At high O2 tensions, as encountered prehistorically, Chlamydomonas reinhardtii elevated levels of NPQ-related proteins and LHCSR3 had an important function in protecting PSI.
Keywords: Carboniferous, electrophile, evolution, non-photochemical quenching, photoinhibition, photosynthesis, reactive oxygen species, stress, qE
Abstract
Non-photochemical quenching (NPQ) helps dissipate surplus light energy, preventing formation of reactive oxygen species (ROS). In Chlamydomonas reinhardtii, the thylakoid membrane protein LHCSR3 is involved in pH-dependent (qE-type) NPQ, lacking in the npq4 mutant. Preventing PSII repair revealed that npq4 lost PSII activity faster than the wild type (WT) in elevated O2, while no difference between strains was observed in O2-depleted conditions. Low Fv/Fm values remained 1.5 h after moving cells out of high light, and this qH-type quenching was independent of LHCSR3 and not accompanied by losses of maximum PSII activity. Culturing cells in historic O2 atmospheres (30–35%) increased the qE of cells, due to increased LHCSR1 and PsbS levels, and LHCSR3 in the WT, showing that atmospheric O2 tensions regulate qE capacity. Colony growth of npq4 was severely restricted at elevated O2, and npq4 accumulated more reactive electrophile species (RES) than the WT, which could damage PSI. Levels of PsaA (PSI) were lower in npq4 grown at 35% O2, while PsbA (PSII) levels remained stable. We conclude that even at high O2 concentrations, the PSII repair cycle is sufficient to maintain net levels of PSII. However, LHCSR3 has an important function in protecting PSI against O2-mediated damage, such as via RES.
Introduction
Molecular oxygen is a photosynthetic by-product from the water-splitting activity of PSII. Oxygen started accumulating in the atmosphere 2.4 billion years ago (bya) due to photosynthesis (Lyons, 2014), which enabled the evolution of oxidative phosphorylation, contributing to the evolution of sex and multicellular life (Hörandl and Speijer, 2018). Today, sunlight drives almost all primary productivity on this planet, and PSII is considered as ‘the engine of life’ (Barber, 2003). However, O2 also forms unstable radical and non-radical reactive oxygen species (ROS), and the initial accumulation of O2 is thought to have caused the first major extinction event of our planet (Lane, 2002). For plants, photosynthesis is a major source of ROS that have to be dealt with (Halliwell, 2006), especially in response to increasing light intensity (Roach et al., 2015a).
Singlet oxygen (1O2) is a highly destructive ROS produced by PSII (Krieger-Liszkay, 2005; Fischer et al., 2013; Telfer, 2014). In higher plants, very high levels of 1O2 activate programmed cell death (Op den Camp et al., 2003). At lower and more typical physiological levels, 1O2 peroxidizes membrane lipids, which break down and release aldehydes that include reactive electrophile species (RES), such as acrolein (Fischer et al., 2012; Mano, 2012; Roach et al., 2017; Yalcinkaya et al., 2019). Several studies have shown that ROS and RES contribute directly to PSII damage (Hideg et al., 1994; Chan et al., 2012; Roach et al., 2013; Kale et al., 2017), while other studies have shown that ROS inhibit the repair of the PSII reaction centres (Nishiyama et al., 2001; Murata et al., 2007). PSI is much more stable than PSII. Photoinhibition of PSI has only been reported for certain chilling-sensitive species and under fluctuating light for mutants affected in cyclic electron transport (e.g. pgr5, pgrl1, and crr). Mechanisms of PSI photoinhibition are not fully resolved, but ROS production has been shown to be involved (Takagi et al., 2016). Importantly, photoinhibition of PSI is a very costly process for the plant since no fast repair cycle exists (Sonoike, 2011). The 4Fe4S clusters FA, FB, and Fx have been identified as the site of damage in PSI (Sonoike et al., 1995; Tiwari et al., 2016).
To protect against photoinhibition, photosynthetic organisms require ways of safely dissipating excess light energy. In part, this is achieved by non-photochemical quenching (NPQ) that regulates light energy use efficiency (Müller et al., 2001). Mutants deficient in NPQ of the higher plant Arabidopsis thaliana and the chlorophytic green alga Chlamydomonas reinhardtii produce more ROS than comparative wild types (WTs) under high light (Havaux et al., 2000; Baroli et al., 2004; Roach and Krieger-Liszkay, 2012; Allorent et al., 2013; Roach et al., 2015a). Further to just dissipating excess energy and preventing ROS production, NPQ regulates photosynthetic electron flow, contributing to plant and algal growth (Cardol et al., 2009; Tikkanen et al., 2011; Kromdijk et al., 2016).
NPQ consists of several processes, the pH-dependent component (qE), state transitions (qT), photoinhibition (qI), and a further type of sustained quenching (qH) that is not associated with qI. qE is rapidly inducible within seconds and is activated in response to a low pH in the thylakoid lumen (Horton et al., 2005; Holzwarth et al., 2009). In higher plants, the four-helix light-harvesting complex (LHC)-related PsbS protein is involved in qE (Li et al., 2000). In C. reinhardtii and the moss Physcomitrella patens, the PsbS protein also contributes to qE (Alboresi et al., 2010; Correa-Galvis et al., 2016; Tibiletti et al., 2016), alongside three-helix LHC-type proteins called ‘light-harvesting-complex-stress-related’ (LHCSR) that are absent in higher plants. Under increased light exposure, LHCSR expression levels are up-regulated, which is in contrast to the typical down-regulation of other LHC genes (Büchel, 2015; Niyogi and Truong, 2013). Chlamydomonas reinhardtii has two closely related genes, LHCSR3.1 and LHCSR3.2, that both encode the same LHCSR3 protein, and LHCSR1 that is 82% identical to LHCSR3 (Peers et al., 2009). LHCSR3.1, LHCSR3.2, and LHCSR1 genes have minor differences in their sequence and promoter regions, leading to distinct regulation (Maruyama et al., 2014). While blue light is stronger in up-regulating LHCSR3.1 and LHCSR3.2 (Petroutsos et al., 2016), UV-B radiation strongly up-regulates LHCSR1 and PSBS, whose corresponding proteins provide photoprotection (Allorent et al., 2016; Dinc et al., 2016; Tilbrook et al., 2016). Of the various C. reinhardtii NPQ mutants, including npq1 (deficient in violaxanthin deepoxidase and therefore in antheraxanthin and zeaxanthin), npq2 (deficient in zeaxanthin epoxidase), stt7-9 (deficient in STT7 kinase and therefore state transitions), and lhcsr1 (deficient in LHCSR1), the npq4 mutant (deficient in LHCSR3) has the lowest qE capacity (Niyogi et al., 1997; Peers et al., 2009; Allorent et al., 2013, 2016). Dissipating excess light energy via LHCSR3 involves protonation of luminal residues at the C-terminus, connecting the low pH of the thylakoid lumen to activation of qE (Bonente et al., 2011; Liguori et al., 2013; Ballottari et al., 2016). However, LHCSR deficiency does not necessarily lead to PSII photoinhibition under constant light approaching a saturating intensity (Cantrell and Peers, 2017). LHCSR3 has been identified in the PSI antenna of C. reinhardtii (Allorent et al., 2013; Bergner et al., 2015), where it may potentially quench the excitation energy of LHCII, thereby decreasing the excitation pressure of PSI, as shown in the moss P. patens (Pinnola et al., 2015) and C. reinhardtii (Girolomoni et al., 2019). Photoinhibition of PSI occurred within a few hours of high light treatment in the C. reinhardtii pgrl1npq4 double mutant, deficient in LHCSR3 and pgrl1-mediated cyclic electron flow, but not in the pgrl1 single mutant (Bergner et al., 2015; Chaux et al., 2017). Overall, this indicates that LHCSR3 can protect PSI from photodamage.
Historically, atmospheric oxygen peaked ~0.3 bya in the carboniferous period at a level of 30–35% (Holland, 2006), and algae of this period may have benefited from a complexity of photoprotective qE mechanisms (LHCSR1, LHCSR3, and PsbS). Despite current atmospheric levels of 21%, the water column that algae inhabit can become highly oxygenated at peak light intensities due to high photosynthetic rates (Roach et al., 2015b).
The aim of this study was to investigate the importance of LHCSR3 for C. reinhardtii to cope with the combination of elevated O2 and high light. We used the LHCSR3-deficient npq4 mutant of C. reinhardtii alongside two WT strains: WT-4A, the WT parent of npq4; and WT-D66 that has higher LHCSR3 levels than WT-4A. Cells were cultivated photoautotrophically in O2 tensions putatively encountered ~0.3 bya (35% O2) and compared with cells at a lower O2 tension (17% O2). LHCSR3 could protect both PSII and PSI from O2-dependent damage but, due to efficient repair of PSII, only PSI levels decreased in npq4 in 35% O2. Tolerance to 1O2, and levels of LHCSR1 and RES were elevated in npq4, particularly in cells cultivated in 35% O2. Since the RES acrolein strongly up-regulated LHSCR1, alongside transcription of many other light stress-associated genes, we discuss the Jekyll and Hyde nature of RES, which on the one hand contribute to retrograde signalling, leading to elevated qE capacity, while on the other hand cause damage, including photoinhibition.
Materials and methods
Strains and growth conditions
Chlamydomonas reinhardtii WT-4A+ (CC-4051) npq4+ (CC-4614; positive mating type npq4) were used in all experiments, and, when indicated, npq4− (CC-4615; negative mating type npq4) was also included. Strains were purchased from the Chlamydomonas Centre (www.chlamycollection.org). When indicated, the WT strain D66 (CC-4425) was also used (a gift from L. Michelet, CEA Saclay, France). Cultures were initiated in Tris-acetate-phosphate (TAP) liquid medium, pH 7.0, and grown photoheterotrophically under low light (50 µmol photons m−2 s−1). To transfer cells to photoautotrophic conditions, TAP cultures were pelleted for 2 min at 1600 g and resuspended in Tris-HCl-phosphate (THP) medium (identical except the pH was adjusted to 7.0 with HCl rather than acetic acid) and cultivated under low light while being bubbled with sterile air, using a 0.22 µm air filter. Cells were in THP for at least 24 h before experiments began, which is well beyond the time for residual acetate to be consumed that can affect 1O2 production by PSII (Roach et al., 2013). Liquid cultures were rotated at 80 rpm at 20 °C, kept in the exponential growth phase, and adjusted to 10 µg chlorophyll ml−1 before starting each experiment. Chlorophyll was measured according to Porra et al. (1989) in 80% acetone.
Elevated oxygen growth tests
A 10 µl aliquot of TAP cultures at 1×106 cells ml−1 was spotted onto THP medium containing 1.5% agar and the medium was dried off in a sterile air flow over 0.5 h. The agar was transferred onto a plastic insert that was held in the neck of an upside down 1 litre clear glass jar. The O2 content of the jar was increased with pure O2 gas to the desired concentration, as measured with O2 optode sensor spots (PreSens, Regensburg, Germany) placed on the inside of the sealed jars. The sensors were calibrated with pure O2 and N2 gases. Jars were placed in an incubator at 25 °C and 250 µmol photons m−2 s−1 on a 16/8 h (day/night) diurnal cycle for 7 d. The lids were opened after 3 d and gases exchanged. In a subsequent experiment for LHCSR1, LHCSR3, and PsbS protein analyses, cells were cultivated as above, except that the O2 level was adjusted to 35% and 17% using pure O2 and N2, respectively, so that gas displacement led to the same CO2 levels (0.033%) in both conditions. Cells were removed for analyses 6–8 h after the onset of light.
High light and gas treatments of liquid cultures
High light was provided by a 250 W horticultural compact fluorescent lamp (Envirolite, 6400K) and cultures were kept between 20 °C and 25 °C with fan-assisted cooling. The light intensity measured at the top and bottom of liquid cultures was 300 µmol photon m−2 s−1 and 200 µmol photon m−2 s−1, respectively (from here on 250 µmol photon m−2 s−1), which was a 5-fold increase over the growth light intensity. Liquid cultures were pre-high light treated for 2 h in the absence of air bubbling to induce the production of LHCSR3 in WT cells, and then recovered for 2 h at 30 µmol photons m−2 s−1 to enable recovery of any photoinhibitory effects of the pre-high light treatment. After this, the Fv/Fm of WT-4A, WT-D66, and npq4 were 0.63±0.01, 0.65±0.02, and 0.61±0.01, respectively, and net O2 production rates under saturating light (PSII activity) were 218±31, 169±8, and 149±32 µmol mg−1 chlorophyll h−1, respectively (n=3±SD). Subsequently, for measuring rates of photoinhibition, cells were re-treated with high light in the presence of 2.5 mM lincomycin and in the presence of 5 mM NaHCO3, either constantly bubbled with either N2 or O2 gas, or without any gas bubbling, as indicated in the figure legends.
Photosynthetic measurements
Net O2 production at saturating light intensity (1500 µmol photons m−2 s−1) was measured using a Fibox 3 optode dipping probe (PreSens, Regensburg, Germany) in the presence of 1 mM NaHCO3 with constant stirring. For measuring chlorophyll fluorescence of liquid cultures, a cuvette-adapted Aquapen-C was used (Photon System Instruments, Drasov, Czech Republic). A 2 ml aliquot of culture was diluted to 0.5 µg ml−1 chlorophyll, and Fo (background fluorescence) and Fm (maximum fluorescence) were measured before and during a 2 s saturating pulse of 3000 µmol photons m−2 s−1. Maximum and relative quantum yields of PSII (Fv/Fm and ФPSII, respectively) were calculated via (Fo–Fm)/Fm, whereby Fm was measured before (ФPSII) or after (Fv/Fm) 1.5 h dark recovery to allow the majority of Fm quenching to relax, while maintaining cells in the physiological state closest to that at the end of the light treatment. qE was measured using the NPQ_1 program (2 min at 1000 µmol photons m−2 s−1) and calculated with (Fo–Fm')/Fm', with Fm' measured during actinic light. For measuring chlorophyll fluorescence of agar-grown cultures, a CCD camera (FluorCam 701MF, Photon System Instruments) was used. Fm was measured with a 600 ms saturating pulse of 2300 µmol photons m−2 s−1. For measuring qE, actinic light was provided by two red LED panels, providing 200 µmol photons m−2 s−1 and measured after 2 min light treatment. The redox state of PSI was measured by near infra-red absorption with a Dual-PAM-100 (Heinz Walz, Effeltrich, Germany) according to the method of Klughammer and Schreiber (1994). The maximum P700 change from the dark-adapted to fully oxidized level (P700+) obtained 20 ms after a saturating light pulse was calculated manually in Excel after exporting raw data as the difference between 0–5 ms before and 19–21 ms after starting the saturating pulse. Cultures with a chlorophyll content of 80 µg ml−1 were dark adapted for 2 h and vigorously stirred immediately before measurement. A minimum of three technical replicate measurements were averaged for each biological replicate.
Analysis of LHCSR3, LHCSR1, PsbS, PsaA, and PsbA protein levels
Proteins were extracted from the same cells, or pool of cells, and non-invasively measured (e.g. chlorophyll fluorescence) for data that are shown within the same figure, or related supplementary figure. Total cellular proteins were either extracted in 2% SDS, in 50 mM Tris-HCl, pH 6.8 (for agar-grown cultures), or in 7 M urea, 2 M thiourea, 20 mM Tris–HCl with 0.2% (v/v) Triton X-100 (for liquid cultures), in both cases with protease inhibitor cocktail (Complete Mini, Roche Diagnostics, Switzerland), with protein quantification via the bicinchoninic acid or Bradford assay, for the two extraction methods, respectively. Before loading, proteins were denaturated at 85 °C with 0.1 M DTT and separated by PAGE using 12% acrylamide gels at 40 mA in Tris-glycine-SDS running buffer. For semi-dry western blotting, separated proteins were transferred to nitrocellulose membranes at 40 mA per gel for 1 h, which were subsequently blocked in 5% fat-free milk powder before incubating with either LHCSR3 (AS14-2766), LHCSR1 (AS14-2819), or PsaA (AS06-172) antibodies at 1:10 000 dilution, PsbA antibody (AS05-084) at 1:50 000 dilution (Agrisera, Sweden), or PsbS (a gift from Stefano Caffarri, Aix Marseille University) at 1:2000 dilution, for 1 h at room temperature. The peroxidase-coupled secondary antibodies (Sigma-Aldrich, St Louis, MO, USA) were visualized with enhanced chemiluminescence (Amersham, GE Healthcare, UK) and light-sensitive film (Amersham, GE Healthcare, UK). Blots were scanned for densitometry in ImageJ (Schneider et al., 2012).
Quantification of RES and other aldehydes via LC-MS/MS
Aldehydes were measured according to Roach et al. (2018). Briefly, cultures grown on agar were carefully scraped off the agar, weighed, and immediately suspended in 1 ml of acetonitrile with 0.5 µM 2-ethylhexanal (as internal standard) and 0.05% (w/v) of butylated hydroxytoluene. After centrifugation, aldehydes in the supernatant were derivatized with 2,4-dinitrophenylhydrazine (DNPH) in the presence of formic acid and diluted 50:50 with ultra-pure H2O before injection. Separation was carried out using a reversed-phase column (NUCLEODUR C18 Pyramid, EC 50/2, 50×2 mm, 1.8 µm, Macherey-Nagel, Düren, Germany), with an ekspert ultraLC 100 UHPLC system (AB SCIEX, Framingham, MA, USA) coupled to a QTRAP 4500 mass spectrometer for quantification of 2,4-DNPH-RES. Peak areas of selected ions were normalized relative to the internal standard, and concentrations were calculated to absolute amounts according to the calibration curves using external standards, which were treated and derivatized in the same way as samples. For other aldehydes that were not injected as external standards, peak areas of the DNPH-aldehyde were normalized to dry weight and shown as relative levels, as shown for the WT and npq4 cultured in 17% and 35% O2.
Singlet oxygen resistance test
Resistance to 1O2 was performed according to Fischer et al. (2012). Briefly, 1 ml of liquid culture at 2×106 cells ml−1 was pipetted into a 24-well flat-bottomed culture plate and Rose Bengal was added to the final concentrations indicated. Chlorophyll contents were used as an indicator of cell growth after 24 h at 50 μmol photons m−2 s−1. Data were normalized to the amount of chlorophyll in the absence of Rose Bengal for each interval of high light treatment and for WT and npq4 separately. In another experiment, a similar Rose Bengal treatment was given to cells pre-exposed to high light or high light in 80% O2. After 4 min intervals, 10 µl of culture was pipetted onto TAP agar and cultivated for 5 d at low light intensity <10 µmol m−2 s−1.
Statistics
Statistical analysis of data was carried out with the SPSS software package (v. 23) via one- or two-way ANOVA using Tukey’s post-hoc test. Univariate ANOVA-derived P-values for differences between factors or treatments are either given in the figures or, for simplicity, denoted by different letters above bars or symbols when P<0.05.
Results
LHCSR3 enables growth in an oxygenated atmosphere
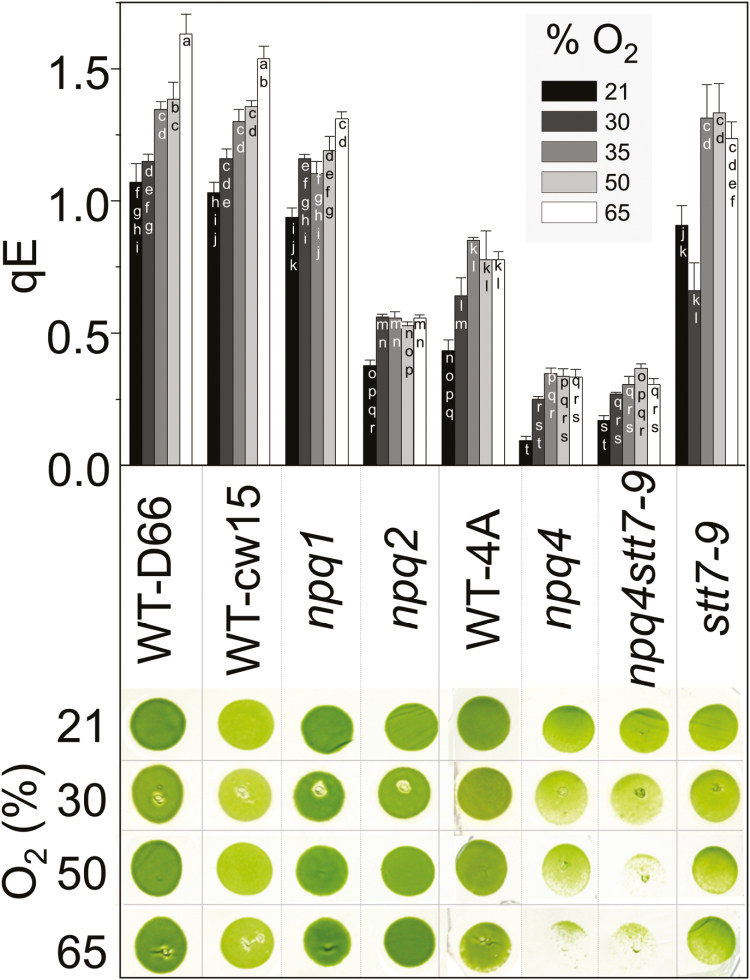
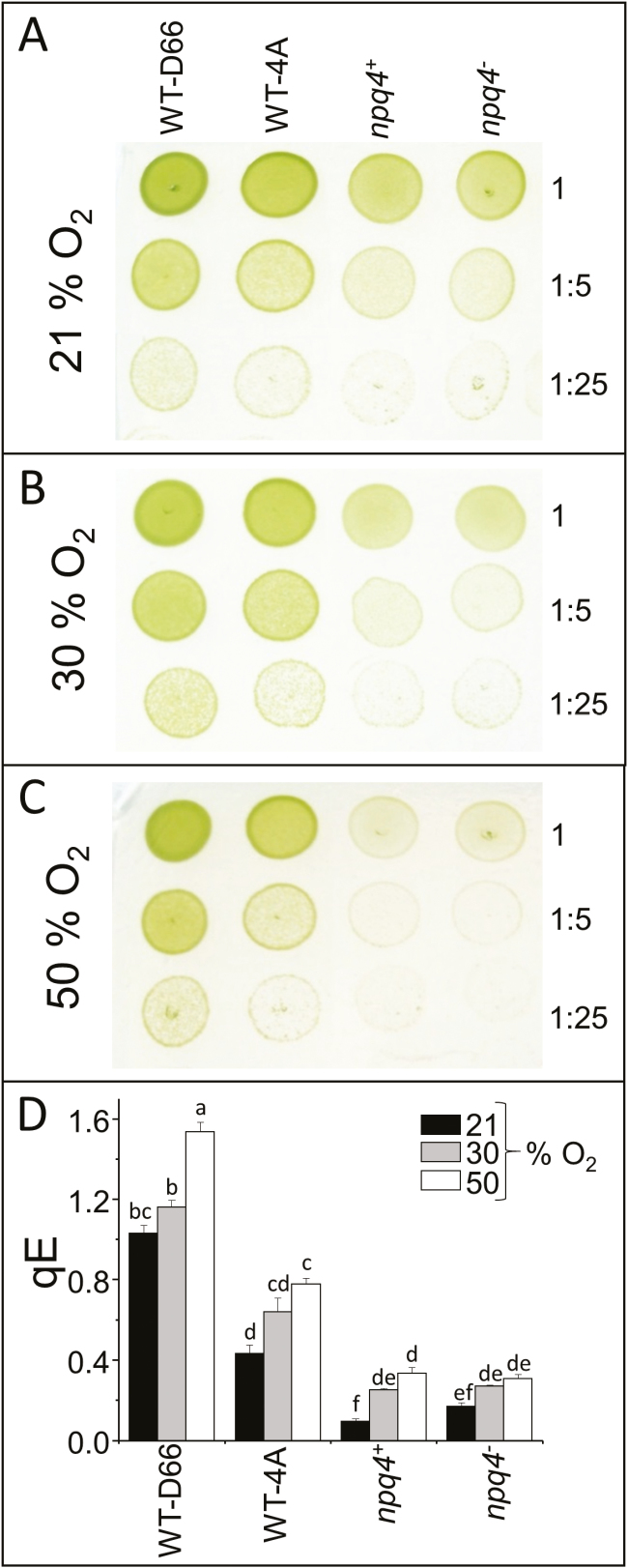
Chlamydomonas reinhardtii mutants affected in NPQ (npq1, npq2, npq4, stt7-9, and the npq4stt7-9 double mutant) with their corresponding WTs, WT-cw15 (npq1, npq2, and stt7-9) and WT-4A (npq4), as well as WT-D66, were screened for the effect of elevated O2 on growth and qE. All strains increased qE capacity in elevated O2, but only LHCSR3-deficient mutants had growth-sensitive phenotypes (Fig. 1). Three of the strains were selected to further investigate the relationship between colony growth in elevated O2 and qE capacity: npq4, its parental WT (WT-4A), and WT-D66 that had the highest qE capacity (Fig. 1). After 7 d, colony growth of npq4 was slowed in an atmosphere of 30% O2, whereas growth of WT-4A and WT-D66 was hardly affected (Fig. 2A, B). In a constant atmosphere of 50% O2, colony growth of WT-D66 was equal to growth at 21% O2, whereas WT-4A was marginally less and npq4 was considerably weakened (Fig. 2C). In agreement with Fig. 1, the qE capacity of all strains was increased by growth in elevated O2, but the differences between the genotypes were maintained (Fig. 2). At all O2 tensions, the qE of npq4 was between 2- and 2.5-fold lower than of WT-4A (Figs 1, 2).
Fig. 1.
C. reinhardtii mutants deficient in LHCSR3 show sensitivity to culture initiation in elevated O2. Mutant strains (see text for details) and corresponding WTs were cultured for 3 d on 1.5% agar medium in photoautotrophic conditions in 21–65% O2 under continuous light (250 µmol photons m−2 s−1). Before measurement of qE, cells were allowed to recover in the dark for 1.5 h, n=3±SD. Colony growth was imaged after a subsequent 3 d culturing at 100 µmol photons m−2 s−1 in 21% O2. (This figure is available in colour at JXB online.)
Fig. 2.
Higher qE capacity permits faster colony growth in elevated O2. WT-D66 (high LHCSR3), WT-4A (medium LHCSR3), and LHCSR3-deficient npq4+ and npq4− were cultured on 1.5% agar medium in photoautotrophic conditions for 7 d under 250 µmol photons m−2 s−1, and a 16/8 h light/dark cycle, in (A) 21, (B) 30, or (C) 50% O2 environments. Before growth, culture spots, initiated from 10 µl of liquid culture at 15 µg ml−1 chlorophyll, were non-diluted or diluted 5-fold or 25-fold, as indicated to the right. (D) qE of cells after 7 d growth shown in (A–C). Before measurement of qE, cells were allowed to recover in the dark for 1.5 h, n=3±SD. (This figure is available in colour at JXB online.)
Prevention of PSI photoinhibition in an oxygenated environment by LHCSR3
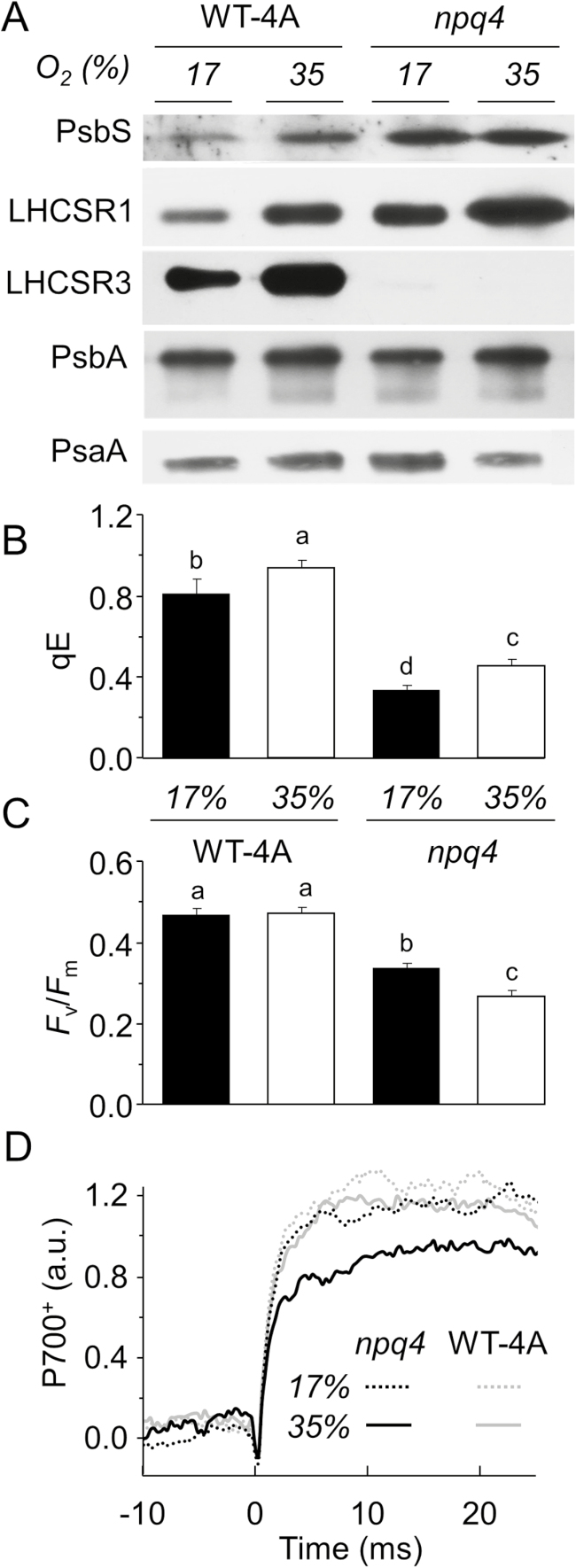
In order to address the potential relevance of the O2-dependent increase in qE capacity, WT-4A and npq4 were cultivated on agar in 35% or 17% O2, in either case with equal CO2 availability of 0.033%. Cultivating WT-4A in 35% O2 increased levels of LHCSR1, LHCSR3, and PsbS compared with cultivation in 17% O2 (Fig. 3A). In npq4, PsbS and LHCSR1 were higher than in the WT at either 35% or 17% O2 (Fig. 3A), indicating compensation for the absence of LHCSR3. In npq4, the amount of LHCSR1 was higher in 35% O2 than in 17% O2. Noticeably, qE values of WT-4A and npq4 were higher under 17% than 21% O2 (Figs 2, 3B), which is attributed to lower CO2 levels (0.033%) in 17% O2, due to gas displacement by adding N2 to lower O2 levels. Low CO2 elevates transcription of LHCSR1 and LHCSR3.1 (Maruyama et al., 2014). Importantly, however, CO2 levels were equal in 17% and 35% O2 treatments. Densitometric quantifications of band intensity of western blots from three independent experiments showed no influence of O2 concentration on levels of the PSII reaction centre, as shown by the amount of PsbA in either genotype (Supplementary Fig. S1 at JXB online), despite lower Fv/Fm values found in npq4 (Fig. 3B). However, levels of the PSI reaction centre, as shown by the amount of PsaA, were on average, 26% lower in npq4 cultivated in 35% O2, relative to cultivation in 17% O2, whereas PsaA levels in the WT were less altered by the O2 concentration (Supplementary Fig. S1). This agreed with only npq4 grown in 35% O2 having lowered maximum P700+ levels (Fig. 3D). In summary, C. reinhardtii adjusted to oxygenated atmospheres by increasing its qE capacity via producing LHCSR1, LHCSR3, and PsbS. In contrast, npq4, despite increasing LHCSR1 and PsbS, showed a lower level of PsaA when grown in 35% O2.
Fig. 3.
Growth in elevated O2 raises qE-related protein levels and qE capacity, but lowers PsaA and maximum P700+ levels in npq4. WT-4A and npq4 were grown on 1.5% agar medium under photoautotrophic conditions in a 17% or 35% O2 atmosphere for 5 d under a 16/8 h light/dark cycle (250 µmol photons m−2 s−1). (A) The same protein extracts were used for all five blots. (B and C) Measurements of chlorophyll fluorescence were made after 1.5 h low light recovery, n=4±SD with different letters denoting significant differences (P<0.05). (D) P700-dependent absorption changes in WT-4A and npq4 (see key). The increase in signal during a saturating pulse starting at time 0 corresponds to accumulation of P700+. Kinetics were averaged from four biological replicates each measured four times.
Prevention of PSII photoinhibition in an oxygenated environment by LHCSR3
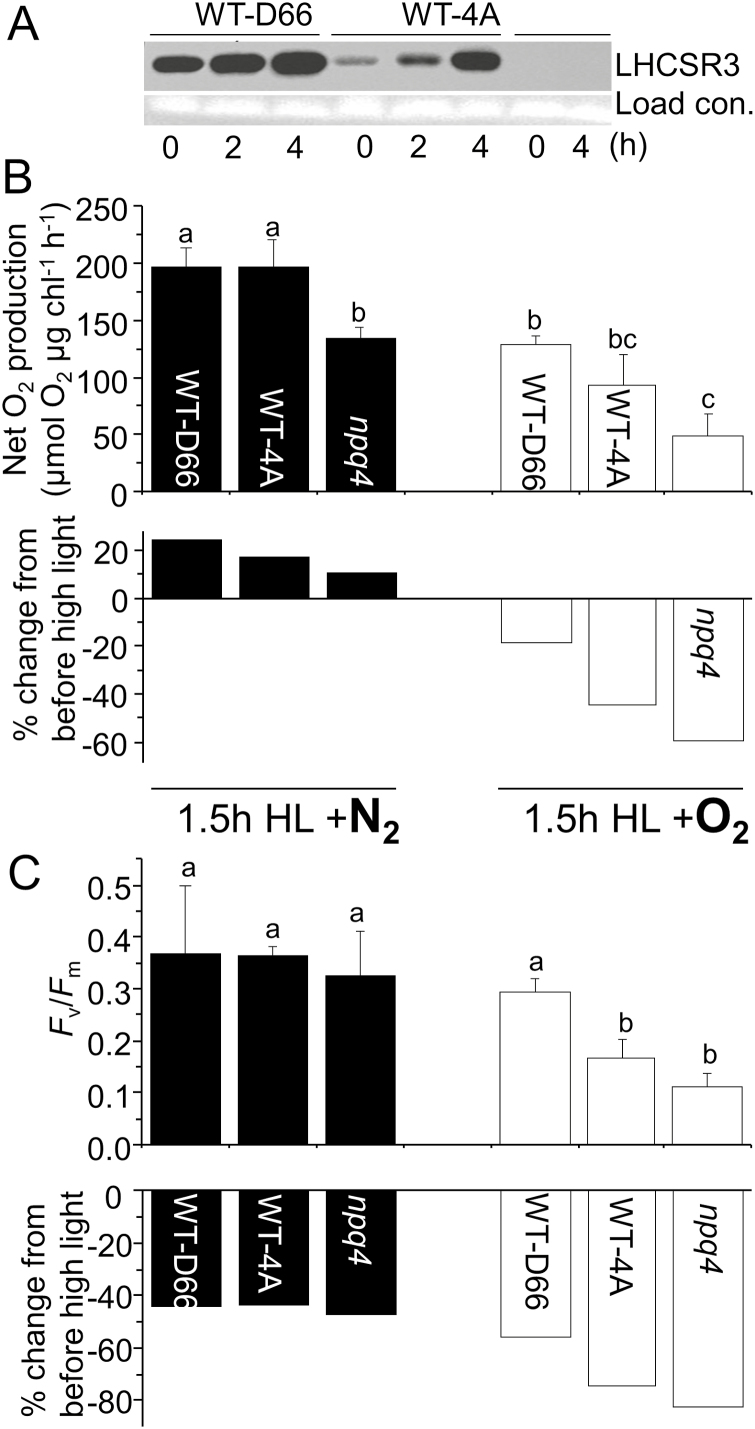
For investigating the protection LHCSR3 affords against O2-mediated photoinhibition of PSII, liquid cultures were used, enabling the use of lincomycin to block PSII repair. Without lincomycin, high light (250 µmol photons m−2 s−1) treatment of WT and npq4 photoautotrophic cultures in ambient O2 led to a steady increase in maximal PSII activity (from here on referred to as PSII activity), as monitored by net O2 production under saturating light without any CO2 restriction (Supplementary Fig. S2). In the presence of lincomycin, PSII activity was lost within 2 h high light (Supplementary Fig. S2), but there were limited differences between npq4 and WT-4A. Therefore, in the next experiment, cultures were pre-treated with high light for 2 h to induce accumulation of LHCSR proteins. After this pre-treatment, LHCSR1 became detectable, and in a higher amount in npq4 than in WT-4A (Supplementary Fig. S3), while WT cells accumulated more LHCSR3 (Fig. 4A). After allowing cultures to recover from the first high light treatment, cells were further treated with 1.5 h high light with lincomycin. In these conditions, losses of PSII activity in the three strains were prevented when the culture was constantly purged with N2 gas during high light treatment (Fig. 4B), in agreement with no loss of PsbA in the WT or npq4 (Supplementary Fig. S4). In contrast, purging cultures with O2 gas significantly decreased PSII activity (Fig. 4C), which correlated with a progressive loss of PsbA (Supplementary Fig. S4). The largest decrease of PSII activity occurred in LHCSR3-deficient npq4 and the smallest decrease in WT-D66, the WT with the highest level of LHCSR3 (Fig. 4A). After 1–5 h recovery, Fv/Fm values of cultures light treated in the presence of lincomycin decreased in both N2 and O2 purging to values of 0.33–0.36 and 0.10–0.28, respectively (Fig. 4C).
Fig. 4.
Oxygen accelerates PSII photoinhibition depending on LHCSR3 amounts. (A) Influence of high light (250 µmol photons m−2 s−1) on LHCSR3 levels in WT-D66, WT-4A, and npq4. High light-acclimated cultures were further high light treated for 1.5 h in the presence of 2.5 mM lincomycin and 5 mM NaHCO3 while purging with pure N2 (left panel; black) or pure O2 (right panel; white). PSII activity was measured after 1.5 h recovery by (B) O2 production at saturating light in the presence of 1 mM NaHCO3, and (C) Fv/Fm, n=3±SD. Data are from three independent experiments, with different letters denoting significant differences (P<0.05). The percentage changes from before to after high light treatment are indicated beneath (B) and (C).
To investigate the repair process of PSII, npq4, WT-4A, and WT-D66 were treated with an even higher light intensity (500 µmol m−2 s−1) in photoautotrophic liquid medium for 16 h without gas purging (ambient O2), which induced a loss of PsbA in npq4 (Supplementary Fig. S5). Immediately after light treatment, the quantum yield of PSII of npq4 was <0.2, which increased along with increased PsbA levels during 1 h recovery. The Fv/Fm of npq4 reached WT values within 4 h when recovered in the presence of light, but not in the dark (Supplementary Fig. S5). In WT-D66, light had no effect on the recovery of Fv/Fm, showing that synthesis of D1 protein, reassembly of the PSII reaction centre, and photoactivation (i.e. light-dependent assembly of the Mn cluster) were not needed. Light influenced the recovery of Fm in WT-4A, showing some photoinhibition under these conditions, but less than occurred in npq4 (Supplementary Fig. S5). Nonetheless, a rapid increase of Fm occurred in npq4 when recovery was under low light, indicating an efficient PSII repair cycle.
Light stress-associated production of reactive electrophile species and other aldehydes
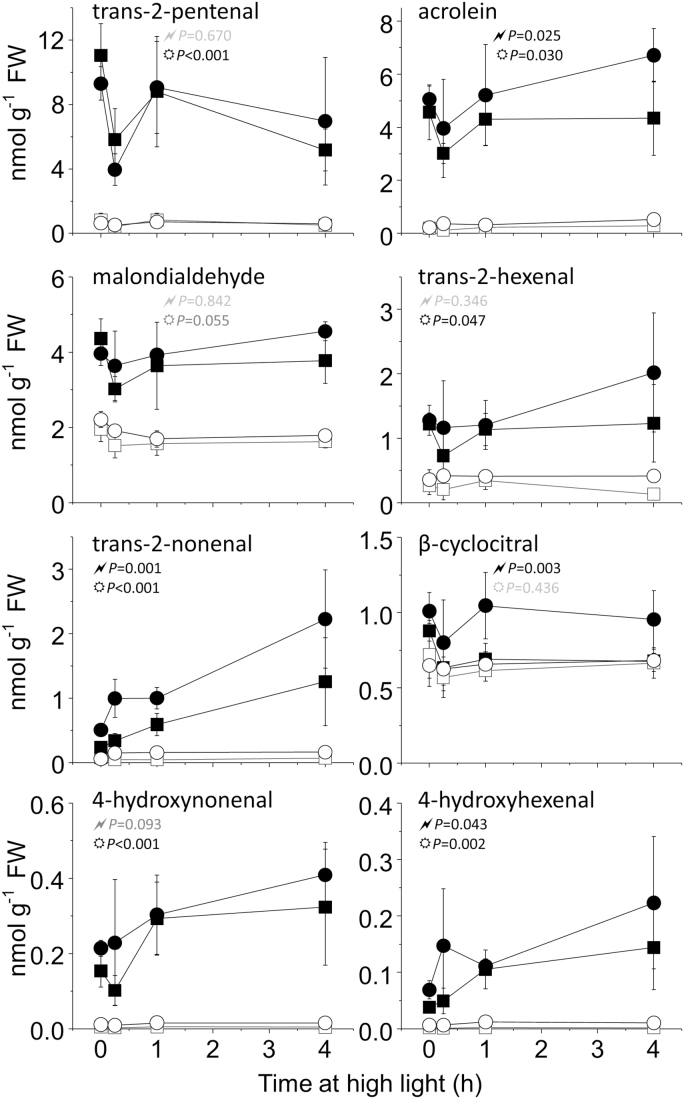
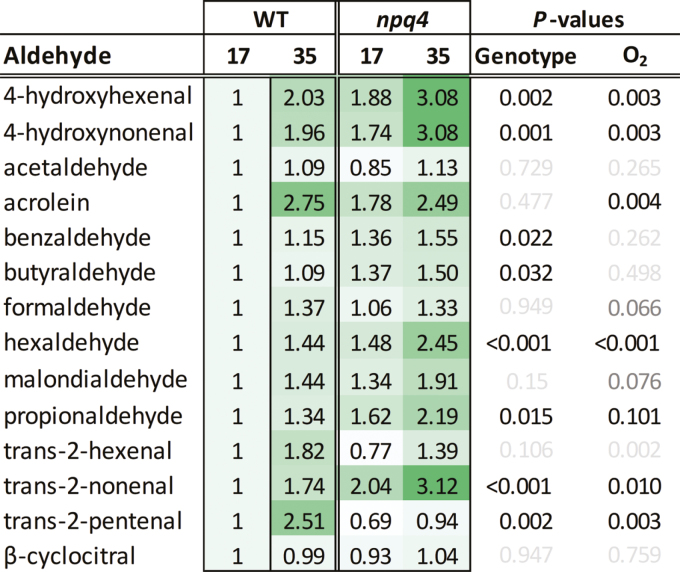
Photoautotrophic C. reinhardtii cells are under more light stress and produce more 1O2 compared with mixotrophic cells grown with an organic carbon source, such as acetate (Roach et al., 2013, 2017). Aldehydes and carbonyls, including RES, are produced under light stress as downstream products of lipid peroxidation (Mano, 2012). The concentrations of RES were elevated in photoautotrophic cells compared with mixotrophic cells, both before and during 4 h high light treatment (Fig. 5). Overall, npq4 produced a higher level of RES than the WT, with an ANOVA revealing a significant interaction for genotype (npq4 or WT-4A) and medium (photoautotrophic or mixotrophic) for acrolein, trans-2-nonenal, β-cyclocitral, and 4-hydroxyhexanal. Levels of aldehydes, including the aforementioned RES, were also measured in npq4 or WT-4A cultivated in 17% or 35% O2, under the same conditions used in Fig. 3. For most aldehydes, cultivation in 35% O2 led to a significantly higher level than growth in 17% O2, and half of the determined aldehydes were significantly higher in npq4 than in the WT (Fig. 6). Exogenously treating cells with acrolein for 4 h showed a concentration-dependent loss of maximum P700+ levels (Supplementary Fig. S6), revealing that it affects PSI. Furthermore, treating WT cells for 3 h with high light in the presence of the PSII inhibitor bromoxynil led to a slight, but significant (α=0.026, t-test), decline in maximum P700+ levels relative to dichlorophenyl dimethylurea (DCMU) treatment (Supplementary Fig. S6), whereby bromoxynil promotes more 1O2 production within PSII than does DCMU (Fufezan et al., 2002). RES are also signalling molecules that contribute to 1O2 signalling. For example, acrolein and other RES induced tolerance to the 1O2-producing photosensitizer Rose Bengal (Fischer et al., 2012; Roach et al., 2018). Treating cultures with high light significantly enhanced tolerance of npq4 to Rose Bengal more than WT-4A, while high light treatment in elevated O2 further induced Rose Bengal tolerance (Fig. S7).
Fig. 5.
Light stress is associated with production of RES. WT-4A (squares) and npq4 (circles) were grown photoautotrophically (filled symbols) or mixotrophically (open symbols) on 1.5% agar plates at 50 µmol photons m−2 s−1 before exposure to high light (250 µmol photons m−2 s−1). 2,4-DNPH-derivatized RES were measured by LC-MS/MS. The P-values from a two-way ANOVA of interaction between ‘genotype and media’ and ‘genotype and high light exposure time’ are represented by ‘ ’ and ‘
’ and ‘ ’, respectively, in black (P<0.05) or grey (P>0.05), n=4±SD.
’, respectively, in black (P<0.05) or grey (P>0.05), n=4±SD.
Fig. 6.
High oxygen tensions during culturing lead to higher RES and other aldehyde levels, and more in npq4 than in the WT. Cells were grown photoautotrophically on 1.5% agar under photoautotrophic conditions in a 17% or 35% O2 atmosphere for 5 d under a 16/8 h light/dark cycle (250 µmol photons m−2 s−1). 2,4-DNPH-derivatized aldehydes, including several RES, were measured by LC-MS/MS, normalized to dry weight, and shown as fold change relative to the WT at 17% O2, as also indicated by shading. For each aldehyde, the P-values from a two-way ANOVA of genotype or percentage O2 are given in black (P<0.05) or grey (P>0.05), n=4±SD. (This figure is available in colour at JXB online.)
Acrolein-induced changes in photosynthesis-related transcription
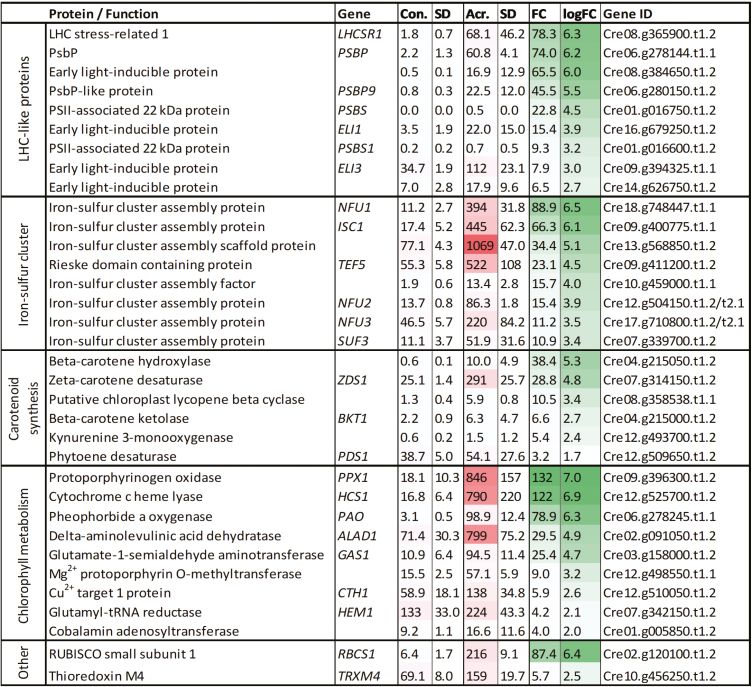
Previously, an RNA sequencing (RNA-seq) analysis of acrolein-treated C. reinhardtii focused upon the up-regulation of genes involved in redox defences, specifically thiol–disulfide exchanges (Roach et al., 2018). Acrolein has been used as an example RES, but, most probably, the overall RES load is important, since various RES are able to activate the same ERE transcription factors (Fischer et al., 2013). Further investigation of the RNA-seq data set of Roach et al. (2018) shows that up-regulated genes in response to a non-toxic acrolein dose include LHCSR1 (78-fold) and PSBS (23-fold), as well as transcripts encoding several early light-inducible proteins (ELIPs) that are involved in photosystem assembly (Beck et al., 2017), and proteins for carotenoid biosynthesis (Fig. 7). Furthermore, many iron–sulfur assembly protein-encoding genes were significantly up-regulated, such as NFU1 (89-fold), ISC1 (66-fold), and TEF5 (23-fold).
Fig. 7.
Transcription affected by the RES acrolein associated with NPQ, carotenoid synthesis, iron–sulfur clusters, and other selected chloroplast-associated pathways. Data were retrieved from RNA-seq of agar-grown cells treated with 600 ppm (atmospheric) volatile acrolein (Acr.) compared with non-treated cells (Con.) (Roach et al., 2018). Intensity of shading indicates the relative reads per kilobase of transcript per million mapped reads (RPKM; dark shading=higher value) and fold changes (FC). All listed genes have significant differential expression (false-discovery-rate P<0.01) relative to control, n=3. (This figure is available in colour at JXB online.)
Discussion
The production of ROS is inevitable during photosynthesis, and dissipation of excess light energy via NPQ prevents further ROS formation. Photoinhibition of PSII, here defined as a decrease in maximal O2 production and PsbA levels, only occurred at a high O2 tension (Fig. 4), indicating that ROS production is an important contributor to this process. The higher sensitivity of npq4 to photoinhibition in elevated O2 further supports previous observations that qE, and especially LHCSR3, prevents ROS production (Allorent et al., 2013; Roach et al., 2015a). Singlet oxygen has been shown to be the major ROS involved in photooxidative damage to plants (Triantaphylidès et al., 2008), but evidently other ROS and RES also attack PSII (Chan et al., 2012; Kale et al., 2017; Roach et al., 2017).
PSII is a particularly labile protein complex, which has a very efficient repair mechanism (Lidholm et al., 1987; Aro et al., 1993; Vass, 2012). Protein synthesis in the chloroplast, including the D1 reaction centre of PSII, has been shown to be inhibited by ROS, contributing to photoinhibition (Nishiyama et al., 2001; Murata et al., 2007). However, npq4 maintained a high level of PSII repair (Supplementary Fig. S5), responding to a 5-fold increase in light intensity by increasing net O2 production (Supplementary Fig. S2). In the absence of repair (with lincomycin), PSII activity was completely lost within 2 h (Supplementary Fig. S2). Photoinhibition is often measured by a reduction in Fv/Fm values, which can occur from a lower Fm or higher Fo value. In green algae, such as C. reinhardtii, a high level of state transitions (qT) can cause major changes in Fm that are not due to photoinhibition (Allorent et al., 2013; Roach and Na, 2017). In addition, other types of sustained quenching exist, which cannot be attributed to either qT, photoinhibition of PSII (qI), or qE (Brooks et al., 2013). High light treatment in the absence of O2 induced a sustained quenching of chlorophyll fluorescence, leading to lowered Fv/Fm values (Fig. 4C). However, this did not correspond to lowered PSII activity (Fig. 4B), and is therefore not qI. A strong quenching of Fm in anaerobic conditions, which did not correlate with a loss of O2 evolution, was also observed in thylakoid membranes from higher plants (Kirilovsky et al., 1994). In A. thaliana, a sustained non-qI quenching localized to the peripheral antenna (LHCII) of PSII, involving a plastid lipocalin (LCNP), was described as qH (Malnoë et al., 2018). Under cold and high light stress, mutants either deficient in or overexpressing LCNP produced more or fewer lipid peroxides than the WT, respectively, showing that qH is photoprotective (Malnoe et al., 2018). Here, light treatment in the absence of O2 led to ~40% lower Fv/Fm values after 1.5 h recovery, despite the fact that the maximal PSII activity was not affected (Fig. 4B). Therefore, we propose that a similar qH quenching mechanism exists in C. reinhardtii, although probably via a different protein, since a BLAST search in C. reinhardtii failed to reveal any sequence similarity to LCNP. Since this O2-independent quenching occurred equally in both WTs and npq4 (Fig. 4C), it is independent of LHCSR3. Sustained quenching in plants can also occur from retained zeaxanthin in LHC, termed qZ (Verhoeven et al., 1996; Nilkens et al., 2010), but in C. reinhardtii zeaxanthin is not a major contributor to chlorophyll fluorescence quenching (Quaas et al., 2015).
Historically, atmospheric oxygen peaked ~300 mya at a level of 30–35% (Holland, 2006), but it is noteworthy that some algae living today in an atmosphere of 21% O2 have to tolerate much higher O2 tensions in highly oxygenated water columns. Water-borne photosynthetic organisms can raise O2 concentrations, which coincide with elevated H2O2 concentrations and the highest qE levels (Roach et al., 2015b). Chlamydomonas reinhardtii increased NPQ-related protein levels in response to elevated oxygen tensions (Fig. 3A). In 35% O2, the npq4 mutant compensated for the lack of LHCSR3 by elevating LHCSR1, which probably contributed to its increased qE capacity induced by high light at high O2 tensions. However, the qE capacity was always compromised in the npq4 mutant by at least 2-fold relative to the WT (Figs 1, 2).
In contrast to PSII, PSI has a less efficient repair system (Sonoike, 2011). Under an elevated light intensity, C. reinhardtii responds by decreasing PSI levels, including the PSI antenna (Bonente et al., 2012). The protection afforded by LHCSR3 to PSI at high oxygen tensions (Fig. 3) is intriguing since the protein interacts with LHCII, the major antenna of PSII (Tokutsu and Minagawa, 2013). An over-reduced plastoquinone pool, as could be expected in npq4 under high light, activates phosphorylation of LHCII that can subsequently migrate to PSI during qT (Lemeille et al., 2010). After a transition to state II, a high proportion of LHCII becomes an antenna of PSI (Drop et al., 2014). LHCSR3 has been identified in the PSI antenna of C. reinhardtii (Allorent et al., 2013; Bergner et al., 2015), where it may potentially quench the excitation energy of LHCII, thereby decreasing the excitation pressure of PSI, as previously shown in the moss P. patens (Pinnola et al., 2015) and C. reinhardtii (Girolomoni et al., 2019). It should also be considered that LHCSR3 may also indirectly affect PSI activity in C. reinhardtii by, for example, affecting other photoprotective mechanisms, such as qT (Roach and Na, 2017), or preventing PSII-derived 1O2 formation and the downstream production of RES.
Previously, we have shown that npq4 suffers from more photoinhibition than WT-4A when cultivated on photoautotrophic agar medium, and has higher levels of protein carbonylation (Roach et al., 2017). This agrees with the higher RES levels detected in photoautotrophic npq4 compared with WT-4A (Fig. 5). Acrolein treatment damaged PSI (Supplementary Fig. S6; Roach et al., 2017), possibly targeting the iron–sulfur clusters of PSI, which are known to be damaged in PSI photoinhibition (Sonoike et al., 1995; Tiwari et al., 2016). The npq4 mutant showed the tendency of higher RES production on photoautotrophic media, especially when cultivated in 35% O2 (Fig. 6), alongside a loss of photoinducible P700+ formation (Fig. 3D). Therefore, we suggest that RES may contribute to PSI photoinhibition.
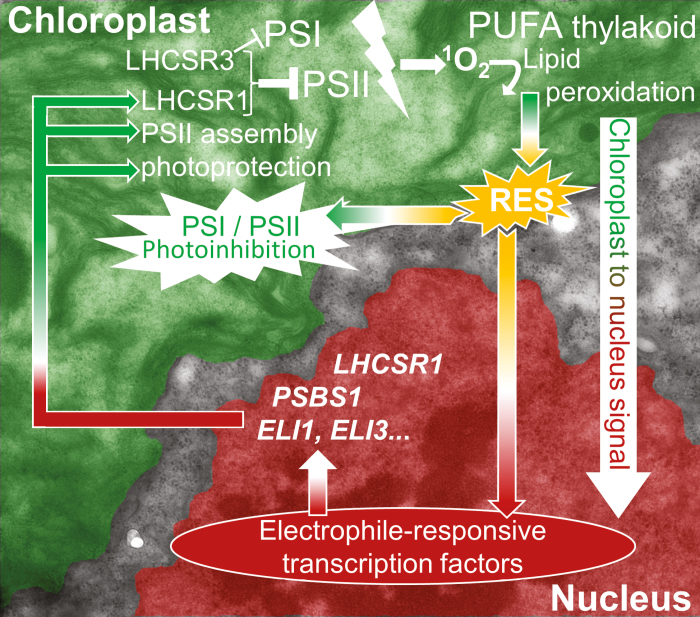
It is increasingly accepted that RES also contribute to stress signalling and can be involved in ROS signal propagation (Roach et al., 2018; Mano et al., 2019). Treatment of WT cells with acrolein increased the expression of genes involved in iron–sulfur cluster assembly (Fig. 7). Other up-regulated genes included LHCSR1 (78-fold) and PSBS1 (23-fold), which both function in qE (Allorent et al., 2016; Dinc et al., 2016; Tilbrook et al., 2016), helping mitigate light stress and prevent further RES production. Higher RES levels would explain why npq4 and the WT contained more LHCSR1 and PsbS (in the WT) when cultivated in 35% O2 (Figs 3A, 6). As summarized by Fig. 8, on the one hand RES cause damage in the chloroplast, attacking proteins and contributing to photoinhibition, while on the other hand function in 1O2-mediated chloroplast to nucleus signalling during high light acclimation.
Fig. 8.
The involvement of reactive electrophile species (RES) in the Jekyll and Hyde high light stress responses of C. reinhardtii. Excess light increases the formation of singlet oxygen (1O2) from PSII, which can induce lipid peroxidation of the thylakoid membrane lipids in the chloroplast (green). Lipid peroxides decay to release RES (yellow) that attack photosystems, contributing to photoinhibition, but are also sensed by specific nuclear transcription factors, such as SOR1, to affect transcription in the nucleus (red), whereby RES act as chloroplast-to-nucleus ‘retrograde’ signals. Up-regulated transcripts (white italics) are involved in helping mitigate excess light energy (LHCSR1, PSBS1), or contribute to photosystem assembly (ELI1, ELI3). The pathway, modified with permission from Roach et al. (2018) is superimposed over a false-coloured electron micrograph of an algal cell. The non-coloured region is the cytosol.
Conclusions
We showed that O2 availability influences how important qE is in protecting the photosynthetic apparatus from photoinhibition, and that in C. reinhardtii the O2 tension is a regulator of qE capacity. This may partially be due to the elevated levels of RES, which up-regulated expression of LHCSR1 and PSBS. Since npq4 was suffering a heavier RES load at elevated O2 concentrations, LHCSR3 protects against oxygen-mediated stress. This may include PSI photoinhibition, although the relative contribution of RES to this remains to be elucidated, as LHCSR3 also quenches excitation energy in the PSI antenna. Even though the PSII repair cycle was sufficient to prevent a net loss of PSII levels in npq4, elevated PSII repair rates would have energetic costs that may also affect growth rates in elevated O2 tensions. The lack of indifference in phenotype between npq4 and the WT light treated with low O2 availability shows that a multilevel qE mechanism was only necessary when O2 was highly abundant. Therefore, an O2-rich atmosphere can be considered a potential driver in the evolution of qE mechanisms.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Influence of 35% or 17% O2 during growth on levels of PsaA and PsbA protein levels in WT-4A and npq4.
Fig. S2. Effect of high light in the presence and absence of lincomycin on maximum PSII activity in WT-4A and npq4.
Fig. S3. Effect of high light on NPQ and LHCSR1 levels in WT-4A and npq4.
Fig. S4. Changes in PsbA protein levels in WT-4A and npq4 during high light treatment in the presence of lincomycin with N2 or O2 gas purging.
Fig. S5. The effect of light during post-high light recovery on relative and PSII quantum yields and PsaA protein levels in WT-D66 and npq4.
Fig. S6. Effect of acrolein, bromoxynil, and DCMU on P700-dependent absorption changes, indicating maximum P700+ levels.
Fig. S7. High light-treated npq4 has higher tolerance to 1O2 than the WT, and high light treatment in a high O2 atmosphere induces greater tolerance than in ambient O2.
Acknowledgements
We thank Stefano Caffari (Aix-Marseille Université, France) for providing the PsbS antibody, and Theresa Baur and Chantal Kruckenhauser for helping with LC-MS/MS measurements of RES. Travel support from The Bundesministerium für Bildung, Wissenschaft und Forschung (FR09/2018) is gratefully acknowledged.
References
- Alboresi A, Gerotto C, Giacometti GM, Bassi R, Morosinotto T. 2010. Physcomitrella patens mutants affected on heat dissipation clarify the evolution of photoprotection mechanisms upon land colonization. Proceedings of the National Academy of Sciences, USA 107, 11128–11133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allorent G, Lefebvre-Legendre L, Chappuis R, Kuntz M, Truong TB, Niyogi KK, Ulm R, Goldschmidt-Clermont M. 2016. UV-B photoreceptor-mediated protection of the photosynthetic machinery in Chlamydomonas reinhardtii. Proceedings of the National Academy of Sciences, USA 113, 14864–14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allorent G, Tokutsu R, Roach T, et al.. 2013. A dual strategy to cope with high light in Chlamydomonas reinhardtii. The Plant Cell 25, 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro EM, McCaffery S, Anderson JM. 1993. Photoinhibition and D1 protein degradation in peas acclimated to different growth irradiances. Plant Physiology 103, 835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballottari M, Truong TB, De Re E, Erickson E, Stella GR, Fleming GR, Bassi R, Niyogi KK. 2016. Identification of pH-sensing sites in the light harvesting complex stress-related 3 protein essential for triggering non-photochemical quenching in Chlamydomonas reinhardtii. Journal of Biological Chemistry 291, 7334–7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber J. 2003. Photosystem II: the engine of life. Quarterly Reviews of Biophysics 36, 71–89. [DOI] [PubMed] [Google Scholar]
- Baroli I, Gutman BL, Ledford HK, Shin JW, Chin BL, Havaux M, Niyogi KK. 2004. Photo-oxidative stress in a xanthophyll-deficient mutant of Chlamydomonas. Journal of Biological Chemistry 279, 6337–6344. [DOI] [PubMed] [Google Scholar]
- Beck J, Lohscheider JN, Albert S, Andersson U, Mendgen KW, Rojas-Stütz MC, Adamska I, Funck D. 2017. Small One-Helix proteins are essential for photosynthesis in arabidopsis. Frontiers in Plant Science 8, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergner SV, Scholz M, Trompelt K, et al.. 2015. STATE TRANSITION7-dependent phosphorylation is modulated by changing environmental conditions, and its absence triggers remodeling of photosynthetic protein complexes. Plant Physiology 168, 615–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonente G, Ballottari M, Truong TB, Morosinotto T, Ahn TK, Fleming GR, Niyogi KK, Bassi R. 2011. Analysis of LhcSR3, a protein essential for feedback de-excitation in the green alga Chlamydomonas reinhardtii. PLoS Biology 9, e1000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonente G, Pippa S, Castellano S, Bassi R, Ballottari M. 2012. Acclimation of Chlamydomonas reinhardtii to different growth irradiances. Journal of Biological Chemistry 287, 5833–5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks MD, Sylak-Glassman EJ, Fleming GR, Niyogi KK. 2013. A thioredoxin-like/β-propeller protein maintains the efficiency of light harvesting in Arabidopsis. Proceedings of the National Academy of Sciences, USA 110, E2733–E2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C. 2015. Evolution and function of light harvesting proteins. Journal of Plant Physiology 172, 62–75. [DOI] [PubMed] [Google Scholar]
- Cantrell M, Peers G. 2017. A mutant of Chlamydomonas without LHCSR maintains high rates of photosynthesis, but has reduced cell division rates in sinusoidal light conditions. PLoS One 12, e0179395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardol P, Alric J, Girard-Bascou J, Franck F, Wollman FA, Finazzi G. 2009. Impaired respiration discloses the physiological significance of state transitions in Chlamydomonas. Proceedings of the National Academy of Sciences, USA 106, 15979–15984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T, Shimizu Y, Pospíšil P, et al.. 2012. Quality control of photosystem II: lipid peroxidation accelerates photoinhibition under excessive illumination. PLoS One 7, e52100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaux F, Johnson X, Auroy P, Beyly-Adriano A, Te I, Cuiné S, Peltier G. 2017. PGRL1 and LHCSR3 compensate for each other in controlling photosynthesis and avoiding photosystem I photoinhibition during high light acclimation of Chlamydomonas cells. Molecular Plant 10, 216–218. [DOI] [PubMed] [Google Scholar]
- Correa-Galvis V, Redekop P, Guan K, Griess A, Truong TB, Wakao S, Niyogi KK, Jahns P. 2016. Photosystem II subunit PsbS is involved in the induction of LHCSR protein-dependent energy dissipation in Chlamydomonas reinhardtii. Journal of Biological Chemistry 291, 17478–17487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinc E, Tian L, Roy LM, Roth R, Goodenough U, Croce R. 2016. LHCSR1 induces a fast and reversible pH-dependent fluorescence quenching in LHCII in Chlamydomonas reinhardtii cells. Proceedings of the National Academy of Sciences, USA 113, 7673–7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drop B, Yadav K N S, Boekema EJ, Croce R. 2014. Consequences of state transitions on the structural and functional organization of photosystem I in the green alga Chlamydomonas reinhardtii. The Plant Journal 78, 181–191. [DOI] [PubMed] [Google Scholar]
- Fischer BB, Hideg É, Krieger-Liszkay A. 2013. Production, detection, and signaling of singlet oxygen in photosynthetic organisms. Antioxidants & Redox Signaling 18, 2145–2162. [DOI] [PubMed] [Google Scholar]
- Fischer BB, Ledford HK, Wakao S, Huang SG, Casero D, Pellegrini M, Merchant SS, Koller A, Eggen RI, Niyogi KK. 2012. SINGLET OXYGEN RESISTANT 1 links reactive electrophile signaling to singlet oxygen acclimation in Chlamydomonas reinhardtii. Proceedings of the National Academy of Sciences, USA 109, E1302–E1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fufezan C, Rutherford AW, Krieger-Liszkay A. 2002. Singlet oxygen production in herbicide-treated photosystem II. FEBS Letters 532, 407–410. [DOI] [PubMed] [Google Scholar]
- Girolomoni L, Cazzaniga S, Pinnola A, Perozeni F, Ballottari M, Bassi R. 2019. LHCSR3 is a nonphotochemical quencher of both photosystems in Chlamydomonas reinhardtii. Proceedings of the National Academy of Sciences, USA 116, 4212–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. 2006. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiology 141, 312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Bonfils JP, Lütz C, Niyogi KK. 2000. Photodamage of the photosynthetic apparatus and its dependence on the leaf developmental stage in the npq1 Arabidopsis mutant deficient in the xanthophyll cycle enzyme violaxanthin de-epoxidase. Plant Physiology 124, 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideg E, Spetea C, Vass I. 1994. Singlet oxygen production in thylakoid membranes during photoinhibition as detected by EPR spectroscopy. Photosynthesis Research 39, 191–199. [DOI] [PubMed] [Google Scholar]
- Holland HD. 2006. The oxygenation of the atmosphere and oceans. Philosophical Transactions of the Royal Society B: Biological Sciences 361, 903–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzwarth AR, Miloslavina Y, Nilkens M, Jahns P. 2009. Identification of two quenching sites active in the regulation of photosynthetic light-harvesting studied by time-resolved fluorescence. Chemical Physics Letters 483, 262–267. [Google Scholar]
- Hörandl E,, Speijer D. 2018. How oxygen gave rise to eukaryotic sex. Proceedings of the Royal Society B: Biological Sciences 285, 20172706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P, Wentworth M, Ruban A. 2005. Control of the light harvesting function of chloroplast membranes: the LHCII-aggregation model for non-photochemical quenching. FEBS Letters 579, 4201–4206. [DOI] [PubMed] [Google Scholar]
- Kale R, Hebert AE, Frankel LK, Sallans L, Bricker TM, Pospíšil P. 2017. Amino acid oxidation of the D1 and D2 proteins by oxygen radicals during photoinhibition of Photosystem II. Proceedings of the National Academy of Sciences, USA 114, 2988–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilovsky D, Rutherford AW, Etienne AL. 1994. Influence of DCMU and ferricyanide on photodamage in photosystem II. Biochemistry 33, 3087–3095. [DOI] [PubMed] [Google Scholar]
- Klughammer C, Schreiber U. 1994. An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700+-absorbance changes at 830 nm. Planta 192, 261–268. [Google Scholar]
- Krieger-Liszkay A. 2005. Singlet oxygen production in photosynthesis. Journal of Experimental Botany 56, 337–346. [DOI] [PubMed] [Google Scholar]
- Kromdijk J, Głowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP. 2016. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354, 857–861. [DOI] [PubMed] [Google Scholar]
- Lane B. 2002. Oxygen: the molecule that made the world. Oxford: Oxford University Press. [Google Scholar]
- Lemeille S, Turkina MV, Vener AV, Rochaix JD. 2010. Stt7-dependent phosphorylation during state transitions in the green alga Chlamydomonas reinhardtii. Molecular & Cellular Proteomics 9, 1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XP, Björkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK. 2000. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403, 391–395. [DOI] [PubMed] [Google Scholar]
- Lidholm J, Gustafsson P, Öquist G. 1987. Photoinhibition of photosynthesis and its recovery in the green alga Chlamydomonas reinhardii. Plant & Cell Physiology 28, 1133–1140. [Google Scholar]
- Liguori N, Roy LM, Opacic M, Durand G, Croce R. 2013. Regulation of light harvesting in the green alga Chlamydomonas reinhardtii: the C-terminus of LHCSR is the knob of a dimmer switch. Journal of the American Chemical Society 135, 18339–18342. [DOI] [PubMed] [Google Scholar]
- Lyons TW, Reinhard CT, Planavsky NJ. 2014. The rise of oxygen in Earth’s early ocean and atmosphere. Nature 506, 307–315. [DOI] [PubMed] [Google Scholar]
- Malnoë A, Schultink A, Shahrasbi S, Rumeau D, Havaux M, Niyogi KK. 2018. The plastid lipocalin LCNP is required for sustained photoprotective energy dissipation in arabidopsis. The Plant Cell 30, 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano J. 2012. Reactive carbonyl species: their production from lipid peroxides, action in environmental stress, and the detoxification mechanism. Plant Physiology and Biochemistry 59, 90–97. [DOI] [PubMed] [Google Scholar]
- Mano J, Biswas MS, Sugimoto K. 2019. Reactive carbonyl species: a missing link in ROS signaling. Plants 8, e391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama S, Tokutsu R, Minagawa J. 2014. Transcriptional regulation of the stress-responsive light harvesting complex genes in Chlamydomonas reinhardtii. Plant & Cell Physiology 55, 1304–1310. [DOI] [PubMed] [Google Scholar]
- Müller P, Li XP, Niyogi KK. 2001. Non-photochemical quenching. A response to excess light energy. Plant Physiology 125, 1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI. 2007. Photoinhibition of photosystem II under environmental stress. Biochimica et Biophysica Acta 1767, 414–421. [DOI] [PubMed] [Google Scholar]
- Nilkens M, Kress E, Lambrev P, Miloslavina Y, Müller M, Holzwarth AR, Jahns P. 2010. Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. Biochimica et Biophysica Acta 1797, 466–475. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y, Yamamoto H, Allakhverdiev SI, Inaba M, Yokota A, Murata N. 2001. Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. The EMBO Journal 20, 5587–5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Bjorkman O, Grossman AR. 1997. Chlamydomonas xanthophyll cycle mutants identified by video imaging of chlorophyll fluorescence quenching. The Plant Cell 9, 1369–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Truong TB. 2013. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Current Opinion in Plant Biology 16, 307–314. [DOI] [PubMed] [Google Scholar]
- op den Camp RG, Przybyla D, Ochsenbein C, et al.. 2003. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. The Plant Cell 15, 2320–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers G, Truong TB, Ostendorf E, Busch A, Elrad D, Grossman AR, Hippler M, Niyogi KK. 2009. An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 462, 518–521. [DOI] [PubMed] [Google Scholar]
- Petroutsos D, Tokutsu R, Maruyama S, et al.. 2016. A blue-light photoreceptor mediates the feedback regulation of photosynthesis. Nature 537, 563–566. [DOI] [PubMed] [Google Scholar]
- Pinnola A, Cazzaniga S, Alboresi A, Nevo R, Levin-Zaidman S, Reich Z, Bassi R. 2015. Light-harvesting complex stress-related proteins catalyze excess energy dissipation in both photosystems of Physcomitrella patens. The Plant Cell 27, 3213–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. 1989. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochimica et Biophysica Acta 975, 384–394. [Google Scholar]
- Quaas T, Berteotti S, Ballottari M, Flieger K, Bassi R, Wilhelm C, Goss R. 2015. Non-photochemical quenching and xanthophyll cycle activities in six green algal species suggest mechanistic differences in the process of excess energy dissipation. Journal of Plant Physiology 172, 92–103 [DOI] [PubMed] [Google Scholar]
- Roach T, Krieger-Liszkay A. 2012. The role of the PsbS protein in the protection of photosystems I and II against high light in Arabidopsis thaliana. Biochimica et Biophysica Acta 1817, 2158–2165. [DOI] [PubMed] [Google Scholar]
- Roach T, Baur T, Stöggl W, Krieger-Liszkay A. 2017. Chlamydomonas reinhardtii responding to high light: a role for 2-propenal (acrolein). Physiologia Plantarum 161, 75–87. [DOI] [PubMed] [Google Scholar]
- Roach T, Miller R, Aigner S, Kranner I. 2015a Diurnal changes in the xanthophyll cycle pigments of freshwater algae correlate with the environmental hydrogen peroxide concentration rather than non-photochemical quenching. Annals of Botany 116, 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach T, Na CS. 2017. LHCSR3 affects de-coupling and re-coupling of LHCII to PSII during state transitions in Chlamydomonas reinhardtii. Scientific Reports 7, 43145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach T, Na CS, Krieger-Liszkay A. 2015b High light-induced hydrogen peroxide production in Chlamydomonas reinhardtii is increased by high CO2 availability. The Plant Journal 81, 759–766. [DOI] [PubMed] [Google Scholar]
- Roach T, Sedoud A, Krieger-Liszkay A. 2013. Acetate in mixotrophic growth medium affects photosystem II in Chlamydomonas reinhardtii and protects against photoinhibition. Biochimica et Biophysica Acta 1827, 1183–1190. [DOI] [PubMed] [Google Scholar]
- Roach T, Stöggl W, Baur T, Kranner I. 2018. Distress and eustress of reactive electrophiles and relevance to light stress acclimation via stimulation of thiol/disulphide-based redox defences. Free Radical Biology & Medicine 122, 65–73. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoike K. 2011. Photoinhibition of photosystem I. Physiologia Plantarum 142, 56–64. [DOI] [PubMed] [Google Scholar]
- Sonoike K, Terashima I, Iwaki M, Itoh S. 1995. Destruction of photosystem I iron–sulfur centers in leaves of Cucumis sativus L. by weak illumination at chilling temperatures. FEBS Letters 362, 235–238. [DOI] [PubMed] [Google Scholar]
- Takagi D, Takumi S, Hashiguchi M, Sejima T, Miyake C. 2016. Superoxide and singlet oxygen produced within the thylakoid membranes both cause Photosystem I photoinhibition. Plant Physiology 171, 1626–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer A. 2014. Singlet oxygen production by PSII under light stress: mechanism, detection and the protective role of β-carotene. Plant & Cell Physiology 55, 1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibiletti T, Auroy P, Peltier G, Caffarri S. 2016. Chlamydomonas reinhardtii PsbS protein is functional and accumulates rapidly and transiently under high light. Plant Physiology 171, 2717–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikkanen M, Grieco M, Aro EM. 2011. Novel insights into plant light-harvesting complex II phosphorylation and ‘state transitions’. Trends in Plant Science 16, 126–131. [DOI] [PubMed] [Google Scholar]
- Tilbrook K, Dubois M, Crocco CD, Yin R, Chappuis R, Allorent G, Schmid-Siegert E, Goldschmidt-Clermont M, Ulm R. 2016. UV-B perception and acclimation in Chlamydomonas reinhardtii. The Plant Cell 28, 966–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A, Mamedov F, Grieco M, Suorsa M, Jajoo A, Styring S, Tikkanen M, Aro EM. 2016. Photodamage of iron–sulphur clusters in photosystem I induces non-photochemical energy dissipation. Nature Plants 2, 16035. [DOI] [PubMed] [Google Scholar]
- Tokutsu R, Minagawa J. 2013. Energy-dissipative supercomplex of photosystem II associated with LHCSR3 in Chlamydomonas reinhardtii. Proceedings of the National Academy of Sciences, USA 110, 10016–10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantaphylidès C, Krischke M, Hoeberichts FA, Ksas B, Gresser G, Havaux M, Van Breusegem F, Mueller MJ. 2008. Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiology 148, 960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass I. 2012. Molecular mechanisms of photodamage in the Photosystem II complex. Biochimica et Biophysica Acta 1817, 209–217. [DOI] [PubMed] [Google Scholar]
- Verhoeven AS, Adams WW, Demmig-Adams B. 1996. Close relationship between the state of the xanthophyll cycle pigments and photosystem II efficiency during recovery from winter stress. Physiologia Plantarum 96, 567–576. [Google Scholar]
- Yalcinkaya T, Uzilday B, Ozgur R, Turkan I, Mano J. 2019. Lipid peroxidation-derived reactive carbonyl species (RCS): their interaction with ROS and cellular redox during environmental stresses. Environmental and Experimental Botany 165, 139–149. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.