Abstract
Transplantation of fully allogeneic organs into immunocompetent recipients invariably elicits T cell and B cell responses that lead to the production of donor-specific antibodies (DSA). When immunosuppression is inadequate donor-specific T cell and B cell responses escape, leading to T cell-mediated rejection (TCMR), antibody mediated (ABMR) rejection, or mixed rejection (MR) exhibiting features of both TCMR and ABMR. Current literature suggests that ABMR is a major cause of late graft loss, and that new therapies to curtail the donor-specific humoral response are necessary. The majority of research into B cell responses elicited by allogeneic allografts in both preclinical models and clinical studies, has focused on the function of B cells as antibody-secreting cells and the pathogenic effects of DSA as mediators of ABMR. However, it has long been recognized that the DSA response to allografts is T cell-dependent, and that B cells engage in cognate interactions with T cells that provide “help” and promote B cell differentiation into antibody-secreting cells (ASCs). This review focusses the function of B cells as antigen-presenting cells (APCs) to T cells in lymphoid organs, how they may be critical APCs to T cell in the allograft, and the functional consequences of these interactions.
Introduction
Under current standard of care, a majority of transplant recipients do not enjoy indefinite allograft survival. The median graft survival for primary kidney allografts from deceased donors in the United States from 2008-2015 (ttps://optn.transplant.hrsa.gov/data/view-data-reports/national-data/) was 93.2% for 1 year but declined to 74.4 % for 5 years, while the outcomes for organs such as the lung and small bowel are considerably lower. Concerted efforts have been made to accurately diagnose the cause of graft loss and mechanism of rejection, by examining histological changes in graft biopsies, and more recently, by using molecular signatures (1–3). T cell-mediated rejection (TCMR) has been identified as occurring more frequently in the early post-transplantation months or at later times due to inadequate immunosuppression, whereas late graft rejection has been attributed primarily to chronic antibody-mediated rejection (ABMR) (4, 5). A number of drugs can effectively target TCMR while chronic ABMR is less responsive to current immunosuppression, lending weight to the notion that ABMR is a more important cause for graft loss and for which therapies capable of reversing chronic ABMR are critically necessary (6, 7). As a result, research has focused on improving the diagnosis of donor-specific antibodies (DSA), understanding the pathogenic properties of DSA upon binding to allograft endothelium, and gaining therapeutic insights into the immunobiology of plasma cells (PC) producing DSA.
In addition to B cells differentiating into antibody-secreting cells (ASCs), and where antibodies function as opsonins to facilitate DC activation and T cell responses (8–10), B cells can function as antigen presenting cells (APCs) that regulate T cell function and potentially, TCMR. While the ability of B cells to function as APCs was described in the early 1980’s, dendritic cells (DCs) are now considered to be best at activating naïve T cells (11–13). As a result, focus has shifted away from the investigation of B cells as APCs to naïve T cells, and indeed, a revised hypothesis is that naïve B cells lacking the expression of co-stimulatory molecules might actually function as mediators of T cell anergy (14–16). Nevertheless, there continues to be evidence for B cells functioning as APCs to naïve T cells. Recently, Shen et al. reported that upon immunization with antigens displayed on a virus-like nanoparticle, B cells are the dominant and necessary APC activating naïve CD4+ T cells, while DCs are not necessary(17). These B cells promote antigen-specific T cell expansion and their differentiation into T follicular helper (Tfh) cells. Finally, it has been argued that conditions of low antigen concentrations might hinder efficient antigen uptake and presentation by DCs, whereas expanded populations of memory B cells expressing the appropriate BCRs may be able to capture low concentrations of antigens and present to memory T cells (17, 18). These observations raise the possibility that B cells play a dominant role during recall responses.
In this review, we will focus our discussion on the function of B cells as APCs to T cells in the context of promoting transplantation rejection or tolerance. Specifically, I will discuss presentation of alloantigen by recipient B cells leading to the stimulation of alloreactive T cells via the indirect pathway, and discuss the role of B cells as APCs in secondary lymphoid organs (SLOs). I will also review the potential role of B cells in the thymus as mediators of T cell deletion, discuss the antibody-independent role of B cells in a preclinical model of chronic allograft rejection, and their recently documented role as APC to T cells in rejecting human kidney biopsies.
Indirect alloantigen presentation of alloantigen by recipient B cells to recipient CD4+ T cells
Alloreactive T cells can directly recognize intact donor MHC presented on donor-derived cells, or indirectly when recipient APCs acquire donor antigens, process and then present donor-derived peptides in the context of recipient Class I or Class II (reviewed (19, 20). In addition, alloreactive T cells can be activated by the semi-direct pathway whereby recipient APCs acquire intact donor MHC molecules through a number of processes that are cell contact-dependent (e.g. trogocytosis, or nibbling) or cell contact-independent through cross-dressing, i.e., decoration of recipient APCs with extracellular vesicles/exosomes shed from donor cells (reviewed in (21, 22)). There is increasing evidence suggesting that the semi-direct pathway is an important means for eliciting CD8+ T cell responses capable of recognizing donor Class I, because the donor-derived DCs capable of priming alloreactive CD8+ T cells by the direct pathway rapidly disappear post-transplantation. Instead, persistence of cross-dressed DCs in the draining lymph nodes (LNs) allows for the priming of alloreactive CD8+ T cell that recognize intact donor MHC Class I. In this scenario, donor Class I cross-dressed DCs that are simultaneously presenting donor-derived peptides in the context of recipient Class II to donor-specific CD4+ T cells (via the indirect pathway) will receive licensing signals that allow the DCs to productively activate CD8+. As a result, CD8+ T cells with direct donor class I specificity can be generated under a three-cell scenario at any time post-transplantation (Fig 1A), with the caveat that donor parenchymal cells transfer intact Class I to recipient DCs, and these CD8+ T cells are then able to recognize donor cells within the allograft and mediate their destruction (23–25).
Fig 1.
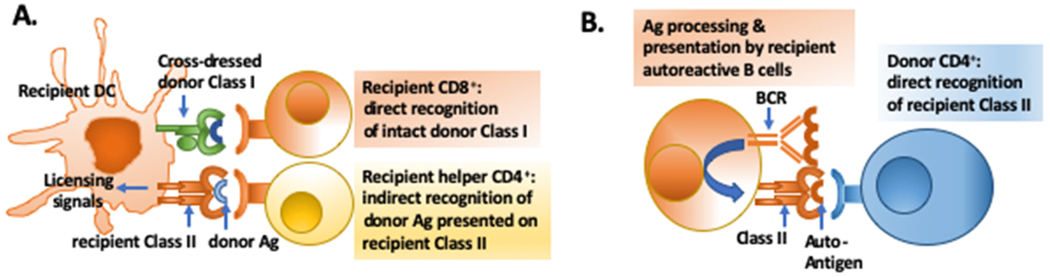
A. Three cell model for the activation of recipient CD8+ T cells with direct specificity for intact donor Class I. Cross-dressed recipient DCs presenting donor antigen on recipient Class II are licensed by indirect recognizing allogeneic CD4+; these licensed DCs, cross-dressed with intact donor Class I are then able to stimulate donor Class I-specific CD8+ T cells. Cross-dressing has not been reported for B cells so recipient B cells cannot stimulate direct CD8+ T cell responses. B. Autoantibody produced by recipient B cells receiving help from passenger donor CD4+ T cells that directly recognize host allogeneic Class II expressed by recipient B cells.
Recipient DCs are capable of indirect as well as semi-direct presentation, whereas recipient B cells have only been reported to be capable of indirect presentation. Indeed, the ability of recipient B cells to participate in indirect presentation is critical to their ability to engage in cognate interactions with recipient T cells that express T cell receptors (TCR) with indirect alloantigen specificity. Alloantigen engagement of the BCR results in the activation of the B cells and the processing and presentation of alloantigens in the context of Class II antigens (26). In contrast, exogenously derived alloantigen presentation on recipient Class I requires specialized antigen processing pathways that deliver antigen to the endoplasmic reticulum, so that the alloantigen can be loaded onto recipient MHC I and ultimately be cross-presented to recipient CD8+ T cells. Specialized subsets of conventional DCs, liver sinusoidal endothelial cells, Kupffer cells, and hepatocytes are capable of cross-presentation (reviewed in (27), and the ability of B cells to cross-present antigens has been reported, when the antigen is internalized by the TLR9 pathway or introduced through a gene gun approach (28, 29). Whether alloantigens shed by an allograft can be cross-presented by B cells remains to be determined. As a result, current evidence suggests that in fully-mismatched transplantation, host alloreactive B cells can engage in cognate interactions with recipient CD4+ but not CD8+ T cells, and only via the indirect pathway (Fig 2). An exception is the report by Win et al. (30) where autoantibody detected following Class II-mismatched allograft transplantation was produced by recipient B cells acquiring help from passenger donor CD4+ T cells directly recognizing allogeneic Class II on the recipient B cells (Fig 1B).
Fig 2.
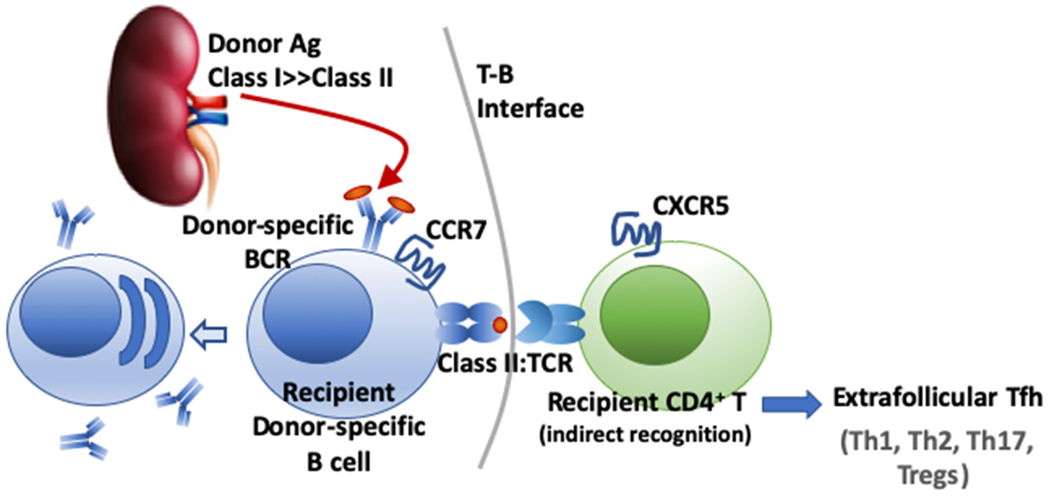
Class II-restricted antigen presentation by alloreactive B cells to CD4+ T cells with indirect specificity results in their cognate interaction with T follicular helper cells (Tfh) at the T:B interface and in the germinal center, leading to the elaboration of DSA responses. It is possible that B cells also engage in cognate interactions with non-Tfh T cells, including Th1, Th2, Th17 and Tregs, but where this occurs within the lymph node or spleen requires further clarification. The ability of B cells to cross-present exogenous donor antigens on MHC Class I has not been described, so B cells most likely cannot engage in cognate interactions with alloreactive CD8+ T cells that have indirect specificity.
B cells as APCs to T follicular helper cells in secondary lymphoid organs
B cells constantly recirculate between SLOs in search of cognate antigens that have been captured and displayed on specialized macrophages in the subcapsular sinus of LN or the marginal zone of spleens, or on follicular dendritic cells embedded within the center of B cell follicles (reviewed in (31)). Binding of alloantigens by BCRs, together with engagement of complement or toll-like co-receptors results in B cell activation as well as the internalization, processing and presentation of alloantigen on MHC Class II to CD4+ T cells. In a T-dependent B cell response, the activated B cell migrates to the T-B interface where in engages in stable cognate interactions with Tfh cells that had been pre-activated by encounter with DCs. The consequence of the T:B interaction is the extrafollicular differentiation of B cells into memory B cells or short-lived PCs, or entry into a germinal center (GC) response (reviewed in (32)). Within the GC, B cells undergo class-switching, rounds of somatic hypermutation, while limiting access to Tfh cells results in the selection of the highest affinity B cells (33, 34). Critical components of cognate T cell help to B cells include CD40:CD154, CD28:B7 and ICOS:ICOSL interactions (31), while PD-1:PD1L interactions constrain CXCR5 and CXCR3 expression leading to Tfh cells being restricted to the GC, optimize B cell competition and increase stringency of B cell affinity maturation (35). The necessity of these molecular interactions to sustaining GC responses suggests therapeutic strategies for reversing ongoing alloantibody responses (36, 37). Finally, it is now recognized that lower-affinity B cells emerge early from the GC as memory B cells, whereas higher-affinity B cells will emerge later as ASCs (38–40), underscoring the notion that the BCR repertoire between memory B cells and plasma cells is clonally related by not identical ((41), reviewed (42)). These observations raise the possibility that successful treatment of ABMR and reversal of DSA responses may not have prevented the generation of memory B cells, and that vigilant control to prevent recall responses may be necessary.
As summarized above, the tightly choreographed series of cognate interactions between T and B cells leading to humoral immune responses and alloantibody production is increasingly well understood, however, more work is required to test whether B cells function as APCs exclusively to Tfh cells, or also to other non-Tfh cell subsets, including Th1, Th2, Th17, Fox3+ regulatory T cells. As described by Hong et al. (17), the accumulation of non-Tfh T cells following nanoparticle vaccination requires the presence of B cells and the presence of DCs is not sufficient. However, the rules that dictate a necessary role for B cells over DCs as APCs were not elucidated. In addition, regulatory B cells (Bregs) producing IL-10 have been reported to restrain T cell responses during viral and bacterial infections, to promote the recovery from autoimmunity and to play an essential role in the induction of transplantation tolerance (reviewed in (43, 44)). Whether these Bregs express antigen-specific B cell receptors and are involved in cognate interactions with the T cells was recently addressed (45). Following transfer of Bregs from IL‐10‐green fluorescent protein (GFP) reporter mice and pulsed with NP-OVA, together with OVA-specific OT-I or OT-II cells, cognate interactions between Bregs and OVA-specific T cells were observed at the T-B interface. Interactions were more frequent between Bregs and OVA-specific T cells, compared to non-Bregs, and were antigen-specific. Finally, following encounter with Bregs, the OVA-specific T cells exhibited reduced interactions with DCs suggesting a mechanism of action for Bregs limiting T cell activation. Whether the sustained interactions between Bregs and T cells leads to Breg differentiation into plasma cells capable of secreting antigen-specific antibodies, remain unanswered.
B cells as APCs in the thymus effect clonal deletion of autoreactive T cells
Dendritic cells, B cells and medullary thymic epithelial cells (mTECs) are the three major APC subsets that play a central role in the clonal deletion of self-reactive T cells within the thymus. While B cells represent only 0.3% of all cells in the thymus, they outnumber DCs (reviewed (46)). Thymic B cells are divided into two main subsets: Type A are class-switched B cells that immigrated from the blood, are autoreactive and present self-antigens, and Type B cells are AIRE-expressing and enriched in the thymus of young mice. Perera J et al. (47) first reported that the thymus harbored B cells that co-localized with DCs and mTECs in the thymic medulla, expressed high levels of MHC Class II and mediated the negative selection of autoreactive T cell receptor (TCR)-transgenic CD4+ T cells. In a follow-up study, they went on to show that the self-antigen driven thymic B cells expanded and underwent class-switching intra-thymically and were thymus-resident (48). Likewise, Yamano et al (49) reported that thymic B cells, with elevated levels of MHC class II and constitutively expressed CD80, were licensed to present self-antigens and mediated the clonal deletion of autoreactive CD4+ thymocytes. Finally, recent transcriptome analysis of APCs in the human thymus revealed that DCs expressed highest levels of HLA Class I and Class II genes and HLA class I and II antigen presentation pathway genes. In contrast, the transcription factors, AIRE and FEZF2, implicated in thymic program of tissue-restricted antigen expression, were primarily expressed in mTECs. Finally, “tissue enriched genes” including autoantigens were expressed in all APC subsets (mTEC > B cells > DCs) (50). Thus collectively, these data are consistent with B cells playing a role in the deletion of auto- and tissue-reactive T cells in the thymus, and raise the possibility that in the context of a hematopoietic cell-induced transplantation tolerance, allogeneic B cells may migrate into the thymus to mediate the clonal deletion of T cells specific for donor MHC Class I and Class II (51, 52).
B cells as APCs in mediating chronic allograft rejection
While the focus on B cells in transplantation has focused on alloantibody production, Zeng et al. (53) reported that B cells can mediate chronic allograft rejection independently of antibody production, thus suggesting a role for B cells as APCs driving T cell-mediated rejection. Using a model of chronic allograft vasculopathy (CAV) involving BALB/c heart grafts into C57BL/6 recipients and treatment with anti-CD154 plus CLTA4-Ig, they showed that recipients lacking B cells or recipients that had a normal complement of B cells that were unable to secrete antibodies, were resistant to CAV. Further mechanistic studies revealed that the in vivo priming of alloreactive CD4+ and CD8+ T cells in SLOs, and the number of graft-infiltrating T cells, were reduced in the absence of B cells but restored in the presence of B cells even in the absence of alloantibodies. Their data suggest that the presence of B cells (and not antibodies) was necessary for the optimal priming of alloreactive T cells, and that both antigen-specific and non-antigen-specific functions of B cells were implicated in the priming of alloreactive Th1 and CD8+ T cell responses. Whether the priming of CD8+ T cell responses was mediated directly by B cells or indirectly through “help” provided by B cell-activated CD4+ T cells to license DCs, and where these T:B interactions were occurring, were not delineated. Indeed, these interactions could be occurring in the SLO where B cells are directly involved in CD4+ T cell priming, and/or within the allograft where B cells are promoting the reactivation of T cells.
B cells as APCs to Tfh cells within the kidney allografts
In 2003, Sarwal et al. (54) published a seminal paper that the presence of a B cell gene signature and dense clusters of B cells in the kidney allograft biopsies were strongly associated with glucocorticoid-resistant TCMR and graft loss. They proposed an unanticipated hypothesis at that time, that infiltrating B cells play a pivotal role in TCMR by functioning as APCs to T cells within the allograft. This hypothesis was subsequently confirmed by Liarski et al. (55), who used novel computer-assisted cell distance mapping to identify B cell-Tfh cognate interactions within human renal biopsies. Cognate interactions were defined by the nuclei of Tfh (CD4+ICOS+PD-1+) and B cells being <0.54 μm apart. By examining renal biopsies classified as undergoing TCMR or mixed rejection (MR), they reported that Tfh cells were observed in 64.2% and 50% of biopsies, respectively, and that the frequencies per high powered field were similar (14.0 versus 12.5 cells, respectively). However, the conjugate rates between Tfh and B cells were significantly different in TCMR and mixed rejection (MR, biopsies exhibiting cellular rejection and c4d deposition) In MR, almost 80% of Tfh cells were within 0.54 μm of a B cell, but this number was only 15% in TCMR. Furthermore, segregating MR renal rejection cases on the basis of mean density of infiltrating B cells revealed no significant change in conjugate rates confirming that the identification of cognate interaction was not affected by B cell density, and suggesting that the immunobiology of Tfh cells in TCMR and MR were distinct. In contrast to Tfh, the fraction of B cells at ≤.54 μm from a Tfh cell was disproportionally lower (23.4%) in both TCMR and MR. Thus, the majority of Tfh cells were interacting with B cells in MR, but the majority of B cells were not interacting with Tfh cells in TCRM and MR; whether B cells were interacting with non-Tfh T cells was not clarified in those studies. Finally, Liarski et al. (55) confirmed that in situ Tfh:B cell interactions with kidney allografts undergoing MR compared to TCMR were associated with functional Tfh cells that expressed higher levels of Bcl-6 and IL-2. It is tempting to speculate that Tfh:B cell interactions in MR prompt the in situ differentiation of B cells into ASC, thereby facilitating local production of DSA, antibody deposition and complement activation – hallmarks of ABMR and MR that are absent in TCMR. Future studies defining the specificity of B cells in MR versus TCMR, and as well as gene expression analysis, will provide further insights into the function of intrarenal B cells in TCMR and MR.
Summary and Conclusions
The contribution of B cells functioning as APCs, independently of antibody production, in rejection and tolerance induction is an under-investigated area. There is an abundance of evidence that B cells engage in cognate interactions with Tfh cells in SLOs and within the allograft, and there is tantalizing evidence that they may be important APCs for non-Tfh CD4+ T cells. However, the rules that determine B cell necessity and sufficiency over the contributions of DCs to T cell priming are not known, and whether the dichotomy between B cells and DCs as APCs determines the type of allograft rejection remains to be clarified.
Acknowledgments
Grant Support
This work was supported in part by grants (R01 AI 142747, P01AI097113) from the National Institute of Allergy, Immunology and Infectious Diseases, National Institutes of Health.
Abbreviations
- ABMR
Antibody-mediated rejection
- APC
antigen presenting cells
- ASC
antibody secreting cells
- BCR
B cell receptor
- Bregs
regulatory B cells
- CAV
chronic allograft vasculopathy
- DC
dendritic cells
- DSA
donor specific antibody
- GC
germinal center
- LN
lymph node
- MR
mixed rejection
- mTECs
medullary thymic epithelial cells
- PC
plasma cells
- SLO
secondary lymphoid organs
- TCMR
T cell-mediated rejection
- TCR
T cell receptor
- Tfh
T follicular helper
References
- 1.Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D et al. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 2018;18(2):293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loupy A, Lefaucheur C, Vernerey D, Chang J, Hidalgo LG, Beuscart T et al. Molecular microscope strategy to improve risk stratification in early antibody-mediated kidney allograft rejection. J Am Soc Nephrol 2014;25(10):2267–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkes MD, Aliabadi AZ, Cadeiras M, Crespo-Leiro MG, Deng M, Depasquale EC et al. An integrated molecular diagnostic report for heart transplant biopsies using an ensemble of diagnostic algorithms. J Heart Lung Transplant 2019;38(6):636–646. [DOI] [PubMed] [Google Scholar]
- 4.Halloran PF, Chang J, Famulski K, Hidalgo LG, Salazar ID, Merino Lopez M et al. Disappearance of T Cell-Mediated Rejection Despite Continued Antibody-Mediated Rejection in Late Kidney Transplant Recipients. J Am Soc Nephrol 2015;26(7):1711–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halloran PF, Reeve JP, Pereira AB, Hidalgo LG, Famulski KS. Antibody-mediated rejection, T cell-mediated rejection, and the injury-repair response: new insights from the Genome Canada studies of kidney transplant biopsies. Kidney Int 2014;85(2):258–264. [DOI] [PubMed] [Google Scholar]
- 6.Bouatou Y, Viglietti D, Pievani D, Louis K, Duong Van Huyen JP, Rabant M et al. Response to treatment and long-term outcomes in kidney transplant recipients with acute T cell-mediated rejection. Am J Transplant 2019;19(7):1972–1988. [DOI] [PubMed] [Google Scholar]
- 7.Bohmig GA, Eskandary F, Doberer K, Halloran PF. The therapeutic challenge of late antibody-mediated kidney allograft rejection. Transpl Int 2019;32(8):775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns AM, Chong AS. Alloantibodies prevent the induction of transplantation tolerance by enhancing alloreactive T cell priming. J Immunol 2011;186(1):214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns AM, Ma L, Li Y, Yin D, Shen J, Xu J et al. Memory alloreactive B cells and alloantibodies prevent anti-CD154-mediated allograft acceptance. J Immunol 2009;182(3):1314–1324. [DOI] [PubMed] [Google Scholar]
- 10.Bjorck P, Beilhack A, Herman EI, Negrin RS, Engleman EG. Plasmacytoid dendritic cells take up opsonized antigen leading to CD4+ and CD8+ T cell activation in vivo. J Immunol 2008;181(6):3811–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashwell JD, DeFranco AL, Paul WE, Schwartz RH. Can resting B cells present antigen to T cells? Fed Proc 1985;44(8):2475–2479. [PubMed] [Google Scholar]
- 12.Jenkins MK, Burrell E, Ashwell JD. Antigen presentation by resting B cells. Effectiveness at inducing T cell proliferation is determined by costimulatory signals, not T cell receptor occupancy. J Immunol 1990;144(5):1585–1590. [PubMed] [Google Scholar]
- 13.Cassell DJ, Schwartz RH. A quantitative analysis of antigen-presenting cell function: activated B cells stimulate naive CD4 T cells but are inferior to dendritic cells in providing costimulation. J Exp Med 1994;180(5):1829–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eynon EE, Parker DC. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med 1992;175(1):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho WY, Cooke MP, Goodnow CC, Davis MM. Resting and anergic B cells are defective in CD28-dependent costimulation of naive CD4+ T cells. J Exp Med 1994;179(5):1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalai SK, Mirshahidi S, Morrot A, Zavala F, Sadegh-Nasseri S. Anergy in memory CD4+ T cells is induced by B cells. J Immunol 2008;181(5):3221–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong S, Zhang Z, Liu H, Tian M, Zhu X, Zhang Z et al. B Cells Are the Dominant Antigen-Presenting Cells that Activate Naive CD4(+) T Cells upon Immunization with a Virus-Derived Nanoparticle Antigen. Immunity 2018;49(4):695–708 e694. [DOI] [PubMed] [Google Scholar]
- 18.Ronchese F, Hausmann B. B lymphocytes in vivo fail to prime naive T cells but can stimulate antigen-experienced T lymphocytes. J Exp Med 1993;177(3):679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afzali B, Lombardi G, Lechler RI. Pathways of major histocompatibility complex allorecognition. Curr Opin Organ Transplant 2008;13(4):438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siu JHY, Surendrakumar V, Richards JA, Pettigrew GJ. T cell Allorecognition Pathways in Solid Organ Transplantation. Front Immunol 2018;9:2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Nolasco B, Wang M, Prunevieille A, Benichou G. Emerging role of exosomes in allorecognition and allograft rejection. Curr Opin Organ Transplant 2018;23(1):22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morelli AE, Bracamonte-Baran W, Burlingham WJ. Donor-derived exosomes: the trick behind the semidirect pathway of allorecognition. Curr Opin Organ Transplant 2017;22(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrera OB, Golshayan D, Tibbott R, Salcido Ochoa F, James MJ, Marelli-Berg FM et al. A novel pathway of alloantigen presentation by dendritic cells. J Immunol 2004;173(8):4828–4837. [DOI] [PubMed] [Google Scholar]
- 24.Smyth LA, Lechler RI, Lombardi G. Continuous Acquisition of MHC:Peptide Complexes by Recipient Cells Contributes to the Generation of Anti-Graft CD8(+) T Cell Immunity. Am J Transplant 2017;17(1):60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harper SJ, Ali JM, Wlodek E, Negus MC, Harper IG, Chhabra M et al. CD8 T-cell recognition of acquired alloantigen promotes acute allograft rejection. Proc Natl Acad Sci U S A 2015;112(41):12788–12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conlon TM, Saeb-Parsy K, Cole JL, Motallebzadeh R, Qureshi MS, Rehakova S et al. Germinal center alloantibody responses are mediated exclusively by indirect-pathway CD4 T follicular helper cells. J Immunol 2012;188(6):2643–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blander JM. Regulation of the Cell Biology of Antigen Cross-Presentation. Annu Rev Immunol 2018;36:717–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hon H, Oran A, Brocker T, Jacob J. B lymphocytes participate in cross-presentation of antigen following gene gun vaccination. J Immunol 2005;174(9):5233–5242. [DOI] [PubMed] [Google Scholar]
- 29.Heit A, Huster KM, Schmitz F, Schiemann M, Busch DH, Wagner H. CpG-DNA aided cross-priming by cross-presenting B cells. J Immunol 2004;172(3):1501–1507. [DOI] [PubMed] [Google Scholar]
- 30.Win TS, Rehakova S, Negus MC, Saeb-Parsy K, Goddard M, Conlon TM et al. Donor CD4 T cells contribute to cardiac allograft vasculopathy by providing help for autoantibody production. Circ Heart Fail 2009;2(4):361–369. [DOI] [PubMed] [Google Scholar]
- 31.Cyster JG, Allen CDC. B Cell Responses: Cell Interaction Dynamics and Decisions. Cell 2019;177(3):524–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The generation of antibody-secreting plasma cells. Nat Rev Immunol 2015;15(3):160–171. [DOI] [PubMed] [Google Scholar]
- 33.Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell 2010;143(4):592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwun J, Burghuber C, Manook M, Ezekian B, Park J, Yoon J et al. Successful desensitization with proteasome inhibition and costimulation blockade in sensitized nonhuman primates. Blood Adv 2017;1(24):2115–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi J, Hou S, Fang Q, Liu X, Liu X, Qi H. PD-1 Controls Follicular T Helper Cell Positioning and Function. Immunity 2018;49(2):264–274 e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J, Yin H, Xu J, Wang Q, Edelblum KL, Sciammas R et al. Reversing endogenous alloreactive B cell GC responses with anti-CD154 or CTLA-4Ig. Am J Transplant 2013;13(9):2280–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Chen J, Young JS, Wang Q, Yin D, Sciammas R et al. Tracing Donor-MHC Class II Reactive B cells in Mouse Cardiac Transplantation: Delayed CTLA4-Ig Treatment Prevents Memory Alloreactive B-Cell Generation. Transplantation 2016;100(8):1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weisel FJ, Zuccarino-Catania GV, Chikina M, Shlomchik MJ. A Temporal Switch in the Germinal Center Determines Differential Output of Memory B and Plasma Cells. Immunity 2016;44(1):116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krautler NJ, Suan D, Butt D, Bourne K, Hermes JR, Chan TD et al. Differentiation of germinal center B cells into plasma cells is initiated by high-affinity antigen and completed by Tfh cells. J Exp Med 2017;214(5):1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kometani K, Nakagawa R, Shinnakasu R, Kaji T, Rybouchkin A, Moriyama S et al. Repression of the transcription factor Bach2 contributes to predisposition of IgG1 memory B cells toward plasma cell differentiation. Immunity 2013;39(1):136–147. [DOI] [PubMed] [Google Scholar]
- 41.Lavinder JJ, Wine Y, Giesecke C, Ippolito GC, Horton AP, Lungu OI et al. Identification and characterization of the constituent human serum antibodies elicited by vaccination. Proc Natl Acad Sci U S A 2014;111(6):2259–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chong AS, Ansari MJ. Heterogeneity of memory B cells. Am J Transplant 2018;18(4):779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stolp J, Turka LA, Wood KJ. B cells with immune-regulating function in transplantation. Nat Rev Nephrol 2014;10(7):389–397. [DOI] [PubMed] [Google Scholar]
- 44.Lykken JM, Candando KM, Tedder TF. Regulatory B10 cell development and function. Int Immunol 2015;27(10):471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohib K, Cherukuri A, Zhou Y, Ding Q, Watkins SC, Rothstein DM. Antigen-dependent interactions between regulatory B cells and T cells at the T:B border inhibit subsequent T cell interactions with DCs. Am J Transplant 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamano T, Steinert M, Klein L. Thymic B Cells and Central T Cell Tolerance. Front Immunol 2015;6:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perera J, Meng L, Meng F, Huang H. Autoreactive thymic B cells are efficient antigen-presenting cells of cognate self-antigens for T cell negative selection. Proc Natl Acad Sci U S A 2013;110(42):17011–17016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perera J, Zheng Z, Li S, Gudjonson H, Kalinina O, Benichou JIC et al. Self-Antigen-Driven Thymic B Cell Class Switching Promotes T Cell Central Tolerance. Cell Rep 2016;17(2):387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamano T, Nedjic J, Hinterberger M, Steinert M, Koser S, Pinto S et al. Thymic B Cells Are Licensed to Present Self Antigens for Central T Cell Tolerance Induction. Immunity 2015;42(6):1048–1061. [DOI] [PubMed] [Google Scholar]
- 50.Gabrielsen ISM, Helgeland H, Akselsen H, HC DA, Sundaram AYM, Snowhite IV et al. Transcriptomes of antigen presenting cells in human thymus. PLoS One 2019;14(7):e0218858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fehr T, Haspot F, Mollov J, Chittenden M, Hogan T, Sykes M. Alloreactive CD8 T cell tolerance requires recipient B cells, dendritic cells, and MHC class II. J Immunol 2008;181(1):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fehr T, Wang S, Haspot F, Kurtz J, Blaha P, Hogan T et al. Rapid deletional peripheral CD8 T cell tolerance induced by allogeneic bone marrow: role of donor class II MHC and B cells. J Immunol 2008;181(6):4371–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng Q, Ng YH, Singh T, Jiang K, Sheriff KA, Ippolito R et al. B cells mediate chronic allograft rejection independently of antibody production. J Clin Invest 2014;124(3):1052–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarwal M, Chua MS, Kambham N, Hsieh SC, Satterwhite T, Masek M et al. Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N Engl J Med 2003;349(2):125–138. [DOI] [PubMed] [Google Scholar]
- 55.Liarski VM, Kaverina N, Chang A, Brandt D, Yanez D, Talasnik L et al. Cell distance mapping identifies functional T follicular helper cells in inflamed human renal tissue. Sci Transl Med 2014;6(230):230ra246. [DOI] [PMC free article] [PubMed] [Google Scholar]


