Abstract
Background
NAC transcription factors play important roles in the regulation of plant growth, development, abiotic and biotic stress responses. The transcriptional level of MdNACs in different tissues and under various biotic and abiotic stress treatments was determined to provide a solid foundation for studying the function of MdNACs.
Methods
Thirty-two full-length cDNA sequences of Md NACs were isolated by homologous comparison and RT-PCR confirmation, and the obtained cDNA sequences and the deduced amino acid sequences were analyzed with bioinformatics methods. The prediction of subcellular locations of MdNAC proteins was performed using CELLO v.2.5, PSORT, and SoftBerry ProtComp 9.0. Expression levels of MdNACs were detected in 16 different tissues using an array. Expression patterns of MdNACs were detected in response to Alternaria alternata apple pathotype (AAAP) infection using RNA-seq, and the expression of MdNACs was analyzed under NaCl and mannitol treatments using RT-qPCR.
Results
The sequencing results produced 32 cDNAs (designated as MdNAC24-39, MdNAC54-65, and MdNAC67-70 with GenBank accession No. MG099861–MG099876, MG099891–MG099902, and MG099904–MG099907, respectively). Phylogenetic analysis revealed that MdNAC34 belonged to the ATAF group, MdNAC63 belonged to the AtNAC3 group, MdNAC24, MdNAC26-30, MdNAC32-33, MdNAC35, MdNAC37-39, MdNAC56-57, MdNAC59-62, MdNAC64-65, and MdNAC67-70 belonged to the NAM group, and MdNAC25, MdNAC36, MdNAC54-55, and MdNAC58 belonged to the VND group. Predictions of subcellular localization showed that MdNAC24-27, MdNAC29-30, MdNAC33-37, MdNAC39, MdNAC54-65, and MdNAC67-70 proteins were located in the nucleus, MdNAC28 proteins were located in the cytoplasm, MdNAC31-32 proteins were located in the nucleus and cytoplasm, and MdNAC38 proteins were located in the nucleus and plasma membrane. Array results indicated that 32 MdNACs were expressed in all examined tissues at various expression levels. RNA-seq results showed that expression levels of MdNAC26-28, MdNAC33-34, MdNAC60, MdNAC62-65, and MdNAC68 were induced, but MdNAC24, MdNAC32, and MdNAC58 were down-regulated in response to AAAP infection. Under salt treatment, MdNAC24, MdNAC27, MdNAC29, MdNAC34, MdNAC37, MdNAC39, MdNAC54, MdNAC59, and MdNAC63 transcription levels were induced. Under mannitol treatment, MdNAC32 and MdNAC54 transcription levels were induced, but MdNAC24, MdNAC28, MdNAC30, MdNAC33, MdNAC35, MdNAC37, MdNAC55, MdNAC56, MdNAC58, and MdNAC59 were down-regulated. Taken together, the results indicated that the cloned MdNAC genes were expressed constitutively in all examined tissues. These genes were up-regulated or down-regulated in response to AAAP infection and to salt or mannitol, which suggested they may be involved in the regulation of growth, development, and stress response in apple.
Keywords: Apple, MdNAC, Expression analysis, Gene cloning, Biotic stress, Abiotic stress, NAC transcription factor
Introduction
The plant-specific NAC transcription factors (TFs) constitute a major TF family in plants, and play important roles in the growth, development, and responses to biotic and abiotic stress (Puranik et al., 2012). The name NAC refers to the first letter of three genes: NAM from Pharbifis nil, and ATAF1/2 and CUC2 from Arabidopsis thaliana (Aida et al., 1997). These genes are characterized by a conserved NAC domain of approximately 150 amino acids in length that is located at the N-terminal region. The NAC domain can be divided further into five subdomains: A, B, C, D, and E; A, C, and D subdomains are highly conserved, but the B and E subdomains are variable in different plants. The C-terminal sequence of the NAC TF is the transcriptional regulatory region, which determines transcriptional activation or inhibitory activity (Puranik et al., 2012).
NAC TFs are involved in the regulation of plant growth, development, and morphogenesis, which included seed germination (Kim et al., 2008), formation of lateral roots (He et al., 2005), formation of meristems and organ boundaries (Aida et al., 1997; Mao et al., 2007), secondary wall thickening (Mitsuda et al., 2007), floral organ development (Yoo et al., 2007), fruit maturation (Ríos et al., 2017), and plant senescence (Sperotto et al., 2009). The most in-depth and extensive research on the biological function of NAC TFs involves plant response to stress. Multiple NAC TFs are involved in plant abiotic stress responses to drought, high salinity, high and low temperatures, and waterlogging. NAC TFs, such as ATAF1, ANAC019, ANAC055, ANAC072 in Arabidopsis, and SNAC1, OsNAC5, SNAC2/OsNAC6, OsNAC10, and ONAC022 in Oryza sativa L., are involved in plant response to drought and high salt stress, and the functions of the corresponding mutants or overexpressed lines indicate that these genes play an important role in salt and drought resistance (Tran et al., 2004; Hu et al., 2006; Lu et al., 2007; Nakashima et al., 2007; Hu et al., 2008; Wu et al., 2009; Jeong et al., 2010; Takasaki et al., 2010; Hong et al., 2016).
NAC TFs were also involved in resistance to disease. NAC TFs positively or negatively regulated the resistance of rice and Arabidopsis to different types of diseases; rice OsNAC6 and Arabidopsis ANAC019 and ANAC055 positively regulated the resistance of rice to blast disease (Nakashima et al., 2007) and Arabidopsis to Botrytis cinerea (Bu et al., 2008), respectively. Rice RIM1 and Arabidopsis ATAF1 and ATAF2 negatively regulated the resistance of rice to dwarf virus (Yoshii et al., 2010) and Arabidopsis to Fusarium oxysporum (Delessert et al., 2005), respectively. In addition, transgenic Arabidopsis plants that overexpressed Triticum aestivum TaNAC2, TaNAC67, and TaNAC29 increased tolerance to drought and high salinity (Mao et al., 2012; Mao et al., 2014; Huang et al., 2015). Transgenic plants of Artemisia annua L. and Arabidopsis that overexpressed AaNAC1 increased drought resistance and resistance to B. cinerea (Lv et al., 2016a; Lv et al., 2016b), and transgenic Ipomoea batatas plants that overexpressed IbNAC1 had greatly increased sporamin expression by binding the SWRE motif against mechanical wounding and herbivore attack (Chen et al., 2016a). Finally, transgenic Solanum lycopersicum plants that overexpressed SlNAC4 and SlJUB1 enhanced drought tolerance (Zhu et al., 2014a; Thirumalaikumar et al., 2018).
Although apple (Malus ×domestica Borkh.) is one of the most economically important fruit trees in the world, there have been few studies on NAC TFs in apple compared with the in-depth studies of NAC TFs in model plants like Arabidopsis and rice. Recent studies have shown that overexpression of apple NAC TFs MdNAC029 and MdNAC047 in transgenic apple calli and Arabidopsis increased tolerance to cold and salt stress, respectively (An et al., 2017; An et al., 2018). Overexpression of MdNAC1 conferred the dwarf phenotype in transgenic apple (Jia et al., 2018). Through genome-wide analysis, a total of 180 MdNAC genes were identified in the apple genome that were clustered phylogenetically into six groups (I–VI) with the NAC genes from Arabidopsis and rice (Su et al., 2013). In view of the important role of NAC TFs in plant growth, development, and regulation of stress response, our study removed 23 MdNAC genes that already existed in the GenBank database through sequence alignment, cloned 32 MdNAC genes, analyzed their evolutionary relationships and subcellular localization of MdNAC, and studied the expression patterns in different tissues and organs under abiotic and biotic stress treatments. Our study provides a theoretical foundation for further study of the regulation of growth, development, and stress response by NAC TFs in apple.
Materials & Methods
Plant materials
The apple cultivar ‘Gala’ (Malus ×domestica cv. Gala) was used as material under stress conditions. In vitro seedlings of ‘Gala’ were cultivated on basic subculture medium (MS medium + 0.2 mg L1 indole-3-acetic acid (IAA) + 0.8 mg L−1 6-benzylaminopurine (6-BA) + 30 g L−1 sucrose + 7g L−1 agrose) that was changed every 30 d. The cultivation conditions were under 14-h light/10-h dark and a temperature of 24 ± 2 °C. On the 20th day on the basic subculture medium, seedlings of similar growth and size (30 per treatment) were selected and transplanted to different media. The basic subculture medium was used as the control. We added 150 mmol L−1 NaCl or 300 mmol L−1 mannitol to the basic subculture medium to create different treatments (Li et al., 2019a; Li et al., 2019b).
Gene cloning and sequence analysis
RNA was extracted in the fully expanded leaves of ‘Zihong Fuji’ apple (Malus ×domestica cv. Zihong Fuji) using the hot boric acid method, then cDNA was synthesized using a PrimeScript TM 1STStrand cDNA Synthesis Kit (Takara, Dalian, China). Based on the nucleotide sequence of identified members in the MdNAC gene family, we designed primers for PCR (Table S1). PCR reaction conditions were 94 °C for 5 min, then 35 cycles for 94 °C for 1 min 20 s, 56–61 °C for 1 min 20 s, 72 °C for 3 min, and a final extension at 72 °C for 10 min. PCR products were purified and cloned into pMD19-T vectors to construct recombinant plasmids. The recombinant plasmids were transformed into the competent cells of E. coli DH5 α, and then the positive clones were selected.
The cDNA sequences that we obtained were used as queries in BLASTX searches against NCBI (https://www.ncbi.nlm.nih.gov/). Functional annotations (Gene Ontology) were analyzed with Blast2GO software (https://www.blast2go.com/). The open reading frame (ORF) and amino acid sequences were analyzed by DNAMAN 6.0, and a phylogenetic tree was constructed using MEGA 6 software, according to the NJ method (execution parameters: poission correction, pairwise deletion, and bootstrap (1000 replicates)). The conserved domains were predicted by Pfam 26.0 (http://pfam.xfam.org/) and the Conserved Domains program in NCBI (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). CELLO v.2.5 (http://cello.life.nctu.edu.tw/), PSORT (https://psort.hgc.jp/form.html), and SoftBerry ProtComp 9.0 (http://linux1.softberry.com/) were used to predict subcellular locations (Dong et al., 2018a; Dong et al., 2018b; Dong et al., 2018c; Hao & Qiao, 2018).
Expression analysis of MdNAC genes
Expression data for the MdNAC gene family in different tissues were obtained from Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/) in NCBI (https://www.ncbi.nlm.nih.gov/), with GEO accession No. GSE42873 (Celton et al., 2014; Cui et al., 2015; Chen et al., 2016b; Zhang et al., 2016; Chen et al., 2017; Fan et al., 2017; Chen et al., 2018; Liu et al., 2018; Zuo et al., 2018; Li et al., 2019a; Li et al., 2019b; Zuo et al., 2019). These data included a set of expression arrays from 16 different apple tissues, with two biological replicates for each tissue, and an array probe was used as the MDP identification number in apple genome database V1.0. The RNA-seq data for MdNAC response to AAAP was from (Zhu et al., 2017). The treatment method was as follows: 2-year-old “Starking Delicious” cultivated apple plants grafted on Malus robusta rootstock were used as treatment materials. The fourth and fifth youngest opened leaves were collected from above plants and were inoculated with Alternaria alternata apple pathotype (AAAP) fungus. The AAAP fungus were grown on PDA medium for 5 days at 26 °C under dark conditions. In each treatment, leaves were inoculated with six cakes of mycelium applied to both side of the midrib of the abaxial leaf surfaces; the mock-inoculation of leaves using PDA medium cakes instead of mycelia was also carried out as a control. Thus, five groups of leaves were inoculated with mycelia or PDA cakes at 36, 18, 6, and 4 h intervals and then incubated at 25 °C under a 14 h light/10 h dark cycle in sterilized plastic chambers. The groups were then sampled simultaneously when the first set of inoculated leaves reached the 72 h post inoculation (HPI) time point; these five groups represent the five infection stage, 72, 36, 18, 12, and 8 HPI (Zhu et al., 2017).The RT-qPCR primers (Table S1) were designed based on the 3′- or 5′-UTR of MdNAC genes, and then RT-qPCR was conducted using a 3-step method by BIO-RAD IQ5 (USA) with MdMDH RNA as the internal reference gene (Perini et al., 2014). All RT-qPCR reactions were repeated three times. Each 20 µL RT-qPCR reaction mixture consisted of SYBR Green Master I 10 µL, 5 µmol L−1 forward prime 1 µL, 5 µmol L−1 reverse prime 1 µL, template 1 µL, and ddH2O 7 µL. RT-qPCR conditions were 95 °C for 3 min, then 40 cycles for 95 °C for 10 s, 58.5 °C for 30 s, 72 °C for 15 sand, after annealing to 55 °C, the temperature was increased 0.5 °C every 7 s till 95 °C, with 81 cycles in total. The 2−ΔΔCT method was used to analyze the data (Livak & Schmittgen, 2001).
Results
Cloning of MdNAC genes
A total of 32 MdNAC genes were cloned. Their gene names, gene IDs, GenBank accessions, genomic positions, lengths, molecular weights, isoelectric point (pI)-values, and information about Gene Ontology (GO) annotations were summarized for these MdNAC proteins (Table 1; Table S2). Homology alignment for the amino acid information showed that all MdNAC proteins contained a NAC conserved domain, and the NAC conserved domains could be divided further into A, B, C, D, and E subdomains (Fig. 1).
Table 1. The MdNAC genes in apple.
(a) V1.0 gene ID represents gene ID from apple V1.0 database (Velasco et al., 2010) (b) GDDH13 gene ID represents gene ID from apple GDDH13 v1.1 database.
| Gene name | V1.0 gene IDa | GDDH13 gene IDb | GenBank accession | GDDH13 Chromosome location | ORF | Amino acid | MW | pI |
|---|---|---|---|---|---|---|---|---|
| MdNAC24 | MDP0000204582 | MD10G1217500 | MG099861 | Chr10: 31583926-31588221 | 1,056 | 351 | 39.736 | 4.645 |
| MdNAC25 | MDP0000221977 | MD00G1117000 | MG099862 | Chr00: 24815722-24817459 | 1,119 | 372 | 43.189 | 7.021 |
| MdNAC26 | MDP0000852271 | MD05G1037700 | MG099863 | Chr05: 6012065-6015907 | 1,728 | 575 | 64.63 | 4.692 |
| MdNAC27 | MDP0000276765 | MD17G1051600 | MG099864 | Chr17: 4033937-4035671 | 1,056 | 351 | 39.929 | 6.397 |
| MdNAC28 | MDP0000655623 | MD16G1062600 | MG099865 | Chr16: 4451167-4452234 | 696 | 231 | 26.122 | 4.722 |
| MdNAC29 | MDP0000238035 | MD10G1030400 | MG099866 | Chr10: 4011026-4014329 | 1,167 | 388 | 43.005 | 4.953 |
| MdNAC30 | MDP0000240094 | MD08G1222300 | MG099867 | Chr08: 28493191-28494535 | 621 | 206 | 23.557 | 4.714 |
| MdNAC31 | MDP0000258167 | MD07G1073100 | MG099868 | Chr07: 6943268-6950293 | 1,965 | 654 | 70.762 | 5.835 |
| MdNAC32 | MDP0000276982 | MD09G1279900 | MG099869 | Chr09: 35666413-35671178 | 2,088 | 695 | 79.241 | 5.678 |
| MdNAC33 | MDP0000231845 | MD07G1163200 | MG099870 | Chr07: 23835271-23837822 | 1,071 | 356 | 40.353 | 5.01 |
| MdNAC34 | MDP0000334047 | MD15G1136600 | MG099871 | Chr15: 10025017-10026934 | 924 | 307 | 35.461 | 6.61 |
| MdNAC35 | MDP0000387787 | MD14G1243700 | MG099872 | Chr14: 32218641-32221015 | 783 | 260 | 29.841 | 5.066 |
| MdNAC36 | MDP0000563165 | MD07G1158300 | MG099873 | Chr07: 23095230-23097200 | 1,269 | 422 | 48.159 | 6.202 |
| MdNAC37 | MDP0000575835 | MD07G1164000 | MG099874 | Chr07: 23874863-23877677 | 1,590 | 529 | 59.787 | 6.537 |
| MdNAC38 | MDP0000266267 | None | MG099875 | None | 1,134 | 377 | 42.459 | 5.253 |
| MdNAC39 | MDP0000852815 | MD01G1095100 | MG099876 | Chr01: 21019852-21023573 | 1,668 | 555 | 63.297 | 5.342 |
| MdNAC54 | MDP0000176447 | MD15G1247800 | MG099891 | Chr15: 20579332-20584307 | 1,038 | 345 | 39.816 | 4.713 |
| MdNAC55 | MDP0000140229 | MD14G1137900 | MG099892 | Chr14: 22516958-22518874 | 1,206 | 401 | 46.146 | 6.191 |
| MdNAC56 | MDP0000130686 | MD06G1135700 | MG099893 | Chr06: 28061855-28063088 | 681 | 226 | 25.56 | 9.08 |
| MdNAC57 | MDP0000282616 | MD07G1163700 | MG099894 | Chr07: 23860144-23863461 | 1,536 | 511 | 57.647 | 6.15 |
| MdNAC58 | MDP0000226701 | MD11G1195600 | MG099895 | Chr11: 28030484-28032942 | 966 | 321 | 37.071 | 6.378 |
| MdNAC59 | MDP0000189394 | MD08G1250000 | MG099896 | Chr08: 31430542-31432937 | 1,434 | 477 | 54.342 | 6.924 |
| MdNAC60 | MDP0000184069 | MD14G1001200 | MG099897 | Chr14: 256059-258347 | 1,449 | 482 | 53.792 | 7.179 |
| MdNAC61 | MDP0000224592 | MD13G1046600 | MG099898 | Chr13: 3226751-3230841 | 1,371 | 456 | 51.427 | 4.792 |
| MdNAC62 | MDP0000130123 | MD16G1125800 | MG099899 | Chr16: 9127788-9129766 | 768 | 255 | 28.863 | 8.951 |
| MdNAC63 | MDP0000180683 | MD14G1001000 | MG099900 | Chr14: 196247-198440 | 1,122 | 373 | 41.941 | 8.803 |
| MdNAC64 | MDP0000266908 | MD00G1096600 | MG099901 | Chr00: 20298949-20303133 | 1,572 | 523 | 58.422 | 5.09 |
| MdNAC65 | MDP0000240855 | MD04G1043700 | MG099902 | Chr04: 5142167-5148131 | 1,644 | 547 | 60.763 | 4.508 |
| MdNAC67 | MDP0000272823 | MD01G1092800 | MG099904 | Chr01: 20755672-20758434 | 1,500 | 499 | 55.384 | 5.097 |
| MdNAC68 | MDP0000238773 | MD15G1032200 | MG099905 | Chr15: 1983295-1985648 | 1,080 | 359 | 40.37 | 6.518 |
| MdNAC69 | MDP0000238035 | MD05G1029800 | MG099906 | Chr05: 4849511-4852998 | 1,191 | 396 | 44.016 | 4.757 |
| MdNAC70 | MDP0000206212 | MD12G1030200 | MG099907 | Chr12: 3262786-3264615 | 846 | 281 | 31.643 | 6.361 |
Figure 1. Sequence analysis of the NAC conserved domain in apple NAC proteins.
Sequence alignment was generated by DNAMAN 6.0 software. The locations of A, B, C, D and E subdomains are indicated on top of the sequences.
Evolutionary analysis of MdNAC proteins
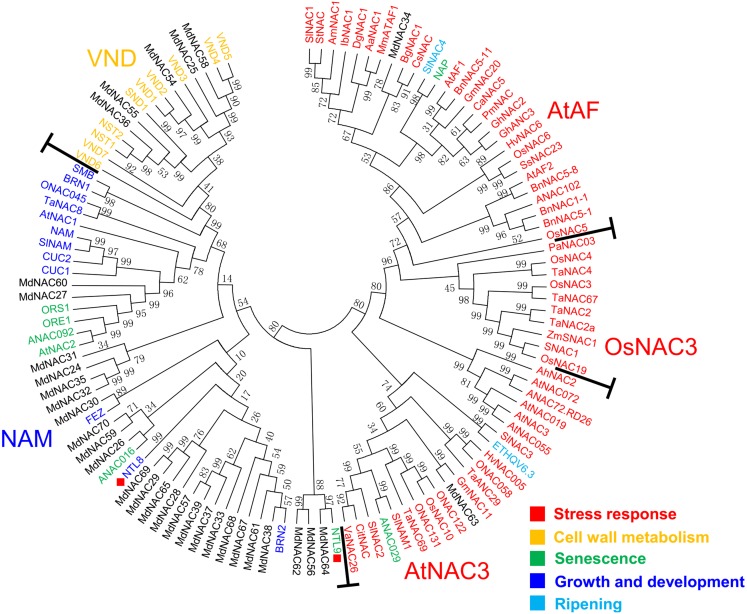
To understand the evolutionary relationships and potential biological functions among apple NAC proteins, cluster analysis of 32 apple NAC proteins and 86 other plant NAC proteins with known functions was performed using MEGA6 software. The result showed that 118 NAC proteins were divided into five subgroups: ATAF, OsNAC3, AtNAC3, NAM, and VND (Fig. 2). The NAC members of the ATAF, OsNAC3, and AtNAC3 subgroups mostly participated in stress response, the members of the NAM subgroup mostly regulated plant growth, development, and senescence, and the members of the VND subgroup were primarily involved in cell wall metabolism (Ooka et al., 2003; Zhu et al., 2014b). Further, proteins MdNAC34 and MdNAC63 were clustered into the ATAF and AtNAC3 subgroups, respectively; proteins MdNAC24, MdNAC26-30, MdNAC32-33, MdNAC35, MdNAC37-39, MdNAC56-57, MdNAC59-62, MdNAC64-65, and MdNAC67-70 were clustered into the NAM subgroup, and proteins MdNAC25, MdNAC36, MdNAC54-55, and MdNAC58 were clustered into the VND subgroup (Fig. 2).
Figure 2. Phylogenetic relationships from NAC proteins of apple and known function of other plant species.
Unrooted Neighbour Joining (NJ) phylogenetic tree was constructed with MEGA 6 software using full-length amino acid sequences from NAC proteins of apple and known function of other plant species. Colours indicate protein function: red, stress response; orange, cell wall metabolism; green, senescence; blue, plant growth and development; light blue, fruit ripening. Accession numbers and references of the NAC protein of each species are shown in File S2.
Analysis of subcellular localization of MdNAC proteins
Identification of subcellular locations of MdNAC proteins was performed using SoftBerry ProtComp 9.0. Predicted values for MdNAC24-27, MdNAC29-30, MdNAC33-37, MdNAC39, MdNAC54-65, and MdNAC67-70 to be located in the nucleus were the highest (a total of 10 points: higher value indicates greater credibility); the highest predictive value for MdNAC28 was in the cytoplasm, for MdNAC31-32 in the nucleus and cytoplasm, and for MdNAC38 in the nucleus and plasma membrane (Table 2). The subcellular localization of MdNAC proteins was analyzed further using online software CELLO and PORST. The prediction results showed that the localization of MdNAC proteins was consistent with the above results. All predictions indicated that MdNAC24-27, MdNAC29-30, MdNAC33-37, MdNAC39, MdNAC54-65, and MdNAC67-70 were located in the nucleus; MdNAC28, MdNAC31-32, and MdNAC38 were located in the cytoplasm, the nucleus and the cytoplasm, and the nucleus and plasma membrane, respectively.
Table 2. The information in predicting MdNAC subcellular localization.
| Location | Nuclear | Plasma membrane | Extracellular | Cytoplasmic | Mitochondrial | Endoplasm. retic | Peroxisomal | Golgi | Chloroplast | Vacuolar |
|---|---|---|---|---|---|---|---|---|---|---|
| MdNAC24 | 9.99 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MdNAC25 | 9.99 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 |
| MdNAC26 | 6.59 | 0 | 0.7 | 0.33 | 0.63 | 0.6 | 0 | 0 | 1.16 | 0 |
| MdNAC27 | 9.99 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MdNAC28 | 0.61 | 0.51 | 0 | 6.47 | 2.42 | 0 | 0 | 0 | 0 | 0 |
| MdNAC29 | 0.96 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0.04 | 0 |
| MdNAC30 | 9.99 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 |
| MdNAC31 | 3.46 | 0.54 | 0 | 3.89 | 1.44 | 0 | 0.39 | 0 | 0.28 | 0 |
| MdNAC32 | 3.36 | 0.65 | 0.36 | 3.28 | 1.04 | 0.13 | 0.2 | 0 | 0.62 | 0.36 |
| MdNAC33 | 6.73 | 0.3 | 0.71 | 1.09 | 0.97 | 0 | 0.21 | 0 | 0 | 0 |
| MdNAC34 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MdNAC35 | 9.99 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MdNAC36 | 9.99 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 |
| MdNAC37 | 9.03 | 0 | 0.33 | 0 | 0.55 | 0 | 0.04 | 0 | 0.05 | 0 |
| MdNAC38 | 4.09 | 4.98 | 0 | 0 | 0 | 0 | 0.3 | 0 | 0.45 | 0.19 |
| MdNAC39 | 9.61 | 0 | 0.07 | 0 | 0.23 | 0 | 0.01 | 0 | 0.08 | 0 |
| MdNAC54 | 9.99 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MdNAC55 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MdNAC56 | 9.95 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.05 | 0 |
| MdNAC57 | 9.31 | 0 | 0 | 0.09 | 0.42 | 0 | 0 | 0 | 0.18 | 0 |
| MdNAC58 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MdNAC59 | 8.75 | 0.15 | 0.09 | 0.34 | 0.57 | 0 | 0.13 | 0 | 0 | 0.01 |
| MdNAC60 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MdNAC61 | 9.86 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.14 | 0.01 |
| MdNAC62 | 9.98 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.02 | 0 |
| MdNAC63 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MdNAC64 | 4.31 | 0.97 | 0.32 | 2.25 | 1.46 | 0.3 | 0.08 | 0 | 0.21 | 0.1 |
| MdNAC65 | 4.02 | 1.77 | 1.91 | 1.33 | 0 | 0.11 | 0.18 | 0 | 0.28 | 0.41 |
| MdNAC67 | 6.66 | 0.57 | 0.69 | 0.59 | 0.97 | 0 | 0 | 0 | 0.53 | 0 |
| MdNAC68 | 4.18 | 1.68 | 0.55 | 0.67 | 2.14 | 0.05 | 0.62 | 0.01 | 0.09 | |
| MdNAC69 | 9.98 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0.02 | 0 |
Expression analysis of MdNAC genes
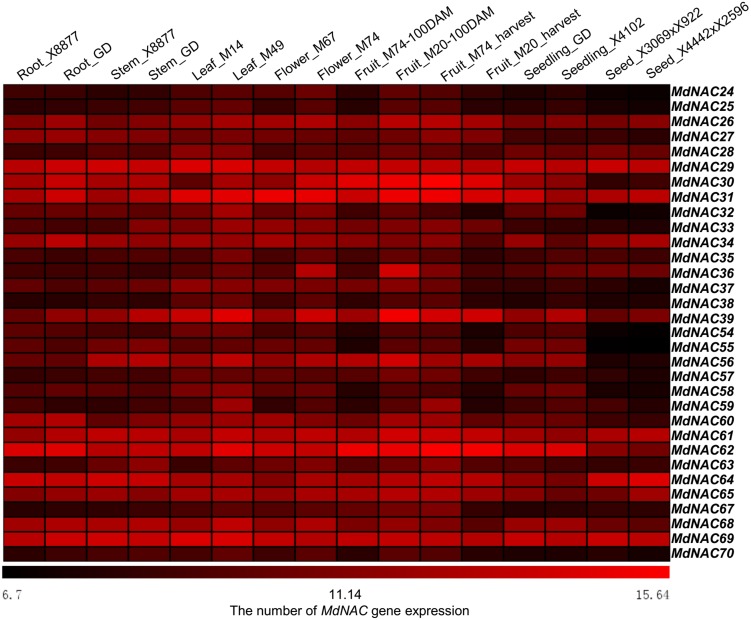
The array (GSE42873) in 16 different apple tissues in GEO (https://www.ncbi.nlm.nih.gov/geo/) was used to evaluate the expression level of MdNACs in different tissues (Fig. 3). Thirty two MdNAC genes were expressed at different levels in 16 tissues of apple, and expression levels of MdNAC29, MdNAC31, MdNAC61, MdNAC64, and MdNAC69 were relatively higher in all tested tissues, levels of MdNAC26, MdNAC30, MdNAC39, MdNAC56, MdNAC62, MdNAC65, and MdNAC68 were relatively higher in multiple examined tissues, and levels of MdNAC24, MdANC25, MdNAC35, MdNAC54, MdNAC55, MdNAC57, MdNAC67, and MdNAC70 were relatively lower in the tested tissues (Fig. 3).
Figure 3. Expression profiles of apple MdNAC genes in various tissues.
The data of apple MdNAC expression (GSE42873) in 16 different were searched at GEO database in NCBI. The heat map of apple MdNAC genes was generated by TIGR MeV v4.8.1 software. Each horizontal row represents a number of gene expression with its MdNAC, and the vertical columns represent different tissues from left to right.
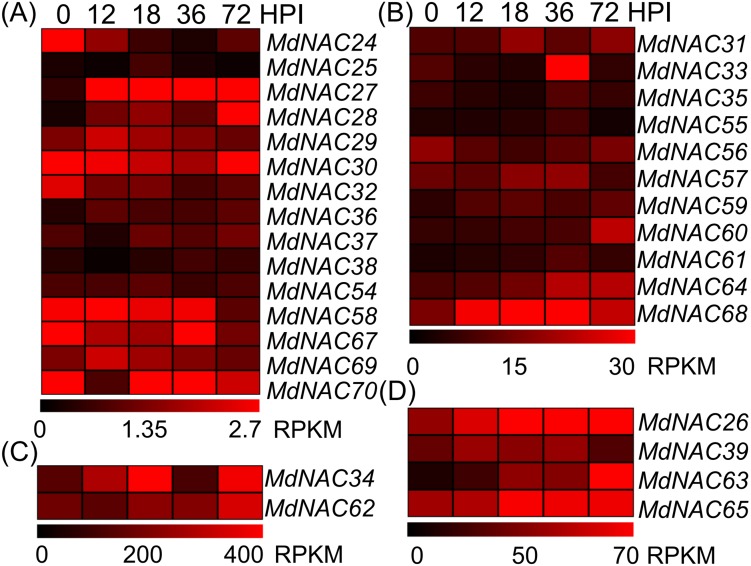
Further, we detected the expression level of the response of MdNAC genes to AAAP infection. Expression levels of MdNAC26-28, MdNAC33-34, MdNAC60, MdNAC62-65, and MdNAC68 were all up-regulated in the response of apples’ AAAP infection (Fig. 4). Particularly, the expression levels of MdNAC27, MdNAC28, and MdNAC63 increased significantly, which was 17.2, 15.9, and 10.5-folds by 72 h post inoculation (HPI), respectively. Expression levels of MdNAC24, MdNAC32, and MdNAC58 decreased, and the relative expression for the other MdNAC genes did not change significantly (Fig. 4).
Figure 4. Expression profiles of apple MdNAC genes in response to Alternaria alternata apple pathotype infection.
According to the RPKM values in RNA-seq, the expression data of apple MdNAC genes in response to AAAP infection were divided into four groups (A, B, C and D).The expression data of apple MdNAC genes in response to AAAP infection were obtained from supplementary data of previously published study (Zhu et al., 2017). The heat map of apple MdNAC genes was generated by TIGR MeV v4.8.1 software. Each horizontal row represents a RPFM with its MdNAC, and the vertical columns represent 0, 12, 18, 36, and 72 from left to right.
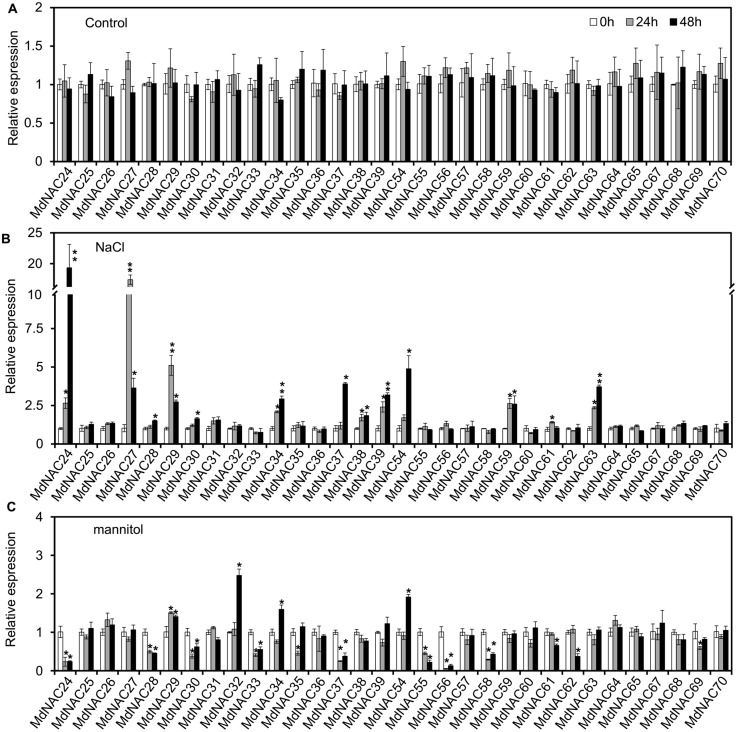
Expression levels of MdNAC genes in ‘Gala’ seedlings under mannitol and NaCl stress were analyzed by RT-qPCR. Under mannitol treatment, 10 MdNAC members were down-regulated, which included MdNAC24, MdNAC28, MdNAC30, MdNAC33, MdNAC35, MdNAC37, MdNAC55, MdNAC56, MdNAC58, and MdNAC59. Among them, expression of MdNAC56 decreased only 0.05-fold when treated for 24 h compared with that of the control (Fig. 5). However, expression levels of MdNAC32 and MdNAC54 increased 2.47 and 1.91-folds when treated for 48 h compared with that of the control, respectively. Relative expression levels of other MdNAC genes did not change significantly under mannitol treatment (Fig. 5). Under NaCl treatment, expression levels of MdNAC24, MdNAC27, MdNAC29, MdNAC34, MdNAC37, MdNAC39, MdNAC54, MdNAC59, and MdNAC63 were up-regulated compared with that of the control. Among them, relative expression levels of MdNAC27 and MdNAC24 increased by 12.28 and 19.33-folds when treated for 24 h and 48 h, respectively, and relative expression levels of other MdNAC genes were not changed significantly (Fig. 5).
Figure 5. Expression analysis of apple MdNAC genes under normal growth, salt and mannitol treatments.
(A) Expression analysis of apple MdNAC genes under normal growth; (B) expression analysis of apple MdNAC genes under salt treatment; (C) expression analysis of apple MdNAC genes under mannitol treatment. The reference gene used in qRT-PCR was MdMDH. Three independent biological replicates were used for calculations. Error bars indicate standard deviation. ∗ and ∗∗ indicate statistically significant differences, as determined by Student’s t tests, at p < 0.05 and p < 0.01, respectively.
Discussion
Based on the highly conserved domain in NAC TFs of plants and the draft genome sequence of the domesticated apple, 180 MdNAC genes were identified in the apple genome database v 1.0 (Velasco et al., 2010; Su et al., 2013). However, compared with other species, there has been little research on NAC transcription factors in apple. In this study, we cloned 32 MdNAC genes and detected their expression patterns in tissues and organs and their response patterns to abiotic and biotic stress. The multiple sequence alignment analysis showed that the NAC domain of the cloned MdNAC transcription factors in this study was highly conserved, and the C-terminus of their transcriptional regulatory domains was highly variable (Fig. 1). This suggested that MdNAC transcription factors played different important roles in regulating the growth, development, and response to abiotic and biotic stress in apple.
NAC transcription factors are one of the largest transcription factor families in plants; they have a large number of members, which determines their complex classification. Using different species of NAC proteins for cluster analysis and different software or algorithms may cause some differences in the results of evolutionary analysis of NAC transcription factors (Ooka et al., 2003; Rushton et al., 2008; Lv et al., 2016a; Lv et al., 2016b). The cluster analysis of 105 and 75 NAC transcription factors in Arabidopsis and rice, respectively, showed that NAC transcription factors could be divided into two groups: group I and group II, based on the sequence characteristics of NAC domains. Group I could be divided further into 14 subgroups, and group II could be divided into four subgroups (Ooka et al., 2003). Rushton et al. (2008) studied the evolution of 450 NAC proteins in Arabidopsis, rice, tobacco, poplar, and solanaceous plants, and divided the NAC proteins into seven subfamilies, of which six subfamilies were shared by all plants, and the other subfamily was unique to Solanaceae. The NAC proteins identified from poplar and watermelon genomes were clustered with NAC proteins from Arabidopsis and rice, and similar results were obtained; these NAC proteins could be divided into 18 groups (Hu, Qi & Kong, 2010; Lv et al., 2016a; Lv et al., 2016b). In addition, NAC transcription factors that were clustered in the same subgroup had similar functions (Fang et al., 2008; Su et al., 2013). For example, cluster analysis of the rice NAC families revealed that the NAC proteins associated with stress response belonged to class III, and the NAC proteins that were related to growth and development belonged to subclasses I-2, I-3, and I-4 (Fang et al., 2008). In this study, based on the results from previous studies (Ooka et al., 2003; Fang et al., 2008; Rushton et al., 2008; Zhu et al., 2014b), we conducted an evolutionary analysis of the NAC transcriptional factors that were reported to be related to plant growth, development, and stress response that included 32 MdNAC proteins (Fig. 2). The results showed that most members in groups ATAF, AtNAC3, and OsNAC3 were involved in stress response. Moreover, MdNAC34 and MdNAC63 belonged to ATAF and AtNAC3 groups, respectively, which suggested that the two MdNAC members were involved in stress response (Tran et al., 2004; Hu et al., 2006; Lu et al., 2007; Nakashima et al., 2007; Hu et al., 2008; Wu et al., 2009; Jeong et al., 2010; Takasaki et al., 2010; Hong et al., 2016; Lv et al., 2016a; Lv et al., 2016b; Chen et al., 2016a). The transcriptional levels of MdNAC34 and MdNAC63 were up-regulated under the treatment of AAAP and NaCl stress (Figs. 4 and 5), which indicated that MdNAC34 and MdNAC63 play important regulatory roles in response to biotic or abiotic stress, but further experimental verification is needed.
The results of phylogenetic analysis and functional verification of multiple NAC genes showed that NAC members in the NAM and VND groups were mostly related to plant growth and development (He et al., 2005; Mitsuda et al., 2007; Yoo et al., 2007; Kim et al., 2008; Sperotto et al., 2009; Ríos et al., 2017). In this study, we also cloned multiple MdNAC genes that belonged to the NAM and VND groups. Although previous studies have shown that these two groups of NAC members are mostly involved in plant growth, development, and morphogenesis. In this study, after biotic and abiotic stress treatments, the transcriptional levels of multiple MdNAC members in NAM and VND groups exhibited various expression characteristics (Figs. 4 and 5). For example, the transcriptional level of MdNAC24 in the NAM group was up-regulated under NaCl treatment, but down-regulated by AAAP infection and mannitol treatment. The transcription level of MdNAC32 in the NAM group was induced by mannitol treatment, but down-regulated by AAAP infection. The transcription level of MdNAC32 in the NAM group was induced under NaCl treatment, but down-regulated under mannitol treatment. The transcription level of MdNAC54 in the VND group was induced under NaCl and mannitol treatment, but exhibited no response to AAAP infection. These results suggested that the members of the NAM and VND groups participated not only in growth and development processes, but also in the plant’s response to environmental stress.
Recent studies have shown that several NAC TFs are membrane-bound transcription factors (MTFs), which are involved in plant growth, development, and various stress responses (Seo, Kim & Park, 2008; Seo, 2014; Yao, Deng & Zeng, 2017). For instance, overexpression of a TM-deleted, truncated a Arabidopsis NAC gene NTL8 (NTM1-like 8) form exhibit delayed flowering by suppressing the transcript level of FLOWERING LOCUS T ( FT ) (Kim et al., 2008; Seo, Kim & Park, 2008). The transcript level of NTL8 was triggered by high salinity and GA biosynthetic inhibitor paclabutrazol (PAC), and was repressed by GA. ntl8-1 mutants were insensitive to high salinity and PCA, indicating a role in GA regulation of salt signaling in seed germination (Kim et al., 2008; Seo, Kim & Park, 2008). The expression level of another Arabidopsis NAC gene NTL9 (NTM1-like 9) was induced by osmotic stress. Some senescence-associated genes were down-regulated in NTL9 knockout mutant but up-regulated in transgenic plants overexpressing a TM-deleted, transcriptionally active NTL9 form, indicating that NTL9 mediates osmotic stress responses during leaf senescence (Seo, Kim & Park, 2008; Yoon et al., 2008) . In this study, MdNAC38, NTL8, and NTL9 belonged to the NAM subgroup, MdNAC38 was predicted to be located in the nucleus and plasma membrane, and was slightly induced by salt stress, indicating MdNAC38 as MTF may be involved in salt response. However, further subcellular localization and functional analyses are needed to analysis this possible molecular mechanism.
Conclusions
Thirty-two novel MdNAC genes have been successfully isolated from Malus domestica, belonging to subgroup ATAF, OsNAC3, AtNAC3, NAM, and VND of this plant transcription factor family. Array, RNA-seq, and qRT-PCR-based transcription profiling indicated that 32 MdNAC genes were expressed in all examined tissues at different expression levels, and responded differentially to various stresses, suggesting that these genes may be involved in the regulation of growth, development, and stress responses in apple. These results serve as the theoretical basis for understanding the biological function and regulation of apple NAC transcription factors.
Supplemental Information
Acknowledgments
The authors would like to thank Thomas Alan Gavin, Professor Emeritus, Cornell University, and Xu Yi, for help in revising our English composition.
Funding Statement
This study was supported by the Shandong Provincial Natural Science Foundation, China (Grant No. ZR2019MC071), the Shandong Agricultural Good Cultivar Project (Grant No. 2016LZGC034), and the Agricultural Science and Technology Innovation Project of SAAS (CXGC2016A03 and CXGC2018F03). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Huifeng Li, Email: sdiplhf79@126.com.
Song Shi, Email: shisong@njau.edu.cn.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Huifeng Li conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Kun Ran conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Qinglong Dong and Qiang Zhao performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Song Shi conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
DNA Deposition
Data Availability
The following information was supplied regarding data availability:
The expression level of MdNACs in different tissues is available at NCBI GEO: GSE42873. The expression level of the response of MdNAC genes to AAAP infection is available at https://www.frontiersin.org/articles/10.3389/fpls.2017.00022/full.
References
- Aida et al. (1997).Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M. Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. The Plant Cell. 1997;9(6):841–857. doi: 10.1105/tpc.9.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An et al. (2017).An JP, Li R, Qu FJ, You CX, Wang XF, Hao YJ. An apple NAC transcription factor negatively regulates cold tolerance via CBF-dependent pathway. Journal of Plant Physiology. 2017;221:74–80. doi: 10.1016/j.jplph.2017.12.009. [DOI] [PubMed] [Google Scholar]
- An et al. (2018).An JP, Yao JF, Xu RR, You CX, Wang XF, Hao YJ. An apple NAC transcription factor enhances salt stress tolerance by modulating the ethylene response. Physiologia Plantarum. 2018;164(3):279–289. doi: 10.1111/ppl.12724. [DOI] [PubMed] [Google Scholar]
- Bu et al. (2008).Bu QY, Jiang HL, Li CB, Zhai QZ, Zhang J, Wu XY, Sun JQ, Xie Q, Li CY. Role of the Arabidopsis thaliana NAC transcription factors ANAC019 and ANAC055 in regulating jasmonic acid-signaled defense responses. Cell Research. 2008;18(7):756–767. doi: 10.1038/cr.2008.53. [DOI] [PubMed] [Google Scholar]
- Celton et al. (2014).Celton JM, Gaillard S, Bruneau M, Pelletier S, Aubourg S, Martin-Magniette ML, Navarro L, Laurens F, Renou JP. Widespread anti-sense transcription in apple is correlated with siRNA production and indicates a large potential for transcriptional and/or post-transcriptional control. New Phytologist. 2014;203(1):287–299. doi: 10.1111/nph.12787. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2016b).Chen H, Shao H, Fan S, Ma J, Zhang D, Han M. Identification and phylogenetic analysis of the POLYGALACTURONASE gene family in apple. Horticultural Plant Journal. 2016b;2(5):241–252. doi: 10.1016/j.hpj.2017.01.004. [DOI] [Google Scholar]
- Chen et al. (2017).Chen H, Shao H, Li K, Zhang D, Fan S, Li Y, Han M. I Genome-wide identification, evolution, and expression analysis of GATA transcription factors in apple (Malus× domestica Borkh.) Gene. 2017;627:460–472. doi: 10.1016/j.gene.2017.06.049. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2018).Chen H, Zuo X, Shao H, Fan S, Ma J, Zhang D, Zhao C, Yan X, Liu X, Han M. Genome-wide analysis of carotenoid cleavage oxygenase genes and their responses to various phytohormones and abiotic stresses in apple (Malus domestica) Plant Physiology and Biochemistry. 2018;123:81–93. doi: 10.1016/j.plaphy.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2016a).Chen SP, Lin IW, Chen X, Huang YH, Chang SC, Lo HS, Lu HH, Yeh KW. Sweet potato NAC transcription factor, IbNAC1, up-regulates sporamin gene expression by binding the SWRE motif against mechanical wounding and herbivore attack. The Plant Journal. 2016a;86(3):234–248. doi: 10.1111/tpj.13171. [DOI] [PubMed] [Google Scholar]
- Cui et al. (2015).Cui HR, Zhang ZR, Lv W, Xu JN, Wang XY. Genome-wide characterization and analysis of F-box protein-encoding genes in the Malus domestica genome. Molecular Genetics and Genomics. 2015;290(4):1435–1446. doi: 10.1007/s00438-015-1004-z. [DOI] [PubMed] [Google Scholar]
- Delessert et al. (2005).Delessert C, Kazan K, Wilsom L, Straetend D, Manners J, Dennis ES, Dolferus R. The transcription factor ATAF2 represses the expression of pathogenesis-related genes in Arabidopsis. The Plant Journal. 2005;43(5):745–757. doi: 10.1111/j.1365-313X.2005.02488.x. [DOI] [PubMed] [Google Scholar]
- Dong et al. (2018a).Dong Q, Duan D, Zhao S, Xu B, Luo J, Wang Q, Huang D, Liu C, Li C, Gong X, Mao K, Ma F. Genome-wide analysis and cloning of the apple stress-associated protein gene family reveals MdSAP15, which confers tolerance to drought and osmotic stresses in transgenic Arabidopsis. International Journal of Molecular Sciences. 2018a;19(9):2478. doi: 10.3390/ijms19092478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong et al. (2018b).Dong Q, Mao K, Duan D, Zhao S, Wang Y, Wang Q, Huang D, Li C, Liu C, Gong X, Ma F. Genome-wide analyses of genes encoding FK506-binding proteins reveal their involvement in abiotic stress responses in apple. BMC Genomics. 2018b;19(1):707. doi: 10.1186/s12864-018-5097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong et al. (2018c).Dong Q, Zhao S, Duan D, Tian Y, Wang Y, Mao K, Zhou Z, Ma F. Structural and functional analyses of genes encoding VQ proteins in apple. Plant Science. 2018c;272:208–219. doi: 10.1016/j.plantsci.2018.04.029. [DOI] [PubMed] [Google Scholar]
- Fan et al. (2017).Fan S, Zhang D, Zhang L, Gao C, Xin M, Tahir MM, Li Y, Ma J, Han M. Comprehensive analysis of GASA family members in the Malus domestica genome: identification, characterization, and their expressions in response to apple flower induction. BMC Genomics. 2017;18:827. doi: 10.1186/s12864-017-4213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang et al. (2008).Fang YJ, You J, Xie K, Xie WB, Xiong LZ. Systematic sequence analysis and identification of tissue-specific orstress-responsive genes of NAC transcription factor family in rice. Molecular Genetics and Genomics. 2008;280(6):547–563. doi: 10.1007/s00438-008-0386-6. [DOI] [PubMed] [Google Scholar]
- Hao & Qiao (2018).Hao L, Qiao X. Genome-wide identification and analysis of the CNGC gene family in maize. PeerJ. 2018;6:e5816. doi: 10.7717/peerj.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He et al. (2005).He XJ, Mu RL, Cao WH, Zhang ZG, Zhang JS, Chen SY. AtNAC2, a transcription factor downstream of ethylene and auxin signaling pathways, is involved in salt stress response and lateral root development. The Plant Journal. 2005;44(6):903–916. doi: 10.1111/j.1365-313X.2005.02575.x. [DOI] [PubMed] [Google Scholar]
- Hong et al. (2016).Hong Y, Zhang H, Huang L, Li D, Song F. Overexpression of a stress-responsive NAC transcription factor gene ONAC022 improves drought and salt tolerance in rice. Frontiers in Plant Science. 2016;7:4. doi: 10.3389/fpls.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu et al. (2006).Hu H, Dai M, Yao J, Xiao B, Li X, Zhang Q, Xiong L. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(35):12987–12992. doi: 10.1073/pnas.0604882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu et al. (2008).Hu H, You J, Fang Y, Zhu X, Qi Z, Xiong L. Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. Plant Molecular Biology. 2008;67(1–2):169–181. doi: 10.1007/s11103-008-9309-5. [DOI] [PubMed] [Google Scholar]
- Hu, Qi & Kong (2010).Hu R, Qi G, Kong Y. Comprehensive analysis of NAC domain transcription factor gene family in Populus trichocarpa. BMC Plant Biology. 2010;10:145. doi: 10.1186/1471-2229-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. (2015).Huang Q, Yan W, Li B, Chang J, Chen M, Li K, Yang G, He G. TaNAC29, a NAC transcription factor from wheat, enhances salt and drought tolerance in transgenic Arabidopsis. BMC Plant Biology. 2015;15(1):268. doi: 10.1186/s12870-015-0644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong et al. (2010).Jeong JS, Kim YS, Baek KH, Jung H, Ha S, Do Choi Y, Kim M, Reuzeau C, Kim J. Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiology. 2010;153(1):185–197. doi: 10.1104/pp.110.154773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia et al. (2018).Jia D, Gong X, Li M, Li C, Sun T, Ma F. Overexpression of a novel apple NAC transcription factor gene, MdNAC1, confers the dwarf phenotype in transgenic apple (Malus domestica) Gene. 2018;9(5):229. doi: 10.3390/genes9050229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2008).Kim SG, Lee AK, Yoon HK, Park CM. A membrane-bound NAC transcription factor NTL8 regulates gibberellic acid-mediated salt signaling in Arabidopsis seed germination. The Plant Journal. 2008;55(1):77–88. doi: 10.1111/j.1365-313X.2008.03493.x. [DOI] [PubMed] [Google Scholar]
- Li et al. (2019a).Li H, Dong Q, Zhao Q, Ran K. Genome-wide identification, expression profiling, and protein-protein interaction properties of ovate family proteins in apple. Tree Genetics and Genomes. 2019a;15:45. doi: 10.1007/s11295-019-1354-5. [DOI] [PubMed] [Google Scholar]
- Li et al. (2019b).Li R, Ge H, Dai Y, Yuan L, Liu X, Sun Q, Wang X. Genome wide analysis of homeobox gene family in apple (Malus domestica Borkh.) and their response to abiotic stress. Journal of Genetics. 2019b;98:13. doi: 10.1007/s12041-018-1049-y. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2018).Liu X, Li R, Dai Y, Chen X, Wang X. Genome-wide identification and expression analysis of the B-box gene family in the apple (Malus domestica Borkh.) genome. Molecular Genetics and Genomics. 2018;293(2):303–315. doi: 10.1007/s00438-017-1386-1. [DOI] [PubMed] [Google Scholar]
- Livak & Schmittgen (2001).Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu et al. (2007).Lu PL, Chen NZ, An R, Su Z, Qi BS, Ren F, Chen J, Wang XC. A novel drought-inducible gene, ATAF1, encodes a NAC family protein that negatively regulates the expression of stress-responsive genes in Arabidopsis. Plant Molecular Biology. 2007;63(2):289–305. doi: 10.1007/s11103-006-9089-8. [DOI] [PubMed] [Google Scholar]
- Lv et al. (2016a).Lv X, Lan S, Guy K, Yang J, Zhang M, Hu Z. Global expressions landscape of NAC transcription factor family and their responses to abiotic stresses in Citrullus lanatus. Scientific Reports. 2016a;6:30574. doi: 10.1038/srep30574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv et al. (2016b).Lv Z, Wang S, Zhang F, Chen L, Hao X, Pan Q, Fu X, Li L, Sun X, Tang K. Overexpression of a novel NAC domain-containing transcription factor (AaNAC1) enhances the content of artemisinin and increases tolerance to drought and Botrytis cinerea in Artemisia annua. Plant and Cell Physiology. 2016b;57(9):1961–1971. doi: 10.1093/pcp/pcw118. [DOI] [PubMed] [Google Scholar]
- Mao et al. (2007).Mao C, Ding W, Wu Y, Yu J, He X, Shou H, Wu P. Overexpression of a NAC-domain protein promotes shoot branching in rice. New Phytologist. 2007;176(2):288–298. doi: 10.1111/j.1469-8137.2007.02177.x. [DOI] [PubMed] [Google Scholar]
- Mao et al. (2014).Mao X, Chen S, Li A, Zhai C, Jing R. Novel NAC transcription factor TaNAC67 confers enhanced multi-abiotic stress tolerances in Arabidopsis. PLOS ONE. 2014;9(1):e84359. doi: 10.1371/journal.pone.0084359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao et al. (2012).Mao X, Zhang H, Qian X, Li A, Zhao G, Jing R. TaNAC2, a NAC-type wheat transcription factor conferring enhanced multiple abiotic stress tolerances in Arabidopsis. Journal of Experimental Botany. 2012;63(8):2933–2946. doi: 10.1093/jxb/err462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuda et al. (2007).Mitsuda N, Iwase A, Yamamoto H, Yoshida M, Seki M, Shinozaki K, Ohme-Takagi M. NAC transcription factors, NST1 and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis. The Plant Cell. 2007;19(1):270–280. doi: 10.1105/tpc.106.047043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima et al. (2007).Nakashima K, Tran LSP, Van Nguyen D, Fujita M, Maruyama K, Todaka D, Ito Y, Hayashi N, Shinozaki K, Yamaguchi-Shinozaki K. Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. The Plant Journal. 2007;51(4):617–630. doi: 10.1111/j.1365-313X.2007.03168.x. [DOI] [PubMed] [Google Scholar]
- Ooka et al. (2003).Ooka H, Satoh K, Doi K, Nagata T, Otomo Y, Murakami K, Matsubara K, Osato N, Kawai J, Carninci P, Hayashizaki Y, Suzuki K, Kojima K, Takahara Y, Yamamoto K, Kikuchi S. Comprehensive analysis of NAC family genes in Oryzasativa and Arabidopsis thaliana. DNA Research. 2003;10(6):239–247. doi: 10.1093/dnares/10.6.239. [DOI] [PubMed] [Google Scholar]
- Perini et al. (2014).Perini P, Pasquali G, Margis-Pinheiro M, De Oliviera PRD, Revers LF. Reference genes for transcriptional analysis of flowering and fruit ripening stages in apple (Malus × domestica Borkh) Molecular Breeding. 2014;34(3):829–842. doi: 10.1007/s11032-014-0078-3. [DOI] [Google Scholar]
- Puranik et al. (2012).Puranik S, Sahu PP, Srivastava PS, Prasad M. NAC proteins: regulation and role in stress tolerance. Trends in Plant Science. 2012;17(6):369–381. doi: 10.1016/j.tplants.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Ríos et al. (2017).Ríos P, Argyris J, Vegas J, Leida C, Kenigswald M, Tzuri G, Troadec C, Bendahmane A, Katzir N, Picó B, Monforte AJ, Garcia-Mas J. ETHQV6.3 is involved in melon climacteric fruit ripening and is encoded by a NAC domain transcription factor. The Plant Journal. 2017;91(4):671–683. doi: 10.1111/tpj.13596. [DOI] [PubMed] [Google Scholar]
- Rushton et al. (2008).Rushton PJ, Bokowiec MT, Han SC, Zhang HB, Brannock JF, Chen XF, Laudeman TW, Timko MP. Tobacco transcription factors: novel insights into transcriptional regulation in the Solanaceae. Plant Physiology. 2008;147(1):280–295. doi: 10.1104/pp.107.114041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo (2014).Seo PJ. Recent advances in plant membrane-bound transcription factor research: emphasis on intracellular movement. Journal of Integrative Plant Biology. 2014;56(4):334–342. doi: 10.1111/jipb.12139. [DOI] [PubMed] [Google Scholar]
- Seo, Kim & Park (2008).Seo PJ, Kim S, Park C. Membrane-bound transcription factors in plants. Trends in Plant Science. 2008;13:550–556. doi: 10.1016/j.tplants.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Sperotto et al. (2009).Sperotto RA, Ricachenevsky FK, Duarte GL, Boff T, Lopes KL, Sperb ER, Grusak MA, Fett JP. Identification of up-regulated genes in flag leaves during rice grain filling and characterization of OsNAC5, a new ABA-dependent transcription factor. Planta. 2009;230(5):985–1002. doi: 10.1007/s00425-009-1000-9. [DOI] [PubMed] [Google Scholar]
- Su et al. (2013).Su H, Zhang S, Yuan X, Chen C, Wang XF, Hao YJ. Genome-wide analysis and identification of stress-responsive genes of the NAM-ATAF1, 2-CUC2 transcription factor family in apple. Plant Physiology and Biochemistry. 2013;71(2):11–21. doi: 10.1016/j.plaphy.2013.06.022. [DOI] [PubMed] [Google Scholar]
- Takasaki et al. (2010).Takasaki H, Maruyama K, Kidokoro S, Ito Y, Fujita Y, Shinozaki K, Yamaguchi-Shinozaki K, Nakashima K. The abiotic stress-responsive NAC-type transcription factor OsNAC5 regulates stress-inducible genes and stress tolerance in rice. Molecular Genetics and Genomics. 2010;284(3):173–183. doi: 10.1007/s00438-010-0557-0. [DOI] [PubMed] [Google Scholar]
- Thirumalaikumar et al. (2018).Thirumalaikumar VP, Devkar V, Mehterov N, Ozqur R, Turkan I, Mueller-Roeber B, Balazadeh S. NAC transcription factor JUNGBRUNNEN1 enhances drought tolerance in tomato. Plant Biotechnology Journal. 2018;16(2):354–366. doi: 10.1111/pbi.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran et al. (2004).Tran LSP, Nakashima K, Sakuma Y, Simpson SD, Fujita Y, Maruyama K, Fujita M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. The Plant Cell. 2004;16(9):2481–2498. doi: 10.1105/tpc.104.022699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco et al. (2010).Velasco R, Zharkikh A, Affourtit J, Dhingra A, Cestaro A, Kalyanaraman A, Fontana P, Bhatnagar SK, Troggio M, Pruss D, Salvi S, Pindo M, Baldi P, Castelletti S, Cavaiuolo M, Coppola G, Costa F, Cova V, Dal Ri A, Goremykin V, Komjanc M, Longhi S, MagnArgonaute P, Malacarne G, Malnoy M, Micheletti D, Moretto M, Perazzolli M, Si-Ammour A, Vezzulli S, Zini E, Eldredge G, Fitzgerald LM, Gutin N, Lanchbury J, Macalma T, Mitchell JT, Reid J, Wardell B, Kodira C, Chen Z, Desany B, Niazi F, Palmer M, Koepke T, Jiwan D, Schaeffer S, Krishnan V, Wu C, Chu VT, King ST, Vick J, Tao Q, Mraz A, Stormo A, Stormo K, Bogden R, Ederle D, Stella A, Vecchietti A, Kater MM, Masiero S, Lasserre P, Lespinasse Y, Allan AC, Bus V, Chagne D, Crowhurst RN, Gleave AP, Lavezzo E, Fawcett JA, Proost S, Rouze P, Sterck L, Toppo S, Lazzari B, Hellens RP, Durel CE, Gutin A, Bumgarner RE, Gardiner SE, Skolnick M, Egholm M, Van de Peer Y, Salamini F, Viola R. The genome of the domesticated apple (Malus× domestica Borkh.) Nature Genetics. 2010;42(10):833–839. doi: 10.1038/ng.654. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2009).Wu Y, Deng Z, Lai J, Zhang Y, Yang C, Yin B, Zhao Q, Zhang L, Li Y, Yang C. Dual function of Arabidopsis ATAF1 in abiotic and biotic stress responses. Cell Research. 2009;19(11):1279–1290. doi: 10.1038/cr.2009.108. [DOI] [PubMed] [Google Scholar]
- Yao, Deng & Zeng (2017).Yao S, Deng L, Zeng K. Genome-wide in silico identification of membrane-bound transcription factors in plant species. PeerJ. 2017;5:e4051. doi: 10.7717/peerj.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo et al. (2007).Yoo SY, Kim Y, Kim SY, Lee JS, Ahn JH. Control of flowering time and cold response by a NAC-domain protein in Arabidopsis. PLOS ONE. 2007;2(7):e642. doi: 10.1371/journal.pone.0000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon et al. (2008).Yoon HK, Kim SG, Kim SY, Park CM. Regulation of leaf senescence by NTL9-mediated osmotic stress signaling in Arabidopsis. Molecules and Cells. 2008;25(3):438–445. [PubMed] [Google Scholar]
- Yoshii et al. (2010).Yoshii M, Yamazaki M, Rakwal R, Kishi-Kaboshi M, Miyao A, Hirochika H. The NAC transcription factor RIM1of rice is a new regulator of jasmonate signaling. The Plant Journal. 2010;61(5):804–815. doi: 10.1111/j.1365-313X.2009.04107.x. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2016).Zhang Z, Cui H, Xing S, Liu X, Chen X, Wang X. Genome-wide identification and characterization of transcription factors of Basic Leucine Zipper family in Malus domestica. bioRxiv. 2016 doi: 10.1101/075994. [DOI]
- Zhu et al. (2017).Zhu L, Ni W, Liu S, Cai B, Xing H, Wang S. Transcriptomics analysis of apple leaves in response to Alternaria alternate apple pathotype infection. Frontiers in Plant Science. 2017;8:22. doi: 10.3389/fpls.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al. (2014a).Zhu M, Chen G, Zhang J, Zhang Y, Xie Q, Zhao Z, Pan Y, Hu Z. The abiotic stress-responsive NAC-type transcription factor SlNAC4 regulates salt and drought tolerance and stress-related genes in tomato (Solanum lycopersicum) Plant Cell Reports. 2014a;33(11):1851–1863. doi: 10.1007/s00299-014-1662-z. [DOI] [PubMed] [Google Scholar]
- Zhu et al. (2014b).Zhu M, Hu Z, Zhou S, Wang L, Dong T, Pan Y, Chen G. Molecular characterization of six tissue-specific or stress-inducible genes of NAC transcription factor family in tomato (Solanum lycopersicum) Journal of Plant Growth Regulation. 2014b;33(4):730–744. doi: 10.1007/s00344-014-9420-6. [DOI] [Google Scholar]
- Zuo et al. (2019).Zuo C, Liu Y, Guo Z, Mao J, Chu M, Chen B. Genome-wide annotation and expression responses to biotic stresses of the WALL-ASSOCIATED KINASE—RECEPTOR-LIKE KINASE (WAK-RLK) gene family in apple (Malus domestica) European Journal of Plant Pathology. 2019;153(3):771–785. doi: 10.1007/s10658-018-1591-8. [DOI] [Google Scholar]
- Zuo et al. (2018).Zuo C, Zhang W, Ma Z, Chu M, Ma J, An Z, Chen B. Genome-wide identification and expression analysis of the CrRLK1L gene family in apple (Malus domestica) Plant Molecular Biology Reporter. 2018;36(5–6):844–857. doi: 10.1007/s11105-018-1125-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The expression level of MdNACs in different tissues is available at NCBI GEO: GSE42873. The expression level of the response of MdNAC genes to AAAP infection is available at https://www.frontiersin.org/articles/10.3389/fpls.2017.00022/full.