Abstract
Methamphetamine (METH) abusers are prone to develop a variety of comorbidities, including cognitive disabilities, and the immunological responses have been recognized as an important component involved in the toxicity of this drug. Cytokines are among the key mediators between systemic inflammatory status and tissue responses. One of these, interleukin 1 (IL-1), has been hypothesized to be involved in cognitive functions and also appears to play a pivotal role among inflammatory molecules. In the present study, we demonstrate that exposure of mice to METH markedly increased the protein level of IL-1β in hippocampal tissue. Additionally, METH administration induced a decline in spatial learning as determined by the Morris water maze test. We next evaluated the hypothesis that blocking IL-1β signaling can protect against METH-induced loss of cognitive functioning. The results indicated that METH-induced impaired spatial learning abilities were attenuated by co-administration of mouse IL-1 Trap, a dimeric fusion protein that incorporates the extracellular domains of both of the IL-1 receptor components required for IL-1 signaling (IL-1 receptor type 1 and IL-1 receptor accessory protein), linked to the Fc portion of murine IgG2a. This effect was associated with a decrease in hippocampal IL-1β level. The current study indicates for the first time that the loss of METH-related cognitive decline can be attenuated by neutralizing IL-1 signaling. Our findings suggest a potential new therapeutic pathway for treatment of altered cognitive abilities that occur in METH abusing individuals.
1. Introduction
As recently reported by the United Nations Office on Drugs and Crime, there is a global increasing trend in drug use, with as many as 255 million adults admitting to taking drugs within the past year (United Nations Office on Drugs and Crime, 2017). Abuse of synthetic drugs, such as amphetamines and prescription stimulants, lags behind only the plant-based drugs (cocaine, opiates and cannabis), reaching 37 million users globally. A growing use of these drugs has been observed in North America, South-West Asia and parts of Europe. Methamphetamine (METH), the most popular stimulant drug, is characterized by a high neurotoxicity due to excessive release of dopamine, dysfunction of the ubiquitin-proteasome system, increased protein nitration and reticular stress, blood-brain barrier disruption, and overproduction of inflammatory cytokines (Yu et al., 2015). All of these mechanisms seem to orchestrate METH toxicity and may contribute to cognitive decline frequently associated with METH abuse (Soontornniyomkij et al., 2016). However, it is not clear which factor (if any) is mediating this machinery. Identification of such primary factors would provide promising targets for intervention to attenuate toxicity associated with METH abuse.
Immunological responses may play an important role in METH toxicity, as increased levels of proinflammatory cytokines, such as tumor necrosis factor, interferons and interleukins are routinely observed in METH-exposed animals or cells. In addition, it was reported that METH-induced neurotoxicity was attenuated in IL-6 knockout mice (Ladenheim et al., 2000), suggesting a potential casual role of this cytokine. IL-6 is a cytokine that can be upregulated by other proinflammatory molecules, such as IL-1, via the AKT pathway and NFκB transcription factor (Cahill and Rogers, 2008).
IL-1 is a major proinflammatory cytokine that can induce a number of other inflammatory factors to stimulate immune responses. Its two isoforms, IL-1α and IL-1β, occur in the form of precursor proteins that are cleaved to their mature forms by calpain or caspase 1, respectively. Pro-IL-1α, IL-1α and mature IL-1β are biologically active, exerting physiological effect by binding to the same IL-1 receptor (IL-1R). IL-1 is produced mainly by macrophages; in addition, microglia appear to be the major source of this cytokine in the CNS. Apart from its role in regulating inflammatory and host defense responses, IL-1β has also been implicated in learning and memory (Rizzo et al., 2018). While adequate levels of IL-1β are required for proper synaptic plasticity and learning processes, elevated IL-1β, recognized as a hallmark of neuroinflammation, adversely impacts multiple learning and memory systems, contributing to excitotoxicity and neurodegeneration (Rizzo et al., 2018).
In the present study, we indicate that chronic exposure to METH results in increased levels of IL-1β, an effect that was linked to impaired neurogenesis (Park et al., 2016). We further explored this finding by showing that inhibition of binding of IL-1 to its receptor is sufficient to protect against loss of spatial learning abilities in mice exposed to METH.
2. Materials and methods
2.1. Project design and drug treatment
All animals were provided by the Animal House of the Department for Experimental Medicine, Medical University of Silesia, Katowice, Poland, and were treated in accordance to the Directive 2010/63/EU for animal experiments using the protocols approved and monitored by the Local Ethics Committee for Animal Experimentation in Katowice.
13 week old C57BL/6NCrL male mice were divided into the following experimental groups (n = 12 per group) in a weight matched manner: (i) METH exposed, (ii) METH exposed and co-administered with murine IL-1 Trap (mIL1T), (iii) controls treated with saline and co-administered with mIL1T, and (iv) controls treated only with saline. Exposure to METH was accomplished by i.p. injections with METH (methamphetamine hydrochloride, M8750, Sigma-Aldrich, MO, US) solution in saline three times per day for 4 days with an escalating dose regimen (starting with 0.2 mg/kg to the final dose of 2.4 mg/kg), using a step-wise increase of 0.2 mg/kg with each injection. Then, mice were exposed for one more day to a binge-like high METH dosage which consisted of 3 successive injections of 4.0 mg/kg METH at 3 h intervals. This escalating regimen of METH dosing was adapted from our earlier study (Park et al., 2016). Control mice were injected in the same manner with saline.
Mice received mIL1T (granted by Regeneron Pharmaceuticals Inc., Tarrytown, NY) twice, both at 30 mg/kg. The first dose was given i.p. 15 min before the first METH injection, and the second dose was given after three days of METH administration, i.e., before the last two days of METH exposure. The dosing was established based on the Tmax and Cmax for mIL1T provided by the literature (Rydgren et al., 2013).
Eight randomly chosen animals from each group were allocated for the behavioral tests. Four remaining mice from each group were sacrificed by decapitation 24 h after the last METH dose. The sera, hippocampi and cortices were collected in these animals for ELISA and immunoblotting.
2.2. ELISA and immunoblotting
On the day of euthanasia, ∼0.5 mL of orbital sinus blood was collected. The blood was allowed to clot by leaving it for 30 min on ice. The samples were then centrifuged (2000×g for 15 min), and the serum was collected and stored at −80 °C. Individual hippocampal and cortical samples were homogenized in RIPA lysis buffer (Millipore, MS, US) supplemented with protease and phosphatase inhibitor cocktail (Thermo Scientific, MA, US). The homogenates were centrifuged at 14,000×g for 15 min, and the supernatants were used for either ELISA or immunoblotting. Protein concentrations were determined using RotiQuant assay (Carl Roth, DE). Levels of IL-1β and IL-6 were measured with the commercially available ELISA kit (Mouse IL-1beta ELISA Kit, ab100705; Mouse IL-6 ELISA Kit, ab100713; both from Abcam, UK).
For immunoblotting, the samples were separated on 4–15% SDSPAGE and transferred onto PVDF membranes (Bio-Rad Laboratories, CA, US). Membranes were blocked for 2 h at room temperature in Casein Blocking Buffer (Sigma-Aldrich, MO, US), and incubated overnight at 4 °C (10% casein in TBST) with rabbit anti-IL-1 RAcP (1:1000; Abcam, ab8110), rabbit anti-IL-1 Receptor 1 (IL-1R1) antibody (1:1000; Abcam, ab238457) or rabbit anti-β-actin (1:5000; Sigma-Aldrich). After washing, the membranes were incubated with secondary donkey anti-rabbit IgG antibody (Abcam, ab205722). Individual immunoblots were visualized by Clarity Western ECL Blotting Substrates (Bio-Rad) and detected by ChemiDoc™ Touch Imaging System (Bio-Rad). Targeted proteins were quantified by ImageLab Software 6.0.1 (Bio-Rad) by blinded observer. Western blots were repeated twice, and the results were normalized to actin, expressed as percentage of mean control. The values from each blot were averaged.
Cell culture samples were processed for immunoblotting as described earlier (Park et al., 2013; Skowronska et al., 2018). The following antibodies were used for detection of NeuN, GFAP, and β-tubulin (internal control): rabbit anti-NeuN (1:1000; Abcam, ab177487), rabbit anti-GFAP (1:1000; Abcam, ab7260), and rabbit anti-beta tubulin (1:1000; Cell Signaling Technology, 2136). In addition, the following secondary antibodies were applied: RDye® 800CW goat antirabbit IgG (Li-Cor, P/N 925–32211) and IRDye® 680LT goat anti-rabbit IgG (Li-Cor, P/N 925–68022) for detection by Licor CLX Imaging System. The signal quantification was performed using Image Studio 4.0 software (Licor).
2.3. Morris water maze (MWM) test
Hippocampus-dependent cognitive processes, such as spatial learning and memory, were measured by the MWM test in two paradigms according to well-established protocols (Vorhees and Williams, 2006). The first paradigm (initial spatial acquisition learning) started four days after the last day of METH or saline administration. Based on spatial cues, the mice learned the location of a hidden platform (11 cm in diameter) for five constitutive days (D1-D5), during four 1-minutelong trials per day. On the day D5 the additional trial was done to strengthen memory retention before the upcoming reversal learning phase. On the 6th day (the probe trial, D6), the platform was removed, and the animals were allowed to swim for 1 min to evaluate retention memory.
In the second paradigm, the platform was located in the opposite quadrant of the pool to evaluate cognitive flexibility in the reversal relearning mode of the MWM test. This part of the test lasted for five days of learning (R1-R5), followed by the day of the probe trial (R6), similar to the previous test.
The MWM consisted of a round pool (90 cm in diameter) filled with water (25 °C) containing non-toxic white tempera paint, to make the water opaque. The experiment was performed in a square room (2 × 2 m). The following parameters were evaluated: a) average latency to reach the platform, b) proximity measure (cumulative distance in centimeters of mice from the center of the platform location recorded 5 times per sec. until the animal reached the platform or for 60 s during the probe trial), c) swimming speed, and d) percent quadrant time (amount of time mice spent in individual quadrant during the probe trial searching for the removed platform).
2.4. Open field test
The open field test, which measures locomotor and exploratory activity, was performed two days after the last day of METH or saline injections. Mice were placed for 10 min in a transparent square cage (40 × 40 cm) located in a new, unknown room. The movements of animals were recorded by infrared detectors (TruScan, Coulbourn Instruments, PA, US), and the movements, total distance, and velocity were calculated.
2.5. Differentiation of neural progenitor cells (NPCs)
Primary NPCs, derived from cells isolated from embryonic day 14 mouse cortex, were purchased from Stemcell Technologies (Vancouver, Canada) and cultured according to the technical manual provided by the company on poly-D-lysine (100 μg/ml; Sigma) and laminin (1 μg/ml; Sigma) coated dishes. Neurospheres were allowed to expand to 100–150 μm in diameter, followed by passage or harvesting. The cells were used in less than fifth passage. To maintain NPCs in an undifferentiated/proliferating state, Complete Proliferating Medium (Stemcell Technologies), containing 20 ng/ml of recombinant human epidermal growth factor (rhEGF), was used. In order to differentiate NPCs, the medium was switched to Complete Differentiation Medium (Stemcell Technologies), and cells were allowed to differentiate for 14 days. During the differentiation process, cells were treated with METH 10 μM and/or IL-1β 1 ng/mL. Cell culture media were changed every other day, and during that time new aliquots of METH 1 and/or IL-1β were added to the media.
2.6. Statistical analysis
Prism 7.0 (GraphPad Software, CA, US) was used for statistical analyses and figure generation. The water maze results were analyzed by two-way repeated measures ANOVA, followed by Neuman-Keuls post hoc test, which has a correction for multiple comparison. For probe trials, percent of time spend in individual quadrants was tested via nonparametric Kruskal–Wallis test with Dunn’s post hoc and the proximity results were examined via a two-way ANOVA. Cell culture experiments were analyzed using one-way ANOVA, followed by the Tukey test. The above mentioned data was presented as a mean ± S.E. ELISA data was compared with two-way ANOVA following Tukey test, and Pearson correlation (r) was used to measure dependence between cytokines levels. Western Blot data from tissue homogenates was presented as median ± min to max and compared by non-parametric Kruskal–Wallis test with Dunn’s post hoc. In all analyses, the p value less than 0.05 was considered to be statistically significant.
3. Results
3.1. METH administration increases levels of IL-1β in the hippocampus
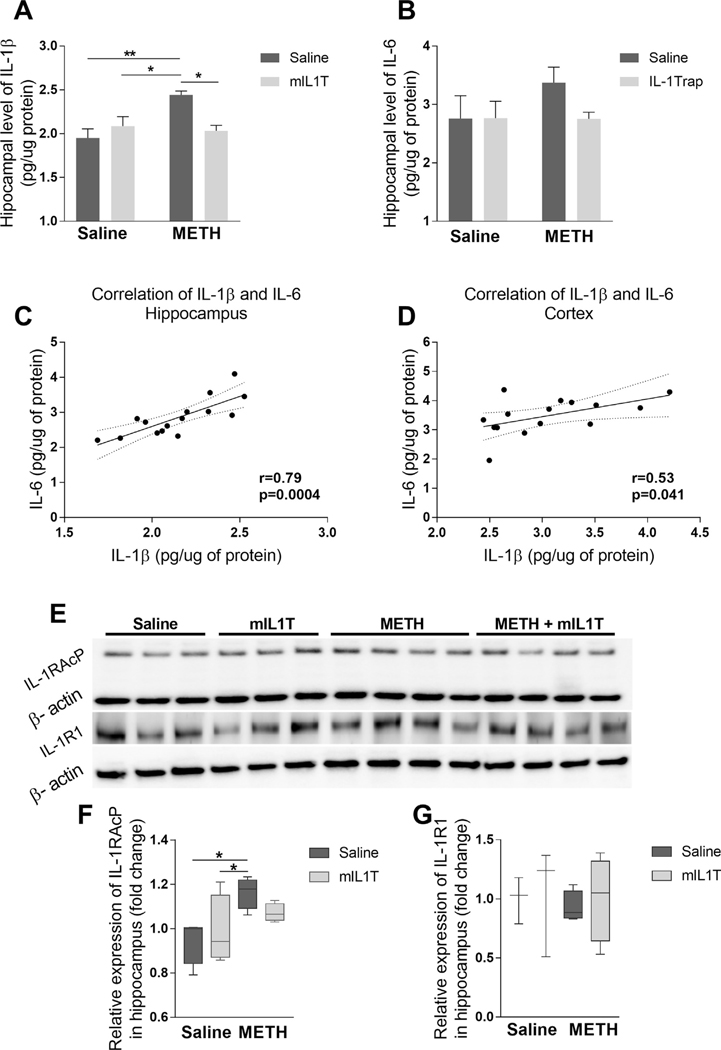
IL-1β has been implicated in the alterations of learning and memory (Rizzo et al., 2018); therefore, we measured IL-1β levels in the sera, hippocampal and cortical homogenates of METH-exposed and control mice using highly sensitive ELISA kit. Elevated hippocampal protein levels of IL-1β were observed in METH-exposed mice (two-way ANOVA; METH treatment for (F (1, 12) = 6.739, p = 0.0234)) (Fig. 1A), while serum and cortical levels of IL-1β did not differ among groups (Table 1). To demonstrate the specificity of these responses, mice were co-administered mIL1T. Two doses of mIL1T administered during METH exposure were sufficient to normalize hippocampal levels of IL-1β (interaction F (1, 12) = 10.47, p = 0.0071) (Fig. 1A). Additionally, we measured IL-6 levels in the hippocampal (Fig. 1B) and cortical (Table 1) homogenates. No statistically significant differences were observed in the concentrations of IL-6 in the analyzed brain samples between METH-treated and control mice. However, the Pearson correlation coefficient revealed positive correlations between IL-1β and IL-6 levels in the hippocampal (r = 0.79, p = 0.0004; Fig. 1C) and cortical (r = 0.53, p = 0.041; Fig. 1D) samples.
Fig. 1.
Impact of METH and/or mIL1T on IL-1β, IL-6, IL-1RacP, and IL-1R1 expression. Mice were administered with METH and/or mIL1T as described in the Materials and Methods section, and hippocampal protein levels of IL-1β (A) and IL-6 (B) were measured by ELISA. The results were analyzed by two-way-ANOVA followed by Tukey post hoc test; mean ± S.E. The positive Pearson’s correlation was observed among IL-1β and IL-6 levels in the hippocampi (C) and cortices (D) of examined mice. In addition, hippocampal protein expression of IL-1RacP (E, F) and IL-1R1 (E, G) were evaluated by immunoblotting. The blots were normalized to βactin levels and analyzed by the Kruskal–Wallis test with Dunn’s post hoc test; the results are median ± min to max. (E) Representative images, and (F and G) quantitative data from these experiments. All measurements were performed 24 h after the last METH injection (n = 3–4; *p < 0.05, **p < 0.01).
Table 1.
Levels of interleukin IL-1β and IL-6 in serum and cortex (mean ± S.E.).
| Protein | Control | mIL1T | METH | METH + mIL1T |
|---|---|---|---|---|
| Serum IL-1β (pg/ml) | 82.6 (15.68) | 81.4 (23.1) | 81.41 (17.14) | 65.36 (11.64) |
| Cortex IL-1β (pg/mg of protein) | 3.08 (0.41) | 3.61 (0.25) | 3.81 (0.26) | 3.58 (0.28) |
| Cortex IL-6 (pg/mg of protein) | 3.05 (0.21) | 3.276 (0.33) | 3.37 (0.27) | 2.76 (0.11) |
Because mIL1T acts partially by attenuating binding to the IL-1 receptor accessory protein (IL-1RAcP), we measured the expression of this protein in the hippocampal homogenates (Fig. 1E, F). The mean levels of IL-1RAcP were elevated in METH treated animals as compared to saline (p = 0.01) or mIL1T (p = 0.03) exposed mice, indicating increased production of this protein in response to METH administration (Fig. 1F). However, this METH-induced effect was attenuated by co-administration of mIL1T. In contrast to IL-1RAcP expression, we did not observe any significant differences in the hippocampal levels of the IL-1R1 protein among the experimental groups (Fig. 1E, G).
3.2. METH administration does not affect locomotor performance
In order to evaluate a possible impact of METH and/or mIL1T exposure on the locomotor abilities of mice, we employed the open field test. The studies were performed three days after the last dose of METH. The test evaluated the number of movements, movement time, velocity and total distance traveled. No differences in the measured functional locomotor parameters were observed between the experimental groups (Table 2).
Table 2.
Functional parameters measured by the open field test in mice (mean ± S.E.).
| Control | mIL1T | METH | METH + mIL1T | |
|---|---|---|---|---|
| No. of moves | 92.38 (3) | 90 (2.7) | 92.13 (3) | 89.75 (2.6) |
| Move time (s) | 416 (23) | 414 (21) | 430 (51) | 417 (47) |
| Velocity (cm/s) | 4.2 (0.3) | 4 (0.4) | 4.5 (0.3) | 4.3 (0.3) |
| Distance moved (cm) | 1802 (203) | 1702 (226) | 1963 (201) | 1803 (155) |
3.3. METH abuse deteriorates spatial learning but not memory retention. Inhibition of IL-1 signaling attenuates METH-induced impairment of spatial learning
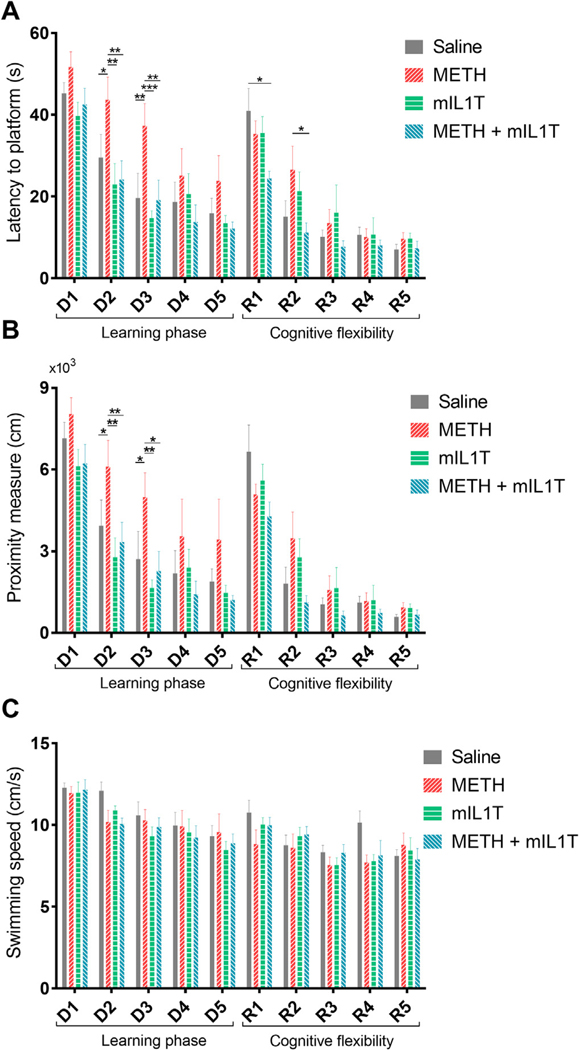
Altered cognitive function may be one of the most important long-term outcomes of METH abuse. Therefore, the MWM test to measure spatial learning and memory was performed in mice four days after the last doses of METH. Two-way-repeated measures ANOVA revealed overall significant differences for time spend to reach the platform (time F (9, 252) =40.12, p < 0.0001; treatment F (3, 28) = 4.705, p = 0.0088) and the total distance to the target (time F (9, 252) = 43.72, p < 0.0001; treatment F (3, 28) =4.198, p = 0.0142). Between-group analysis indicated that METH-administered mice required more time (Fig. 2A) to reach the platform. In addition, the proximity measure that reflects the distance of mice from the center of the platform was higher in mice exposed to METH (Fig. 2B). Importantly, neutralization of the biological impact of IL-1 signaling by mIL1T significantly attenuated these effects. The impact of METH and/or mIL1T was the most apparent at days 2–3 of the test, when statistically significant differences were noted.
Fig. 2.
METH-induced cognitive decline is attenuated by mIL1T. Mice were treated as in Fig. 1, and spatial learning was evaluated by Morris water maze test four days after the last dose of METH. For the first five days (D1-D5; the learning phase of the test) the animals learned the location of the platform. Then, the reversal learning was applied (R1-R5) when the animals relearned a new location of the platform, testing cognitive flexibility. The statistical differences were observed in (A) latency to reach the platform and (B) proximity measure, suggesting that the experimental groups differed in spatial learning abilities. (C) Exposure to METH and/or mIL1T did not affect swimming speed of mice during the training period. All results were analyzed by two-way repeated measures ANOVA with Student Newman-Keuls test; mean ± S.E.; n = 8; *p < 0.05, **p < 0.01, ***p < 0.001).
In the reversal phase of the MWM test (marked by the symbol “R” in Fig. 2A and B), the platform was moved to the opposite quadrant of the pool, allowing to attest memory cognitive flexibility. In this test, METH-exposed animals also showed affected learning abilities. Importantly, mice in the METH+ mIL1T group performed better than the mice in the METH group, with statistical significance observed at day R2 (Fig. 2A). These mice demonstrated the best cognitive flexibility at the first day of the reversal learning phase, and performed significantly better than the saline treated controls.
The MWM test employed in the present study is dependent on distal cues for navigation from the start locations and around the perimeter of an open swimming arena to locate the submerged escape platform. While the test is highly reliable, it relies on the ability of mice to swim. Therefore, it was important that METH and/or mIL1T administration did not affect swimming speed (Fig. 2C). These results were consistent with normal locomotor performance in all treated groups as determined in the open field test (Table 2).
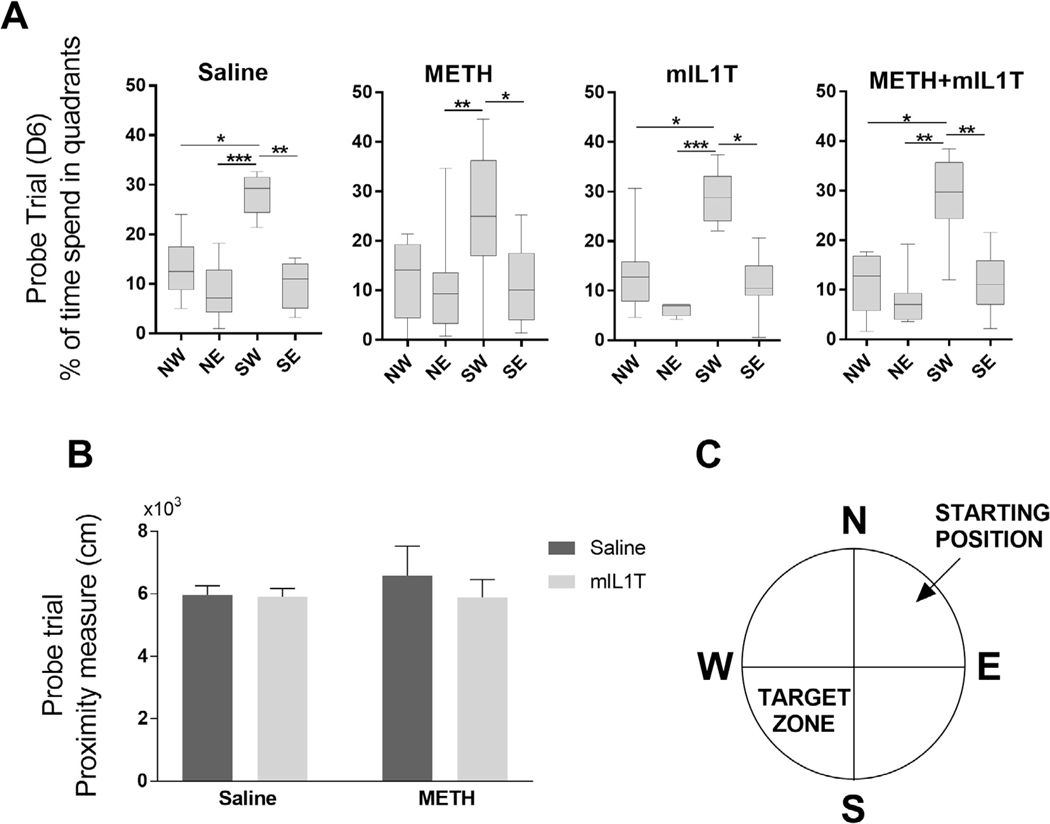
On the trial day (Day 6), the platform was removed, and the animals were evaluated for retention memory by measuring the percentage of time that mice spent in the individual quadrants of the pool (Fig. 3A). As indicated, mice from all groups spent the most time in the quadrant were the platform was located during the learning phase. These results suggest that the long term memory and memory retention were not affected by METH exposure. The lack of the differences in the proximity measure among the experimental groups at D6 (Fig. 3B) additionally supports this conclusion. Moreover, we did not observe any differences during the probe trial performed at R6 (Supplementary information).
Fig. 3.
The long term spatial memory retention is unaffected by METH treatment. Memory retention was evaluated as part of the Morris water maze test by removing the platform during the probe trial (D6) and measuring (A) the time spend in target quadrant were the platform was located during the learning period (Kruskal–Wallis test with Dunn’s post hoc test; the results are median ± min to max) and (B) proximity measure from the location of the removed platform. (A-B) Two-way-ANOVA; mean ± S.E) (n = 8; *p < 0.05, **p < 0.01, ***p < 0.001. (C) Diagram of the Morris water maze pool quadrants with the marked location of the platform and where mice were placed to find the platform.
3.4. IL-1β exposure diminishes NPC differentiation into mature neurons
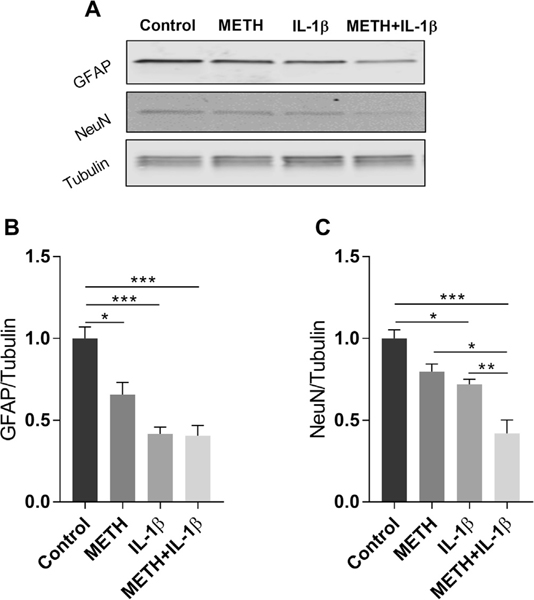
In order to explain the deleterious effect of IL-1β on cognitive function, we next evaluated the impact of this cytokine on differentiation of NPCs. Because NPCs can differentiate to both neurons and astrocytes, NeuN was used as a marker of mature neurons and GFAP as a marker of astrocytes in these experiments. As illustrated in Fig. 4, a 2 week exposure to METH or IL-1β significantly diminished differentiation of NPCs into both astrocytes and neurons. Interestingly, a combined exposure to METH+ IL-1β affected formation of neurons to a higher degree as compared to these factors acting alone. We also analyzed if NPCs can express this cytokine on the gene levels. As demonstrated in Supplementary Fig. 2, non-differentiated mouse NPCs do not express this cytokine.
Fig. 4.
Exposure to METH and/or IL-1β diminishes differentiation of neural progenitor cells (NPCs) to mature astrocytes and neurons. Primary mouse NPCs were induced to differentiate in the presence of METH (10 μM) and/or IL-1β (1 ng/ml) for 2 weeks. Control cells were exposed to vehicle. Then, the protein levels of GFAP and NeuN were assessed by immunoblotting as the markers of astrocytes and mature neurons, respectively. β-tubulin was assessed as the internal control. (A) Representative images, and (B and C) quantitative data from these experiments. The results were analyzed by one-way ANOVA with Tukey post hoc test; mean ± S.E. (n = 3 in each group; p < 0.05, **p < 0.01, ***p < 0.001 compared to control).
4. Discussion
In the present study, we have shown that alterations in cognitive functioning, as defined by spatial learning, in mice exposed to METH can be attenuated by pharmacological neutralization of IL-1 signaling. To our knowledge, this is the first report clearly showing that cognitive decline related to METH exposure may be associated with IL-1 levels. The evidence of this dependency relies on the use of a mouse homolog of Rilonacept, a clinically approved IL-1 inhibitor. The inhibitory impact of mIL1T is accomplished by neutralizing IL-1 signaling. Indeed, mIL1T consists of the extracellular components of the IL-1 receptor (IL1R) and IL-1RacP conjugated with the Fc region of IgG. The trap binds both IL-1α and IL-1β, effectively preventing them from interaction with IL-1R. While mIL1T has a higher affinity to IL-1β than IL-1α, its effects cannot differentiate the biological impact of these IL-1 isoforms as they share the same IL-1R. Because mIL1T is a relatively large molecule, its penetration through the blood-brain barrier appears to be unlikely under regular conditions. However, METH possesses well established blood-brain barrier disruption properties (Northrop and Yamamoto, 2015; Toborek et al., 2013); therefore, it may facilitate penetration of mIL1T into the brain, allowing for IL-1β trapping. Part of the METH-induced BBB alterations is related to the well-recognized susceptibility of the vascular endothelium to oxidative and pro-inflammatory damage (Lee et al., 2004; Lee et al., 2001; Lee et al., 2002; Toborek et al., 1995).
Because of the focus of the present study on the neurotoxicity of METH, we employed an involuntary route of METH administration (namely, escalating doses of i.p. injections) that allowed for a strict control of METH dosage and exposure times. In contrast, self-administration of METH is a more frequent approach in studies on drug addiction. While self-administration models reproduce several features of addiction, they also have limitations, such as implanting an indwelling catheter in the jugular vein and maintaining the catheter, interference with the animals by the personnel conducting the experiments, training to nose-poke for i.v. infusion, and various reinforcement strategies (e.g., food restriction) that may induce compounding effects. In addition, voluntary vs. involuntary METH administration exerts distinctive neurochemical adaptations, such as different patterns of dopamine and glutamate release in the nucleus accumbens (Lominac et al., 2012), with self-administration typically leading to sensitization and protection against neurotoxicity (Marshall and O’Dell, 2012). METH-induced glutamate release evokes inflammatory reactions through stimulation of microglial activation and production of inflammatory cytokines (Salamanca et al., 2014). Therefore, different types of METH administration may induce different patterns of immune response.
The use of only male mice is a study limitation as females and males respond differently to METH abuse (Dluzen and Liu, 2008; Ruda-Kucerova et al., 2015). Women appear to be more dependent on and committed to METH but show diminished dopamine responses and a lower degree of METH toxicity (Dluzen and Liu, 2008). In an experimental model of METH abuse, female rats were found to self-administer significantly lower dose of METH but to be more vulnerable to relapse of METH seeking behavior. This effect was detected in all females, independently of current phase of their estrous cycle (Ruda-Kucerova et al., 2015). It should be noted the model of involuntary METH administration employed in the present study did not include a relapse stage.
Early studies by Yamaguchi et al. observed that METH stimulates expression of IL-1β mRNA in the brain, particularly in the hypothalamus (Yamaguchi et al., 1991a,b), suggesting that this cytokine may be partially involved in the production of the central responses in METH abuse. More than a decade later, in vitro investigations showed that METH can induce production of IL-1β in macrophages (Tipton et al., 2010) and microglial cells (Jiraporn et al., 2010). Our current results, using the same METH administration pattern to mice as reported recently (Park et al., 2016), indicated elevated hippocampal levels of IL1β. In contrast, METH and/or mIL1T administration did not affect levels of IL-1β in serum and the cerebral cortex. Nonetheless, we cannot exclude the presence of METH-induced inflammatory changes in other organs and brain areas, since METH concentration, distribution and metabolism vary between organs (Riviere et al., 2000). We also evaluated the levels of IL-6 and additional components of IL-1 signaling, i.e. the expression of IL-1R1 and IL-1RAcP that serves as its co-receptor required for signal transduction of IL-1/IL-1R complexes (Weber et al., 2010). No differences were shown for IL-1R1 expression, but increased levels of IL-1RAcP were observed as the result of METH treatment. This effect is in line with the study showing that METH can stimulate expression of IL-1RAcP mRNA (Najera et al., 2016). In contrast, it was shown that IL-1β upregulates expression of IL-1RI and IL-1RII, but not IL-1RacP (Huang et al., 2011; Pousset et al., 2001; Yoon and Dinarello, 1998). While the hippocampal IL-6 expression did not differ significantly among the experimental groups, a positive correlation was observed between IL-1β and IL-6 levels in the hippocampus and, to a lesser extent, in the cerebral cortex. These findings are in line with the notion that IL-1β is a potent inflammatory effector of METH action (Xu et al., 2018), with ability to recruit other proinflammatory molecules (Cahill and Rogers, 2008; Eskan et al., 2008) and orchestrate immunotoxic machinery.
A growing body of evidence supports the importance of proinflammatory cytokines in METH-induced neurotoxicity and cognitive decline. It has been shown that IL-1β mediates METH-related sleep disturbances (Schmidt and Wisor, 2012), but not hyperthermia (Seminerio et al., 2012). A recent study focused on the role of the inflammatory response in the occurrence of METH-induced cognitive deficits reported that disturbances in spatial memory may be improved when an anti-inflammatory agent is administered (Jennifer M. Loftis et al., 2013). It was also observed that the METH-related decrease of dopamine and serotonin was diminished in IL-6 knockout mice (Ladenheim et al., 2000).
The spatial learning delays observed in the present study appears to be associated with increased IL-1β, with some prior evidence suggesting that IL-1β interferes mostly with hippocampus-dependent memory (Huang and Sheng, 2010). The fact that the hippocampus is a vulnerable brain structure to IL-1β-mediated impact is supported by the observation that it contains, especially in the dentate gyrus, the highest amount of IL-1 receptor in the CNS (Parnet et al., 2002). In this context, it is important to note that exposure to IL-1β inhibited differentiation of NPCs both into mature neurons and astrocytes (Fig. 4). While there is no clear indication that aberrant neurogenesis played a role in METH-induced alterations of spatial memory, our recently published manuscript, using the same mouse model of METH exposure as that in the present study, indicated alterations of hippocampal neurogenesis (Park et al., 2016). Interestingly, IL-1β is not produced by NPCs (Supplemental Fig. 2), suggesting that the IL-1 β protein detected in the hippocampus (Fig. 1) was derived not from NPCs but from other cell types of the hippocampus, and/or they originated from the periphery and penetrated into the brain via disrupted BBB. Should the latter hypothesis prove correct, it may provide a new therapeutic strategy to protect against METH-induced cognitive alterations.
In the present study, we focused on tasks involving hippocampal dependent mechanisms, because it was proposed that IL-1β signaling modulates predominantly spatial learning (Huang and Sheng, 2010). In addition, exposure to METH disrupted the blood-brain barrier preferentially in the hippocampal region (Toborek et al., 2013). However, both METH and mIL1T administrations were systemic and the improved spatial memory might result from reduction of IL-1β impact also in other brain regions. It is well recognized that METH abuse induces a broad spectrum of cognitive changes, both in humans and laboratory animals, including deficits in memory, attention, and decision-making skills. The extent of cognitive deficits depends on the dose and the time of treatment; thus, it is highly associated with the applied model. Exposing rats to a binge neurotoxic METH regimen results in lasting impairments in their novel object recognition memory, recognition of socially relevant odors, as well as impaired inhibitory control. Cognitive impairment was also observed in motor learning, which may involve hippocampal or striatal dependent mechanisms. For example, poor learning of sequences of turns in the radial arm maze or the Cincinnati water maze, a task that is striatal-dependent and relies on response learning rather than on place learning, was observed in rats exposed to a binge METH regimen (Marshall and O’Dell, 2012). Deficits in tasks determined by hippocampal function appear to be dependent on METH dose and administration regimen. Some authors observed little or no effect of METH exposure on rats’ performance in MWM (Friedman et al., 1998; Herring et al., 2008; Schroder et al., 2003), whereas we and the others (Chen et al., 2012; Loftis et al., 2013; Wu et al., 2003) showed a dysfunction of the spatial parameters measured by this test in METH-exposed mice. Indeed, spatial learning, but not memory retention, appears to be predominantly affected in our model of METH toxicity.
Neuroinflammation, along with hypothalamic functioning may also underpin addictive behavior (Harricharan et al., 2017). While this connection has been studied primarily in the context of alcohol, opioid, or cocaine dependency (Evans and Cahill, 2016; Harricharan et al., 2017; Kane and Drew, 2016), it is likely that activation of the immune system and inflammation can also contribute to METH addiction (Krasnova et al., 2016). Indeed, IL-1β, i.e., the main inflammatory cytokine induced by METH exposure, was demonstrated to induce impairments of long-term potentiation and memory functions, the effects that were linked to altered actin polymerization (Tong et al., 2018). Simultaneously, the development of METH-craving in response to synaptic stimulation relays on actin remodeling, and inhibition of actin polymerization prevents relapse to METH use (Young et al., 2015). The involvement of inflammatory reactions in the development of drug addiction is supported by the observation that mice with targeted deletions of the TNF-α gene self-administered more METH than controls (Loftis and Janowsky, 2014). Finally, it was demonstrated that anti-inflammatory suppression of glia reduced the amount of self-administered METH (Snider et al., 2013) and attenuated relapse (Beardsley et al., 2010).
4.1. Conclusions
Taken together, our data indicate that inhibition of METH-stimulated elevation of IL-1 isoforms may attenuate the loss of cognitive abilities associated with exposure to this drug of abuse. This report brings attention to the importance of IL-1 isoforms in METH-induced toxicity, suggesting a potential new pathway for treatment of altered cognitive abilities that occur in METH abusing individuals.
Supplementary Material
Acknowledgements
We would like to acknowledge Regeneron Pharmaceuticals Inc. (Tarrytown, NY) for providing murine IL-1 Trap (REGN128).
Funding
The study was supported in part by the National Science Centre (NSC) grant 2015/17/B/NZ7/02985 and the National Institutes of Health (NIH), grants DA039576, DA040537, DA044579, MH098891, MH072567, and HL126559.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2019.03.016.
References
- Beardsley PM, Shelton KL, Hendrick E, Johnson KW, 2010. The glial cell modulator and phosphodiesterase inhibitor, AV411 (ibudilast), attenuates prime- and stress-induced methamphetamine relapse. Eur. J. Pharmacol. 637, 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Rogers JT, 2008. Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J. Biol. Chem. 283, 25900–25912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-J, Liu Y-L, Zhong Q, Yu Y-F, Su H-L, Toque HA, Dang Y-H, Chen F, Xu M, Chen T, 2012. Tetrahydropalmatine protects against methamphetam-ineinduced spatial learning and memory impairment in mice. Neurosci. Bull. 28, 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE, Liu B, 2008. Gender differences in methamphetamine use and responses: a review. Gend. Med. 5, 24–35. [DOI] [PubMed] [Google Scholar]
- Eskan MA, Benakanakere MR, Rose BG, Zhang P, Zhao J, Stathopoulou P, Fujioka D, Kinane DF, 2008. Interleukin-1beta modulates proinflammatory cytokine production in human epithelial cells. Infect. Immun. 76, 2080–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CJ, Cahill CM, 2016. Neurobiology of opioid dependence in creating addiction vulnerability. F1000Research 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SD, Castaneda E, Hodge GK, 1998. Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacol. Biochem. Behav. 61, 35–44. [DOI] [PubMed] [Google Scholar]
- Harricharan R, Abboussi O, Daniels WMU, 2017. Addiction: a dysregulation of satiety and inflammatory processes. Prog. Brain Res. 235, 65–91. [DOI] [PubMed] [Google Scholar]
- Herring NR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT, 2008. Effect of +-methamphetamine on path integration learning, novel object recognition, and neurotoxicity in rats. Psychopharmacology 199, 637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Smith DE, Ibanez-Sandoval O, Sims JE, Friedman WJ, 2011. Neuronspecific effects of interleukin-1beta are mediated by a novel isoform of the IL-1 receptor accessory protein. J. Neurosci. 31, 18048–18059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZB, Sheng GQ, 2010. Interleukin-1β with learning and memory. Neurosci. Bull. 26, 455–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiraporn T, Chakkrapong K, Sukumal C, Piyarat G, 2010. Melatonin attenuates methamphetamine-induced overexpression of pro-inflammatory cytokines in microglial cell lines. J. Pineal Res. 48, 347–352. [DOI] [PubMed] [Google Scholar]
- Kane CJM, Drew PD, 2016. Inflammatory responses to alcohol in the CNS: nuclear receptors as potential therapeutics for alcohol-induced neuropathologies. J. Leukoc. Biol. 100, 951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Justinova Z, Cadet JL, 2016. Methamphetamine addiction: involvement of CREB and neuroinflammatory signaling pathways. Psychopharmacology 233, 1945–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenheim B, Krasnova IN, Deng X, Oyler JM, Polettini A, Moran TH, Huestis MA, Cadet JL, 2000. Methamphetamine-induced neurotoxicity is attenuated in transgenic mice with a null mutation for interleukin-6. Mol. Pharmacol. 58 1247 LP1256. [DOI] [PubMed] [Google Scholar]
- Lee YW, Eum SY, Nath A, Toborek M, 2004. Estrogen-mediated protection against HIV Tat protein-induced inflammatory pathways in human vascular endothelial cells. Cardiovasc. Res. 63, 139–148. [DOI] [PubMed] [Google Scholar]
- Lee YW, Kühn H, Hennig B, Neish AS, Toborek M, 2001. IL-4-induced oxidative stress upregulates VCAM-1 gene expression in human endothelial cells. J. Mol. Cell. Cardiol. 33, 83–94. [DOI] [PubMed] [Google Scholar]
- Lee YW, Son KW, Flora G, Hennig B, Nath A, Toborek M, 2002. Methamphetamine activates DNA-binding of specific redox-responsive transcription factors in mouse brain. J. Neurosci. Res. 70, 82–89. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A, 2014. Neuroimmune basis of methamphetamine toxicity. Int. Rev. Neurobiol. 118, 165–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Wilhelm CJ, Vandenbark AA, Huckans M, 2013. Partial MHC/neuroantigen peptide constructs: a potential neuroimmune-based treatment for methamphetamine addiction. PLoS One 8, e56306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac KD, Sacramento AD, Szumlinski KK, Kippin TE, 2012. Distinct neurochemical adaptations within the nucleus accumbens produced by a history of self-administered vs non-contingently administered intravenous methamphetamine. Neuropsychopharmacology 37, 707–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JF, O’Dell SJ, 2012. Methamphetamine influences on brain and behavior: unsafe at any speed? Trends Neurosci. 35, 536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najera JA, Bustamante EA, Bortell N, Morsey B, Fox HS, Ravasi T, Marcondes MCG, 2016. Methamphetamine abuse affects gene expression in brain-derived microglia of SIV-infected macaques to enhance inflammation and promote virus targets. BMC Immunol. 17, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop NA, Yamamoto BK, 2015. Methamphetamine effects on blood-brain barrier structure and function. Front. Neurosci. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Kim H-J, Lim B, Wylegala A, Toborek M, 2013. Methamphetamine-induced occludin endocytosis is mediated by the Arp2/3 complex-regulated actin rearrangement. J. Biol. Chem. 288, 33324–33334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Levine H, Toborek M, 2016. Exercise protects against methamphetamine-induced aberrant neurogenesis. Sci. Rep. 6, 34111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnet P, Kelley KW, Bluthe RM, Dantzer R, 2002. Expression and regulation of interleukin-1 receptors in the brain. Role in cytokines-induced sickness behavior. J. Neuroimmunol. 125, 5–14. [DOI] [PubMed] [Google Scholar]
- Pousset F, Cremona S, Dantzer R, Kelley KW, Parnet P, 2001. IL-10 and IL-4 regulate type-I and type-II IL-1 receptors expression on IL-1 beta-activated mouse primary astrocytes. J. Neurochem. 79, 726–736. [DOI] [PubMed] [Google Scholar]
- Riviere GJ, Gentry WB, Owens SM, 2000. Disposition of methamphetamine and its metabolite amphetamine in brain and other tissues in rats after intravenous administration. J. Pharmacol. Exp. Ther. 292, 1042–1047. [PubMed] [Google Scholar]
- Rizzo FR, Musella A, De Vito F, Fresegna D, Bullitta S, Vanni V, Guadalupi L, Bassi MS, Buttari F, Mandolesi G, Centonze D, Gentile A, Johnson SW, 2018. Tumor necrosis factor and interleukin-1β Modulate synaptic plasticity during neuroinflammation. Neural Plast. 2018, 8430123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruda-Kucerova J, Amchova P, Babinska Z, Dusek L, Micale V, Sulcova A, 2015. Sex differences in the reinstatement of methamphetamine seeking after forced abstinence in Sprague-Dawley rats. Front. Psychiatry 6, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydgren T, Öster E, Sandberg M, Sandler S, 2013. Administration of IL-1 Trap prolongs survival of transplanted pancreatic islets to type 1 diabetic NOD mice. Cytokine 63, 123–129. [DOI] [PubMed] [Google Scholar]
- Salamanca SA, Sorrentino EE, Nosanchuk JD, Martinez LR, 2014. Impact of methamphetamine on infection and immunity. Front. Neurosci. 8, 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MA, Wisor JP, 2012. Interleukin 1 receptor contributes to methamphetamine- and sleep deprivation-induced hypersomnolence. Neurosci. Lett. 513, 209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder N, O’Dell SJ, Marshall JF, 2003. Neurotoxic methamphetamine regimen severely impairs recognition memory in rats. Synapse 49, 89–96. [DOI] [PubMed] [Google Scholar]
- Seminerio MJ, Robson MJ, McCurdy CR, Matsumoto RR, 2012. Sigma receptor antagonists attenuate acute methamphetamine-induced hyperthermia by a mechanism independent of IL-1β mRNA expression in the hypothalamus. Eur. J. Pharmacol. 691, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronska M, McDonald M, Velichkovska M, Leda AR, Park M, Toborek M, 2018. Methamphetamine increases HIV infectivity in neural progenitor cells. J. Biol. Chem. 293, 296–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SE, Hendrick ES, Beardsley PM, 2013. Glial cell modulators attenuate methamphetamine self-administration in the rat. Eur. J. Pharmacol. 701, 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V, Kesby JP, Morgan EE, Bischoff-Grethe A, Minassian A, Brown GG, Grant I, TMARC Group, 2016. Effects of HIV and methamphetamine on brain and behavior: evidence from human studies and animal models. J. Neuroimmune Pharmacol. 11, 495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton DA, Legan ZT, Dabbous MK, 2010. Methamphetamine cytotoxicity and effect on LPS-stimulated IL-1β production by human monocytes. Toxicol. Vitr. 24, 921–927. [DOI] [PubMed] [Google Scholar]
- Toborek M, Barger SW, Mattson MP, McClain CJ, Hennig B, 1995. Role of glutathione redox cycle in TNF-mediated endothelial cell dysfunction. Atherosclerosis 117, 179–188. [DOI] [PubMed] [Google Scholar]
- Toborek M, Seelbach MJ, Rashid CS, Andras IE, Chen L, Park M, Esser KA, 2013. Voluntary exercise protects against methamphetamine-induced oxidative stress in brain microvasculature and disruption of the blood-brain barrier. Mol. Neurodegener. 8, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Prieto GA, Cotman CW, 2018. IL-1beta suppresses cLTP-induced surface expression of GluA1 and actin polymerization via ceramide-mediated Src activation. J. Neuroinflammation 15, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime, 2017. Executive summary. Conclusion and policy implications of the world drug report 2017, World Drug Report; 2017. [Google Scholar]
- Vorhees CV, Williams MT, 2006. Forms of learning and memory. Nat. Protoc. 1, 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A, Wasiliew P, Kracht M, 2010. Interleukin-1 (IL-1) pathway. Sci. Signal. 3, cm1. [DOI] [PubMed] [Google Scholar]
- Wu CF, Liu YL, Song M, Liu W, Wang JH, Li X, Yang JY, 2003. Protective effects of pseudoginsenoside-F11 on methamphetamine-induced neurotoxicity in mice. Pharmacol. Biochem. Behav. 76, 103–109. [DOI] [PubMed] [Google Scholar]
- Xu E, Liu J, Liu H, Wang X, Xiong H, 2018. Inflammasome activation by methamphetamine potentiates lipopolysaccharide stimulation of IL-1beta production in microglia. J. Neuroimmune Pharmacol. 13, 237–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Kuraishi Y, Minami M, Nakai S, Hirai Y, Satoh M, 1991a. Methamphetamine-induced expression of interleukin-1β mRNA in the rat hypothalamus. Neurosci. Lett. 128, 90–92. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Kuraishi Y, Yabuuchi K, Minami M, Satoh M, 1991b. In situ hybridization analysis of the induction of interleukin-1β mRNA by methamphetamine in the rat hypothalamus. Mol. Cell. Neurosci. 2, 259–265. [DOI] [PubMed] [Google Scholar]
- Yoon DY, Dinarello CA, 1998. Antibodies to domains II and III of the IL-1 receptor accessory protein inhibit IL-1 beta activity but not binding: regulation of IL-1 responses is via type I receptor, not the accessory protein. J. Immunol. 160, 3170–3179. [PubMed] [Google Scholar]
- Young EJ, Briggs SB, Miller CA, 2015. The actin cytoskeleton as a therapeutic target for the prevention of relapse to methamphetamine use. CNS Neurol. Disord. Drug Targets 14, 731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Zhu L, Shen Q, Bai X, Di X, 2015. Recent advances in methamphetamine neurotoxicity mechanisms and its molecular pathophysiology. Behav. Neurol. 2015, 103969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.