High CO2 triggers post-deastringency softening in persimmon fruit through interactions between ethylene response factors and NAC9 that lead to activation of the cellulose-degrading gene DkEGase1.
Keywords: DkEGase1, NAC, NAC-ERF complex, persimmon fruit, post-deastringency softening, transcriptomic analysis, transcriptional regulation
Abstract
Most persimmon (Diospyros kaki) cultivars are astringent and require post-harvest deastringency treatments such as 95% CO2 (high-CO2 treatment) to make them acceptable to consumers. High-CO2 treatment can, however, also induce excessive softening, which can be reduced by adding 1-methylcyclopropene (1-MCP). Previous studies have shown that genes encoding the ETHYLENE RESPONSE FACTORS (ERFs) DkERF8/16/19 can trans-activate xyloglucan endotransglycosylase/hydrolase (DkXTH9), which encodes the cell wall-degrading enzyme associated with persimmon fruit softening. In this study, RNA-seq data between three treatments were compared, namely high-CO2, high-CO2+1-MCP, and controls. A total of 227 differentially expressed genes, including 17 transcription factors, were predicted to be related to persimmon post-deastringency softening. Dual-luciferase assays indicated that DkNAC9 activated the DkEGase1 promoter 2.64-fold. Synergistic effects on transcription of DkEGase1 that involved DkNAC9 and the previously reported DkERF8/16 were identified. Electrophoretic mobility shift assay indicated that DkNAC9 could physically bind to the DkEGase1 promoter. Bimolecular fluorescence complementation and firefly luciferase complementation imaging assays indicated protein–protein interactions between DkNAC9 and DkERF8/16. Based on these findings, we conclude that DkNAC9 is a direct transcriptional activator of DkEGase1 that can co-operate with DkERF8/16 to enhance fruit post-deastringency softening.
Introduction
Texture, usually reflected by firmness, is critical for fruit storability and quality (Brummell, 2006; Li et al., 2010; Wang et al., 2018). Fruit texture is not only dependent on the genetic background, but is also influenced by external environments, such as temperature (Yang et al., 2007) and plant hormones (Li et al., 2017b; Zhang et al., 2018). Low-oxygen environments, usually applied in controlled atmospheres, have been used by industry and researchers to efficiently prolonging post-harvest storage and maintenance of firmness in various fruit (McDonald and Harman, 1982; Siddiqui et al., 1996; Yahia, 1998). A particular side effect of high-CO2 (95% CO2, 1% O2, 4% N2) treatment is the accelerated removal of astringency in persimmon (Diospyros kaki) fruit. Although this greatly improves the taste for consumers, it subsequently triggers rapid softening (Wang et al., 2017), which is undesirable. Persimmon fruit therefore provides an interesting model to investigate changes in fruit flavor and texture in response to hypoxia. In many fruits, ripening and quality-related gradients of metabolites can occur in relation to in situ hypoxic areas generated inside the fruit (Pedreschi et al., 2009; Biais et al., 2010). In persimmon, there is a burst in acetaldehyde production under low oxygen, which contributes to deastringency by precipitating the soluble tannins responsible for the astringent taste (Taira et al., 2001; Min et al., 2012; Zhu et al., 2018). It is known that production of acetaldehyde under anoxia is regulated by various transcription factors (TFs) for example, ethylene response factors (ERFs; Min et al., 2012; Zhu et al., 2018) and WRKY (Zhu et al., 2019). However, the mechanism(s) regulating the excessive softening that occurs after deastringency treatment are poorly understood.
Persimmon is a climacteric type of fruit, showing an increase in ethylene production during softening (Harima et al., 2003; Nakatsuka et al., 2011; Yin et al., 2012). As with other fruit produced for consumption, modulations in taste, softening, and texture are the main post-harvest issues. In persimmon, the activities of several enzymes have been shown to be positively correlated with fruit softening, namely polygalacturonase (PG: endo-type, EC 3.2.1.15; exo-type, EC 3.2.1.67), pectin methylesterase (PME, EC 3.1.1.11), β-galactosidase (β-gal, EC 3.2.1.23), and xyloglucan endotransglycosylase/hydrolase (XTH, previously known as xyloglucan endotransglycosylase, XET, EC 2.4.1.207), and endo-1,4-β-d-glucanase (EGase, EC 3.2.1.4) (Cutillas-Iturralde et al., 1994; Luo, 2005). Genes encoding some of these enzymes are believed to be involved in fruit softening, such as DkXTH1/2/8 (Nakatsuka et al., 2011; Zhu et al., 2013; Han et al., 2016) and DkExp3 (Zhang et al., 2012), but little is known about the molecular aspects of persimmon fruit softening.
Unlike the regular softening that occurs during the ripening of persimmon fruit, the extreme softening that occurs post-deastringency is undesirable. As most of the commercialized persimmon belong to the astringent type (Yamada et al., 1994; Wang et al., 1997), which require the removal of astringency to reach an edible standard, this presents a major problem for the industry. Most persimmon post-deastringency fruit softening occurs rapidly after the removal of astringency. In recent years, addition of 1-methylcyclopropene (1-MCP), an inhibitor of ethylene perception, to high-CO2 treatment has been found to be effective for achieving deastringency while maintaining firmness for various persimmon cultivars (Harima et al., 2003; Wang et al., 2017). Initial attempts to investigate the transcriptional regulatory mechanisms of post-deastringency softening, with a limited range of ERFs, identified high-CO2/hypoxia responsive DkERF8/16/19 as activators of the promoter of DkXTH9, which encodes an enzyme related to hemicellulose degradation (Wang et al., 2017). Transcripts of some AP2/ERF TFs have also shown significant correlations with fruit cell wall degradation and softening in various other fruits (Xie et al., 2016), such as MdCBF in apple that activates MdPG1 (Tacken et al., 2010), kiwifruit AdERF9 that acts as a suppressor of the AdXET5 promoter (Yin et al., 2010), and some other AP2/ERF genes in tomato (SlAP2a, Chung et al., 2010; Sl-ERF.B3, Liu et al., 2014). Apart from members of the AP2/ERF family, other TFs have also been found to play significant roles in tomato fruit softening, such as the MADS box TF RIPENING INHIBITOR (RIN) (Fujisawa et al., 2013), SlAN2 (an R2R3-MYB factor) (Meng et al., 2015), and SlNAC1 (NAM, ATAF1/ATAF2, CUC2) (Ma et al., 2014). Hence, the larger-scale screening of TFs would very likely discover new regulators of post-deastringency softening in persimmon fruit.
In this study, RNA-seq was performed with persimmon fruit subjected to different treatments in order to search for more TFs beyond ERFs that are involved in this process. Comparative analyses between different treatments revealed multiple differentially expressed TFs. Dual luciferase assays and electrophoretic mobility shift assays indicated that DkNAC9 could physically bind and transactive the DkEGase1 promoter, and this regulation could be enhanced by the presence of DkERF8/16, which has previously been identified as being involved in post-deastringent softening in persimmon (Wang et al., 2017). However, our assays indicated that these were indirect regulators that functioned by interacting with DkNAC9, rather than with the target gene promoter. Our results thus provide valuable insights into the mechanisms of hypoxia-induced fruit softening.
Materials and methods
Plant material and treatments
Fruit of an astringent persimmon (Diospyros kaki) cultivar, ‘Jingmianshi’, were selected for this study and harvested from a commercial orchard in 2014 at Qingdao, Shandong, China. The experimental treatments have been described in detail in a previous study by Wang et al., (2017). In brief, uniform mature fruit without disease or mechanical wounding were selected and divided into three batches that were treated in air-tight containers. (I) The first batch was treated with high CO2 for 1 d to remove astringency and to initiate rapid post-deastringency softening. (II) The second batch was treated with a combination of high CO2 and 1 μl·L–1 1-MCP for 1 d, which achieved similar removal of astringency while maintaining the firmness of the fruit. (III) The third batch was sealed in air-tight containers for 1 d, as a control. After treatment, the fruit were transferred to storage in air at 20 °C. Collection of physiological data and details of sampling were as described by Wang et al. (2017), and the following experiments were all based on these samples.
RNA extraction and cDNA synthesis
Total RNA extraction was conducted according to the methods described by Yin et al. (2012). Potential genomic DNA contamination was removed using a TURBO DNAse Kit (Ambion). A total of 1 μg RNA was used for cDNA synthesis from each sample, using an iScriptTM cDNA Synthesis Kit (Bio-Rad). All of the RNA extraction and cDNA synthesis reactions were performed with three biological replicates.
Transcriptome analysis
Three batches of samples after 4 d in storage were selected to perform the RNA-seq, using the same RNA for RT-qPCR. The quality of the RNA for library construction was verified using a Qubit 2.0 Flurometer (Life Technologies) and a RNA Nano 6000 Assay Kit (Agilent Technologies). Library constructions, sequencing, and bioinformatics analyses were conducted by Novogene Bioinformatics Institute (Beijing). The clustering of the index-coded samples was performed on a cBot Cluster Generation System using TruSeq PE Cluster Kit v3-cBot-HS (Illumia) according to the manufacturer’s instructions. After cluster generation, the library preparations were sequenced on an Illumina Hiseq 4000 sequencing platform and paired-end reads were generated. For transcriptome analysis without a reference genome, transcriptome assembly was accomplished based on the left.fq and right.fq using Trinity (Grabherr et al., 2011) with min_kmer_cov set to 2 by default and all other parameters set as default. Gene function was annotated based on the following databases: Nr (NCBI non-redundant protein sequences), Nt (NCBI nucleotide sequences), Pfam (Protein family), KOG/COG (eukaryotic Ortholog Groups/Clusters of Orthologous Groups of proteins), Swiss-Prot (a manually annotated and reviewed protein sequence database), KEGG (Kyoto Encyclopedia of Genes and Genomes), and GO (Gene Ontology). The iTAK software was used to predict TFs. Gene expression levels were estimated by using FPKM (fragments per kilobase of transcript sequence per millions base pairs sequenced; Trapnell et al., 2010). Differential expression analysis of two conditions/groups was performed using the DESeq R package (1.10.1; Love et al., 2014). The P-values were adjusted using the Benjamini–Hochberg approach for controlling the false discovery rate. An adjusted P-value<0.05 and |FoldChange|>5 were set as thresholds. KOBAS (Mao et al., 2005) was used to test the statistical enrichment of differentially expressed genes in the KEGG pathways.
RT-qPCR analysis
RT-qPCR was carried out on a CFX96 instrument (Bio-Rad), with SsoFast EvaGreen Supermix (Bio-Rad). The PCR reactions and procedures were performed as described by Wang et al. (2017). Gene-specific oligonucleotide primers were designed using Primer3 and are listed in Supplementary Table S1 at JXB online. The specificity of the primers was double-checked by melting-curve and PCR-product resequencing. The housekeeping gene DkACT (Min et al., 2012) was chosen to monitor the abundance of mRNA. Real-time PCR reactions for each gene were performed at each sampling point of each treatment, with three biological replicates.
Dual-luciferase assay
A dual-luciferase assay was conducted to test the in vivo regulatory effects of TFs on promoters of softening-related genes. Full-length genes were amplified with primers (Supplementary Table S2) and fused to the pGreen II 0029 62-SK vector (SK; Hellens et al., 2005). Full-length of DkERF (DkERF8/16/19) genes had previously been constructed to SK by Min et al. (2012, 2014). Promoters of the cell wall metabolism-related genes (Dkβ-gal1/4, DkEGase1, DkPE1/2, DkPG1, and DkXTH9/10) were originally constructed by Wang et al. (2017), and were integrated into the pGreen II 0800-LUC vector (LUC; Hellens et al., 2005).
All of the recombinant SK and LUC vectors were electroporated into Agrobacterium tumefaciens GV3101. The transfected Agrobacterium were grown in Luria–Bertani (LB) medium plates with 50 μg ml–1 kanamycin and 25 μg ml–1 gentamycin for 2 d and then re-streaked onto new LB plates for 1 d. Agrobacterium samples were resuspended in infiltration buffer (10 mM MES, 10 mM MgCl2, 150 μM acetosyringone, pH 5.6) and adjusted to OD600 of ~0.75. The cultures with the TF and promoter were then mixed (v/v, 10:1; for synergistic effect analysis, the TF1:TF2: promoter was 5:5:1; for gradient dilutions analysis, the TF1:TF2: promoter ratio changed from 10:0:1, 9:1:1 to 0:10:1) and infiltrated into leaves of tobacco (Nicotiana benthamiana) using syringes without needles. At 3d after infiltration, leaf discs were collected from the infiltrated areas and assayed using dual-luciferase assay reagents (Promega). Dual-luciferase assays were conducted with three replicates.
Subcellular localization and phylogenetic analysis
35S-DkERF8/16-GFP (green fluorescent protein) and 35S-DkNAC9-GFP were transiently expressed in leaves of transgenic N. benthamiana with NLS-mCherry by Agrobacterium-mediated infiltration (GV3101) according to the method described by Xu et al. (2014). At 2 d after infiltration, the GFP signals of the leaves were imaged using a Zeiss LSM710NLO confocal laser-scanning microscope. The primers used for GFP constructions are listed in Supplementary Table S2.
The NAC gene (DkNAC9) was aligned with Arabidopsis NAC genes downloaded from the TAIR database (https://www.arabidopsis.org/). The alignment results were visualized using ClustalX (v.1.81) and FigTree (v.1.4.2).
Electrophoretic mobility shift assay
The full-length DkERF8, DkERF16, and DkNAC9 were inserted into the pGEX-4T-1 vector (GE), the constructs were then transformed into Rosetta (DE3) pLys bacteria (Novagen) by heat shock. Isopropyl β-d-1-thiogalactopyranoside (IPTG, 1 mM) was used to induce accumulation of the proteins at 16 °C for 20 h, then a GST-tag Protein Purification Kit (Beyotime Biotechnology) was used to purify the target proteins.
An electrophoretic mobility shift assay (EMSA) was performed using a LightShift Chemiluminescent EMSA kit (ThermoFisher Scientific). The probes used for this assay were synthesized and 3´-biotin labeled by HuaGene (Shanghai, China), and were mixed and annealed to the probe with its complementary chain to form a double strand.
Bimolecular fluorescence complementation assay
The full-length of DkERF8, DkERF16, and DkNAC9 were constructed into either C-terminal or N-terminal fragments of yellow fluorescent protein (YFP) vectors, using the primers listed in Supplementary Table S2 (the same primers were used in each case). All constructs were transiently expressed in leaves of transgenic N. benthamiana with NLS-mCherry by Agrobacterium-mediated infiltration (GV3101) according to previously published methods (Li et al., 2016). The YFP fluorescence of the tobacco leaves was imaged 1 d after infiltration using a Zeiss LSM710NLO confocal laser-scanning microscope.
Firefly luciferase complementation imaging assay
The full-length DkERF8, DkERF16, and DkNAC9 were constructed into both the pCAMBIA1300-nLuc and pCAMBIA1300-cLuc (luciferase) vectors, using the primers listed in Supplementary Table S2. All constructs were transiently expressed in tobacco leaves by Agrobacterium-mediated infiltration (GV3101) as described previously (Li et al., 2017a). The experimental combinations of TFs and the corresponding controls were injected into the same leaf and the luciferase activity of tobacco leaves was imaged 1 d after infiltration using a NightSHADE LB 985 imaging system (Berthold Technologies).
Statistical analysis
Excel was used to conduct t-tests and ANOVA followed by least-significant difference (LSD) analyses. The figures were drawn using GraphPadPrism7 and Adobe Photoshop CS6.
Accession numbers
The transcriptome data generated in this study have been submitted to the NCBI database with the BioProject ID PRJNA562104 and the GEO accession number GSE137588.
Sequence data from this paper are available at GenBank with the accession numbers KY849610, MH253881, MH621298, MK737978, MK737990, MK737999, MK738002, MK838487, MK838489, MK838490, and MK838492.
Results
Transcriptomic analysis and isolation of candidate TFs
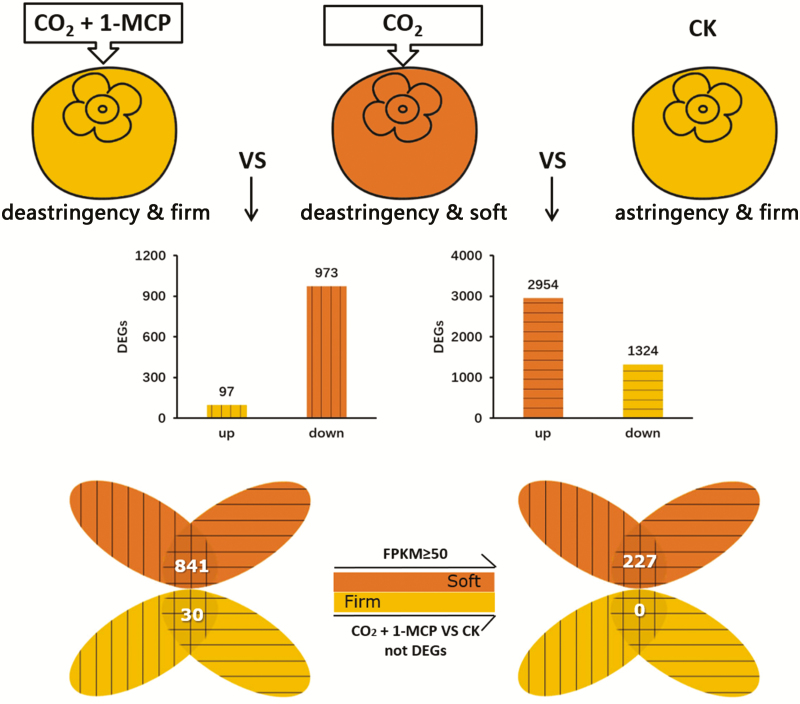
High-CO2+1-MCP maintains firmness in persimmon fruit (Wang et al., 2017), whilst high-CO2 causes rapid softening. Setting the adjusted P-value at <0.05 and |FoldChange| >5 as the thresholds, 2954 genes were found to be up-regulated by high-CO2 treatment (CO2) in ‘Jingmianshi’ persimmon fruit compared with the control (CK), and 1324 genes were down-regulated (Fig. 1). Comparison of the RNA-seq results for the high-CO2+1-MCP treatment (CO2+1-MCP) with the high-CO2 treatment alone indicated that 97 and 973 genes were up- and down-regulated, respectively. Firmness was one of parameters in the comparison (CO2 versus CK = soft versus firm; CO2+1-MCP versus CO2 = firm versus soft), and selection of genes that showed opposite trends in the two sets of samples enabled the number of potential candidate genes for post-deastringency softening to be reduced to 871 (Fig. 1). After removing the differentially expressed genes (DEGs) in the comparison between high-CO2+1-MCP and CK, the remaining DEGs with a maximum FPKM≥50 were then selected as a reference set (227 genes; Fig. 1, Supplementary Table S3) and their role in fruit softening was investigated. Of these 227 genes, 17 unigenes encoded putative TFs (Supplementary Table. S4). After full-length cloning and sequence analysis, 12 genes were obtained, namely DkAlfin-like1 (Alfin-like PHD finger, MK738002), DkbZIP12/13 (MK737990/MK838490), DkERF34/35 (MK737978/MK838487), DkNAC9/21 (MH253881/MK838489), DkPLATZ1 (plant AT-rich sequence- and zinc-binding, MK838492), DkWRKY3 (KY849610), DkYABBY2 (MK737999), DkZAT1 (C2H2 zinc-finger, MH621298) (Fig. 2), and the previously reported softening-related DkERF8 (see Wang et al., 2017).
Fig. 1.
Identification by RNA-seq of differentially expressed genes (DEGs) responding to different treatments in post-harvest ‘Jingmianshi’ persimmon fruit. Mature fruit were treated with 95% CO2 (CO2), 95% CO2+1 μl l–1 1-methylcyclopropene (CO2+1-MCP), or air (control, CK) for 1 d in air-tight containers, and then transferred to air. Samples were analysed 3 d after treatment. Orange indicates softening and yellow indicates that firmness was retained. The numbers of up- and down-regulated DEGs in each comparison are shown in the graphs. The Venn diagram to the left shows genes that had opposite trends in the two sets of comparisons. After removing the DEGs in the comparison between high-CO2+1-MCP and CK, 227 DEGs remained that had maximum FPKM values ≥50.
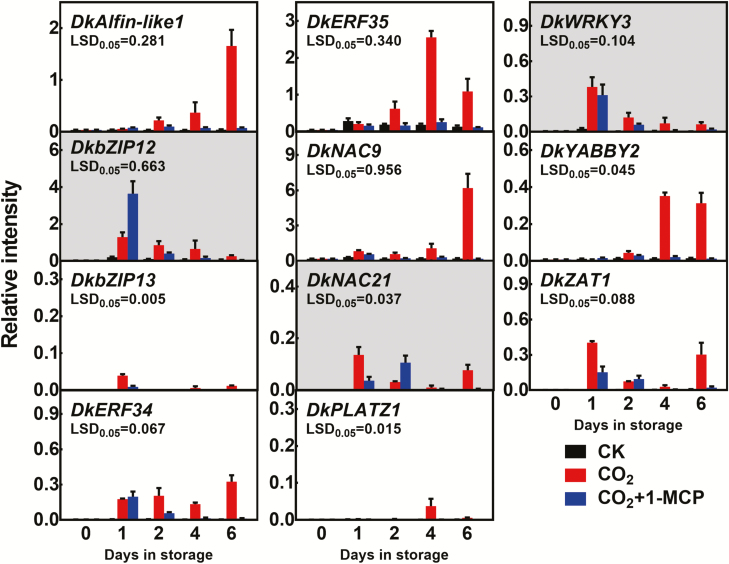
Fig. 2.
Expression of the differentially expressed transcription factors in ‘Jingmianshi’ persimmon fruit. Mature fruit were treated at 0 d in storage with 95% CO2 (CO2), 95% CO2+1 μl l–1 1-methylcyclopropene (CO2+1-MCP), or air (control, CK) for 1 d in air-tight containers, and then transferred to air. Transcript levels were analysed by RT-qPCR. The three genes shown with grey backgrounds were discarded from further investigation, due to their lack of agreement with the RNA-seq results. Relative intensity is the expression relative to that of DkACT. Data are means (±SE) of three biological replicates.
Transcript levels of the 11 newly isolated TFs were verified by reverse-transcription quantitative PCR (RT-qPCR). The expression of DkERF34/35, DkYABBY2, DkNAC9, DkAlfin-like1, DkPLATZ1, DkZAT1, and DkbZIP13 was substantially greater in high-CO2 than in the high-CO2+1-MCP and CK treatments (Fig. 2). DkbZIP12 and DkNAC21 were more abundant in the fruit treated with high-CO2+1-MCP at 1 d in storage (DIS) and 2 DIS respectively, and were not considered for further analysis. DkWRKY3 showed no significant differences at 1 DIS and 2 DIS between high-CO2+1-MCP and high-CO2 and was also not considered further (Fig. 2).
In vivo regulatory effects of selected TFs on promoters of genes involved in cell wall metabolism
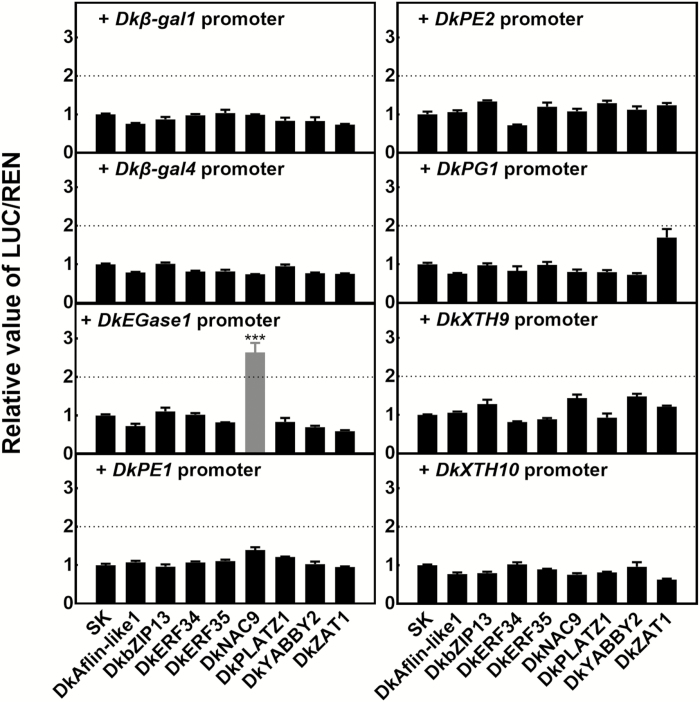
To examine the potential regulatory effects of TFs on fruit-softening genes, the previously isolated promoters of eight genes were selected, namely Dkβ-gal1/4, DkEGase1, DkPE1/2, DkPG1, and DkXTH9/10 (Wang et al., 2017). Dual-luciferase assays indicated there was significant transactivation (2.6-fold) by DkNAC9 on the DkEGase1 promoter, and very limited effects of the other TFs on the other gene promoters tested (Fig. 3).
Fig. 3.
Regulatory effects of transcription factors on the promoters of genes related to cell wall metabolism in persimmon fruit. Dual-luciferase assays were used for analysis of regulatory effects. Values of the ratio of LUC/REN are expressed relative to that of the empty vector (SK) plus promoter, which was set as 1. Data are means (±SE) of three replicates and significant differences were determined using Student’s t-test: ***P<0.001.
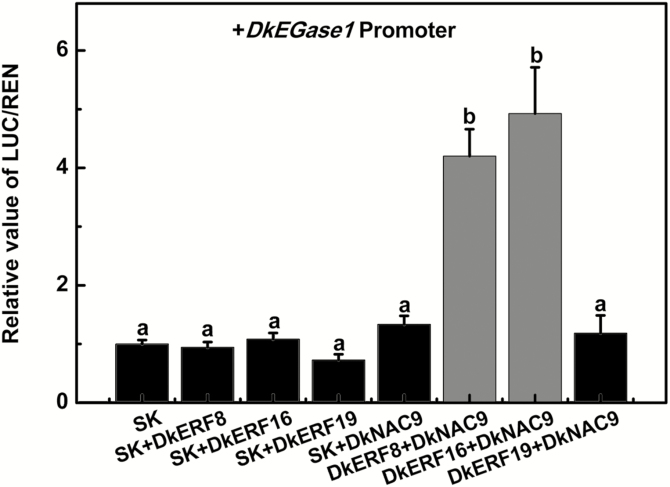
DkERFs 8, 16, and 19 have previously been shown to be positive regulators of the DkXTH9 promoter, thus indicating a role in persimmon post-deastringency softening, but they have little effect on the DkEGase1 promoter (Wang et al., 2017). Using dual-luciferase assays, the synergistic effects of DkERFs and DkNAC9 on the DkEGase1 promoter were investigated. As shown in Fig. 4, the combinations of DkERF8+DkNAC9 and DkERF16+DkNAC9 significantly enhanced the promoter activity of DkEGase1, by 4.2-fold and 4.9-fold, respectively, compared with the effects of individual DkERF8, DkERF16, or DkNAC9. However, the combination of DkERF19 and DkNAC9 failed to show any additive effect (Fig. 4).
Fig. 4.
The synergistic effects of DkNAC9 and DkERFs on the promoter of DkEGase1 in persimmon fruit. Dual-luciferase assays were used to test the synergistic effects of different combinations of transcription factors. Values of the ratio of LUC/REN are expressed relative to that of the empty vector (SK) plus promoter was, which was set as 1. Data are means (±SE) of three replicates. Different letters indicate significant differences between means as determined using ANOVA followed by LSD tests (P<0.05).
The three positively acting TFs were fused to a GFP tag and expressed in tobacco leaves in order to examine their subcellular localization. All three genes produced strong signals in the nucleus, although some DkERF8 signal was also found in plastids and DkERF16 was also found in the cell membrane (Supplementary Fig. S1).
Interactions between the TFs and the DkEGase1 promoter
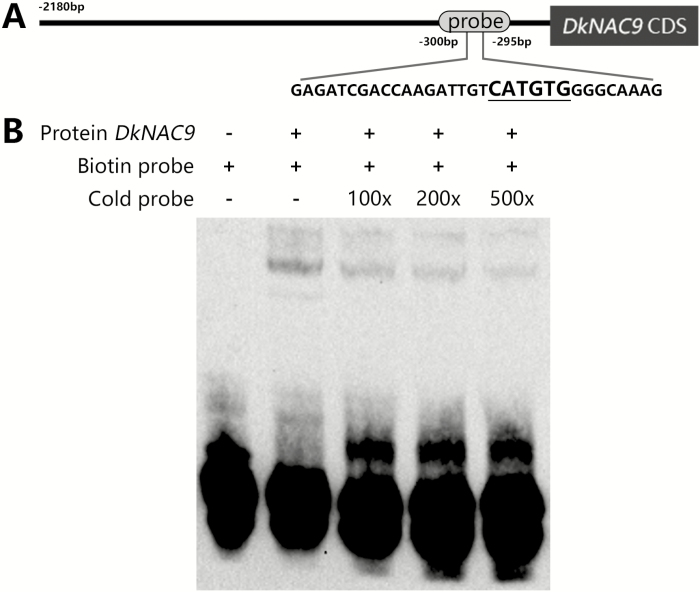
In order to test the physical interactions between TFs and the DkEGase1 promoter, EMSA was conducted. NAC family proteins have been reported to bind to the CATGTG motif (Tran et al., 2004) and there was only one CATGTG motif in the DkEGase1 promoter, which was located between approximately –300 bp and –295 bp (Fig. 5A). EMSAs indicated that DkNAC9 retarded the biotin-labeled probe and that the cold competitor probe (without biotin labeling) could reduce the band intensity (Fig. 5B). This indicated that DkNAC9 could physically bind to the CATGTG motif. DkERF8 and 16 belong to the AP2/ERF family, and this family of proteins usually interact with a GCC box or DRE motif to regulate ethylene-responsive genes (Huang et al., 2004; Zhang et al., 2009). However, neither sequence was found in the DkEGase1 promoter (from approximately –2180 to –1 bp). Some other rare motifs (e.g. ATCTA, CAACA) were also tried; however, the EMSA indicated that these ERF proteins could not interact with them (Supplementary Fig. S2).
Fig. 5.
Analysis of the binding ability of DkNAC9 to the promoter of DkEGase1. (A) The probe sequence used for electrophoretic mobility shift assay (EMSA) in the DkEGase1 promoter. The core sequence for NAC binding is underlined. (B) EMSA analysis. A 3´ biotin-labeled probe was used as the hot probe and the cold probe was without the 3´ biotin label.
Protein–protein interactions between DkNAC9 and DkERF8 and DkERF16
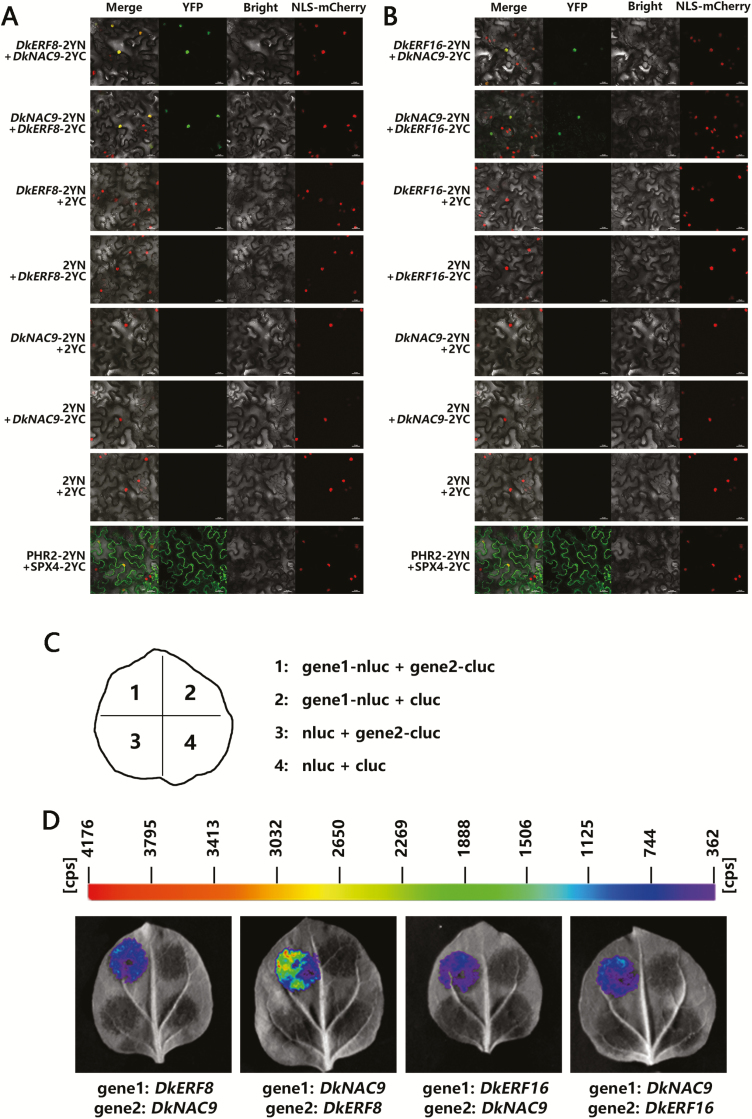
To clarify the synergistic effects of DkERF8 or DkERF16 with DkNAC9, we performed bimolecular fluorescence complementation (BiFC) assays using tobacco leaves. The results showed that co-expression of DkERF8-YFPN and DkNAC9-YFPC or DkNAC9-YFPN and DkERF8-YFPC both produced strong signals in the nucleus (Fig. 6A), while the negative combinations, such as DkERF8-YFPN + YFPC, YFPN + DkNAC9-YFPC, DkNAC9-YFPN+ YFPC, YFPN + DkERF8-YFPC, and YFPN+ YFPC, all failed to exhibit any detectable fluorescence signal. Similar results were also found for interactions between DkERF16 and DkNAC9 (Fig. 6B) and the firefly luciferase complementation imaging (LCI) assay confirmed their interactions. All combinations of DkERF8-nluc + DkNAC9-cluc, DkERF8-cluc + DkNAC9-nluc, DkERF16-nluc + DkNAC9-cluc, or DkERF16-cluc + DkNAC9-nluc emitted fluorescence signals (Fig. 6C, D).
Fig. 6.
Protein–protein interactions between DkNAC9 and DkERF8/16 as analysed by bimolecular fluorescence complementation (BiFC) and luciferase complementation imaging (LCI) assays. Interactions between DkNAC9 and (A) DkERF8 and (B) DkERF16 as determined by BIFC. N- and C-terminal fragments of yellow fluorescent protein (YFP) (2YN and 2YC, respectively) were fused to the C-terminus of DkNAC9 and DkERF8/16, respectively. Combinations of 2YN or 2YC with the corresponding DkNAC9 and DkERF8/16 constructs were used as negative controls. PHR2-2YN + SPX4-2YC was used as the positive control. Fluorescence of YFP represents protein–protein interaction. Scale bars are 25 μm. (C) Schematic diagram of the injections for LCI, showing one experimental group and its three controls in a tobacco leaf. (D) Interactions between DkNAC9 and DkERF8/16 as determined by LCI. Combinations of nluc or cluc with the corresponding DkNAC9 and DkERF8/16 constructs were used as negative controls.
Discussion
The molecular basis for softening has been widely investigated in various fruits and a few important regulators have been reported, such as MADS-RIN (Vrebalov et al., 2002), MaMYB3 (Fan et al., 2018), and AdDof3 (Zhang et al., 2018). Most of these regulators mainly contribute to natural softening. It is well known that non-optimal environments, such as low temperature (Xu et al., 2014) and water stress (Nakano et al., 2002), also have a major influence on fruit texture; however, these have rarely been studied. Post-deastringency softening of persimmon is triggered by high-CO2 treatment (i.e. an extreme low-oxygen environment) and we used this to examine the environment–texture interaction.
Transcriptomic analysis indicates multiple genes associated with post-deastringency softening
The underlying physiological, biochemical, and molecular effects of high-CO2 treatment on persimmon fruit deastringency have been extensively reported. Here, we found more than 4000 DEGs when comparing the high-CO2 treatment with controls (Fig. 1), which far exceeded the known number of deastringency regulators (~15 genes, namely DkADH1/DkPDC2, Min et al., 2012; DkERF9/10/19, Min et al., 2012, 2014; DkERF18/21/22 and DkMYB6/10, Zhu et al., 2018; DkERF23/24/25 and DkWRKY1/7, Zhu et al., 2019).
Wang et al. (2017) demonstrated that treatment with high CO2 combined with 1-MCP has similar effects on the maintenance of firmness in various cultivars of persimmon, which not only provides a potential practical application but also a means by which DEGs related to post-deastringency softening can be identified, by comparing the effects of high-CO2 alone versus high-CO2+1-MCP. Using RNA-seq, we obtained 12 full-length TFs among 227 DEGs, including the previously reported DkERF8 (see Wang et al., 2017). However, DkERF16/19, which have also been previously reported as being involved in fruit softening in persimmon, were not identified as being DEGs. We therefore individually examined the FPKM values of DkERF16/19 and found that their transcript levels were up-regulated by high-CO2 and suppressed by high-CO2+1-MCP (Supplementary Fig. S3). We assume that the failure to identify them as DEGs was due to the variation among the three biological replicates in the initial screening.
In addition to the TFs, it was notable that the 227 DEGs were significantly enriched in anthocyanin biosynthesis, pentose and glucuronate interconversions, phenylpropanoid biosynthesis, monoterpenoid biosynthesis, starch and sucrose metabolism, and plant hormone signal transduction pathways (Supplementary Fig. S4). Our focus was to characterize softening-related genes, so we did not further investigate these other physiological changes. The carotenoid content for astringent persimmon fruit has previously been found to be higher after high-CO2 treatment (Plaza et al., 2012), and the soluble sugar content has also been reported to change significantly during high-CO2 treatment, but without loss of sweetness (Ittah, 1993). However, the molecular mechanisms underlying these phenomena are still unclear, and in this respect our transcriptome data may also provide useful insights for future research.
Involvement of high CO2/hypoxia-responsive DkNAC9 in regulating post-deastringency softening
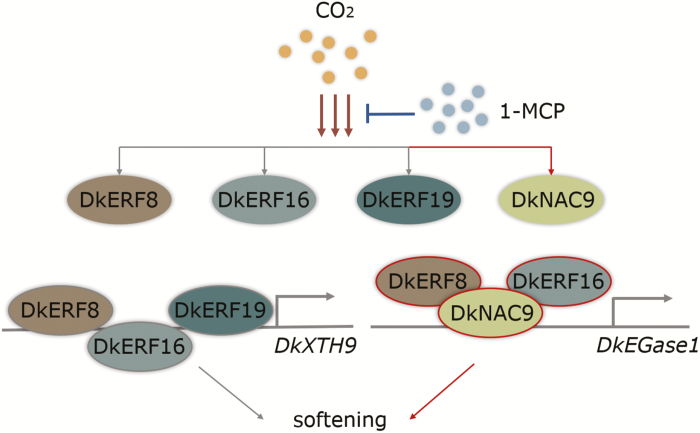
Among the 11 newly isolated high-CO2/hypoxia-responsive TFs, only a NAC TF (DkNAC9) showed significant transactivation of the DkEGase1 promoter, while the other TFs had little effect (Fig. 3). EMSA indicated a physical interaction of DkNAC9 with the CATGTG motif within the DkEGase1 promoter (Fig. 5). These results point to the direct regulation of post-deastringency softening by DkNAC9, via regulation of DkEGase1 (Fig. 7). The NACs belong to a family of plant-specific transcription factors that have been identified in various species (Aida et al., 1997; Berger et al., 2009; Zhong et al., 2010), and most of the reported NAC TFs have been shown to have diverse functions in plant growth, development, and stress responses (He et al., 2005; Christianson et al., 2009; Xia et al., 2010; Min et al., 2015). Moreover, NACs such as SlNAC1 (Ma et al., 2014) and Nor-like genes (Gao et al., 2018) have been reported to have roles in tomato ripening. However, the regulatory mechanisms of these NACs on fruit softening have not been specifically investigated. Our identification of DkNAC9 has not only revealed another regulator for post-deastringency softening of persimmon fruit, but also provides new clues with regards to regulation NAC-induced softening. Phylogenetic analysis indicated that DkNAC9 is a homolog of AtNAC102 (Supplementary Fig. S5), which is involved in the low-oxygen response in germinating seedlings of Arabidopsis (Christianson et al., 2009). It remains unclear whether or not AtNAC102 is related to cell wall metabolism. As AtNAC102 is a hypoxic-response gene, we also examined the role of DkNAC9 in regulation of DkADH1 and DkPDC2, two genes associated with anaerobic respiration (Min et al., 2012), and the results indicated the DkPDC2 promoter was transactivated by DkNAC9 (Supplementary Fig. S6). Thus, DkNAC9 has a broader role in responses induced by low oxygen in persimmon fruit.
Fig 7.
Proposed regulatory model for the role of transcription factors in persimmon fruit post-deastringency softening. Transcripts of DkERF8/16/19 (Wang et al., 2017) and DkNAC9 are activated by high-CO2, but reduced when 1-methylcyclopropene (1-MCP) is also present. DkERF8/16/19 are involved in regulation of DkXTH9, which is related to cell wall metabolism (Wang et al., 2017). DkNAC9 is a direct activator of the DkEGase1 promoter, and DkERF8 and DkERF16 can interact with DkNAC9 to enhance this regulation. Red lines indicate new data from the current study and grey lines indicate previously published results.
EGase is the enzyme that hydrolyses glucosidal bonds in the 1,4-β-glucan backbone, resulting in cellulose degradation. In tomato, the expressions of EGases has been reported to increase during ripening (Gonzalez-Bosch et al., 1996), and similar phenomena have been observed in other fruits, such as strawberry (Knee et al., 1977) and avocado (O’Donoghue and Huber, 1992). Furthermore, the suppression of EGase results in firmer fruit and overexpression of EGase genes accelerates fruit softening (Brummell et al., 1999). DkEGase1 was the only coding gene for an EGase that reached the selection threshold in our previous study (Wang et al., 2017), and therefore it may be regarded as the main contributor to the post-harvest decrease in cellulose. The role of DkNAC9 in DkEGase1 regulation thus provides a new means by which the mechanism of fruit softening in persimmon may be elucidated.
DkERF8 and DkERF16 are indirect regulators of the DkEGase1 promoter via protein–protein interactions with DkNAC9
Our previous research indicated that three ethylene response-factor genes, DkERF8/16/19, are involved in regulation of the promoter of DkXTH9 (Wang et al., 2017). In our current study, we found synergistic interactions between DkNAC9 and both DkERF8 and DkERF16 (Fig. 4), which generated enhanced transactivation of the DkEGase1 promoter. The differences in activation by DkNAC9 that can be seen in Figs 3 and 4 can be explained by the different concentrations used (Supplementary Fig. S7) with a DkNAC9:SK:promoter ratio of 5: 5: 1 (v:v:v) in Fig 4, compared with 10:0:1 in Fig 3. It can be assumed that DkERF8 and DkERF16 are indirect regulators, as all the EMSAs failed to generate band shifts (Supplementary Fig. S2). Moreover, BiFC and LCI assays all indicated potential protein–protein interactions between DkNAC9 and DkERF8 or DkERF16 (Fig. 6). Thus, it can be proposed that the DkEGase1 promoter is directly regulated by DkNAC9, which is also the mediator for interactions with DkERF8/16 (Fig. 7). Our previous study indicated the direct regulation by DkERF8 and DkERF16 on the DkXTH9 promoter (Wang et al., 2017), and hence DkERF8/16 have dual direct and indirect functions in the regulation of two major cell wall genes (i.e. DkXTH9 for hemicellulose degradation and DkEGase1 for cellulose degradation) (Fig. 7).
A few ERFs have been reported as regulators for softening in kiwifruit (AdERF9; Yin et al., 2010), apple (MdCBF1; Tacken et al., 2010), tomato (Sl-ERF.B3; Liu et al., 2014), and banana (MaDEAR1; Fan et al., 2016). With regards to the regulation of ripening, protein complexes involving either ERFs or NACs have been reported in various fruit. For example, MaNAC1/2 interacts with the MaEIL5 transcription factor involved in ripening in banana (Shan et al., 2012), a complex of CitNAC62 and CitWRKY1 contributes to citric acid degradation in citrus fruit (Li et al., 2017a), and AP2/ERF genes have been reported as the partners for MYB transcription factors in the regulation of lignin (EjAP2-1, loquat; Zeng et al., 2015) and anthocyanin (PyERF3, pear; Yao et al., 2017). However, interactions between NAC and ERF transcription factors have rarely been reported for the regulation of fruit ripening. In conclusion, our present research not only suggests the involvement of DkNAC9 in regulating post-deastringency softening of persimmon fruit, but has also revealed the transcriptional complexes DkNAC9–DkERF8/16, which may also prove to be useful in understanding the roles of ripening-related NACs or ERFs in the regulation of fruit texture.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Subcellular localization of DkERF8/16-GFP and DkNAC9-GFP expressed in tobacco leaves.
Fig. S2. Analysis of the binding ability of DkERF8/16 to the promoter of DkEGase1.
Fig. S3. The FPKM values of previously reported DkERFs in response to CO2 and CO2+1-MCP treatments in the ‘Jingmianshi’ cultivar.
Fig. S4. KEGG enrichment analyses of DEGs in response to the different treatments.
Fig. S5. Phylogenetic analyses of persimmon NAC genes.
Fig. S6. Regulatory effects of DkNAC9 with or without DkERF8/16 on the promoters of DkADH1 and DkPDC2.
Fig. S7. Effects of different dilutions of DkNAC9 on the DkEGase1 promoter.
Table S1. Sequences of the primers used for real-time PCR.
Table S2. Sequences of the primers used for vector construction.
Table S3. The expression of 227 DEGs in response to the different treatments.
Table S4. Annotation of the 227 DEGs in response to the different treatments.
Acknowledgements
We would like to thank Prof. Don Grierson (University of Nottingham, UK) for critical reading and revision of the manuscript, and Miss Rong Jin and Mr Xiaoheng Wu (Agricultural Experiment Station, Zhejiang University) for support with facilities. This research was supported by the National Key Research and Development Program (2016YFD0400102), the National Natural Science Foundation of China (31722042; 31672204), the Fok Ying Tung Education Foundation, China (161028), and the Fundamental Research Funds for the Central Universities (2018XZZX002-03). The authors have no conflicts of interest to declare.
Glossary
Abbreviations
- 1-MCP
1-methylcyclopropene
- BiFC
bimolecular fluorescence complementation
- DEGs
differentially expressed genes
- DIS
days in storage
- EGase
endo-1,4-β-d-glucanase
- EMSA
electrophoretic mobility shift assay
- ERF
ethylene response factor
- LCI
luciferase complementation imaging
- TFs
transcription factors
- XTH
xyloglucan endotransglycosylase/hydrolase
- YFP
yellow fluorescent protein
References
- Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M. 1997. Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. The Plant Cell 9, 841–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger Y, Harpaz-Saad S, Brand A, Melnik H, Sirding N, Alvarez JP, Zinder M, Samach A, Eshed Y, Ori N. 2009. The NAC-domain transcription factor GOBLET specifies leaflet boundaries in compound tomato leaves. Development 136, 823–832. [DOI] [PubMed] [Google Scholar]
- Biais B, Beauvoit B, Allwood JW, Deborde C, Maucourt M, Goodacre R, Rolin D, Moing A. 2010. Metabolic acclimation to hypoxia revealed by metabolite gradients in melon fruit. Journal of Plant Physiology 167, 242–245. [DOI] [PubMed] [Google Scholar]
- Brummell DA. 2006. Cell wall disassembly in ripening fruit. Functional Plant Biology 33, 103–119. [DOI] [PubMed] [Google Scholar]
- Brummell DA, Hall BD, Bennett AB. 1999. Antisense suppression of tomato endo-1,4-beta-glucanase Cel2 mRNA accumulation increases the force required to break fruit abscission zones but does not affect fruit softening. Plant Molecular Biology 40, 615–622. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Wilson IW, Llewellyn DJ, Dennis ES. 2009. The low-oxygen-induced NAC domain transcription factor ANAC102 affects viability of Arabidopsis seeds following low-oxygen treatment. Plant Physiology 149, 1724–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MY, Verbalov J, Alba R, Lee J, McQuinn R, Chung JD, Klein P, Giovannoni J. 2010. A tomato (Solanum lycopersicum) APETALA2/ERF gene, SlAP2a, is a negative regulator of fruit ripening. The Plant Journal 64, 936–947. [DOI] [PubMed] [Google Scholar]
- Cutillas-Iturralde A, Zarra I, Fry SC, Lorences EP. 1994. Implication of persimmon fruit hemicellulose metabolism in the softening process. Importance of xyloglucan endotransglycosylase. Physiologia Plantarum 91, 169–176. [Google Scholar]
- Fan ZQ, Ba LJ, Shan W, Xiao YY, Lu WJ, Kuang JK, Chen JY. 2018. A banana R2R3-MYB transcription factor MaMYB3 is involved in fruit ripening through modulation of starch degradation by repressing starch degradation-related genes and MabHLH6. The Plant Journal 96, 1191–1205. [DOI] [PubMed] [Google Scholar]
- Fan ZQ, Kuang JF, Fu CC, et al.. 2016. The banana transcriptional repressor MaDEAR1 negatively regulates cell wall-modifying genes involved in fruit ripening. Frontiers in Plant Science 7, 1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa M, Nakano T, Shima Y, Ito Y. 2013. A large-scale identification of direct targets of the tomato MADS box transcription factor RIPENING INHIBITOR reveals the regulation of fruit ripening. The Plant Cell 25, 371–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Wei W, Zhao X, et al. 2018. A NAC transcription factor, NOR-like1, is a new positive regulator of tomato fruit ripening. Horticulture Research 5, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Bosch C, Brummell DA, Bennett AB. 1996. Differential expression of two endo-1,4-[beta]-glucanase genes in pericarp and locules of wild-type and mutant tomato fruit. Plant Physiology 111, 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabherr MG, Haas BJ, Yassour M, et al.. 2011. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nature Biotechnology 29, 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Ban Q, Li H, Hou Y, Jin M, Han S, Rao J. 2016. DkXTH8, a novel xyloglucan endotransglucosylase/hydrolase in persimmon, alters cell wall structure and promotes leaf senescence and fruit postharvest softening. Scientific Reports 6, 39155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harima S, Nakano R, Yamauchi S, Kitano Y, Yamamoto Y, Inaba A, Kubo Y. 2003. Extending shelf-life of astringent persimmon (Diospyros kaki Thunb.) fruit by 1-MCP. Postharvest Biology and Technology 29, 319–323. [Google Scholar]
- He XJ, Mu RL, Cao WH, Zhang ZG, Zhang JS, Chen SY. 2005. AtNAC2, a transcription factor downstream of ethylene and auxin signaling pathways, is involved in salt stress response and lateral root development. The Plant Journal 44, 903–916. [DOI] [PubMed] [Google Scholar]
- Hellens RP, Allan AC, Friel EN, Bolitho K, Grafton K, Templeton MD, Karunairetnam S, Gleave AP, Laing WA. 2005. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Zhang Z, Zhang X, Zhang H, Huang D, Huang R. 2004. Tomato TERF1 modulates ethylene response and enhances osmotic stress tolerance by activating expression of downstream genes. FEBS Letters 573, 110–116. [DOI] [PubMed] [Google Scholar]
- Ittah Y. 1993. Sugar content changes in persimmon fruits (Diospyros kaki L.) during artificial ripening with CO2: a possible connection to deastringency mechanisms. Food Chemistry 48, 25–29. [Google Scholar]
- Knee M, Sargent JA, Osborne DJ. 1977. Cell wall metabolism in developing strawberry fruits. Journal of Experimental Botany 28, 377–396. [Google Scholar]
- Li SJ, Yin XR, Wang WL, Liu XF, Zhang B, Chen KS. 2017a Citrus CitNAC62 cooperates with CitWRKY1 to participate in citric acid degradation via up-regulation of CitAco3. Journal of Experimental Botany 68, 3419–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SJ, Yin XR, Xie XL, Allan AC, Ge H, Shen SL, Chen KS. 2016. The Citrus transcription factor, CitERF13, regulates citric acid accumulation via a protein–protein interaction with the vacuolar proton pump, CitVHA-c4. Scientific Reports 6, 20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Xu Y, Zhang L, Ji Y, Tan D, Yuan H, Wang A. 2017b The jasmonate-activated transcription factor MdMYC2 regulates ethylene response factor and ethylene biosynthetic genes to promote ethylene biosynthesis during apple fruit ripening. The Plant Cell 29, 1316–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Xu CJ, Korban SS, Chen KS. 2010. Regulatory mechanisms of textural changes in ripening fruits. Critical Reviews in Plant Science 29, 222–243. [Google Scholar]
- Liu MC, Diretto G, Pirrello J, Roustan JP, Li Z, Giuliano G, Regad F, Bouzayen M. 2014. The chimeric repressor version of an Ethylene Response Factor (ERF) family member, Sl-ERF.B3, shows contrasting effects on tomato fruit ripening. New Phytologist 203, 206–218. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZS. 2005. Effects of 1-methylcyclopropene on ripening of postharvest persimmon (Diospyros kaki L.) fruit. LWT – Food Science and Technology 40, 285–291. [Google Scholar]
- Ma N, Feng H, Meng X, Li D, Yang D, Wu C, Meng Q. 2014. Overexpression of tomato SlNAC1 transcription factor alters fruit pigmentation and softening. BMC Plant Biology 14, 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Cai T, Olyarchuk JG, Wei L. 2005. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 21, 3787–3793. [DOI] [PubMed] [Google Scholar]
- McDonald B, Harman JE. 1982. Controlled-atmosphere storage of kiwifruit. I. Effect on fruit firmness and storage life. Scientia Horticulturae 17, 113–123 [Google Scholar]
- Meng X, Yang D, Li X, Zhao S, Sui N, Meng Q. 2015. Physiological changes in fruit ripening caused by overexpression of tomato SlAN2, an R2R3-MYB factor. Plant Physiology and Biochemistry 89, 24–30. [DOI] [PubMed] [Google Scholar]
- Min T, Fang F, Ge H, Shi YN, Luo ZR, Yao YC, Grierson D, Yin XR, Chen KS. 2014. Two novel anoxia-induced ethylene response factors that interact with promoters of deastringency-related genes from persimmon. PLoS ONE 9, e97043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min T, Wang MM, Wang H, Liu X, Fang F, Grierson D, Yin XR, Chen KS. 2015. Isolation and expression of NAC genes during persimmon fruit postharvest astringency removal. International Journal of Molecular Sciences 16, 1894–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min T, Yin XR, Shi YN, Luo ZR, Yao YC, Grierson D, Ferguson IB, Chen KS. 2012. Ethylene-responsive transcription factors interact with promoters of ADH and PDC involved in persimmon (Diospyros kaki) fruit de-astringency. Journal of Experimental Botany 63, 6393–6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano R, Inoue S, Kubo Y, Inaba A. 2002. Water stress-induced ethylene in the calyx triggers autocatalytic ethylene production and fruit softening in ‘Tonewase’ persimmon grown in a heated plastic-house. Postharvest Biology and Technology 25, 293–300. [Google Scholar]
- Nakatsuka A, Maruo T, Ishibashi C, Ueda Y, Kobayashi N, Yamagishi M, Itamura H. 2011. Expression of genes encoding xyloglucan endotransglycosylase/hydrolase in ‘Saijo’ persimmon fruit during softening after deastringency treatment. Postharvest Biology and Technology 62, 89–92. [Google Scholar]
- O’Donoghue EM, Huber DJ. 1992. Modification of matrix polysaccharides during avocado (Persea americana) fruit ripening: an assessment of the role of Cx-cellulase. Physiologia Plantarum 86, 33–42. [Google Scholar]
- Pedreschi R, Franck C, Lammertyn J, Erban A, Joachim K, Hertog M, Verlinden B, Nicolaï B. 2009. Metabolic profiling of ‘Conference’ pears under low oxygen stress. Postharvest Biology and Technology 51, 123–130. [Google Scholar]
- Plaza L, Colina C, Ancos BD, Sánchez-Moreno C, Cano MP. 2012. Influence of ripening and astringency on carotenoid content of high-pressure treated persimmon fruit (Diospyros kaki L.). Food Chemistry 130, 591–597. [Google Scholar]
- Shan W, Kuang JF, Chen L, et al.. 2012. Molecular characterization of banana NAC transcription factors and their interactions with ethylene signalling component EIL during fruit ripening. Journal of Experimental Botany 63, 5171–5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui S, Brackmann A, Streif J, Bangerth F. 1996. Controlled atmosphere storage of apples: cell wall composition and fruit softening. The Journal of Horticultural Science 71, 613–620. [Google Scholar]
- Tacken E, Ireland H, Gunaseelan K, Karunairetnam S, Wang D, Schultz K, Bowen J, Atkinson RG, Johnston JW, Putterill J. 2010. The role of ethylene and cold temperature in the regulation of the apple POLYGALACTURONASE1 gene and fruit softening. Plant Physiology 153, 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira S, Ikeda K, Ohkawa K. 2001. Comparison of insolubility of tannins induced by acetaldehyde vapor in fruit of three types of astringent persimmon. Journal of Japanese Society for Horticultural Science 48, 684–687. [Google Scholar]
- Tran LS, Nakashima K, Sakuma Y, Simpson SD, Fujita Y, Maruyama K, Fujita M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. 2004. Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. The Plant Cell 16, 2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. 2010. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature Biotechnology 28, 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni J. 2002. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science 296, 343–346. [DOI] [PubMed] [Google Scholar]
- Wang D, Yeats TH, Uluisik S, Rose JKC, Seymour GB. 2018. Fruit softening: revisiting the role of pectin. Trends in Plant Science 23, 302–310. [DOI] [PubMed] [Google Scholar]
- Wang MM, Zhu QG, Deng CL, Luo ZR, Sun NJ, Grierson D, Yin XR, Chen KS. 2017. Hypoxia-responsive ERFs involved in postdeastringency softening of persimmon fruit. Plant Biotechnology Journal 15, 1409–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RZ, Yang Y, Li GC. 1997. Chinese persimmon germplasm resources. Acta Horticulturae 436, 43–50. [Google Scholar]
- Xia N, Zhang G, Sun YF, et al. 2010. TaNAC8, a novel NAC transcription factor gene in wheat, responds to stripe rust pathogen infection and abiotic stresses. Physiological and Molecular Plant Pathology 74, 394–402. [Google Scholar]
- Xie XL, Yin XR, Chen KS. 2016. Roles of APETALA2/ethylene-response factors in regulation of fruit quality. Critical Reviews in Plant Sciences 35, 120–130. [Google Scholar]
- Xu Q, Yin XR, Zeng JK, Ge H, Song M, Xu CJ, Li X, Ferguson IB, Chen KS. 2014. Activator- and repressor-type MYB transcription factors are involved in chilling injury induced flesh lignification in loquat via their interactions with the phenylpropanoid pathway. Journal of Experimental Botany 65, 4349–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahia EM. 1998. Modified and controlled atmospheres for tropical fruits. Horticultural Reviews 22, 123–183. [Google Scholar]
- Yamada M, Yamane H, Sato A, Hirakawa N, Wang RZ. 1994. Variations in fruit ripening time, fruit weight and soluble solids content of oriental persimmon cultivars native to Japan. Journal of the Japanese Society for Horticultural Science 63, 485–491. [Google Scholar]
- Yang ZF, Zheng YH, Cao SF, Tang SS, Ma SJ, Li N. 2007. Effects of storage temperature on textural properties of Chinese bayberry fruit. Journal of Textural Studies 38, 166–177. [Google Scholar]
- Yao G, Ming M, Allan AC, et al.. 2017. Map-based cloning of the pear gene MYB114 identifies an interaction with other transcription factors to coordinately regulate fruit anthocyanin biosynthesis. The Plant Journal 92, 437–451. [DOI] [PubMed] [Google Scholar]
- Yin XR, Allan AC, Chen KS, Ferguson IB. 2010. Kiwifruit EIL and ERF genes involved in regulating fruit ripening. Plant Physiology 153, 1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin XR, Shi YN, Min T, Luo ZR, Yao YC, Xu Q, Ferguson I, Chen KS. 2012. Expression of ethylene response genes during persimmon fruit astringency removal. Planta 235, 895–906. [DOI] [PubMed] [Google Scholar]
- Zeng JK, Li X, Xu Q, Chen JY, Yin XR, Ferguson IB, Chen KS. 2015. EjAP2-1, an AP2/ERF gene, is a novel regulator of fruit lignification induced by chilling injury, via interaction with EjMYB transcription factors. Plant Biotechnology Journal 13, 1325–1334. [DOI] [PubMed] [Google Scholar]
- Zhang AD, Wang WQ, Tong Y, Li MJ, Grierson D, Ferguson I, Chen KS, Yin XR. 2018. Transcriptome analysis identifies a zinc finger protein regulating starch degradation in kiwifruit. Plant Physiology 178, 850–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhang H, Quan R, Wang XC, Huang R. 2009. Transcriptional regulation of the ethylene response factor LeERF2 in the expression of ethylene biosynthesis genes controls ethylene production in tomato and tobacco. Plant Physiology 150, 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZK, Fu RS, Huber DJ, Rao JP, Chang XX, Hu MJ, Zhang Y, Jiang NN. 2012. Expression of expansin gene (CDK-Exp3) and its modulation by exogenous gibberellic acid during ripening and softening of persimmon fruit. HortScience 47, 378–381. [Google Scholar]
- Zhong R, Lee C, Ye ZH. 2010. Functional characterization of poplar wood-associated NAC domain transcription factors. Plant Physiology 152, 1044–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu QG, Gong ZY, Huang J, Grierson D, Chen KS, Yin XR. 2019. High-CO2/hypoxia-responsive transcription factors DkERF24 and DkWRKY1 interact and activate DkPDC2 promoter. Plant Physiology 180, 621–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu QG, Gong ZY, Wang MM, Li X, Grierson D, Yin XR, Chen KS. 2018. A transcription factor network responsive to high CO2/hypoxia is involved in deastringency in persimmon fruit. Journal of Experimental Botany 69, 2061–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu QG, Zhang Z, Rao J, Huber DJ, Lv J, Hou Y, Song K. 2013. Identification of xyloglucan endotransglucosylase/hydrolase genes (XTHs) and their expression in persimmon fruit as influenced by 1-methylcyclopropene and gibberellic acid during storage at ambient temperature. Food Chemistry 138, 471–477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.