPP interface, which are two successive steps for unloading (Yan et al., 2019). Thus, we believe more unknown regulators will be discovered as direct evidence is revealed. Furthermore, by adjusting the regulation of the plasmodesmal architecture, it is possible to modulate plant development or other biological processes for agricultural production by directing nutrient allocation within plants. Take PLM as an instance: we may be able to change the proportion of plasmodesmal types at specific cell–cell interfaces by altering the sphingolipid levels to modulate symplastic trafficking, enhancing either nutrient unloading for growth promotion or signal molecule movement for stress responses.
Acknowledgements
We thank Sofia Otero and Bo Xu for constructive comments on the manuscript. This work was supported by the Scientific Research Foundation for Advanced Talents of Henan University.
References
- Amsbury S, Kirk P, Benitez-Alfonso Y. 2017. Emerging models on the regulation of intercellular transport by plasmodesmata-associated callose. Journal of Experimental Botany 69, 105–115. [DOI] [PubMed] [Google Scholar]
- Ayre BG, Keller F, Turgeon R. 2003. Symplastic continuity between companion cells and the translocation stream: long-distance transport is controlled by retention and retrieval mechanisms in the phloem. Plant Physiology 131, 1518–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RF, Braun DM. 2008. Tie-dyed2 functions with Tie-dyed1 to promote carbohydrate export from maize leaves. Plant Physiology 146, 1085–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt DH, Kölling K, Graf A, Pike M, Calder G, Findlay K, Zeeman SC, Smith AM. 2011. Callose synthase GSL7 is necessary for normal phloem transport and inflorescence growth in Arabidopsis. Plant Physiology 155, 328–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Alfonso Y, Jackson D. 2009. Redox homeostasis regulates plasmodesmal communication in Arabidopsis meristems. Plant Signaling & Behavior 4, 655–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman LM, Boevink P, Cruz SS, Palukaitis P, Oparka KJ. 1998. The movement protein of cucumber mosaic virus traffics into sieve elements in minor veins of Nicotiana clevelandii. The Plant Cell 10, 525–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha C, Cross R, Van Bel A, Peter C. 2000. Phloem loading in the sucrose-export-defective (SXD-1) mutant maize is limited by callose deposition at plasmodesmata in bundle sheath–vascular parenchyma interface. Protoplasma 214, 65–72. [Google Scholar]
- Brault ML, Petit JD, Immel F, et al. . 2019. Multiple C2 domains and transmembrane region proteins (MCTPs) tether membranes at plasmodesmata. EMBO Reports 20, e47182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkard JO, Zambryski P. 2019. Plant cell-cell transport via plasmodesmata is regulated by light and the circadian clock. Plant Physiology 181, 1459–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch-Smith TM, Zambryski PC. 2010. Loss of Increased Size Exclusion Limit (ISE)1 or ISE2 increases the formation of secondary plasmodesmata. Current Biology 20, 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrill LC, Overall RL, Goodwin PB. 1999. Cell-to-cell communication via plant endomembranes. Cell Biology International 23, 653–661. [DOI] [PubMed] [Google Scholar]
- Comtet J, Turgeon R, Stroock AD. 2017. Phloem loading through plasmodesmata: a biophysical analysis. Plant Physiology 175, 904–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Lee JY. 2016. Arabidopsis callose synthases CalS1/8 regulate plasmodesmal permeability during stress. Nature Plants 2, 16034. [DOI] [PubMed] [Google Scholar]
- De Storme N, Geelen D. 2014. Callose homeostasis at plasmodesmata: molecular regulators and developmental relevance. Frontiers in Plant Science 5, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J, Ursache R, Campilho A, et al. . 2014. Choline transporter-like1 is required for sieve plate development to mediate long-distance cell-to-cell communication. Nature Communications 5, 4276. [DOI] [PubMed] [Google Scholar]
- Diao M, Ren SL, Wang QN, Qian LC, Shen JF, Liu YL, Huang SJ. 2018. Arabidopsis formin 2 regulates cell-to-cell trafficking by capping and stabilizing actin filaments at plasmodesmata. eLife 7, e36316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel BL. 1994. Plasmodesmata: composition, structure and trafficking. Plant Molecular Biology 26, 1343–1356. [DOI] [PubMed] [Google Scholar]
- Esau K, Cheadle VI. 1959. Size of pores and their contents in sieve elements of dicotyledons. Proceedings of the National Academy of Sciences, USA 45, 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau K, Thorsch J. 1985. Sieve plate pores and plasmodesmata, the communication channels of the symplast: ultrastructural aspects and developmental relations. American Journal of Botany 72, 1641–1653. [Google Scholar]
- Faulkner C. 2018. Plasmodesmata and the symplast. Current Biology 28, R1374–R1378. [DOI] [PubMed] [Google Scholar]
- Faulkner C, Akman OE, Bell K, Jeffree C, Oparka K. 2008. Peeking into pit fields: a multiple twinning model of secondary plasmodesmata formation in tobacco. The Plant Cell 20, 1504–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbon J, Bell K, King E, Oparka K. 2010. Super-resolution imaging of plasmodesmata using three-dimensional structured illumination microscopy. Plant Physiology 153, 1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta KM, Yadav SR, Lehesranta S, et al. . 2014. Arabidopsis NAC45/86 direct sieve element morphogenesis culminating in enucleation. Science 345, 933–937. [DOI] [PubMed] [Google Scholar]
- Gilkey JC, Staehelin LA. 1986. Advances in ultrarapid freezing for the preservation of cellular ultrastructure. Journal of Electron Microscopy Technique 3, 177–210. [Google Scholar]
- Grison MS, Brocard L, Fouillen L, et al. . 2015. Specific membrane lipid composition is important for plasmodesmata function in Arabidopsis. The Plant Cell 27, 1228–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui J, Liu C, Shen J, Li L. 2014. Grain setting defect1, encoding a remorin protein, affects the grain setting in rice through regulating plasmodesmatal conductance. Plant Physiology 166, 1463–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham BK, Lucas WJ. 2017. Phloem-mobile RNAs as systemic signaling agents. Annual Review of Plant Biology 68, 173–195. [DOI] [PubMed] [Google Scholar]
- Heo JO, Blob B, Helariutta Y. 2017. Differentiation of conductive cells: a matter of life and death. Current Opinion in Plant Biology 35, 23–29. [DOI] [PubMed] [Google Scholar]
- Heo JO, Roszak P, Furuta KM, Helariutta Y. 2014. Phloem development: current knowledge and future perspectives. American Journal of Botany 101, 1393–1402. [DOI] [PubMed] [Google Scholar]
- Hofius D, Hajirezaei MR, Geiger M, Tschiersch H, Melzer M, Sonnewald U. 2004. RNAi-mediated tocopherol deficiency impairs photoassimilate export in transgenic potato plants. Plant Physiology 135, 1256–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh TS, Chen YJ, Chang CL, Lee WR, Liou J. 2017. Cortical actin contributes to spatial organization of ER-PM junctions. Molecular Biology of the Cell 28, 3171–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B. 2010. Super-resolution optical microscopy: multiple choices. Current Opinion in Chemical Biology 14, 10–14. [DOI] [PubMed] [Google Scholar]
- Huang B, Bates M, Zhuang X. 2009. Super-resolution fluorescence microscopy. Annual Review of Biochemistry 78, 993–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Sun Y, Ma Z, et al. . 2019. Salicylic acid-mediated plasmodesmal closure via Remorin-dependent lipid organization. Proceedings of the National Academy of Sciences, USA 116, 21274–21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach L, Helariutta Y. 2019. Sieve plate pores in the phloem and the unknowns of their formation. Plants 8, E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar TA, Hell SW. 1999. Subdiffraction resolution in far-field fluorescence microscopy. Optics Letters 24, 954–956. [DOI] [PubMed] [Google Scholar]
- Knoblauch M, Oparka K. 2012. The structure of the phloem – still more questions than answers. The Plant Journal 70, 147–156. [DOI] [PubMed] [Google Scholar]
- Knox JP, Benitez-Alfonso Y. 2014. Roles and regulation of plant cell walls surrounding plasmodesmata. Current Opinion in Plant Biology 22, 93–100. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Otegui MS, Krishnakumar S, Mindrinos M, Zambryski P. 2007. Increased size exclusion limit 2 encodes a putative DEVH box RNA helicase involved in plasmodesmata function during Arabidopsis embryogenesis. The Plant Cell 19, 1885–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komis G, Šamajová O, Ovečka M, Šamaj J. 2015. Super-resolution microscopy in plant cell imaging. Trends in Plant Science 20, 834–843. [DOI] [PubMed] [Google Scholar]
- Kragler F, Lucas WJ, Monzer J. 1998. Plasmodesmata: dynamics, domains and patterning. Annals of Botany 81, 1–10. [Google Scholar]
- Kraner ME, Link K, Melzer M, Ekici AB, Uebe S, Tarazona P, Feussner I, Hofmann J, Sonnewald U. 2017a Choline transporter-like1 (CHER1) is crucial for plasmodesmata maturation in Arabidopsis thaliana. The Plant Journal 89, 394–406. [DOI] [PubMed] [Google Scholar]
- Kraner ME, Müller C, Sonnewald U. 2017b Comparative proteomic profiling of the choline transporter-like1 (CHER1) mutant provides insights into plasmodesmata composition of fully developed Arabidopsis thaliana leaves. The Plant Journal 92, 696–709. [DOI] [PubMed] [Google Scholar]
- Lee JY, Frank M. 2018. Plasmodesmata in phloem: different gateways for different cargoes. Current Opinion in Plant Biology 43, 119–124. [DOI] [PubMed] [Google Scholar]
- Lee JY, Wang X, Cui W, et al. . 2011. A plasmodesmata-localized protein mediates crosstalk between cell-to-cell communication and innate immunity in Arabidopsis. The Plant Cell 23, 3353–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, de Ollas C, Dodd IC. 2018. Long-distance ABA transport can mediate distal tissue responses by affecting local ABA concentrations. Journal of Integrative Plant Biology 60, 16–33. [DOI] [PubMed] [Google Scholar]
- Lim GH, Kachroo A, Kachroo P. 2016. Role of plasmodesmata and plasmodesmata localizing proteins in systemic immunity. Plant Signaling & Behavior 11, e1219829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Patterson GH. 2009. Photoactivatable fluorescent proteins for diffraction-limited and super-resolution imaging. Trends in Cell Biology 19, 555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JE, Molino D, Gissot L, Bellec Y, Hématy K, Marion J, Belcram K, Palauqui JC, Satiat-Jeunemaître B, Faure JD. 2011. Sphingolipids containing very-long-chain fatty acids define a secretory pathway for specific polar plasma membrane protein targeting in Arabidopsis. The Plant Cell 23, 2362–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh R, Nicastro D, Mastronarde D. 2005. New views of cells in 3D: an introduction to electron tomography. Trends in Cell Biology 15, 43–51. [DOI] [PubMed] [Google Scholar]
- Milne RJ, Grof CP, Patrick JW. 2018. Mechanisms of phloem unloading: shaped by cellular pathways, their conductances and sink function. Current Opinion in Plant Biology 43, 8–15. [DOI] [PubMed] [Google Scholar]
- Mullendore DL, Windt CW, Van As H, Knoblauch M. 2010. Sieve tube geometry in relation to phloem flow. The Plant Cell 22, 579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas WJ, Grison MS, Trépout S, Gaston A, Fouché M, Cordelières FP, Oparka K, Tilsner J, Brocard L, Bayer EM. 2017. Architecture and permeability of post-cytokinesis plasmodesmata lacking cytoplasmic sleeves. Nature Plants 3, 17082. [DOI] [PubMed] [Google Scholar]
- Oparka KJ, Cruz SS. 2000. The great escape: phloem transport and unloading of macromolecules. Annual Review of Plant Physiology and Plant Molecular Biology 51, 323–347. [DOI] [PubMed] [Google Scholar]
- Oparka KJ, Roberts AG, Boevink P, Santa Cruz S, Roberts I, Pradel KS, Imlau A, Kotlizky G, Sauer N, Epel B. 1999. Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell 97, 743–754. [DOI] [PubMed] [Google Scholar]
- Oparka KJ, Turgeon R. 1999. Sieve elements and companion cells—traffic control centers of the phloem. The Plant Cell 11, 739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero S, Helariutta Y, Benitez-Alfonso Y. 2016. Symplastic communication in organ formation and tissue patterning. Current Opinion in Plant Biology 29, 21–28. [DOI] [PubMed] [Google Scholar]
- Paes G, Habrant A, Terryn C. 2018. Fluorescent nano-probes to image plant cell walls by super-resolution STED microscopy. Plants 7, E11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Knoblauch J, Oparka K, Jensen KH. 2019. Controlling intercellular flow through mechanosensitive plasmodesmata nanopores. Nature Communications 10, 3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick JW. 1997. Phloem unloading: sieve element unloading and post-sieve element transport. Annual Review of Plant Physiology and Plant Molecular Biology 48, 191–222. [DOI] [PubMed] [Google Scholar]
- Radford JE, White RG. 2011. Inhibitors of myosin, but not actin, alter transport through Tradescantia plasmodesmata. Protoplasma 248, 205–216. [DOI] [PubMed] [Google Scholar]
- Roberts AG. 2018. Plasmodesmal structure and development. Annual Plant Reviews 18, doi: 10.1002/9781119312994.apr0173. [DOI] [Google Scholar]
- Roberts AG, Oparka KJ. 2003. Plasmodesmata and the control of symplastic transport. Plant, Cell & Environment 26, 103–124. [Google Scholar]
- Ross-Elliott TJ, Jensen KH, Haaning KS, et al. . 2017. Phloem unloading in Arabidopsis roots is convective and regulated by the phloem-pole pericycle. eLife 6, e24125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russin WA, Evert RF, Vanderveer PJ, Sharkey TD, Briggs SP. 1996. Modification of a specific class of plasmodesmata and loss of sucrose export ability in the sucrose export defective1 maize mutant. The Plant Cell 8, 645–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert V. 2017. Super-resolution microscopy—applications in plant cell research. Frontiers in Plant Science 8, 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson C, Thomas C, Findlay K, Bayer E, Maule AJ. 2009. An Arabidopsis GPI-anchor plasmodesmal neck protein with callose binding activity and potential to regulate cell-to-cell trafficking. The Plant Cell 21, 581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slewinski TL, Baker RF, Stubert A, Braun DM. 2012. Tie-dyed2 encodes a callose synthase that functions in vein development and affects symplastic trafficking within the phloem of maize leaves. Plant Physiology 160, 1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R, Wright KM, Lauterbach C, Amon G, Gahrtz M, Feuerstein A, Oparka KJ, Sauer N. 2005. Expression of GFP-fusions in Arabidopsis companion cells reveals non-specific protein trafficking into sieve elements and identifies a novel post-phloem domain in roots. The Plant Journal 41, 319–331. [DOI] [PubMed] [Google Scholar]
- Tilsner J, Nicolas W, Rosado A, Bayer EM. 2016. Staying tight: plasmodesmal membrane contact sites and the control of cell-to-cell connectivity in plants. Annual Review of Plant Biology 67, 337–364. [DOI] [PubMed] [Google Scholar]
- Tran TM, McCubbin TJ, Bihmidine S, et al. . 2019. Maize Carbohydrate Partitioning Defective33 encodes an MCTP protein and functions in sucrose export from leaves. Molecular Plant 12, 1278–1293. [DOI] [PubMed] [Google Scholar]
- Turgeon R, Wolf S. 2009. Phloem transport: cellular pathways and molecular trafficking. Annual Review of Plant Biology 60, 207–221. [DOI] [PubMed] [Google Scholar]
- Tylewicz S, Petterle A, Marttila S, et al. . 2018. Photoperiodic control of seasonal growth is mediated by ABA acting on cell-cell communication. Science 360, 212–215. [DOI] [PubMed] [Google Scholar]
- Vatén A, Dettmer J, Wu S, et al. . 2011. Callose biosynthesis regulates symplastic trafficking during root development. Developmental Cell 21, 1144–1155. [DOI] [PubMed] [Google Scholar]
- Vesk M, Dibbayawan TP, Vesk PA, Egan EA. 2000. Field emission scanning electron microscopy of plant cells. Protoplasma 210, 138–155. [Google Scholar]
- Wang X, Sager R, Cui W, Zhang C, Lu H, Lee JY. 2013. Salicylic acid regulates plasmodesmata closure during innate immune responses in Arabidopsis. The Plant Cell 25, 2315–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie B, Wang X, Zhu M, Zhang Z, Hong Z. 2011. CalS7 encodes a callose synthase responsible for callose deposition in the phloem. The Plant Journal 65, 1–14. [DOI] [PubMed] [Google Scholar]
- Yadav SR, Yan D, Sevilem I, Helariutta Y. 2014. Plasmodesmata-mediated intercellular signaling during plant growth and development. Frontiers in Plant Science 5, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Yadav SR, Paterlini A, et al. . 2019. Sphingolipid biosynthesis modulates plasmodesmal ultrastructure and phloem unloading. Nature Plants 5, 604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavaliev R, Ueki S, Epel BL, Citovsky V. 2011. Biology of callose (β-1,3-glucan) turnover at plasmodesmata. Protoplasma 248, 117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]