We prove the relevance of callose priming in mycorrhiza-induced resistance against shoot pathogens and studied the main mechanisms regulating it such as starch mobilization, sugar delivery, and vesicular trafficking.
Keywords: Botrytis cinerea, callose, mycorrhiza-induced resistance, priming, starch degradation, sugar transport
Abstract
Mycorrhizal plants display enhanced resistance to several pathogens. However, the molecular mechanisms regulating mycorrhiza-induced resistance (MIR) are still elusive. We aim to study the mechanisms underlying MIR against Botrytis cinerea and the role of callose accumulation during this process. Mycorrhizal tomato plants inoculated with Rhizoglomus irregularis displayed callose priming upon B. cinerea infection. The callose inhibitor 2-deoxy-d-glucose abolished MIR, confirming the relevance of callose in the bioprotection phenomena. While studying the mechanisms underlying mycorrhiza-induced callose priming, we found that mycorrhizal plants display an enhanced starch degradation rate that is correlated with increased levels of β-amylase1 transcripts following pathogen infection. Starch mobilization in mycorrhizal plants seems coordinated with the increased transcription of sugar transporter and invertase genes. Moreover, the expression levels of genes encoding the vesicular trafficking proteins ATL31 and SYP121 and callose synthase PMR4 were higher in the mycorrhizal plants and further boosted by subsequent pathogen infection. All these proteins play a key role in the priming of callose accumulation in Arabidopsis, suggesting that callose priming is an induced resistance mechanism conserved in different plant species. This evidence highlights the importance of sugar mobilization and vesicular trafficking in the priming of callose as a defence mechanism in mycorrhiza-induced resistance.
Introduction
Beneficial microorganism–plant interactions are widespread in nature. Among such interactions, >80% of plants are associated with arbuscular mycorrhiza fungi (AMF), which are soil-borne obligate biotrophs belonging to the Glomeromycota phylum. AMF are found in practically all agricultural and natural environments. These fungi establish a mutualistic symbiosis with plant roots, forming specialized intracellular structures in the root cortex known as arbuscules, where the interchange of nutrients between the symbionts occurs (Gutjahr and Parniske, 2013). Once symbiosis is well established, the plant provides photosynthates and lipids to the fungus and, in return, the AMF improve the plant mineral nutrients and water uptake. In addition to these benefits, AM plants present enhanced tolerance to abiotic and biotic stresses (Miransari, 2010; Jung et al., 2012; Sánchez-Bel et al., 2016; Rivero et al., 2018).
Several studies have shown that AM plants are more resistant to soil-borne and shoot pathogens. Upon biotic challenges, AM plants mount defences in a faster and more efficient manner, representing a phenomenon known as defence priming (Pozo and Azcón-Aguilar, 2007; Jung et al., 2012; Martinez-Medina et al., 2016; Mauch-Mani et al., 2017). Hence, priming is suggested as the mechanism underlying mycorrhiza-induced resistance (MIR; Jung et al., 2012; Balmer et al., 2015). Earlier studies have revealed systemic protection by mycorrhiza against root pathogens associated with the enhanced accumulation of pathogenesis-related (PR) proteins, phenolic compounds, and callose-containing cell wall appositions at pathogen entrance points (Cordier et al., 1998). Funneliformis mosseae in symbiosis with tomato plants alleviated Alternaria solani disease by priming some defence-related genes, and the authors showed that oxylipin [jasmonic acid (JA)]-dependent defences support MIR since JA-deficient spr2 mutant plants did not display the induced resistance (Song et al., 2015). Oxylipin-related responses have also been reported in Rhizoglomus irregulairs-induced resistance to Botrytis cinerea (Sánchez-Bel et al., 2016).
The benefits of symbiosis have also been reported in AM plants facing abiotic and biotic stresses simultaneously. Non-mycorrhizal (NM) plants usually react to nutritional stress by preparing their metabolism to address the nutrient deficiency at the cost of biotic stress defences. However, AM plants buffer the growth–defence balance by maintaining functional biotic defences under nitrogen starvation (Sánchez-Bel et al., 2018).
One of the most intriguing cellular defence responses against pathogens is the deposition of β-glucan polysaccharide callose. This sugar polymer reinforces plant cell walls against attackers, blocks their entrance, and provides the plant with additional time to activate subsequent defence mechanisms if needed. AM plants infected with Blumeria graminis show increased papillae formation at penetration sites (Mustafa et al., 2017). Moreover, AMF can trigger callose accumulation in wheat following chitosan infiltration (Pérez-de-Luque et al., 2017), although the underlying molecular mechanisms have not been explored.
Callose synthase is the enzyme responsible for the accumulation of callose at the papillae. In Arabidopsis plants, 12 callose synthase genes named GSL1 to GSL12 have been identified. Of these genes, GSL5 (also named PMR4), is the callose synthase expressed upon pathogenic infection. In tomato plants, two orthologues of Arabidopsis PMR4 were found, but only one orthologue has been shown to share the same function (Huibers et al., 2013).
Despite the relevance of callose accumulation in induced defences, knowledge regarding the sugar supply required for callose formation is lacking. It has been suggested that indolic glucosinolates in Arabidopsis (Clay et al., 2009) or indolic benzoxazinoids in maize (Ahmad et al., 2011) may provide precursors for callose accumulation. Recently, we showed that starch degradation and subsequent vesicular sugar transport mediate the priming of callose deposition in Arabidopsis (Gamir et al., 2018). The final steps of callose accumulation at the cell wall are regulated by interactions between SNARE proteins. The SNARE complex is required for papilla formation at the fungal entry site. In Arabidopsis, the overexpression of AtATL31 (Arabidopsis Toxicos en Levadura 31) confers resistance to powdery mildew by an enhancement of callose accumulation at early time points of the infection (Maekawa et al., 2014). Recently, our group has shown that in Arabidopsis, ATL31 and its interactor SYP121 regulate indole-3-carboxylic acid (I3CA) callose priming downstream of BAM1 (Gamir et al., 2018).
The modulation of sugar pools in plants is considered to function as a signal to prime immune responses (Gómez-Ariza et al., 2007). Sugar allocation between source leaves and sink organs is of primary importance for both AM symbiosis and plant–pathogen interactions. In addition to plant–pathogen interactions, specific sugars are proposed to function as priming agents in several plant–pathogen systems (Bolouri Moghaddam and Van den Ende, 2012). Although sugar partitioning in plants is a complex topic, exogenous applications of specific sugars, such as galactinol (Kim et al., 2008), trehalose (Singh et al., 2011), and oligogalacturonides, can trigger PR gene expression among other plant immune responses (Bolouri Moghaddam and Van den Ende, 2012). Recently, the concept of ‘sweet immunity’ has been demonstrated in lettuce against B. cinerea (Tarkowski et al., 2019). In addition, the availability of carbohydrates affects plant resistance against shoot pathogens (reviewed by Trouvelot et al., 2014). Thus, both plants and pathogens have evolved mechanisms to modulate the carbohydrate content and flux targeting different steps of the carbohydrate biosynthesis, transport, and degradation processes (Essmann et al., 2008; Scharte et al., 2009; Asai et al., 2016). In fact, during plant–pathogen interactions, the expression of genes encoding cell wall invertases is induced by elicitors in different plant species (Proels and Hückelhoven, 2014). Thus, invertases and the released sugars may act as signals for defence against pathogens (Kocal et al., 2008).
The sugar and lipids provided by the host to AMF are among the most relevant benefits obtained by the fungus during symbiosis (Smith and Smith, 2011; Rich et al., 2017). Regarding the potential role of sugars in defence, the extent to which the differential response of AM plants to diseases is due to mycorrhizal-related changes in carbohydrate metabolism remains unexplored. Several sugar transporter genes belonging to the SUT family (SUT1, 2, and 4) and SWEET family, some invertase genes (LIN6), and sucrose synthases have higher levels during symbiosis (Schaarschmidt et al., 2007; Roth and Paszkowski, 2017). However, the role of sugar mobilization during symbiosis is far more complex; for example, SUT2 has been suggested to play a sensing role rather than simply playing a role in sugar transport (Barker et al., 2000), although this hypothesis has not been further supported.
In the present study, we prove the relevance of the priming of callose deposition in MIR against the fungal foliar pathogen B. cinerea. Moreover, we shed some light on the mechanisms regulating such an enhanced production of callose, which seems to rely on enhanced starch mobilization and sugar delivery to produce such defence structures. We explored the regulation of a stress-related β-amylase and several invertases and sugar transporters that probably mediate callose deposition priming during MIR. Furthermore, we analysed the relevance and contribution of vesicular transport delivering glucose to the cell wall for this defence mechanism.
Materials and methods
Plant material and AMF inoculation
Tomato seeds (Solanum lycopesicum L. cv. Better Boy) were sterilized with 10% HCl (v/v) and rinsed abundantly with sterile water. Seeds were germinated in sterile vermiculite in a growth chamber with a 16 h light period, 70% relative humidity, and 26 °C during the day and 18 °C during the night. Later, seedlings were transplanted to 200 cm3 pots with sterile vermiculite. The AMF Rhizoglomus irregularis (BEG 121) (formerly Glomus intraradices), which was maintained as a soil–sand-based inoculum, was added into the pots at 15% (v/v). For control plants, a 15% (v/v) mixture of soil and sand without AMF inoculum was added. To homogenize the microbial populations, control plants were irrigated with a filtrate (20 µm) of the AMF inoculum. Photoperiod and temperature were maintained in the growth chamber. Twice a week, tomato plants were watered with Long Ashton solution (Hewitt, 1996) with 25% of the standard phosphorus concentration. Plants grew for an additional 4 weeks before pathogen infection to ensure establishment of symbiosis.
Botrytis cinerea infection
Botrytis cinerea CECT2100 (Spanish collection of type cultures, Universidad de Valencia, Burjassot, Spain) was grown from 2 weeks in PDA (potato dextrose agar) plates supplemented with tomato leaves (40 mg ml–1). Conidia were collected and pre-germinated in Gambor’s B5 medium (Duchefa, Haarlem, The Netherlands) supplemented with 10 mM sucrose and 10 mM KH2PO4 for 2 h in the dark without shaking. Plant infection was performed on intact plants at 100% relative humidity as described by Vicedo et al. (2009). Plants where inoculated by spraying the third and fourth leaf with a 106 ml−1B. cinerea spore suspension.
At 72 hours post-infection (hpi), leaves were collected at −80 °C to assess the gene expression and sugar levels, and some leaves were collected and kept in ethanol to study the callose deposition.
Botrytis cinerea infection and sample collection were carried out during the diurnal part of the day, 3 h after the turning on of the lights in the phytotron.
Brefeldin A and 2-deoxy-d-glucose treatment
Detached leaves of NM and AM plants were used to study the effects of treatments with the vesicular trafficking inhibitor brefeldin A (BFA) and the callose synthase competitive inhibitor 2-deoxy-d-glucose (2DDG).
BFA treatment was performed as follows: the petioles of the third and fourth detached leaves were immersed in a solution of 100 µg ml–1 BFA (Sigma Aldrich) and 5 mM EDTA-Na2 (Panreac Química SA). BFA solution was prepared as described by Steele-King et al. (1999). For 2DDG treatment and uptake, the petioles of the third and fourth detached leaves were immersed in a solution of 1 mM 2DDG (Sigma Aldrich) and 5 mM EDTA-Na2. Control leaves were placed in the same type of tubes with water and 5 mM EDTA-Na2. Plants were treated 24 h before the B. cinerea infection.
For these experiments, the infection was carried out with 5 µl drops of a 106 ml−1B. cinerea spore suspension. Four leaves were used in each treatment, with five drops per leaf, one in each leaflet (n=20). The experiment was repeated twice, giving similar results. At 72 hpi, leaves were collected and put in ethanol to study the lesion diameter.
Mycorrhizal colonization levels
Samples of each plant root system were collected to verify the mycorrhizal colonization. Roots were cut into 2 cm segments and stained using the method described by Vierheilig et al. (1998), to stain the fungal structures inside roots. To assess the percentage of root colonization by the AMF we used the grid-line intersection method (Giovannetti and Mosse, 1980) in a Nikon Eclipse 50i microscope under bright-field conditions.
Callose deposition and fungal lesion
Callose deposition was evaluated at 72 hpi by staining the leaves with aniline blue as described by Ton and Mauch-Mani (2004). Stained leaves were photographed under an epifluorescence microscope with a UV filter (Nikon Eclipse 80i). Callose deposition was determined by the quantification of yellow pixels with respect to total pixels of the leaf using GIMP software (GNU Image Manipulation Program, http://gimp.org).
Fungal lesions in BFA and 2DDG treatments were determined as lesion diameter using GIMP software. Leaves were first stained with lactophenol trypan blue (Saha et al., 1988) and then photographed under an epifluorescence microscope with a UV filter (Nikon Eclipse 80i).
RNA extraction and gene expression analysis
Samples of frozen leaves were ground in liquid nitrogen and stored at −80 °C. RNA was extracted essentially as described (Kiefer et al., 2000) with some changes. In short, 1 ml of Trizol was added to 100 mg of ground fresh leaves. Samples were centrifuged, and the supernatant was collected in a new tube to which 220 µl of CHCl3 were added. Next, a second centrifugation was carried out and the supernatant was collected in a new tube. A 350 µl aliquot of isopropanol and 350 µl of 0.8 M citrate/1.2 mM NaCl were added and mixed. After centrifugation, the supernatant was removed, and the pellet was rinsed twice with 70% ethanol. The pellet was dried and dissolved in 25 µl of nuclease-free water.
RNA samples were treated with DNase I (Thermo Scientific) to remove the remaining DNA. Afterwards, 1 μg of RNA was annealed to oligo(dT) and reverse transcribed using a PrimeScript™ RT Reagent Kit (TAKARA) to synthesize the cDNA. Real-time PCR was performed with a Maxima SYBR Green/ROX qPCR Master Mix (2×) (Thermo Scientific) in a StepOne instrument (Applied Biosystems).
To improve the amplification efficiency, serial dilutions of cDNA were performed to create a standard curve. Three technical replicates were done for each sample. The specificity of quantitative reverse transcription–PCR (RT–qPCR) amplification was verified by the presence of a single peak in the melting temperature curve analysis. mRNA levels were quantified using the comparative 2−Δ(ΔCt) method (Livak and Schmittgen, 2001). Tomato elongation factor 1α (EF-1α) and tomato actin 52 (ACT-52) were used as the housekeeping genes to normalize the expression values. Relative expression of mRNA levels was calculated from the difference in threshold cycle (∆Ct) among the studied genes (BAM1, SUS1, SUS3, LIN6, SUT1, SUT2, SUT4, ATL31, SYP121, SWEET15, SWEET17, SWEET4, and PMR4) and housekeeping genes. Primers are detailed in Supplementary Table S1 at JXB online.
DNA extraction and quantification of Botrytis cinerea infection
To quantify B. cinerea infection, DNA was extracted from infected leaves as described by Edwards et al. (1991). Briefly, extraction buffer (200 mM Tris–HCl pH 7.5, 250 mM NaCl, 25 mM EDTA-Na2, 0.5% SDS) was added to 50 mg of ground material. Samples were centrifuged, and the supernatant was transferred to a new tube containing 300 µl of isopropanol. Another centrifugation was performed, and the supernatant was discarded. The pellet was dried and dissolved in 50 µl of nuclease-free water. Later, DNA was amplified by quantitative PCR and B. cinerea infection was quantified by comparing the levels of the B. cinerea tubulin gene Bc-TUB versus the levels of the Sl-EF1α gene. The primers used for the gene expression analysis and the plant and fungal genomic DNA amplification are detailed in Supplementary Table S1.
Liquid chromatography coupled to ESI mass spectrometry
Targeted hormonal analysis
Hormones were analysed as described as Sánchez-Bel et al. (2018). In brief, a pool of internal standards, which contains dehydrojasmonic acid (dhJA) and jasmonic acid-isoleucine-d6 (JA-Ile-d6), was added to 30 mg of powdered freeze-dried material. Then, external calibration curves of each pure standard (dhJA and JA-Ile-d6) were used for the precise JA and JA-Ile quantification, respectively. The extraction was carried out in a mixer mill. After centrifugation, the supernatant was placed in a new tube, and the pH was adjusted with acetic acid to 2.5–2.7. Diethyl ether was added to create two phases, and a centrifugal evaporator (Speedvac) was used to concentrate both organic fractions until dryness. A 1 ml aliquot of MeOH/H2O with 0.01% HCOOH (10:90) was added, to a final concentration of internal standards of 100 ng ml−1. The targeted hormonal analysis was performed in an Acquity ultraperformance liquid chromatography system (UPLC; Waters, Mildford, MA, USA) coupled to a triple quadrupole mass spectrometer (TQD, Waters, Manchester, UK). The conditions used were described by Sánchez-Bel et al. (2016).
LC-ESI full-scan mass spectrometry for sugar analysis
A 300 mg aliquot of freeze-dried material was powdered and 1 ml of MeOH 10% was added. After centrifugation, the supernatant was collected and filtered with a 0.2 µm filter (Regenerated Cellulose Filter, 0.20 μm, 13 mm D. pk/100; 263 Teknokroma). Samples were kept at −20 °C for further analysis.
Untargeted LC-ESI full-scan MS was done as detailed by Gamir et al. (2014). Briefly, 20 μl of the filtered supernatant were injected in an Acquity UPLC system (Waters, www.waters.com) interfaced with a hybrid quadrupole time-of-flight mass spectrometer (QTOF Premier). The identification of the detected signals, as described by Sánchez-Bel et al. (2018), was carried out by introducing a second fragmentation function into the TOF analyser. To obtain the fragmentation of each analyte, a new function was programmed in a t-wave ranging from 5 eV to 45 eV. Analytes were eluted using a gradient of MeOH and H2O with 0.01% HCOOH.
Sugars were identified by comparing their fragmentation spectrum in the Massbank (https://massbank.eu) or the Metlin databases (https://metlin.scripps.edu).
Bioinformatic processing of metabolomic signals
The Databridge tool was used to transform data from the .raw format, obtained with Masslynx 4.1 software (Masslynx 4.1, Waters), to .cdf files. Then, signals were processed using the software R (http://www.r-project.org/). The XCMS algorithm (www.bioconductor.org; Smith et al., 2006) was used to obtain the peak peaking, grouping, and signal corrections. Metabolite amounts were analysed based on the normalized peak area units relative to the dry weight. A non-parametric Kruskal–Wallis test (P<0.05) was performed to test the differences among treatments.
Statistical analysis
Statgraphics-plus software for Windows V.5 (Statistical Graphics Corp., MD, USA) was used to determine the statistical analysis by ANOVA, using Fisher’s least significant difference (LSD) at 99.5% to compare treatments. Means are shown with their SE. Three pooled plants were sampled for each experiment, and each experiment was repeated a minimum of three times.
Results
Priming of callose deposition plays an important role in mycorrhiza-induced resistance against B. cinerea
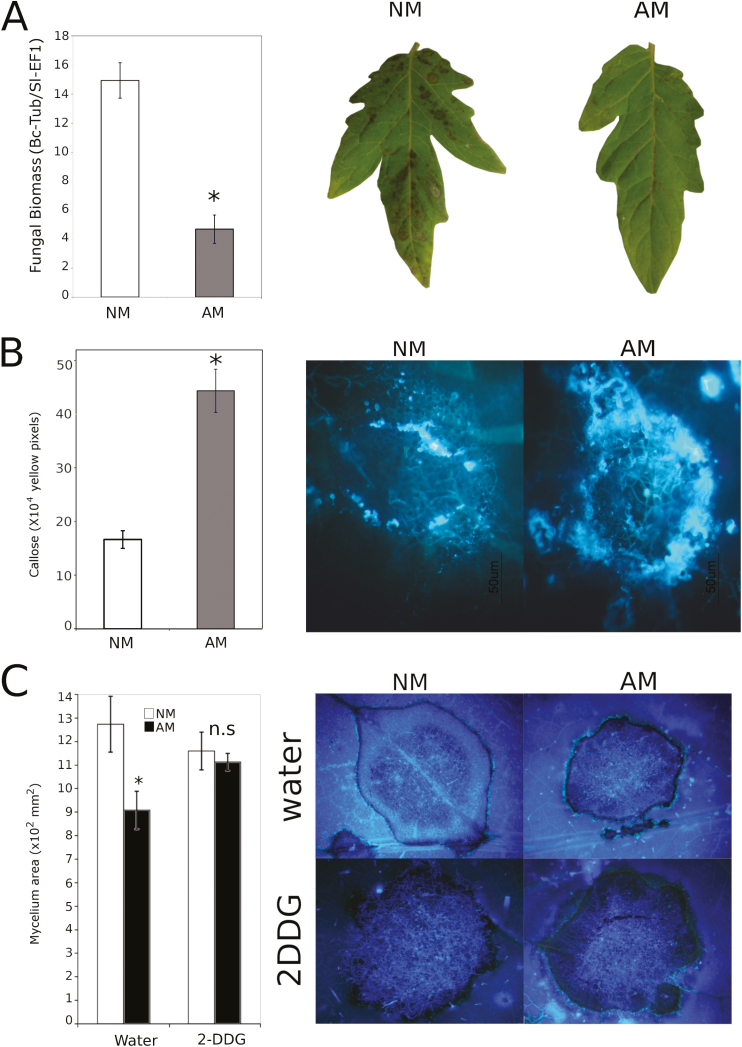
Here, we confirmed that MIR was functional in tomato plants against B. cinerea as previously reported (Sánchez-Bel et al., 2016). Compared with NM plants, the inoculation of tomato plants with the AMF R. irregularis led to a significant reduction in the disease symptoms at 72 hpi (Fig. 1A). The disease incidence was assessed by quantifying the fungal biomass through qPCR measuring the presence of the fungal gene Bc-TUB versus the plant gene Sl-EF1α. The fungal biomass in the AM plants was reduced by 66% compared with that in the NM plants.
Fig. 1.
Mycorrhiza-induced resistance (MIR) and the role of callose deposition priming in MIR. (A) Level of infection with 106 spores ml–1 of Botrytis cinerea at 72 hpi in non-mycorrhizal (NM) and mycorrhizal (AM) plants measured as the ratio of expression of the Tubulin gene (B. cinerea) relative to EF1α (S. lycopersicum). (B) Callose levels in NM and AM plants at 72 hpi. (C) Mycelium diameter after drop inoculation of B. cinerea 72 hpi in NM (white bars) and AM (black bars) plants treated 24 h before infection with 2DDG (2-deoxy-d-glucose) to inhibit callose deposition or treated with water as a control. The experiment was repeated three times with the same results. The error bars represent the SE of the mean, and the statistically significant differences are represented by (*) (t-test; n=18). (This figure is available in colour at JXB online.)
A common plant response to pathogen penetration is the formation of papillae surrounding the infection site, which includes callose. To determine whether MIR against B. cinerea was mediated by enhanced callose deposition, the control and AM plants were infected with the fungus, and callose accumulation was quantified. Compared with the NM plants, the AM tomato plants showed a priming in callose deposition (Fig. 1B). To investigate whether this callose accumulation is an important component in MIR, the plants were infiltrated with 2-DDG, which is a competitive inhibitor of callose synthase, 24 h prior to inoculation with B. cinerea. The infiltration resulted in almost complete inhibition of callose accumulation and the impairment of MIR (Fig. 1C), whereas in the water-infiltrated plants, MIR against B. cinerea was fully functional. Since it has been reported that transient nitrogen starvation of AM tomato plants also results in a partial impairment of MIR against B. cinerea (Sánchez-Bel et al., 2016), we measured the callose deposition rate in NM and AM plants infected with B. cinerea with or without exposure to 48 h of nitrogen starvation prior to the infection (Supplementary Fig. S1). The results showed that under nitrogen starvation, the callose deposition in the NM and AM plants was almost abolished, and, accordingly, these plants were unable to prevent the entrance of the pathogen, resulting in enhanced susceptibility.
Hormonal regulation is known to be among the main plant elements responsive to pathogens. Mycorrhizal symbiosis strongly alters the hormone homeostasis of the plant, and this alteration has been suggested to be involved in MIR against B. cinerea (Sánchez-Bel et al., 2016). Notably, several previous studies suggested that some oxylipins play a regulatory role in callose accumulation, since JA and other oxylipins are thought to regulate the glucan synthase complex in Arabidopsis (Ton and Mauch-Mani, 2004; Flors et al., 2008; García-Andrade et al., 2011). The main defence-related hormones were studied to explore the potential link between altered hormone levels and callose priming. The quantification of the hormones in the oxylipin pathway revealed that the production of all hormones was induced following B. cinerea infection regardless of the mycorrhizal status of the plant. JA displayed a priming profile that was significantly higher in the AM plants upon infection than that in the infected NM plants. However, the accumulation of the hormone conjugate JA-Ile was enhanced upon B. cinerea infection, although the levels in the infected AM plants remained lower than that in the infected NM plants (Supplementary Fig. S2).
Starch mobilization probably fuels the priming of callose deposition
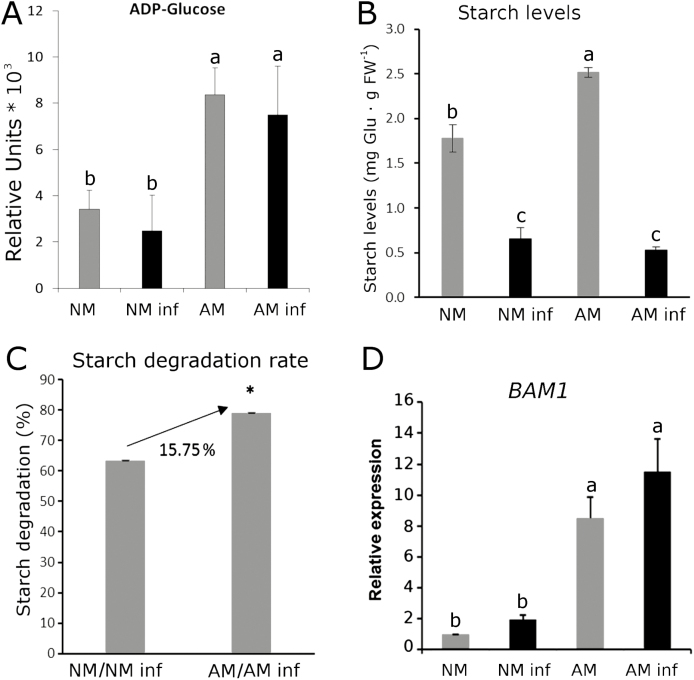
Recently, our research group demonstrated that starch is a possible source of sugars for callose priming mediated by an increase in the β-amylase 1 gene (BAM1) (Gamir et al., 2018). The BAM1 gene encodes a β-amylase responsible for the hydrolysis of starch into maltose in the chloroplast, which is transported to the cytoplasm and hydrolysed into glucose. Since sugar metabolism is among the main targets of AMF, it is plausible that the starch mobilization imposed by symbiosis (Gutjahr et al., 2009) mediates the priming of callose deposition upon a shoot pathogenic infection. To study this hypothesis, we analysed the starch and ADP-glucose content and the BAM1 gene expression level. The levels of ADP-glucose, which is the main precursor of starch biosynthesis, in the AM samples were augmented compared with those in the NM plants regardless of B. cinerea infection status (Fig. 2A). Notably, the starch content in the AM plants was higher than that in the NM plants (Fig. 2B). Interestingly, the infection induced starch mobilization, leading to a significant reduction in both the NM and AM plants, but the rate of starch degradation was higher in the AM plant by 15.75% (Fig. 2C). This stronger degradation correlated with significantly higher levels of BAM1 gene expression in the AM plants compared with that in the NM plants, and the expression was further boosted upon pathogen infection (Fig. 2D).
Fig. 2.
Starch metabolism in the mycorrhizal (AM) plants was higher than that in non-mycorrhizal (NM) plants regardless of the pathogenic infection. (A) Content of the main starch precursor ADP-glucose, (B) starch content, (C) starch degradation represented as the difference between the starch levels before and after the challenge, and (D) relative gene expression of β-amylase 1 (BAM1) in the non-infected (grey bars) and infected (black bars) NM and AM plants after 72 h of B. cinerea infection. BAM1 gene expression data were normalized to the NM controls. Three biological replicates were used in these analyses, and each replicate belonged to a different experiment involving a pool of leaf material from three individual plants per condition. The error bars represent the SE of the mean. Statistically significant differences are indicated in (A), (B), and (D) by different letters (ANOVA, Fisher’s least significant difference test; P<0.05, n=9); and in (C) by an asterisk (t-test; n=9).
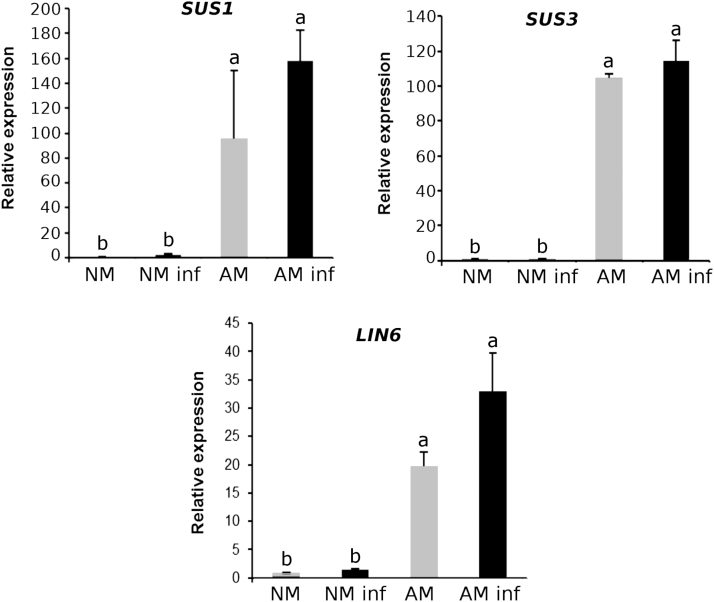
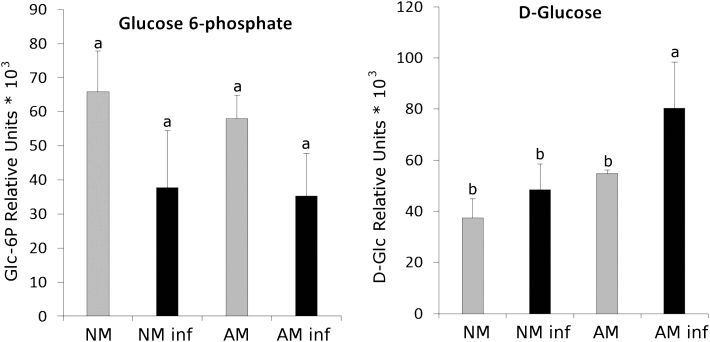
Many sucrose synthases and invertases showed higher levels in the AM plants (Schaarschmidt et al., 2006; Li et al., 2012). The most studied invertase in AM plants is LIN6, which is interestingly a JA-responsive tomato cell wall invertase. To decipher sugar metabolism during MIR and its possible link with primed callose accumulation, we also analysed the most studied sucrose synthase genes in tomato in response to either mycorrhization or pathogenic attack: SUS1 (also known as TOMSSF), SUS3, and LIN6 (García-Rodríguez et al., 2007; Hyun et al., 2011; Ruan, 2014). The SUS1, SUS3, and LIN6 gene expression levels were higher in the AM plants in both the B. cinerea-infected and uninfected plants, whereas the NM plants showed a very low basal expression level of all three genes (the two synthases and the invertase) regardless of the infection status (Fig. 3). Sucrose synthases and invertases are known to be regulated not only at the transcriptional and post-transcriptional level but also at the post-translational level (reviewed by Wan et al., 2018); hence, the study at the gene expression level performed in this work does not provide the complete picture of carbon metabolism. Nevertheless, previous studies support the role of these genes in symbiosis; for example, studies performed in Medicago truncatula showed that knocked down MtSucS1 mutants were impaired in fungal colonization and caused an early collapse of arbuscules (Baier et al., 2010). However, notably, ADP-glucose can be produced not only from ADP-glucose pyrophosphorylase (AGPase) activity but also from cytosolic sucrose synthase activity. Thus, the enhanced expression of the SUS1 and SUS3 genes could support the increase in the ADP-glucose content found in the AM plants regardless of infection with B. cinerea (Fig. 2A). Regarding sugar contents, in the absence of infection, the glucose-6-phosphate levels did not differ between the NM and AM plants, whereas a significant increase in glucose content was observed only in the AM plants upon pathogen infection, suggesting a higher availability to fuel callose accumulation (Fig. 4).
Fig. 3.
Expression levels of sucrose synthase (SUS) and a sugar invertase (LIN6) genes were up-regulated in mycorrhizal (AM) plants. Relative gene expression levels of the sucrose synthases SUS1 and SUS3 and the invertase LIN6 in the non-mycorrhizal (NM) and AM tomato plants upon pathogenic infection (72 hpi, black bars) and control plants (grey bars). Three biological replicates of three different experiments were used in these analyses, and each replicate consisted of a pool of leaf material from three individual plants per condition. The SE of the mean is represented by the error bars, and statistically significant differences are indicated by different letters (ANOVA, Fisher’s least significant differencetest; P<0.05, n=9).
Fig. 4.
Sugar metabolism in mycorrhizal (AM) and non-mycorrhizal (NM) plants before and after B. cinerea infection. Levels of glucose 6-phosphate and d-glucose in the non-infected (grey bars) and infected (black bars) NM and AM tomato plants 72 h after B. cinerea infection. Leaf material from three different plants was pooled for each treatment combination. Each experiment was repeated three times. The error bars represent the SE of the mean. The letters indicate statistically significant differences (ANOVA, Fisher’s least significant difference test; P<0.05, n=9).
Sugar transport in response to pathogens is altered in mycorrhizal plants
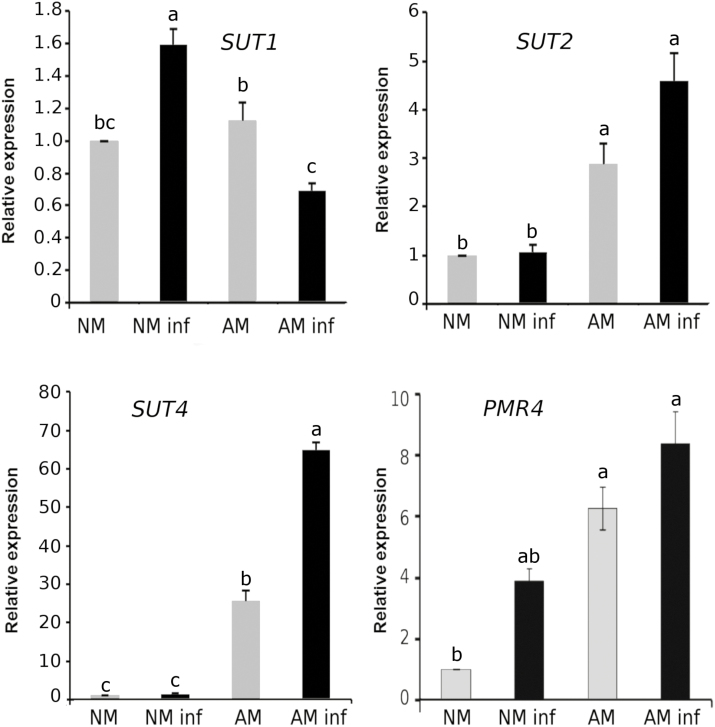
Carbohydrates, such as sucrose, required in organs other than leaves are loaded into the phloem for translocation to the sink organs. In tomato, three sucrose transporter genes have been identified: SlSUT1, SlSUT2, and SlSUT4 (Osorio et al., 2014). SlSUT1 and SlSUT2 are involved in phloem loading and unloading, respectively, whereas SlSUT4 is thought to be a vacuolar sucrose transporter (Endler et al., 2006; Hackel et al., 2006). The basal expression of the genes responsible for phloem unloading (SUT2) and vacuolar transport of sucrose (SUT4) in the AM plants was higher than that in the NM plants. Additionally, the expression of both genes was unaltered by the pathogen in the NM plants but was further boosted upon B. cinerea infection in the AM plants. Interestingly, SUT1 (involved in phloem loading) had a completely different expression profile. SUT1 was not induced by mycorrhiza but, in contrast, was induced in response to the pathogen in the NM plants, and the infected AM plants showed a strong reduction (Fig. 5). This observation suggests that sugars may be retained in the cytoplasm of AM plants following infection rather than being distributed to other metabolic sinks, including the roots. Recently, members of the sugar transporter SWEET family were shown to play an important role in mycorrhizal symbiosis and resistance to B. cinerea since they are involved in the release of sugars to the apoplast (Asai et al., 2016; Manck-Götzenberger and Requena, 2016). In tomato plants, SlSWEET15 has been described as hijacked by B. cinerea to obtain sucrose from the plant during the early stages of infection (Asai et al., 2016). Under our experimental conditions, SWEET15 did not change in the AM plants or in response to the infection (Supplementary Fig. S3). Nevertheless, the other members of the SWEET gene family presented lower (SlSWEET17) and higher levels (SlSWEET4) upon B. cinerea infection regardless of the mycorrhizal status of the plant (Supplementary Fig. S3), suggesting that both respond to the pathogen but are not relevant for MIR.
Fig. 5.
Sugar transport in response to B. cinerea infection in mycorrhizal (AM) and non-mycorrhizal (NM) plants and expression of the callose synthase PMR4 gene. Expression levels of sugar transporter genes (SUT1, SUT2, and SUT4) and the PMR4 gene in NM and AM infected (black bars) and non-infected (grey bars) tomato plants at 72 hpi. Three biological replicates were used in these analyses, and each replicate belonged to a different experiment involving a pool of leaf material from three individual plants per condition. The error bars represent the SE of the mean. and the statistically significant differences are indicated by letters (ANOVA, Fisher’s least significant difference test; P<0.05, n=9).
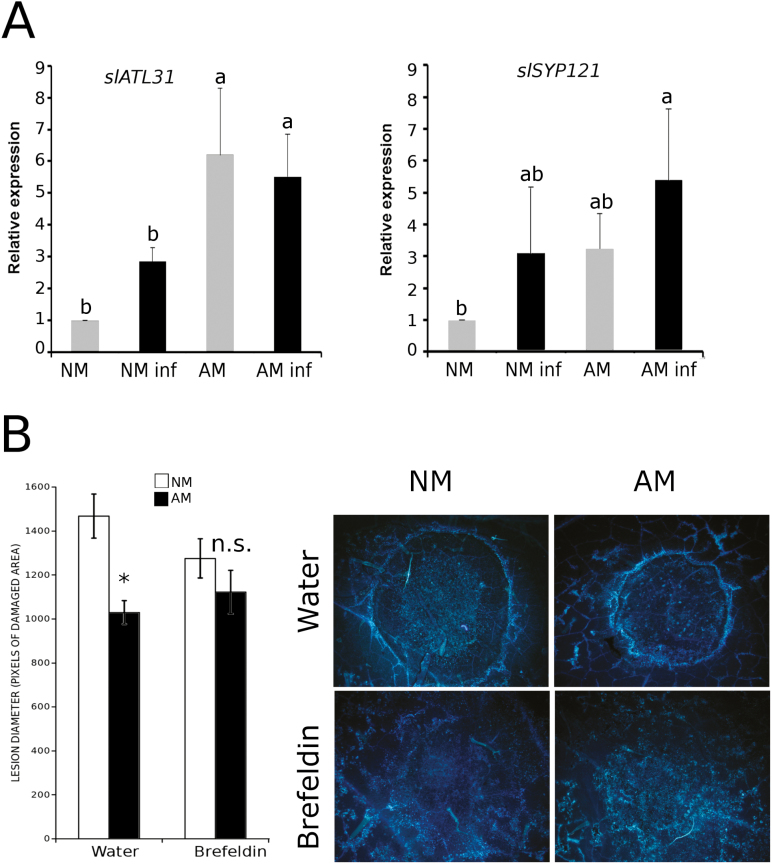
The callose synthase PMR4 is usually regulated at the protein level, but transcriptional regulation has been observed in some specific situations (Ellinger and Voigt, 2014). Here, we observed the transcriptional regulation of callose synthase during symbiosis as the PMR4 gene expression level was >6-fold higher in the AM plants independent of the pathogen infection (Fig. 5). In Arabidopsis, callose synthase has also been demonstrated to be spatially regulated by membrane trafficking by being transported via vesicular trafficking (Xie et al., 2012; Ellinger et al., 2013), and the final distribution of the PMR4 protein in the plasma membrane is driven by the Q-SNARE/R-SNARE complex (Meyer et al., 2009). One of the Q-SNARE proteins, SYP121, has been shown to be involved in the Arabidopsis resistance against Blumeria graminis (Maekawa et al., 2014). In addition, SYP121 interacts with the ubiquitin ligase ATL31, which is also important in fungal penetration. The ATLs are a large family of membrane-associated ubiquitin ligases (Guzmán, 2012). In Arabidopsis, two members of this family, namely ATL6 and ATL31, are specifically involved in callose accumulation during the plant immune response. Under our experimental conditions, the expression level of the SlATL31 gene was enhanced in the AM plants compared with that in the NM plants, but the expression remained at similar levels upon pathogen infection. Nevertheless, SlSYP121 gene expression was significantly enhanced only in the infected AM plants compared with the NM plants (Fig. 6A), suggesting a higher vesicular trafficking in AM plants in response to pathogen infection. To further investigate the role of vesicular trafficking in sugar transport to fuel callose priming during MIR, we treated the samples with BFA, which is an inhibitor of vesicular trafficking, 24 h before the leaf infection. The water-infiltrated leaves from the AM plants displayed MIR and callose priming. However, the BFA infiltration abolished normal callose accumulation in both the NM and AM plants, although some spots of callose were still observed in the AM plants (Fig. 6B). Remarkably, MIR was completely lost in the AM plants, supporting the key role of callose priming and vesicular trafficking in MIR.
Fig. 6.
Vesicular trafficking role during MIR. (A) Expression level of the Q-SNARE SYP121 gene and the ubiquitin ligase ATL31 gene in non-infected (grey bars) or infected (black bars) non-mycorrhizal (NM) or mycorrhizal (AM) plants. The analysis was replicated three times in which three individual plants per treatment were pooled. The error bars represent the SE. The significant differences are indicated by letters (ANOVA, Fisher’s least significant difference test; P<0.05, n=9). (B) Lesion diameter caused by a drop in B. cinerea 72 hpi in NM (white bars) and AM (black bars) plants treated with brefeldin A to inhibit vesicular trafficking or with water as a control 24 h before infection. The error bars represent the SE of the mean, and (*) represents statistically significant differences (t-test; n=18). (This figure is available in colour at JXB online.)
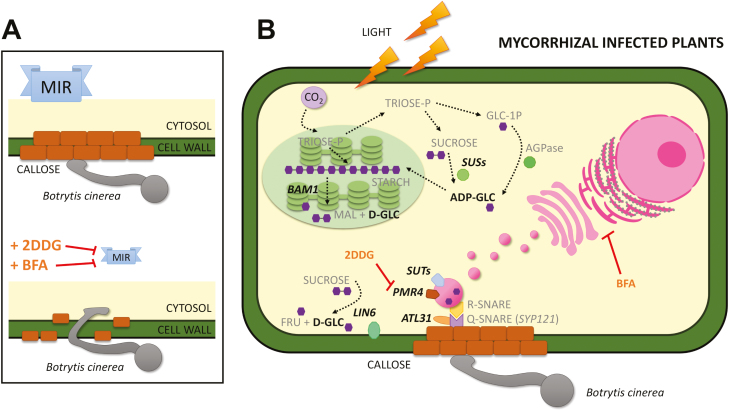
Proposed model of the priming of callose deposition during mycorrhiza-induced resistance
Based on all the results presented above, we propose the model presented in Fig. 7. MIR triggers callose deposition in the cell wall, contributing to a reduction in fungal penetration (Fig. 7A, top); however, treatments with a PMR4 callose synthase inhibitor (2DDG) or a vesicular trafficking inhibitor (BFA) block callose accumulation, facilitating pathogen penetration (Fig. 7A, bottom).
Fig. 7.
Proposed model of the priming of callose deposition in mycorrhizal tomato plants. (A) Role of callose in mycorrhizal-induced resistance (MIR). Callose deposition in the cell wall prevents fungal penetration during MIR (on top); however, the application of 2DDG (a PMR4 callose synthase inhibitor) or BFA (a vesicular trafficking inhibitor) blocks callose accumulation, allowing pathogen penetration. (B) Model depicting the proposed molecular mechanisms underlying the priming of callose deposition during MIR. 2DDG and BFA inhibitors are shown next to their targets. The words in bold show the enzymes and sugars that are accumulated in the mycorrhizal-infected plants compared with the non-mycorrhizal-infected plants. Monosaccharides and disaccharides are represented by hexagons. The dotted arrows could represent several metabolic steps. Abbreviations: 2-DDG, 2-deoxy-d-glucose; ADP-GLC, ADP-glucose; AGPase, ADP-glucose pyrophosphorylase; BAM1, ββamylase 1; BFA, brefeldin A; D-GLC, d-glucose; FRU, fructose; GLC-1P, glucose-1-phosphate; MAL, maltose; SUSs, sucrose synthases, SUT, sucrose transporter. (This figure is available in colour at JXB online).
A more detailed model illustrating the potential regulatory elements in the priming of callose deposition during MIR revealed in this work is presented in Fig. 7B. The higher gene expression and sugars levels in the infected AM plants compared with those in the infected NM plants are represented in bold. The gene expression of β-amylase 1 in the infected AM plants is higher than that in the infected NM plants, improving the rate of starch degradation (Fig. 2). The sugar metabolism-related enzymes sucrose synthases, the invertase LIN6, and sucrose transporters are also more highly expressed in the AM infected plants (Figs 3, 5), which may lead to higher sugar mobilization that might contribute to higher callose deposition. ADP-glucose, which is the product of sucrose degradation by sucrose synthases, is increased in infected AM plants (Fig. 2A). d-Glucose, which is derived from either starch or sucrose degradation, also shows higher levels upon pathogen infection in these plants (Fig. 4). In addition, the infected AM plants displayed enhanced ubiquitin ligase ATL31 gene expression (Fig. 6), strongly suggesting the importance of vesicular trafficking in callose accumulation during MIR.
These data are summarized in Fig. 7B, where the higher gene expression and sugar levels in the infected AM plants compared with those in the infected NM plants are represented in bold. The impairment of MIR by 2DDG and BFA and the targeted processes are also represented, supporting the key role of callose deposition priming in induced resistance.
Discussion
Mycorrhiza-induced resistance against several biotic stressors, such as biotrophic and necrotrophic pathogens, has been investigated (Fiorilli et al., 2011; Nair et al., 2015; Sánchez-Bel et al., 2016). In this study, we found a more pronounced accumulation of callose in leaves of AM plants upon infection by the fungal pathogen B. cinerea. The inhibition of this primed callose response by previous infiltration with the callose synthase inhibitor 2DDG abolished the protection by AMF against B. cinerea confirming the relevance of callose priming in MIR (Fig. 1). Remarkably, simultaneous nitrogen depletion and infection by the necrotrophic fungus also abolish callose priming, explaining the partial MIR impairment observed under these conditions (Sánchez-Bel et al., 2016; Supplementary Fig. S1). These results strongly suggest that priming of callose deposition is critical for MIR against B. cinerea; however, in the absence of callose priming, MIR may be still functional. Notably, the priming of callose deposition has been reported as the main mechanism underlying the induced resistance achieved after chemical priming with compounds, such as I3CA and β-aminobutyric acid (BABA), against the necrotrophic pathogens B. cinerea and P. cucumerina (Nishimura et al., 2003; Ton and Mauch-Mani, 2004; Gamir et al., 2018). To the best of our knowledge, only one report investigated the role of callose deposition in MIR (Lee et al., 2005). The authors observed that the priming of callose deposition seems to be the mechanism underlying the enhanced resistance against Colletotrichum orbiculare in mycorrhizal cucumber plants.
MIR against B. cinerea in tomato plants is mostly under the control of the JA signalling pathway (Sánchez-Bel et al., 2016). Here, we also observed the priming profile of JA (Supplementary Fig. S2), supporting its likely implication in enhanced callose accumulation. In addition, the JA-regulated invertase LIN6 showed a priming profile, and the expression of the vesicular sugar transporter SUT4 gene was primed in the AM plants following infection (Figs 3, 5). Altogether, we collected evidence supporting the role of sugar mobilization and vesicular transport in callose accumulation, which may reconfigure the priming of the callose pathway in AM plants upon fungal infection.
It is commonly accepted that callose is accumulates at the plasma membrane by a glucan synthase complex which is thought to contain a GTPase, UDP-glucose transferase, and sucrose synthase, among other interactors (Ellinger and Voigt, 2014). According to this hypothesis, most aspects related to carbohydrate metabolism and transport might be important in the callose priming pathway. Recently, several studies investigated the mechanisms and molecular events controlling the callose synthesis and callose priming pathway (Maekawa et al., 2014; Gamir et al., 2018; Singh et al., 2018). In the present study, the AM plants showed more active carbohydrate metabolism. ADP-glucose, which is the main precursor of starch biosynthesis, was accumulated more in AM plants than NM plants. Similarly, the starch levels in the AM plants were higher than those in the NM plants. Upon infection, a higher starch degradation rate is likely to be the most relevant event in AM plants contributing to callose priming (Fig. 2). Degradation may supply the sugars that need to be transported and polymerized to accumulate callose at the cell wall interface. To test this hypothesis, the sugar metabolism and expression of the most relevant sugar transport and synthesis genes were analysed.
Host sugar reallocation is an important event during AM symbiosis. AMF take up hexoses mostly as glucose derived from sucrose degradation by the invertases and sucrose synthases in the host cell (Pfeffer et al., 1999; Bago et al., 2003). AMF achieve this presumably via mechanisms similar to those by which pathogenic fungi hijack the sucrose degradation and transport machinery of the host cell (Proels and Hückelhoven, 2014). Therefore, some invertases and their hexose products have been suggested to play a key regulatory role linking the plant sugar signalling pathways and AM symbiosis (Schaarschmidt et al., 2007).
In this study, the expression levels of the genes encoding the sucrose synthases SUS1 and SUS3 and the invertase LIN6 were higher in the AM plants (Fig. 3). LIN6 was previously shown to be induced in shoots and roots of AM tomato plants and is thought to be responsible for the hydrolysis of the sucrose delivered to the shared apoplast between the AMF and the plant (García-Rodríguez et al., 2007). Under our experimental conditions, we did not observe any LIN6 induction in the NM infected plants, while this induction was significantly higher in the AM plants upon B. cinerea infection. This result suggests that the higher glucose content found in the B. cinerea-infected AM plants (Fig. 4) may result from both the different carbohydrate metabolism between NM and AM plants (higher starch hydrolysis and sucrose degradation) and the lower consumption of glucose by B. cinerea due to a lower infection rate. Hexose pools and the sucrose:trehalose-6-phosphate ratio are emerging as important events in cell signalling determining cellular responses (Bolouri Moghaddam and Van den Ende, 2012; Lunn et al., 2014; Figueroa and Lunn, 2016). Although glucose, sucrose, and trehalose-6-phosphate are the sugars most studied as signalling metabolites in plants, UDP-glucose (UDP-Glc) is emerging as an intracellular mediator of reactive oxygen species (ROS) signalling and plant cell death in plants (Janse van Rensburg and Van den Ende, 2017). Considering that UDP-Glc is the main substrate of callose synthase, it is reasonable to hypothesize that under our experimental conditions, the UDP-Glc levels in the AM and NM plants could be different and may play a role in stress signalling. Notably, we found an enhanced callose accumulation rate in the B. cinerea-infected AM plants compared with that in the NM infected plants, which could indicate higher PMR4 activity upon B. cinerea infection and, therefore, a greater use of UDP-Glc in these plants.
Consistent with this hypothesis, the elevated PMR4 gene expression level found in the AM plants did not correlate with any callose accumulation in the uninfected leaves (Figs 1, 5), suggesting that post-transcriptional and/or post-translational regulation of PMR4, as well as the spatial regulation by vesicular trafficking, may define the final output of callose accumulation. In Arabidopsis, PMR4 is transported to the plasma membrane through intracellular vesicles (Xie et al., 2012; Ellinger et al., 2013) and redistributed to the penetration sites during fungal infection (Meyer et al., 2009). The fusion of the tethered vesicles to the plasma membrane is mediated by specific binding between donor membrane-associated proteins, known as R-SNAREs, and plasma membrane-associated proteins, known as Q-SNAREs (Gu et al., 2017). In tomato, an orthologue of the vesicular trafficking-associated ATL31 has been characterized, suggesting that both Arabidopsis and tomato share the same mechanism (Lu et al., 2016). In the experiments presented in this work, SlATL31 and SlSYP121 (a Q-SNARE) expression was induced in the AM plants. Furthermore, SlSYP121 expression was further boosted upon B. cinerea infection, suggesting that both genes contribute to callose deposition during MIR. In fact, the inhibition of membrane trafficking by the BFA treatments completely abolished callose deposition and consequently MIR, confirming that functional vesicular trafficking is needed for callose priming during MIR (Fig. 6).
The transport and sugar level imbalances suggest that AM plants upon infection may orchestrate starch degradation and sugar transport into vesicles, mobilizing sugars within the cell through BAM1, LIN6, and SUT4. Thus, AM plants have a higher availability of carbohydrates probably due to both a higher rate of photosynthesis (Sánchez-Bel et al., 2018) and more active starch degradation metabolism. At the end of the cellular pathway leading to callose deposition, membrane trafficking (estimated through SlATL31 and SlSYP121 gene expression levels) is also enhanced in AM-infected plants. Finally, PMR4 gene expression was also higher in the AM plants with the AMF–host plant interaction being one of the specific cases in which PMR4 transcriptional regulation is found. Notably, the higher basal levels of PMR4 could allow faster activation of the response by post-transcriptional modifications (Fig. 7). Interestingly, our results reveal an important overlap between the priming of the callose pathway described in Arabidopsis (Gamir et al., 2018) and that occurring in AM tomato plants, suggesting that callose priming is a conserved mechanism underlying defence priming triggered by different priming stimuli in different plant species.
Supplementary data
Supplementary data are available at JXB online.
Table S1. List of primers used for qPCR analysis.
Fig. S1. Callose deposition under nitrogen starvation and normal fertilization in infected non-mycorrhizal and mycorrhizal tomato plants.
Fig. S2. Targeted analysis of oxylipin hormones levels in non-mycorrhizal and mycorrhizal plants at 72 hpi.
Fig. S3. Expression levels of different SWEET sugar transporter genes during MIR.
Acknowledgements
We thank the SCIC of the Universitat Jaume I and specially Cristian Barrera for their technical support. This work was funded by the Universitat Jaume I by the Plan de promoción de la Investigación program (P1-1B2015-33; UJI-B22016-43), and the Spanish Ministry Ministerio de Economia y Empresa-MINECO (AGL2015-64990-C2-2; RTI2018-094350-B-C33). NS received a PhD grant from Spanish Ministry MINECO (BES-2016-077208).
References
- Ahmad S, Veyrat N, Gordon-Weeks R, et al. 2011. Benzoxazinoid metabolites regulate innate immunity against aphids and fungi in maize. Plant Physiology 157, 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai Y, Kobayashi Y, Kobayashi I. 2016. Increased expression of the tomato SISWEET15 gene during grey mold infection and the possible involvement of the sugar efflux to apoplasm in the disease susceptibility. Journal of Plant Pathology & Microbiology 07, 1–8. [Google Scholar]
- Bago B, Pfeffer PE, Abubaker J, Jun J, Allen JW, Brouillette J, Douds DD, Lammers PJ, Shachar-Hill Y. 2003. Carbon export from arbuscular mycorrhizal roots involves the translocation of carbohydrate as well as lipid. Plant Physiology 131, 1496–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier MC, Keck M, Gödde V, Niehaus K, Küster H, Hohnjec N. 2010. Knockdown of the symbiotic sucrose synthase MtSucS1 affects arbuscule maturation and maintenance in mycorrhizal roots of Medicago truncatula. Plant Physiology 152, 1000–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer A, Pastor V, Gamir J, Flors V, Mauch-Mani B. 2015. The ‘prime-ome’: towards a holistic approach to priming. Trends in Plant Science 20, 443–452. [DOI] [PubMed] [Google Scholar]
- Barker L, Kühn C, Weise A, Schulz A, Gebhardt C, Hirner B, Hellmann H, Schulze W, Ward JM, Frommer WB. 2000. SUT2, a putative sucrose sensor in sieve elements. The Plant Cell 12, 1153–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolouri Moghaddam MR, Van den Ende W. 2012. Sugars and plant innate immunity. Journal of Experimental Botany 63, 3989–3998. [DOI] [PubMed] [Google Scholar]
- Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM. 2009. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323, 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordier C, Pozo MJ, Barea JM, Gianinazzi S, Gianinazzi-Pearson V. 1998. Cell defense responses associated with localized and systemic resistance to Phytophtora induced in tomato by an arbuscular mycorrhizal fungus. Molecular Plant-Microbe Interaction 11, 1017–1028. [Google Scholar]
- Edwards K, Johnstone C, Thompson C. 1991. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Research 19, 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger D, Naumann M, Falter C, Zwikowics C, Jamrow T, Manisseri C, Somerville SC, Voigt CA. 2013. Elevated early callose deposition results in complete penetration resistance to powdery mildew in Arabidopsis. Plant Physiology 161, 1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger D, Voigt CA. 2014. Callose biosynthesis in Arabidopsis with a focus on pathogen response: what we have learned within the last decade. Annals of Botany 114, 1349–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endler A, Meyer S, Schelbert S, Schneider T, Weschke W, Peters SW, Keller F, Baginsky S, Martinoia E, Schmidt UG. 2006. Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiology 141, 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essmann J, Bones P, Weis E, Scharte J. 2008. Leaf carbohydrate metabolism during defense. Plant Signaling & Behavior 3, 885–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa CM, Lunn JE. 2016. A tale of two sugars: trehalose 6-phosphate and sucrose. Plant Physiology 172, 7–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorilli V, Catoni M, Francia D, Cardinale F, Lanfranco L. 2011. The arbuscular mycorrhizal symbiosis reduces disease severity in tomato plants infected by Botrytis cinerea. Journal of Plant Pathology 93, 237–242. [Google Scholar]
- Flors V, Ton J, van Doorn R, Jakab G, García-Agustín P, Mauch-Mani B. 2008. Interplay between JA, SA and ABA signalling during basal and induced resistance against Pseudomonas syringae and Alternaria brassicicola. The Plant Journal 54, 81–92. [DOI] [PubMed] [Google Scholar]
- Gamir J, Pastor V, Kaever A, Cerezo M, Flors V. 2014. Targeting novel chemical and constitutive primed metabolites against Plectosphaerella cucumerina. The Plant Journal 78, 227–240. [DOI] [PubMed] [Google Scholar]
- Gamir J, Pastor V, Sánchez-Bel P, Agut B, Mateu D, García-Andrade J, Flors V. 2018. Starch degradation, abscisic acid and vesicular trafficking are important elements in callose priming by indole-3-carboxylic acid in response to Plectosphaerella cucumerina infection. The Plant Journal 96, 518–531. [DOI] [PubMed] [Google Scholar]
- García-Andrade J, Ramírez V, Flors V, Vera P. 2011. Arabidopsis ocp3 mutant reveals a mechanism linking ABA and JA to pathogen-induced callose deposition. The Plant Journal 67, 783–794. [DOI] [PubMed] [Google Scholar]
- García-Rodríguez S, Azcón-Aguilar C, Ferrol N. 2007. Transcriptional regulation of host enzymes involved in the cleavage of sucrose during arbuscular mycorrhizal symbiosis. Physiologia Plantarum 129, 737–746. [Google Scholar]
- Giovannetti M, Mosse B. 1980. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytologist 84, 489–500. [Google Scholar]
- Gómez-Ariza J, Campo S, Rufat M, Estopà M, Messeguer J, San Segundo B, Coca M. 2007. Sucrose-mediated priming of plant defense responses and broad-spectrum disease resistance by overexpression of the maize pathogenesis-related PRms protein in rice plants. Molecular Plant-Microbe Interactions 20, 832–842. [DOI] [PubMed] [Google Scholar]
- Gu Y, Zavaliev R, Dong X. 2017. Membrane trafficking in plant immunity. Molecular Plant 10, 1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutjahr C, Novero M, Guether M, Montanari O, Udvardi M, Bonfante P. 2009. Presymbiotic factors released by the arbuscular mycorrhizal fungus Gigaspora margarita induce starch accumulation in Lotus japonicus roots. New Phytologist 183, 53–61. [DOI] [PubMed] [Google Scholar]
- Gutjahr C, Parniske M. 2013. Cell and developmental biology of arbuscular mycorrhiza symbiosis. Annual Review of Cell and Developmental Biology 29, 593–617. [DOI] [PubMed] [Google Scholar]
- Guzmán P. 2012. The prolific ATL family of RING-H2 ubiquitin ligases. Plant Signaling & Behavior 7, 1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackel A, Schauer N, Carrari F, Fernie AR, Grimm B, Kühn C. 2006. Sucrose transporter LeSUT1 and LeSUT2 inhibition affects tomato fruit development in different ways. The Plant Journal 45, 180–192. [DOI] [PubMed] [Google Scholar]
- Hewitt EJ. 1996. Sand and water culture methods used in the study of plant nutrition. Technical Communication No. 22. East Malling, UK: Commonwealth Bureau of Horticulture and Plantation Crops. [Google Scholar]
- Huibers RP, Loonen AE, Gao D, Van den Ackerveken G, Visser RG, Bai Y. 2013. Powdery mildew resistance in tomato by impairment of SlPMR4 and SlDMR1. PLoS One 8, e67467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun TK, Eom SH, Rim Y, Kim JS. 2011. Alteration of the expression and activation of tomato invertases during Botrytis cinerea infection. Plant Omics Journal 4, 413–417. [Google Scholar]
- Janse van Rensburg HC, Van den Ende W. 2017. UDP-glucose: a potential signaling molecule in plants? Frontiers in Plant Science 8, 2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SC, Martinez-Medina A, Lopez-Raez JA, Pozo MJ. 2012. Mycorrhiza-induced resistance and priming of plant defenses. Journal of Chemical Ecology 38, 651–664. [DOI] [PubMed] [Google Scholar]
- Kiefer E, Heller W, Ernst D. 2000. A simple and efficient protocol for isolation of functional RNA from plant tissues rich in secondary metabolites. Plant Molecular Biology Reporter 18, 33–39. [Google Scholar]
- Kim MS, Cho SM, Kang EY, Im YJ, Hwangbo H, Kim YC, Ryu CM, Yang KY, Chung GC, Cho BH. 2008. Galactinol is a signaling component of the induced systemic resistance caused by Pseudomonas chlororaphis O6 root colonization. Molecular Plant-Microbe Interactions 21, 1643–1653. [DOI] [PubMed] [Google Scholar]
- Kocal N, Sonnewald U, Sonnewald S. 2008. Cell wall-bound invertase limits sucrose export and is involved in symptom development and inhibition of photosynthesis during compatible interaction between tomato and Xanthomonas campestris pv vesicatoria. Plant Physiology 148, 1523–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-S, Lee Y-J, Yong-Chull J. 2005. Observations of infection structures on the leaves of cucumber plants pre-treated with arbuscular mycorrhiza Glomus intraradices after challenge inoculation with Colletotrichum orbiculare. The Plant Pathology Journal 21, 237–243. [Google Scholar]
- Li Y, Zou YN, Huang YM, Wu QS. 2012. Influence of arbuscular mycorrhizal fungi on sucrose metabolism of citrus seedlings. Advanced Materials Research 610–613, 3406–3409. [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lu Y, Yasuda S, Li X, Fukao Y, Tohge T, Fernie AR, Matsukura C, Ezura H, Sato T, Yamaguchi J. 2016. Characterization of ubiquitin ligase SlATL31 and proteomic analysis of 14-3-3 targets in tomato fruit tissue (Solanum lycopersicum L.). Journal of Proteomics 143, 254–264. [DOI] [PubMed] [Google Scholar]
- Lunn JE, Delorge I, Figueroa CM, Van Dijck P, Stitt M. 2014. Trehalose metabolism in plants. The Plant Journal 79, 544–567. [DOI] [PubMed] [Google Scholar]
- Maekawa S, Inada N, Yasuda S, Fukao Y, Fujiwara M, Sato T, Yamaguchi J. 2014. The carbon/nitrogen regulator ARABIDOPSIS TOXICOS EN LEVADURA31 controls papilla formation in response to powdery mildew fungi penetration by interacting with SYNTAXIN OF PLANTS121 in Arabidopsis. Plant Physiology 164, 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manck-Götzenberger J, Requena N. 2016. Arbuscular mycorrhiza symbiosis induces a major transcriptional reprogramming of the potato SWEET sugar transporter family. Frontiers in Plant Science 7, 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Medina A, Flors V, Heil M, Mauch-Mani B, Pieterse CMJ, Pozo MJ, Ton J, van Dam NM, Conrath U. 2016. Recognizing plant defense priming. Trends in Plant Science 21, 818–822. [DOI] [PubMed] [Google Scholar]
- Mauch-Mani B, Baccelli I, Luna E, Flors V. 2017. Defense priming: an adaptive part of induced resistance. Annual Review of Plant Biology 68, 485–512. [DOI] [PubMed] [Google Scholar]
- Meyer D, Pajonk S, Micali C, O’Connell R, Schulze-Lefert P. 2009. Extracellular transport and integration of plant secretory proteins into pathogen-induced cell wall compartments. The Plant Journal 57, 986–999. [DOI] [PubMed] [Google Scholar]
- Miransari M. 2010. Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biology 12, 563–569. [DOI] [PubMed] [Google Scholar]
- Mustafa G, Khong NG, Tisserant B, Randoux B, Fontaine J, Magnin-Robert M, Reignault P, Sahraoui ALH. 2017. Defence mechanisms associated with mycorrhiza-induced resistance in wheat against powdery mildew. Functional Plant Biology 44, 443–454. [DOI] [PubMed] [Google Scholar]
- Nair A, Kolet SP, Thulasiram HV, Bhargava S. 2015. Role of methyl jasmonate in the expression of mycorrhizal induced resistance against Fusarium oxysporum in tomato plants. Physiological and Molecular Plant Pathology 92, 139–145. [Google Scholar]
- Nishimura MT, Stein M, Hou BH, Vogel JP, Edwards H, Somerville SC. 2003. Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301, 969–972. [DOI] [PubMed] [Google Scholar]
- Osorio S, Ruan YL, Fernie AR. 2014. An update on source-to-sink carbon partitioning in tomato. Frontiers in Plant Science 5, 516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-de-Luque A, Tille S, Johnson I, Pascual-Pardo D, Ton J, Cameron DD. 2017. The interactive effects of arbuscular mycorrhiza and plant growth-promoting rhizobacteria synergistically enhance host plant defences against pathogens. Scientific Reports 7, 16409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer PE, Douds Jr DD, Becard G, Shachar-Hill Y. 1999. Carbon uptake and the metabolism and transport of lipids in an arbuscular mycorrhiza. Plant Physiology 120, 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo MJ, Azcón-Aguilar C. 2007. Unraveling mycorrhiza-induced resistance. Current Opinion in Plant Biology 10, 393–398. [DOI] [PubMed] [Google Scholar]
- Proels RK, Hückelhoven R. 2014. Cell-wall invertases, key enzymes in the modulation of plant metabolism during defence responses. Molecular Plant Pathology 15, 858–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich MK, Nouri E, Courty PE, Reinhardt D. 2017. Diet of arbuscular mycorrhizal fungi: bread and butter? Trends in Plant Science 22, 652–660. [DOI] [PubMed] [Google Scholar]
- Rivero J, Álvarez D, Flors V, Azcón-Aguilar C, Pozo MJ. 2018. Root metabolic plasticity underlies functional diversity in mycorrhiza-enhanced stress tolerance in tomato. New Phytologist 220, 1322–1336. [DOI] [PubMed] [Google Scholar]
- Roth R, Paszkowski U. 2017. Plant carbon nourishment of arbuscular mycorrhizal fungi. Current Opinion in Plant Biology 39, 50–56. [DOI] [PubMed] [Google Scholar]
- Ruan YL. 2014. Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annual Review of Plant Biology 65, 33–67. [DOI] [PubMed] [Google Scholar]
- Saha DC, Jakson MA, Johnson-Cicalese JM. 1988. A rapid staining method for detection of endophytic fungi in turf and forage grasses. Phytopathology 78, 237–239. [Google Scholar]
- Sánchez-Bel P, Sanmartín N, Pastor V, Mateu D, Cerezo M, Vidal-Albalat A, Pastor-Fernández J, Pozo MJ, Flors V. 2018. Mycorrhizal tomato plants fine tunes the growth–defence balance upon N depleted root environments. Plant, Cell & Environment 41, 406–420. [DOI] [PubMed] [Google Scholar]
- Sanchez-Bel P, Troncho P, Gamir J, Pozo MJ, Camañes G, Cerezo M, Flors V. 2016. The nitrogen availability interferes with mycorrhiza-induced resistance against Botrytis cinerea in tomato. Frontiers in Microbiology 7, 1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaarschmidt S, Kopka J, Ludwig-Müller J, Hause B. 2007. Regulation of arbuscular mycorrhization by apoplastic invertases: enhanced invertase activity in the leaf apoplast affects the symbiotic interaction. The Plant Journal 51, 390–405. [DOI] [PubMed] [Google Scholar]
- Schaarschmidt S, Roitsch T, Hause B. 2006. Arbuscular mycorrhiza induces gene expression of the apoplastic invertase LIN6 in tomato (Lycopersicon esculentum) roots. Journal of Experimental Botany 57, 4015–4023. [DOI] [PubMed] [Google Scholar]
- Scharte J, Schön H, Tjaden Z, Weis E, von Schaewen A. 2009. Isoenzyme replacement of glucose-6-phosphate dehydrogenase in the cytosol improves stress tolerance in plants. Proceedings of the National Academy of Sciences, USA 106, 8061–8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Lee S, Ortega L, Ramu VS, Senthil-Kumar M, Blancaflor EB, Rojas CM, Mysore KS. 2018. Two chloroplast-localized proteins: AtNHR2A and AtNHR2B, contribute to callose deposition during nonhost disease resistance in Arabidopsis. Molecular Plant-Microbe Interactions 31, 1280–1290. [DOI] [PubMed] [Google Scholar]
- Singh V, Louis J, Ayre BG, Reese JC, Pegadaraju V, Shah J. 2011. TREHALOSE PHOSPHATE SYNTHASE11-dependent trehalose metabolism promotes Arabidopsis thaliana defense against the phloem-feeding insect Myzus persicae. The Plant Journal 67, 94–104. [DOI] [PubMed] [Google Scholar]
- Smith CA, Want EJ, O’Maille G, Abagyan R, Siuzdak G. 2006. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Analytical Chemistry 78, 779–787. [DOI] [PubMed] [Google Scholar]
- Smith SE, Smith FA. 2011. Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annual Review of Plant Biology 62, 227–250. [DOI] [PubMed] [Google Scholar]
- Song YY, Chen DM, Lu K, Sun ZX, Zeng RS. 2015. Enhanced tomato disease resistance primed by arbuscular mycorrhizal fungus. Frontiers in Plant Science 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele-King CG, Evans DE, Satiat-Jeunemaitre B, Hawes C. 1999 Plant cells show an acquired insensitivity to brefeldin A. Journal of Experimental Botany 50, 1465–1469. [Google Scholar]
- Tarkowski L, Van de Poel B, Höfte M, Van den Ende W. 2019. Sweet immunity: inulin boosts resistance of lettuce (Lactuca sativa) against grey mold (Botrytis cinerea) in an ethylene-dependent manner. International Journal of Molecular Sciences 20, 1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton J, Mauch-Mani B. 2004. Beta-amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. The Plant Journal 38, 119–130. [DOI] [PubMed] [Google Scholar]
- Trouvelot S, Héloir MC, Poinssot B, Gauthier A, Paris F, Guillier C, Combier M, Trdá L, Daire X, Adrian M. 2014. Carbohydrates in plant immunity and plant protection: roles and potential application as foliar sprays. Frontiers in Plant Science 5, 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicedo B, Flors V, de la O Leyva M, Finiti I, Kravchuk Z, Real MD, García-Agustín P, González-Bosch C. 2009. Hexanoic acid-induced resistance against Botrytis cinerea in tomato plants. Molecular Plant-Microbe Interactions 22, 1455–1465. [DOI] [PubMed] [Google Scholar]
- Vierheilig H, Coughlan AP, Wyss U, Piche Y. 1998. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Applied Environmental Microbiology 64, 5004–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, Wu L, Yang Y, Zhou G, Ruan YL. 2018. Evolution of sucrose metabolism: the dichotomy of invertases and beyond. Trends in Plant Science 23, 163–177. [DOI] [PubMed] [Google Scholar]
- Xie B, Deng Y, Kanaoka MM, Okada K, Hong Z. 2012. Expression of Arabidopsis callose synthase 5 results in callose accumulation and cell wall permeability alteration. Plant Science 183, 1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.