Abstract
It has recently been shown that the loss of the Hippo signaling effectors Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) in adrenocortical steroidogenic cells impairs the postnatal maintenance of the adrenal gland. To further explore the role of Hippo signaling in mouse adrenocortical cells, we conditionally deleted the key Hippo kinases large tumor suppressor homolog kinases 1 and -2 (Lats1 and Lats2, two kinases that antagonize YAP and TAZ transcriptional co-regulatory activity) in steroidogenic cells using an Nr5a1-cre strain (Lats1flox/flox;Lats2flox/flox;Nr5a1-cre). We report here that developing adrenocortical cells adopt characteristics of myofibroblasts in both male and female Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice, resulting in a loss of steroidogenic gene expression, adrenal failure and death by 2 to 3 weeks of age. A marked accumulation of YAP and TAZ in the nuclei of the myofibroblast-like cell population with an accompanying increase in the expression of their transcriptional target genes in the adrenal glands of Lats1flox/flox;Lats2flox/flox;Nr5a1-cre animals suggested that the myofibroblastic differentiation could be attributed in part to YAP and TAZ. Taken together, our results suggest that Hippo signaling is required to maintain proper adrenocortical cell differentiation and suppresses their differentiation into myofibroblast-like cells.
Keywords: LATS1, LATS2, adrenocortical cells, development, adrenal gland
In mice, the adrenal cortex is derived from the intermediate mesoderm and arises from the proliferation of coelomic epithelial cells. The latter initially form the adrenogonadal primordium (AGP) at embryonic day 9.5 (e9.5), a structure from which the gonads also develop (1, 2). Around e10.5, the AGP separates into 2 distinct tissues as the adrenal progenitor cells migrate dorsomedially to form the adrenal primordium (AP) at e11.0 (3, 4). Shortly thereafter, at e12.5, chromaffin cells derived from the neural crest invade the AP to form the adrenal medulla (5, 6), while mesenchymal cells surrounding the developing adrenal gland start to condense to form its capsule (7). Development of the definitive adrenal cortex starts after encapsulation at e14.5, with zonation being first observed around e17.5. Adrenal cortex development is then completed after birth (7, 8). While the definitive cortex expands, the fetal zone decreases in size, and after birth it forms the transient X-zone located in the innermost part of the cortex (9). Despite this 2-step developmental process, lineage-tracing experiments have shown that both the fetal cortex and the definitive cortex derive from a common embryonic population of nuclear receptor subfamily 5, group A, member 1 (NR5A1)-expressing cells (8, 10). Though numerous transcription factors (including GATA binding protein 4 and 6 (GATA4/6) (11), NR5A1 (1, 12, 13) and Wilm’s tumor 1 (WT1) (4) as well as signaling pathways such as insulin/insulin-like growth factor (14), WNT/beta-catenin (15), Hedgehog (16) and protein kinase A (PKA) (17) are all known to be necessary for different stages of adrenal development and maintenance, additional pathways are likely involved.
Hippo is an evolutionarily conserved signaling pathway with well-established roles in cell fate determination, differentiation, and proliferation during embryonic development (reviewed in (18, 19). It consists of a kinase cascade that regulates 2 functionally redundant transcriptional co-activators, Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ). In response to various extracellular signals, the mammalian STE20-like protein kinases 1 and 2 (MST1, MST2) are activated and phosphorylate the large tumor suppressor homolog kinases 1 and 2 (LATS1, LATS2) which in turn phosphorylates YAP and TAZ. YAP and TAZ phosphorylation leads to their inactivation by sequestration in the cytoplasm and/or by proteasome-mediated degradation. When the cascade is inactivated, YAP and TAZ accumulate in the nucleus and interact with transcriptional factors to regulate the transcription of genes involved in cell growth, apoptosis, and proliferation (19, 20).
A role for Hippo signaling in the adrenal gland was first suggested in a study that showed that elevated YAP expression was associated with poor outcome of adrenocortical tumors in pediatric patients (21). More recently, using a genetic model, the concomitant loss of Yap and Taz in steroidogenic adrenocortical cells was shown to lead to the progressive degeneration of the adrenal cortex (22). In the present study, to further characterize the role of the Hippo signaling pathway in adrenal cortex development and maintenance, we generated a mouse model in which Lats1 and/or Lats2 were conditionally deleted in NR5A1-positive steroidogenic cells.
Material and Methods
Ethics
All animal procedures were approved by the Comité d’Éthique de l’Utilisation des Animaux of the Université de Montréal (protocol numbers Rech-1739 and Rech-1909) and conformed to the guidelines of the Canadian Council on Animal Care.
Transgenic mouse strains
Nr5a1-cre mice (FVB-Tg-Nr5a1Cre7Lowl/J, RRID:IMSR_JAX:012462) were obtained from the Jackson Laboratory and maintained by crossing Cre-positive males with wild-type females (C57BL/6J, RRID:IMSR_JAX:000664). Lats1flox/flox (Lats1tm1.1JFm/RjoJ, RRID:MGI:5568587) and Lats2flox/flox (Lats2tm1.1JFm/RjoJ, RRID:MGI:5568590) mice were obtained from Dr Randy L. Johnson (MD Anderson Cancer Center, Houston, TX). Mice were selectively bred over several generations to obtain Lats1flox/flox;Nr5a1-cre, Lats2flox/flox;Nr5a1-cre and Lats1flox/flox;Lats2flox/flox;Nr5a1-cre genotypes. Genotype analyses were done on tail biopsies by polymerase chain reaction (PCR) as previously described for Cre (23) and Lats1/2 (24).
Histopathology, immunohistochemistry, and immunofluorescence
Whole embryos or isolated adrenal glands for light microscopy histopathologic analysis were fixed in 4% paraformaldehyde for 4 hours (whole embryos, 1 day postpartum (dpp) adrenals) or in formalin overnight (adrenals from 2 week-old mice). Tissues were embedded in paraffin, sectioned (5 μm), and stained with hematoxylin and eosin stain (H&E). Immunohistochemistry was done on formalin-fixed, paraffin-embedded, 5 μm-thick tissue sections using VectaStain Elite avidin–biotin complex method kits (Vector Laboratories) or the mouse on mouse (M.O.M.) elite peroxidase kit (Vector Laboratories) as directed by the manufacturer. Sections were probed with primary antibodies against bromodeoxyuridine (BrdU) [1:100, Dako Corp (25)], cleaved caspase-3 (CAS3) [1:100, Cell Signaling Technology (26)], cytochrome P450, family 11, subfamily B, polypeptide 1 (CYP11B1) [1:200 (27)], alpha smooth muscle actin (α-SMA) [1:100, BioGenex (28)], steroid acute regulatory protein (STAR) [1:200, Santa Cruz biotech (29)], TAZ [1:500, Sigmal-Aldrich (30)], tyrosine hydroxylase (TH) [1:200, Santa Cruz biotech (31)], YAP [1:100, Cell Signaling (32)], phospho-YAP [1:100, Cell Signaling (33)] and vimentin [1:100, Cell Signaling (34)]. Staining was done using the 3,3´-diaminobenzidine peroxidase substrate kit (Vector Laboratories). Immunofluorescence was done on formalin-fixed, paraffin-embedded, 5 μm-thick tissue sections. Sections were probed with a primary antibody for 20-alpha-hydroxysteroid dehydrogenase (20αHSD) [1:2500, a gift from F. Beuschlein (35)]. Staining was performed using ImmPress polymer (Vector Laboratories) followed by the Alexa Fluor 555 Tyramide superboost kit (ThermoFisher scientific) and counterstaining with DAPI vectashield (Vector Laboratories). Negative controls consisted of slides for which the primary antibody was omitted.
Hormone measurements
Blood samples for plasma collection were collected between 9:30 and 10:00 in 2K-EDTA microvette tubes (Sarstedt) and centrifuged at 2000g for 15 minutes at 4°C. Plasma samples were transferred to polypropylene tubes and stored at −80°C until analysis. Adrenocorticotropic hormone (ACTH) levels in the plasma were determined by IMMULITE 2000 (Siemens Healthineers Global). Corticosterone levels were determined by RIA (MP Biomedical). Assays were performed by the Center for Research in Reproduction at the Ligand Assay and Analysis Core Laboratory of the University of Virginia.
Reverse transcription-quantitative PCR
Total RNA from adrenal glands of e14.5, e17.5, and 1 day-old animals was extracted using the Total RNA Mini Kit (FroggaBio) according to the manufacturer’s protocol. Total RNA was reverse transcribed using 100 ng of RNA and the SuperScriptVilo™ cDNA synthesis kit (Thermo Fisher Scientific). Real-time PCR reactions were run on a CFX96 Touch instrument (Bio-Rad), using Supergreen Advanced qPCR MasterMix (Wisent, St-Bruno, Canada). Each PCR reaction consisted of 7.5 μl of Power SYBR Green PCR Master Mix, 2.3 μl of water, 4 μl of cDNA sample and 0.6 μl (400 nmol) of gene-specific primers. PCR reactions run without complementary cDNA (water blank) served as negative controls. A common thermal cycling program (3 minutes at 95°C, 40 cycles of 15 seconds at 95°C, 30 seconds at 60°C and 30 seconds at 72°C) was used to amplify each transcript. To quantify relative gene expression, the Ct of genes of interest was compared with that of Rpl19, according to the ratio R = [ECt Rpl19/ECt target] where E is the amplification efficiency for each primer pair. Rpl19 Ct values did not change significantly between tissues, and Rpl19 was therefore deemed suitable as an internal reference gene. The specific primer sequences used are listed (36).
BrdU incorporation assay
Pregnant mice were injected intraperitoneally at gestational day 17.5 with 100 mg/kg body weight BrdU (Sigma-Aldrich) and euthanized 4 hours after the injection. The embryos were collected, fixed in 4% PFA for 4 hours and embedded in paraffin. The BrdU-labeled DNA was detected by immunohistochemistry as described above.
Statistical analyses
All statistical analyses were performed with Prism software version 6.0d (GraphPad Software Inc., RRID: SCR_002798). All the data sets were subjected to the F test to determine the equality of variances. Student t test was used for all comparisons between genotypes, except for ACTH results for which a Mann-Whitney test was used because of the variance between samples. Means were considered significantly different when P value was < 0.05. All data are presented as means ± standard error of the mean (SEM).
Results
Loss of Lats1 and Lats2 causes loss of adrenocortical cell identity
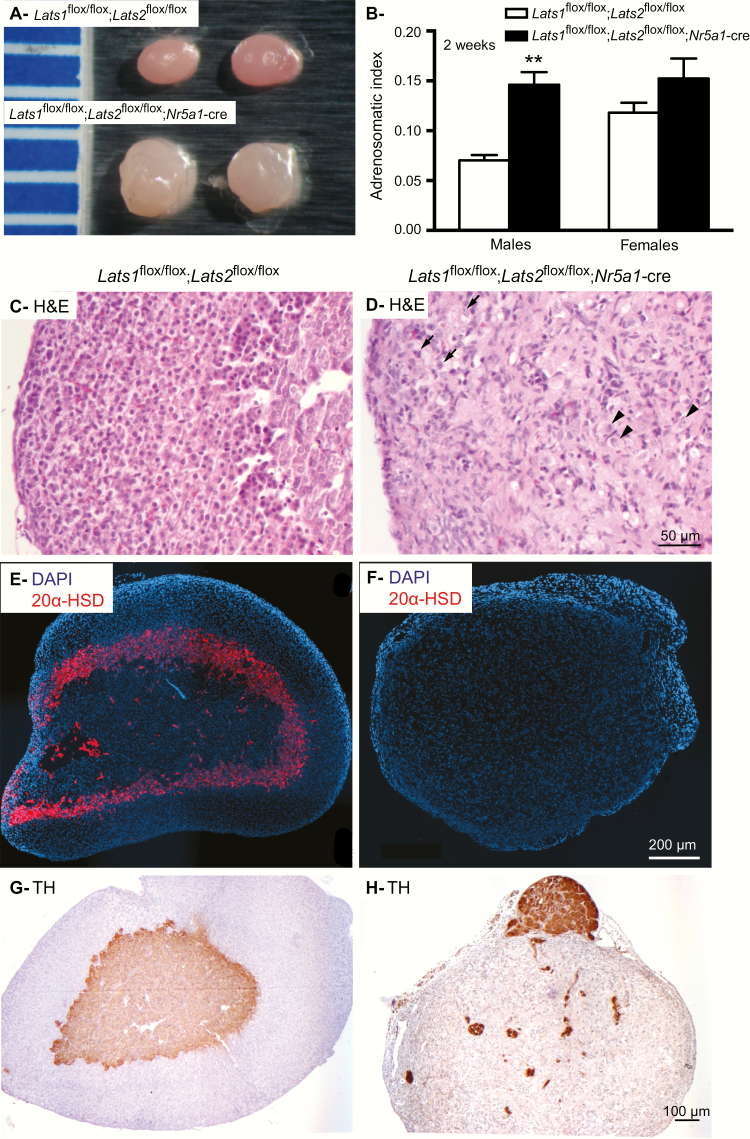
To investigate the role of LATS1 and LATS2 in the adrenal cortex, mice bearing floxed alleles for Lats1 and/or Lats2 were crossed with the Nr5a1-cre strain, which targets steroidogenic cells including those of the fetal and of the definitive adrenal cortex. Lats1flox/flox; Nr5a1-cre and Lats2flox/flox; Nr5a1-cre mice had adrenal glands that appeared normal at the gross and histologic levels (data not shown) and a normal lifespan, and for these reasons were not further studied. Conversely, Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice died between 2 and 3 weeks of age, most likely from adrenocortical failure, as suggested by a marked decrease in circulating corticosterone levels (below the assay detection threshold in the mutant animals) and a concomitant increase in circulating ACTH levels in 10- to 12-day-old mutant mice of both sexes (36). Adrenal glands from 2-week-old Lats1flox/flox;Lats2flox/flox;Nr5a1-cre males (Fig. 1A and 1B) were larger (P < 0.01), more irregular, and paler than the adrenal glands from the control Lats1flox/flox;Lats2flox/flox animals, whereas adrenal glands from 2-week-old mutant females tended to be larger (P = 0.09) (Fig. 1B). Histopathologic analyses (Fig. 1C and 1D; (36)) revealed striking abnormalities in the adrenals of both male (Fig. 1D) and female (36) mutant mice. First, zonation of the adrenal cortex was not apparent in the mutant animals, and the majority of the presumptive adrenocortical cells adopted a spindle shape, with less cytoplasm and smaller, elongated nuclei reminiscent of fibroblasts or myofibroblasts (Fig. 1D). Interspersed among these spindle-shaped cells, a less abundant cell population featuring large nuclei with an eosinophilic cytoplasm (Fig 1D, arrowheads) and a rare cell population featuring a pale cytoplasm containing lipid droplets typical of steroidogenic cells were also observed (Fig. 1D, arrows). Furthermore, 20αHSD-positive cells (Fig. 1E and 1F) were not detected in the adrenal glands of the mutant animals (Fig. 1F), indicating that the X-zone was absent. These results suggest that both the fetal and the definitive adrenal cortex were altered during development.
Figure 1.
Adrenal glands of Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice are larger than controls and are histomorphologically abnormal. (A) Photographs of adrenal gland from 12 dpp Lats1flox/flox;Lats2flox/flox (control) and Lats1flox/flox;Lats2flox/flox;Nr5a1-cre males. Ruler graduations are in millimeters. (B) Adrenosomatic index (adrenal gland weight/corporal weight) comparing male Lats1flox/flox;Lats2flox/flox (n = 12) with Lats1flox/flox; Lats2flox/flox;Nr5a1-cre (n = 8) and female Lats1flox/flox;Lats2flox/flox (n = 10) with Lats1flox/flox;Lats2flox/flox;Nr5a1-cre (n = 8) mice. Data are expressed as means (columns) ± SEM (error bars). Asterisks = significantly different from control (** P < 0.01). (C, D) Photomicrographs comparing the adrenal glands of 12 dpp male (C)Lats1flox/flox;Lats2flox/flox and (D)Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice. (E, F) Immunofluorescence analysis of 20αHSD expression in adrenal glands from 12 dpp female (E)Lats1flox/flox;Lats2flox/flox and (F)Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice. (G, H) Immunohistochemical analysis of TH expression in adrenal glands from 12 dpp male (G)Lats1flox/flox;Lats2flox/flox and (H)Lats1flox/flox;Lats2flox/flox; Nr5a1-cre mice. Abbreviations: H&E, hematoxylin and eosin stain; TH, tyrosine hydroxylase. Arrow = cells with lipid droplets in their cytoplasm. Arrowhead = cells with a large nucleus and eosinophilic cytoplasm. Scale bar in D is valid for C, scale bar in F is valid for E and scale bar in G is valid for H.
Finally, cortex-medulla disorganization was also apparent in the adrenal glands of the Lats1flox/flox;Lats2flox/flox;Nr5a1-cre animals, as only a few tyrosine hydroxylase–positive medullary chromaffin cells were identified, scattered haphazardly within the adrenal gland (Fig. 1H). Larger masses of medullary cells were also often found located at the periphery of the adrenal glands (Fig. 1H). These observations also suggest that the formation of the medulla was altered during development.
LATS1 and LATS2 are required for the normal development of the adrenal gland
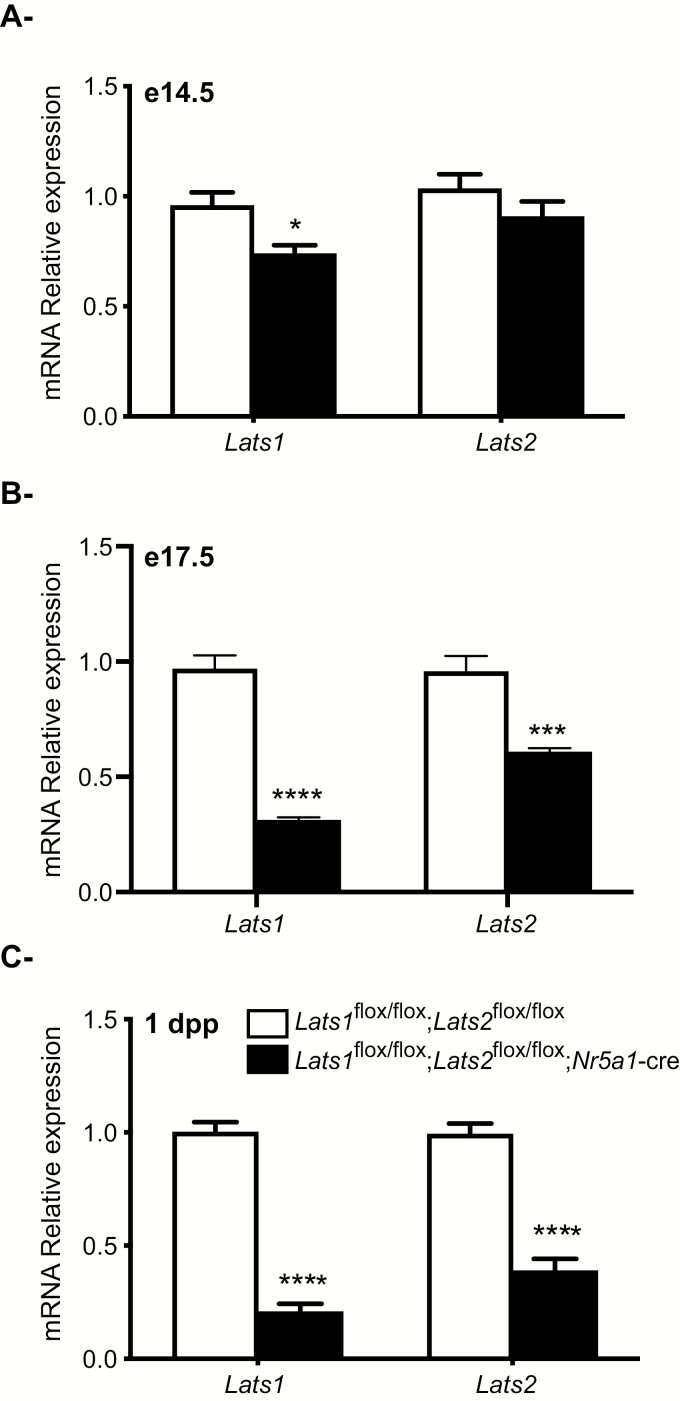
To analyse the onset and evolution of the phenotype observed in Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice, efficiency of the recombination in the developing adrenal gland was evaluated by RT-qPCR at embryonic days e14.5, e17.5, and 1 day postpartum (dpp). RT-qPCR analyses showed a small but significant reduction (23%) of adrenal Lats1 mRNA levels in e14.5 mutant animals (Fig. 2A), 68% and 36% decreases in adrenal Lats1 and Lats2 mRNA levels in e17.5 mutant animals (Fig. 2B), and 80% and 62% decreases in adrenal Lats1 and Lats2 mRNA levels in 1dpp mutant animals (Fig. 2C). These results suggest that the loss of Lats1 and Lats2 was progressive within the adrenal cortex and that recombination is initiated shortly before e14.5.
Figure 2.
Efficiency of Lats1 and Lats2 knockdown in Lats1flox/flox; Lats2flox/flox;Nr5a1-cre mice. (A-C) RT-qPCR analysis of Lats1 and Lats2 mRNA levels in the adrenal glands of (A) e14.5, (B) e17.5 and (C) 1 dpp male mice of the indicated genotypes (n = 6 animals/genotype). All data were normalized to the housekeeping gene Rpl19 and are expressed as means (columns) ± SEM (error bars). Asterisks = significantly different from control (* P < 0.05; *** P < 0.001; **** P < 0.0001).
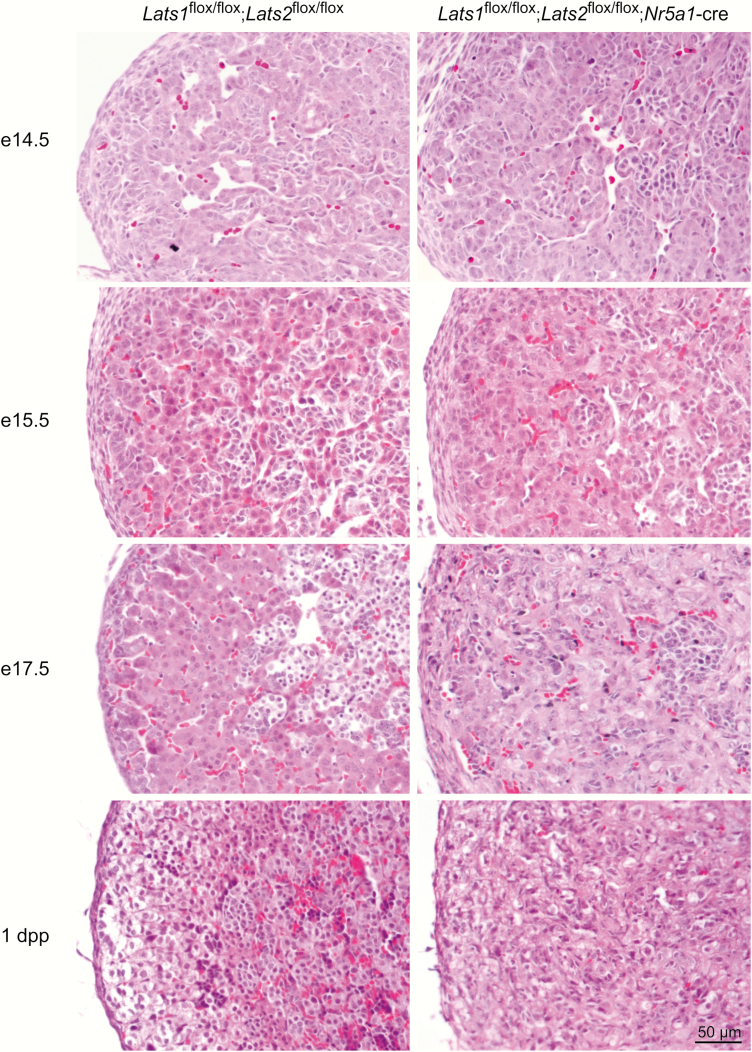
Consistent with the kinetics of the loss of Lats1/2 expression, adrenal glands from e14.5 and e15.5 Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice appeared phenotypically indistinguishable from age-matched controls (Fig. 3) including presence of medullary cells located at the center of the adrenal gland. By e17.5 forward, the adrenal cortex was severely compromised, and no zonation was apparent (Fig. 3). Cells with a large nucleus and an eosinophilic cytoplasm and spindle-shaped cells were observed in the adrenal cortex (Fig. 3 and (31, 36)), as observed in the 2-week-old animals. However, the spindle-shaped cells were less abundant than in the 2-week-old animals. Also similar to what was observed in 2-week-old animals, medullary cells were less abundant, and some medullary cells were observed at the periphery of the adrenal gland (36).
Figure 3.
Progressive appearance of spindle-shaped cells in the adrenal cortex of Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice. Photomicrographs comparing male adrenal gland histology of Lats1flox/flox;Lats2flox/flox;Nr5a1-cre with that of Lats1flox/flox;Lats2flox/flox controls at the indicated ages. Scale bar (lower right) is valid for all images. Hematoxylin and eosin stain.
To characterize the mutant adrenocortical cells, apoptosis and proliferation were evaluated in the developing adrenal gland. Interestingly, no apoptotic cells were observed in the adrenal glands of either control or mutant animals at e14.5 (36) and very rarely were cells observed in the adrenal glands of either control or mutant animals at e17.5 (36). BrdU incorporation assays showed that, in control e17.5 mice, only a few adrenocortical cells were proliferating in the outer cortex, and medullary cells were also proliferating (36). In contrast, proliferative cells were more scattered throughout the cortex in the adrenal glands of Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice, and most of the proliferating cells had round nuclei, whereas the spindled-shaped cells were mostly nonproliferative. Together, the absence of an increase in apoptosis and the absence of proliferation of the spindled-shaped cells suggested that the adrenocortical cells had transdifferentiated into the spindled-shaped cells.
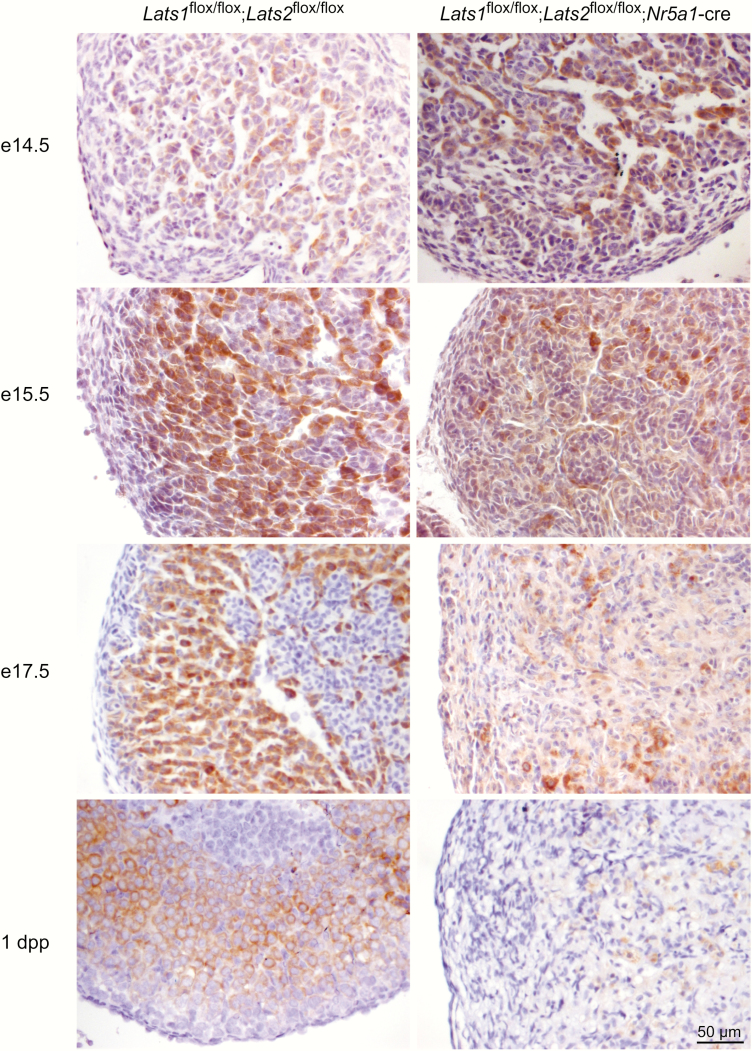
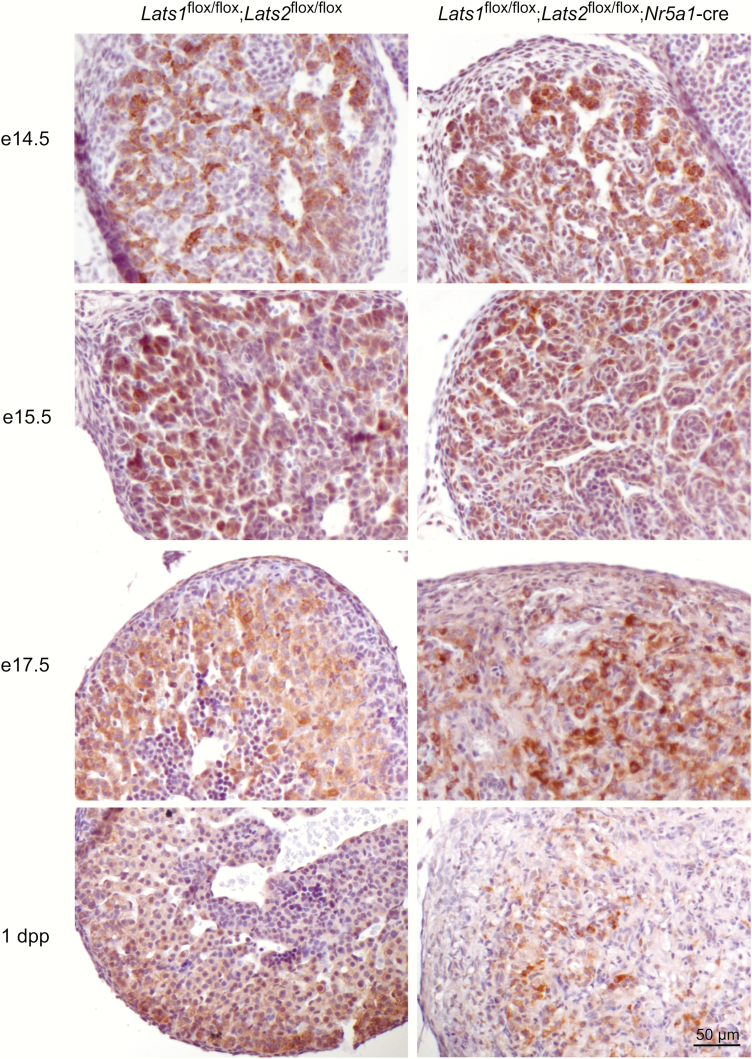
To further evaluate the fate of the adrenocortical cells in the developing adrenal glands of Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice, steroidogenic activity was evaluated by CYP11B1 and STAR immunohistochemistry. At e14.5, the expression of CYP11B1 expression was indistinguishable between control and mutant animals (Fig. 4). At e15.5, the number of CYP11B1+ cells was already considerably reduced (Fig. 4) in the adrenal cortex of Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice, despite being morphologically similar to the adrenal cortex of control animals (Fig. 3). By e17.5, expression of CYP11B1 was only detected in a few cells scattered throughout the adrenal cortex of Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice (Fig 4). Similar to what was observed for CYP11B1, a progressive decrease in STAR+ cells was observed in the adrenal cortex of the Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice. However, this decrease was delayed compared with CYP11B1, as a large proportion of adrenocortical cells still expressed STAR at e17.5 (Fig. 5), and most STAR expression was then gone by 1dpp. These differences in the expression patterns of Cyp11b1 and Star were also observed by RT-qPCR (36). Furthermore, mRNA levels of every steroidogenic genes evaluated were also progressively and severely reduced in the adrenal gland of mutant animals (36). Taken together, these results suggests that the loss of differentiated cell function accompanies the loss of differentiated cell morphology.
Figure 4.
Reduction of CYP11B1 expression in the adrenal cortex of Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice during development. Immunohistochemical analysis of CYP11B1 expression in adrenal glands from e14.5, e15.5, e17.5 and 1 dpp male mice of the indicated genotypes. Scale bar (lower right) is valid for all images.
Figure 5.
Progressive reduction of STAR expression in the adrenal cortex of Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice during development. Immunohistochemical analysis of STAR expression in adrenal glands from e14.5, e15.5, e17.5 and 1 dpp male mice of the indicated genotypes. Scale bar (lower right) is valid for all images.
Loss of hippo signaling causes adrenocortical cells to commit to a myofibroblast-like cell fate
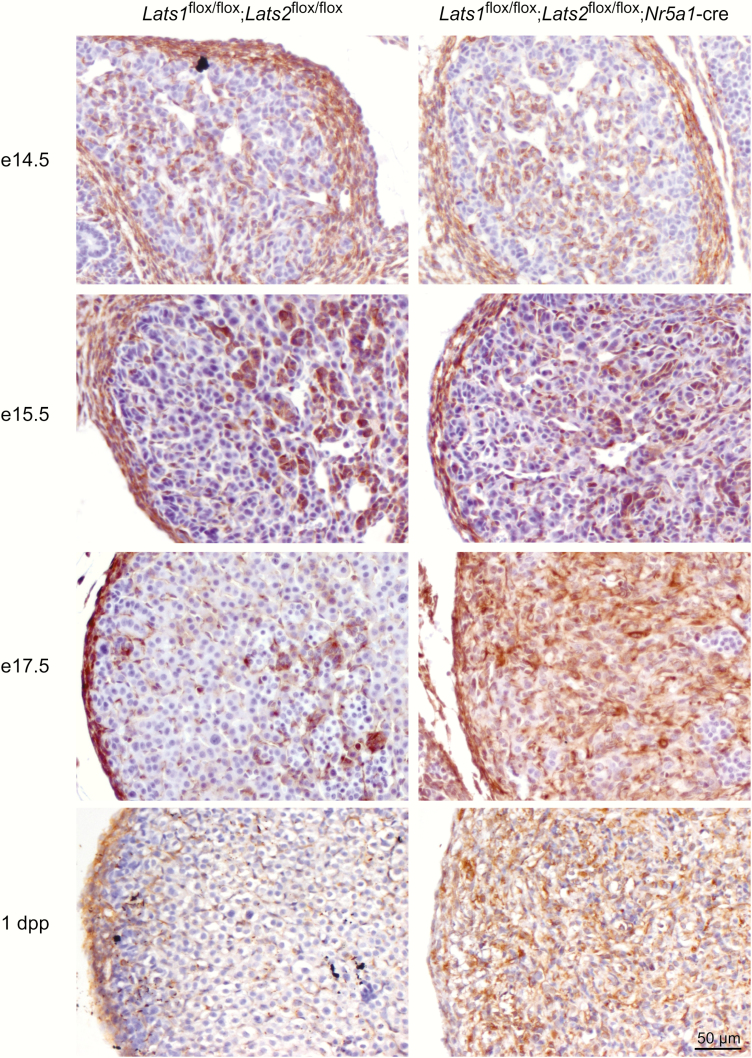
As mentioned above, the spindle-shaped cells observed in the adrenal cortex of the mutant animals were reminiscent of fibroblasts or myofibroblasts. To determine the identity of these cells, adrenal glands of Lats1flox/flox;Lats2flox/flox;Nr5a1-cre were first analyzed for expression of the mesenchymal cell marker vimentin (31). Vimentin was detected in the capsular cells in both control and mutant animals throughout development (Fig. 6). At e14.5, expression of vimentin was similar between the control and the mutant animals, and was predominantly expressed in the medullary cell population at the center of the adrenal gland, with weak expression detected in a few adrenocortical cells and in endothelial cells (Fig. 6). A similar pattern was observed at e15.5; however, a few spindle-shaped cells (Fig. 6, arrow) in the adrenal cortex of Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice also appeared to express vimentin more strongly (Fig. 6). By e17.5, vimentin expression was still detected in scattered adrenocortical cells, as well as in vascular and connective tissues in control animals. However, a marked increase in vimentin expression was observed in the adrenal cortex of Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice (Fig. 6).
Figure 6.
Progressive increase of vimentin expression in the adrenal cortex of Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice during development. Immunohistochemical analysis of vimentin expression in adrenal glands from e14.5, e15.5, e17.5 and 1 dpp male mice of the indicated genotypes. Scale bar (lower right) is valid for all images.
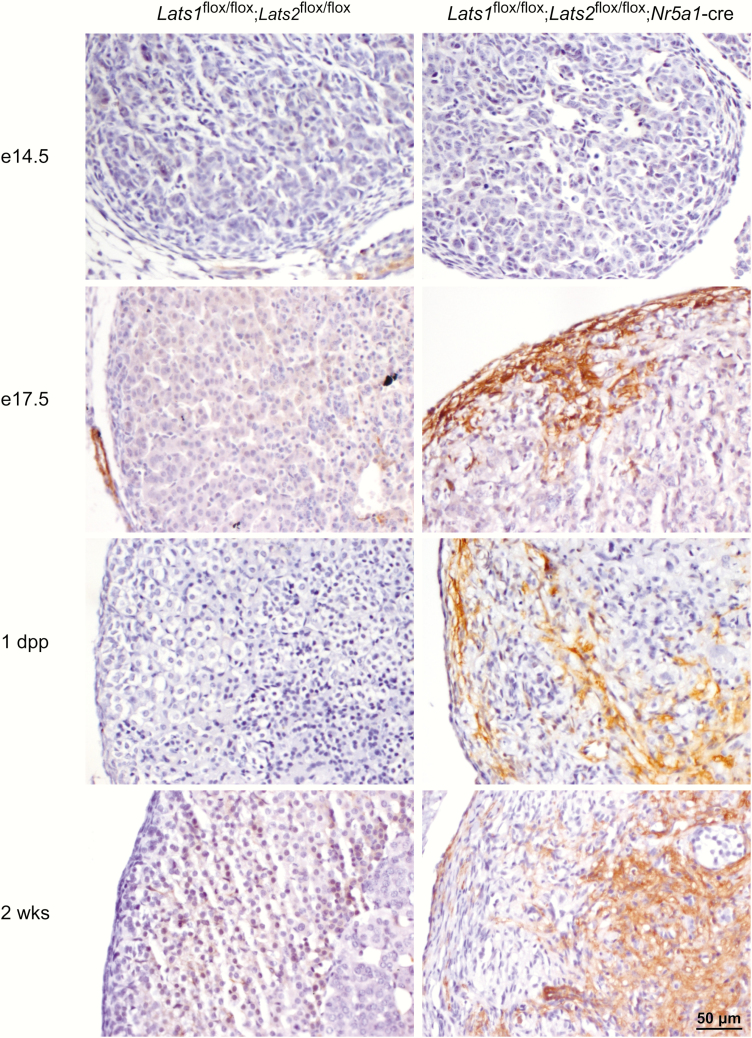
To determine if the cells present in the adrenal cortex of the mutant animals further differentiated into myofibloblasts, alpha smooth muscle actin (α-SMA) expression was evaluated by immunohistochemistry. α-SMA was not detected in the adrenal cortex of control animals (Fig. 7). Conversely, in the adrenal gland of Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice, α-SMA expression was detected in an increasing number of adrenocortical cells, beginning in the subcapsular region at e17.5 (Fig. 7), followed by the center of the adrenal gland in 1-day-old and 2-week-old animals (Fig. 7). This suggests that some cells of the adrenal cortex progressively differentiate into myofibroblast-like cells. Furthermore, an increase in the mRNA levels of actin, alpha 2, smooth muscle, aorta (Acta2, the gene coding for α-SMA), and of the fibrosis/myofibroblast markers caldesmon 1 (Cald1), calponin 1, basic, smooth muscle (Cnn1), snail family zinc finger 1 (Snai1), and secreted phosphoprotein 1 (Spp1) (36) was observed in the adrenal gland of mutant animals. Finally, an accumulation of collagen fibers in the adrenal cortex of mutant animals (36) further confirmed that some adrenocortical cells of the mutant mice acquired features of myofibroblasts, leading to tissue fibrosis. Interestingly, the number of α-SMA-positive cells was more restricted than the pattern of expression of vimentin-positive cells, even in older animals, suggesting either that some fibroblast-like cells remained undifferentiated or differentiated into additional cell types.
Figure 7.
Progressive increase of α-SMA expression in a subpopulation of adrenocortical cells in Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice. Immunohistochemical analysis of α-SMA expression in adrenal glands from e14.5, e17.5, 1 dpp and 12 dpp male mice of the indicated genotypes. Scale bar (lower right) is valid for all images.
Loss of Lats1 and Lats2 causes an increase in the expression of YAP and TAZ downstream transcriptional targets
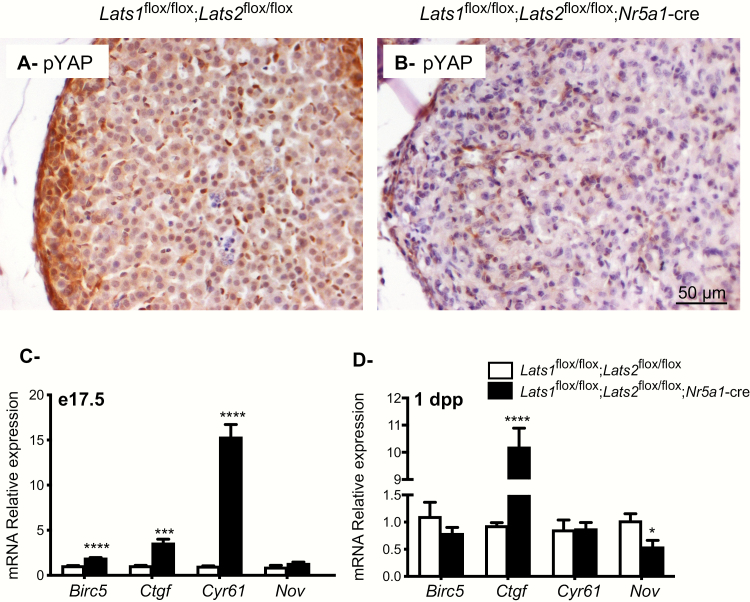
To determine whether the loss of Lats1 and Lats2 lead to the inactivation of Hippo signaling in the adrenal glands of Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice, the phosphorylation of YAP (a main LATS1/2 kinase substrate) was first evaluated by immunohistochemistry. Phospho-YAP was readily detected in the adrenal cortex of control animals at e17.5, with the subcapsular region showing the strongest expression (Fig. 8A). However, phospho-YAP expression was only detected in a few cells in the adrenal cortex of the mutant animals (Fig. 8B), confirming Hippo signaling inactivation. Because the phosphorylation of YAP (and of the functionally redundant TAZ) is normally associated with a decrease in their transcriptional co-activator activity, mRNA levels of the well-established YAP/TAZ target genes Baculoviral IAP repeat containing 5 (Birc5), connective tissue growth factor (Ctgf), cysteine-rich, angiogenic inducer 61 (Cyr61) and nephroblastoma overexpressed (Nov) were quantified by RT-qPCR. A 2-fold, 3-fold, and 15-fold increase, respectively, was observed for Birc5, Ctgf, and Cyr61 in the adrenal cortex of Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice at e17.5, whereas expression of Nov was not modified at this age (Fig. 8C). Conversely, only the expression of Ctgf was strongly increased (12-fold) in the adrenals of newborn mutant animals, and a slight decrease in the expression of Nov was observed (Fig. 8D), confirming that Hippo signaling was inactivated in the adrenal gland of the mutant animals.
Figure 8.
Lats1 and Lats2 deletion causes a decrease in the phosphorylation of YAP and an increase in the expression of YAP and TAZ downstream target genes in Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice. (A, B) Immunohistochemical analysis of phospho-YAP expression in adrenal glands from e17.5 dpp male mice of the indicated genotypes. Scale bar in B is valid for all images. (C, D) RT-qPCR analysis of Hippo target genes mRNA levels in the adrenal glands of (C) e17.5 and (D) 1 dpp male mice of the indicated genotypes (n = 6 animals/genotype). All data were normalized to the housekeeping gene Rpl19 and are expressed as means (columns) ± SEM (error bars). Asterisks = significantly different from control (* P < 0.05; *** P < 0.001; **** P < 0.0001).
To further characterize the expression pattern of YAP and TAZ in the adrenal gland of the mutant animals, immunohistochemistry analyses for YAP and TAZ were also performed on adrenal glands from e17.5 and 1dpp male mice. At both time points, strong YAP expression was detected in the nuclei of capsular and adrenocortical cells of the control animals (36). Strong expression of YAP was also detected in the nuclei of mesenchymal cells present in the adrenal cortex of Lats1flox/flox;Lats2flox/flox;Nr5a1-cre animals (36) at both time points. TAZ was also detected in the nucleus of the capsular cells of the control animals (36). TAZ was detected in the nuclei of adrenocortical cells of control mice at 1dpp (36), but was mostly expressed in the cytoplasm of the adrenocortical cells at e17.5, with the strongest expression being detected in the subcapsular region of the adrenal cortex at e17.5 (36). In the mutant group, strong cytoplasmic and weak nuclear expression of TAZ was detected in the majority of the adrenocortical cells at e17.5 (36). However, by 1dpp, strong nuclear expression of TAZ was also detected in the nuclei of the majority of the adrenocortical cells (36).
Taken together, these data suggest that YAP/TAZ transcriptional co-regulatory activity is increased in the adrenal cortex of Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice and could be involved in the transdifferentiation of adrenocortical cells into myofibroblast-like cells.
Discussion
In recent years, Hippo signaling has been identified as one of the most important signaling pathways involved in tissue development; however, no study has evaluated its function in the development of the adrenal cortex. Here we report that the inactivation of Lats1 and Lats2, the 2 core kinases of the Hippo pathway, leads to the transdifferentiation of adrenocortical cells into myofibroblast-like cells.
Two events could potentially explain the differentiation of the adrenocortical cells into myofibroblast-like cells in the adrenal cortex of the Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice. First, due to the rapid appearance of the spindle-shaped cells, it seems likely that the steroidogenic cells of the adrenal cortex progressively transdifferentiated into the myofibroblast-like cells. The fact that a marked increase in vimentin expression was first observed at a time when STAR but not CYP11B1 was still expressed in numerous cells tends to favor this hypothesis. Interestingly, an increase in the mRNA levels of Snai1, a gene involved in fibrosis and the adoption of the myofibroblast cell fate (37-39) was also observed in the adrenal gland of mutant animals. Increases in vimentin (40) and Snai1 (37, 41) expression are hallmarks of epithelial-to-mesenchymal transition (EMT); a process by which polarized epithelial cells gain mesenchymal properties (42), and that can eventually lead to differentiation into myofibroblasts and fibrosis in late stages of pathological conditions (43-46). Interestingly, the adrenal cortex shares common characteristics with epithelia and expresses numerous epithelial markers such as cytokeratins, laminin I, collagen IV and laminin-associated integrin subunits (47-50). The increases in vimentin and in the mRNA levels of Snai1 suggest that the loss of Hippo signaling could induce the transdifferentiation of the adrenocortical cells and subsequent fibrosis by a process having similitudes to EMT. Interestingly, it was recently suggested that EMT-like processes including increased in vimentin and Snai1 expression could be determinants of the malignancy of adrenocortical carcinoma (51, 52) and childhood-onset adrenocortical tumors (52). Furthermore, suppression of Hippo signaling and increased YAP/TAZ activity play a role in EMT/metastasis of some tumor cells (53, 54), regulate Snai1 expression (55, 56), and are a hallmark of fibrosis in several tissues including the liver (57), the lung (58), the kidney (59, 60), the heart (61), and the Müllerian duct (62). The transdifferentiation of the adrenocortical cells in the Lats1flox/flox;Lats2flox/flox;Nr5a1-cre model could therefore share certain characteristics of these processes.
A second possibility that could explain the phenotype observed in Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice is that Hippo signaling could normally direct a subset of mesenchymal stem cells (migrating from the capsule to form the definitive cortex) towards a steroidogenic cell fate (63, 64), and that the loss of Lats1 and Lats2 interferes with this process. Interestingly, it was shown that, in the developing kidney, the loss of Lats1 and Lats2 in mesenchymal progenitor cells impairs their differentiation into cells of the nephron and induces their differentiation into myofibroblasts (60), suggesting that loss of Lats1 and Lats2 could also impair the differentiation of adrenal stem cells during adrenal development. The fact that α-SMA expression is first specifically detected in the subcapsular region rather than being randomly distributed in the adrenal cortex also suggests that improper differentiation of the mesenchymal stem cells could be involved in the development of the phenotype observed in the Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice. Our model does not permit us to distinguish between these 2 possibilities, and lineage tracing experiments (63, 64) for adrenal cells and capsular stem cells within the Lats1flox/flox;Lats2flox/flox;Nr5a1-cre model would be needed to distinguish between them. However, it seems likely that both processes are involved in the appearance of the myofibroblast-like cells in the adrenal cortex.
As mentioned above, an increase in YAP and TAZ activity has been associated with fibrosis in numerous tissues. It therefore seems likely that an increase in their activity is involved in the transdifferentiation of the adrenocortical cells. Aside from the increase in vimentin and the mRNA levels of Snai1, an increase in the mRNA levels of well-established transcriptional target of YAP and TAZ (65-67) was also observed in the adrenal glands of Lats1flox/flox;Lats2flox/flox;Nr5a1-cre mice. Interestingly, Cyr61 mRNA levels were increased in e17.5 animals, but this increase was not maintained in 1dpp animals. On the other hand, Ctgf mRNA levels continued to be upregulated in 1dpp animals. This suggests that a different set of genes could be regulated by YAP/TAZ depending of the stage of differentiation of the adrenocortical cells. Interestingly, it was previously shown that CYR61 promotes EMT in various tumors (68, 69), and that mRNA levels of Cyr61 increase in profibrogenic liver cells, but decline in transdifferentiated myofibroblasts (70). It was also shown that CYR61 promotes the regression of fibrosis by inducting the senescence of myofibroblasts in the liver and the heart (70-72), suggesting that CYR61 is detrimental for myofibroblast formation. On the other hand, CTGF, which remains strongly expressed in the adrenocortical cells, has been previously described as a key driver of fibrosis (73, 74) and myofibroblast formation (75, 76), suggesting that CTGF could drive the last stage of the adrenocortical cell differentiation. The mechanism whereby Hippo signaling regulates the transition from adrenocortical cells to myofibroblast cells was not evaluated in this study. However, Hippo is known to act in synergy with numerous signaling pathways, including the TGFβ (77-79) and WNT (21, 79-81) pathways, both of which can also regulate Ctgf expression (73, 74, 82-86). It is therefore possible that activation/modulation of the TGFβ and/or WNT pathways is involved in the progressive differentiation of the adrenocortical cells in the Lats1flox/flox;Lats2flox/flox;Nr5a1-cre model. Further experiments will be needed to evaluate the contribution of these pathways. Finally, though we believe that YAP/TAZ is the main driving force behind the observed phenotype, it is important to mention that it was recently shown that LATS1 and LATS2 could bind to other proteins such as estrogen receptor alpha (87) or polycomb repressive complex 2 (88) and act independently of Hippo signaling. It is therefore possible that actions of LATS1/2 on proteins other than YAP/TAZ could also contribute to the observed phenotype. Generation of a Lats1flox/flox;Lats2flox/flox;Yapfloxflox;Tazfloxflox;Nr5a1-cre mouse model will be needed to determine if YAP and TAZ stabilization is solely responsible for the observed phenotype.Another finding in the Lats1flox/flox;Lats2flox/flox;Nr5a1-cre model was that fewer chromaffin cells were present in the adrenal gland, and that they were either scattered throughout the cortex or localized to the periphery of the adrenal gland. Loss of medullary cell growth (89, 90) and their ectopic localization outside of the capsule (16, 91-93) have previously been observed in different genetic mouse models with adrenal cortex development defects, leading several authors to propose that the adrenal cortex is essential for the growth of the medullary cells and their correct localization at the center of the adrenal gland (94, 95). The ectopic positioning of the medullary cells in the Lats1flox/flox;Lats2flox/flox;Nr5a1-cre model and the absence of proliferation of the medullary cells that were able to migrate to the center of the adrenal gland suggests that the transdifferentiated adrenocortical cells are either unable to secrete factors necessary for the migration and/or growth of the medullary cells or, more likely, do not provide the proper structural support for their development.
In summary, we report here a previously unsuspected role of the Hippo pathway in the development of the adrenal cortex, as loss of Lats1/2 causes the developing adrenocortical cells to commit to a myofibroblast-like cell fate. Further studies will be required to define the mechanism of action of Hippo signaling throughout the development of the adrenal gland and its potential role in adrenal gland physiology.
Acknowledgments
The authors would like to thank Manon Salvas for technical assistance, Dr Celso Gomez-Sanchez (University of Mississipi, Medical Center, Jackson, MS) for generously providing the CYP11B1 antibody, Dr Randy L. Johnson (M.D. Anderson Cancer Center, Houston Tx) for generously providing the Lats1/2 floxed mice and mice and Dr Antoine Martinez (Université Clermont-Auvergne, Clermont-Ferrand, France) for letting Adrien Levasseur carry out the immunofluorescence for 20αHSD in his laboratory.
Financial Support: This work was supported by Discovery Grant RGPIN-2014–04358 (to A.B.) from the Natural Sciences and Engineering Research Council of Canada (NESRC). The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development (Specialized Cooperative Centers Program in Reproduction and Infertility Research) Grant P50-HD28934.
Glossary
Abbreviations
- 20αHSD
20-alpha-hydroxysteroid dehydrogenase
- α-SMA
alpha smooth muscle actin
- ACTH
adrenocorticotropic hormone
- AGP
adrenogonadal primordium
- BrdU
bromodeoxyuridine
- dpp
day postpartum
- CTGF
connective tissue growth factor
- e9.5
embryonic day 9.5
- EMT
epithelial-to-mesenchymal transition
- Lats1
large tumor suppressor homolog kinase 1
- Lats2
large tumor suppressor homolog kinase 2
- NR5A1
nuclear receptor subfamily 5, group A, member 1
- PCR
polymerase chain reaction
- SEM
standard error of the mean
- STAR
steroid acute regulatory protein
- TAZ
transcriptional coactivator with PDZ-binding motif
- YAP
Yes-associated protein
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated during this study are included in this article or in the data repositories listed in references.
References
- 1. Hatano O, Takakusu A, Nomura M, Morohashi K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells. 1996;1(7):663-671. [DOI] [PubMed] [Google Scholar]
- 2. Ikeda Y, Shen WH, Ingraham HA, Parker KL. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol. 1994;8(5):654-662. [DOI] [PubMed] [Google Scholar]
- 3. Morohashi K. The ontogenesis of the steroidogenic tissues. Genes Cells. 1997;2(2):95-106. [DOI] [PubMed] [Google Scholar]
- 4. Val P, Martinez-Barbera JP, Swain A. Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development. 2007;134(12):2349-2358. [DOI] [PubMed] [Google Scholar]
- 5. Anderson DJ, Axel R. A bipotential neuroendocrine precursor whose choice of cell fate is determined by NGF and glucocorticoids. Cell. 1986;47(6):1079-1090. [DOI] [PubMed] [Google Scholar]
- 6. Le Douarin NM, Teillet MA. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev Biol. 1974;41(1):162-184. [DOI] [PubMed] [Google Scholar]
- 7. Keegan CE, Hammer GD. Recent insights into organogenesis of the adrenal cortex. Trends Endocrinol Metab. 2002;13(5):200-208. [DOI] [PubMed] [Google Scholar]
- 8. Zubair M, Parker KL, Morohashi K. Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol Cell Biol. 2008;28(23):7030-7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morohashi K, Zubair M. The fetal and adult adrenal cortex. Mol Cell Endocrinol. 2011;336(1-2):193-197. [DOI] [PubMed] [Google Scholar]
- 10. Zubair M, Ishihara S, Oka S, Okumura K, Morohashi K. Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol Cell Biol. 2006;26(11):4111-4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tevosian SG, Jiménez E, Hatch HM, et al. Adrenal Development in Mice Requires GATA4 and GATA6 Transcription Factors. Endocrinology. 2015;156(7):2503-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luo X, Ikeda Y, Lala DS, Baity LA, Meade JC, Parker KL. A cell-specific nuclear receptor plays essential roles in adrenal and gonadal development. Endocr Res. 1995;21(1-2):517-524. [DOI] [PubMed] [Google Scholar]
- 13. Sadovsky Y, Crawford PA, Woodson KG, et al. Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci U S A. 1995;92(24):10939-10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pitetti JL, Calvel P, Romero Y, et al. Insulin and IGF1 receptors are essential for XX and XY gonadal differentiation and adrenal development in mice. Plos Genet. 2013;9(1):e1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim AC, Reuter AL, Zubair M, et al. Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008;135(15):2593-2602. [DOI] [PubMed] [Google Scholar]
- 16. King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci U S A. 2009;106(50):21185-21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dumontet T, Sahut-Barnola I, Septier A, et al. PKA signaling drives reticularis differentiation and sexually dimorphic adrenal cortex renewal. JCI Insight. 2018;3(2):1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maugeri-Saccà M, De Maria R. The Hippo pathway in normal development and cancer. Pharmacol Ther. 2018;186:60-72. [DOI] [PubMed] [Google Scholar]
- 19. Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94(4):1287-1312. [DOI] [PubMed] [Google Scholar]
- 20. Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141(8):1614-1626. [DOI] [PubMed] [Google Scholar]
- 21. Abduch RH, Carolina Bueno A, Leal LF, et al. Unraveling the expression of the oncogene YAP1, a Wnt/beta-catenin target, in adrenocortical tumors and its association with poor outcome in pediatric patients. Oncotarget. 2016;7(51):84634-84644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levasseur A, St-Jean G, Paquet M, Boerboom D, Boyer A. Targeted Disruption of YAP and TAZ Impairs the Maintenance of the Adrenal Cortex. Endocrinology. 2017;158(11):3738-3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dhillon H, Zigman JM, Ye C, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49(2):191-203. [DOI] [PubMed] [Google Scholar]
- 24. Heallen T, Zhang M, Wang J, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332(6028):458-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.RRID: AB_10013660, https://scicrunch.org/resolver/RRID:AB_10013660
- 26.RRID: AB_234188, https://antibodyregistry.org/search.php?q=AB_2341188
- 27.RRID: AB_2687896, https://scicrunch.org/resolver/RRID:AB_2687896
- 28.RRID: AB_2335623, https://scicrunch.org/resolver/RRID:AB_2335623
- 29.RRID: AB_2115937, https://scicrunch.org/resolver/RRID:AB_2115937
- 30.RRID: AB_1080602, https://scicrunch.org/resolver/RRID:AB_1080602
- 31.RRID: AB_671397, https://scicrunch.org/resolver/RRID:AB_671397
- 32.RRID: AB_2650491, https://scicrunch.org/resolver/RRID:AB_2650491
- 33.RRID: AB_2650553, https://scicrunch.org/resolver/AB_2650553
- 34.RRID: AB_10695149, https://scicrunch.org/resolver/RRID:AB_10695459
- 35. Hershkovitz L, Beuschlein F, Klammer S, Krup M, Weinstein Y. Adrenal 20alpha-hydroxysteroid dehydrogenase in the mouse catabolizes progesterone and 11-deoxycorticosterone and is restricted to the X-zone. Endocrinology. 2007;148(3):976-988. [DOI] [PubMed] [Google Scholar]
- 36. Ménard M, Abou Nader N, Levasseur A, et al. Data from: Targeted disruption of Lats1 and Lats2 in mice impairs adrenal cortex development and alters adrenocortical cell fate. Scholars Portal Dataverse Deposited 4 April 2020. https://dataverse.scholarsportal.info/dataset.xhtml?persistentId=doi:10.5683/SP2/XTKLTR [DOI] [PMC free article] [PubMed]
- 37. Grande MT, Sánchez-Laorden B, López-Blau C, et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med. 2015;21(9):989-997. [DOI] [PubMed] [Google Scholar]
- 38. Biswas H, Longmore GD. Action of SNAIL1 in Cardiac Myofibroblasts Is Important for Cardiac Fibrosis following Hypoxic Injury. Plos One. 2016;11(10):e0162636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scarpa M, Grillo AR, Brun P, et al. Snail1 transcription factor is a critical mediator of hepatic stellate cell activation following hepatic injury. Am J Physiol Gastrointest Liver Physiol. 2011;300(2):G316-G326. [DOI] [PubMed] [Google Scholar]
- 40. Battaglia RA, Delic S, Herrmann H, Snider NT. Vimentin on the move: new developments in cell migration. F1000Res. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Y, Shi J, Chai K, Ying X, Zhou BP. The Role of Snail in EMT and Tumorigenesis. Curr Cancer Drug Targets. 2013;13(9):963-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27(55):6958-6969. [DOI] [PubMed] [Google Scholar]
- 43. Masszi A, Speight P, Charbonney E, et al. Fate-determining mechanisms in epithelial-myofibroblast transition: major inhibitory role for Smad3. J Cell Biol. 2010;188(3):383-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yeung KT, Yang J. Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol. 2017;11(1):28-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stone RC, Pastar I, Ojeh N, et al. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016;365(3):495-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110(3):341-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Campbell S, Otis M, Côté M, Gallo-Payet N, Payet MD. Connection between integrins and cell activation in rat adrenal glomerulosa cells: a role for Arg-Gly-Asp peptide in the activation of the p42/p44(mapk) pathway and intracellular calcium. Endocrinology. 2003;144(4):1486-1495. [DOI] [PubMed] [Google Scholar]
- 48. Otis M, Campbell S, Payet MD, Gallo-Payet N. Expression of extracellular matrix proteins and integrins in rat adrenal gland: importance for ACTH-associated functions. J Endocrinol. 2007;193(3):331-347. [DOI] [PubMed] [Google Scholar]
- 49. Virtanen I, Korhonen M, Petäjäniemi N, et al. Laminin isoforms in fetal and adult human adrenal cortex. J Clin Endocrinol Metab. 2003;88(10):4960-4966. [DOI] [PubMed] [Google Scholar]
- 50. Miettinen M, Lehto VP, Virtanen I. Immunofluorescence microscopic evaluation of the intermediate filament expression of the adrenal cortex and medulla and their tumors. Am J Pathol. 1985;118(3):360-366. [PMC free article] [PubMed] [Google Scholar]
- 51. Agosta C, Laugier J, Guyon L, et al. MiR-483-5p and miR-139-5p promote aggressiveness by targeting N-myc downstream-regulated gene family members in adrenocortical cancer. Int J Cancer. 2018;143(4):944-957. [DOI] [PubMed] [Google Scholar]
- 52. Bulzico D, Faria PAS, Maia CB, et al. Is there a role for epithelial-mesenchymal transition in adrenocortical tumors? Endocrine. 2017;58(2):276-288. [DOI] [PubMed] [Google Scholar]
- 53. Xiao H, Jiang N, Zhou B, Liu Q, Du C. TAZ regulates cell proliferation and epithelial-mesenchymal transition of human hepatocellular carcinoma. Cancer Sci. 2015;106(2):151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Oh SH, Swiderska-Syn M, Jewell ML, Premont RT, Diehl AM. Liver regeneration requires Yap1-TGFβ-dependent epithelial-mesenchymal transition in hepatocytes. J Hepatol. 2018;69(2):359-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lu J, Yang Y, Guo G, et al. IKBKE regulates cell proliferation and epithelial-mesenchymal transition of human malignant glioma via the Hippo pathway. Oncotarget. 2017;8(30):49502-49514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhaojie L, Yuchen L, Miao C, et al. Gelsolin-like actin-capping protein has prognostic value and promotes tumorigenesis and epithelial-mesenchymal transition via the Hippo signaling pathway in human bladder cancer. Ther Adv Med Oncol. 2019;11:1758835919841235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mannaerts I, Leite SB, Verhulst S, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol. 2015;63(3):679-688. [DOI] [PubMed] [Google Scholar]
- 58. Zhao X, Sun J, Su W, et al. Melatonin Protects against Lung Fibrosis by Regulating the Hippo/YAP Pathway. Int J Mol Sci. 2018;19(4):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu F, Lagares D, Choi KM, et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2015;308(4):L344-L357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McNeill H, Reginensi A. Lats1/2 Regulate Yap/Taz to Control Nephron Progenitor Epithelialization and Inhibit Myofibroblast Formation. J Am Soc Nephrol. 2017;28(3):852-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xiao Y, Hill MC, Li L, et al. Hippo pathway deletion in adult resting cardiac fibroblasts initiates a cell state transition with spontaneous and self-sustaining fibrosis. Genes Dev. 2019;33(21-22):1491-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. St-Jean G, Tsoi M, Abedini A, et al. Lats1 and Lats2 are required for the maintenance of multipotency in the Mullerian duct mesenchyme. Development 2019;146(20):1-12. [DOI] [PubMed] [Google Scholar]
- 63. Wood MA, Acharya A, Finco I, et al. Fetal adrenal capsular cells serve as progenitor cells for steroidogenic and stromal adrenocortical cell lineages in M. musculus. Development. 2013;140(22):4522-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bandiera R, Vidal VP, Motamedi FJ, et al. WT1 maintains adrenal-gonadal primordium identity and marks a population of AGP-like progenitors within the adrenal gland. Dev Cell. 2013;27(1):5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hiemer SE, Zhang L, Kartha VK, et al. A YAP/TAZ-Regulated molecular signature is associated with oral squamous cell carcinoma. Mol Cancer Res. 2015;13(6):957-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cordenonsi M, Zanconato F, Azzolin L, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147(4):759-772. [DOI] [PubMed] [Google Scholar]
- 67. Thongon N, Castiglioni I, Zucal C, et al. The GSK3β inhibitor BIS I reverts YAP-dependent EMT signature in PDAC cell lines by decreasing SMADs expression level. Oncotarget. 2016;7(18):26551-26566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Huang X, Xiang L, Li Y, et al. Snail/FOXK1/Cyr61 signaling axis regulates the epithelial-mesenchymal transition and metastasis in colorectal cancer. Cell Physiol Biochem. 2018;47(2):590-603. [DOI] [PubMed] [Google Scholar]
- 69. Haque I, Mehta S, Majumder M, et al. Cyr61/CCN1 signaling is critical for epithelial-mesenchymal transition and stemness and promotes pancreatic carcinogenesis. Mol Cancer. 2011;10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Borkham-Kamphorst E, Schaffrath C, Van de Leur E, et al. The anti-fibrotic effects of CCN1/CYR61 in primary portal myofibroblasts are mediated through induction of reactive oxygen species resulting in cellular senescence, apoptosis and attenuated TGF-β signaling. Biochim Biophys Acta. 2014;1843(5):902-914. [DOI] [PubMed] [Google Scholar]
- 71. Kim KH, Chen CC, Monzon RI, Lau LF. Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts. Mol Cell Biol. 2013;33(10):2078-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Meyer K, Hodwin B, Ramanujam D, Engelhardt S, Sarikas A. Essential Role for premature senescence of myofibroblasts in myocardial fibrosis. J Am Coll Cardiol. 2016;67(17):2018-2028. [DOI] [PubMed] [Google Scholar]
- 73. Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5(Suppl 1):S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sakai N, Nakamura M, Lipson KE, et al. Inhibition of CTGF ameliorates peritoneal fibrosis through suppression of fibroblast and myofibroblast accumulation and angiogenesis. Sci Rep. 2017;7(1):5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Garrett Q, Khaw PT, Blalock TD, Schultz GS, Grotendorst GR, Daniels JT. Involvement of CTGF in TGF-beta1-stimulation of myofibroblast differentiation and collagen matrix contraction in the presence of mechanical stress. Invest Ophthalmol Vis Sci. 2004;45(4):1109-1116. [DOI] [PubMed] [Google Scholar]
- 76. Grotendorst GR, Rahmanie H, Duncan MR. Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. Faseb J. 2004;18(3):469-479. [DOI] [PubMed] [Google Scholar]
- 77. Grannas K, Arngården L, Lönn P, et al. Crosstalk between Hippo and TGFβ: Subcellular Localization of YAP/TAZ/Smad Complexes. J Mol Biol. 2015;427(21):3407-3415. [DOI] [PubMed] [Google Scholar]
- 78. Szeto SG, Narimatsu M, Lu M, et al. YAP/TAZ Are Mechanoregulators of TGF-β-Smad Signaling and Renal Fibrogenesis. J Am Soc Nephrol. 2016;27(10):3117-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Attisano L, Wrana JL. Signal integration in TGF-β, WNT, and Hippo pathways. F1000prime Rep. 2013;5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Varelas X, Miller BW, Sopko R, et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010;18(4): 579-591. [DOI] [PubMed] [Google Scholar]
- 81. Azzolin L, Panciera T, Soligo S, et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158(1):157-170. [DOI] [PubMed] [Google Scholar]
- 82. Liu Y, Liu H, Meyer C, et al. Transforming growth factor-β (TGF-β)-mediated connective tissue growth factor (CTGF) expression in hepatic stellate cells requires Stat3 signaling activation. J Biol Chem. 2013;288(42):30708-30719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Parada C, Li J, Iwata J, Suzuki A, Chai Y. CTGF mediates Smad-dependent transforming growth factor β signaling to regulate mesenchymal cell proliferation during palate development. Mol Cell Biol. 2013;33(17):3482-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hiyama A, Morita K, Sakai D, Watanabe M. CCN family member 2/connective tissue growth factor (CCN2/CTGF) is regulated by Wnt-β-catenin signaling in nucleus pulposus cells. Arthritis Res Ther. 2018;20(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jiang L, Yamashita Y, Chew SH, et al. Connective tissue growth factor and β-catenin constitute an autocrine loop for activation in rat sarcomatoid mesothelioma. J Pathol. 2014;233(4):402-414. [DOI] [PubMed] [Google Scholar]
- 86. Piersma B, Bank RA, Boersema M. Signaling in Fibrosis: TGF-beta, WNT, and YAP/TAZ Converge. Front Med. 2015;2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Britschgi A, Duss S, Kim S, et al. The Hippo kinases LATS1 and 2 control human breast cell fate via crosstalk with ERα. Nature. 2017;541(7638):541-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Torigata K, Daisuke O, Mukai S, et al. LATS2 Positively Regulates Polycomb Repressive Complex 2. Plos One. 2016;11(7):e0158562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bland ML, Fowkes RC, Ingraham HA. Differential requirement for steroidogenic factor-1 gene dosage in adrenal development versus endocrine function. Mol Endocrinol. 2004;18(4):941-952. [DOI] [PubMed] [Google Scholar]
- 90. Gut P, Huber K, Lohr J, et al. Lack of an adrenal cortex in Sf1 mutant mice is compatible with the generation and differentiation of chromaffin cells. Development. 2005;132(20):4611-4619. [DOI] [PubMed] [Google Scholar]
- 91. Huang CC, Liu C, Yao HH. Investigating the role of adrenal cortex in organization and differentiation of the adrenal medulla in mice. Mol Cell Endocrinol. 2012;361(1-2):165-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pihlajoki M, Gretzinger E, Cochran R, et al. Conditional mutagenesis of Gata6 in SF1-positive cells causes gonadal-like differentiation in the adrenal cortex of mice. Endocrinology. 2013;154(5):1754-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lee FY, Faivre EJ, Suzawa M, et al. Eliminating SF-1 (NR5A1) sumoylation in vivo results in ectopic hedgehog signaling and disruption of endocrine development. Dev Cell. 2011;21(2):315-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Huber K. The sympathoadrenal cell lineage: specification, diversification, and new perspectives. Dev Biol. 2006;298(2):335-343. [DOI] [PubMed] [Google Scholar]
- 95. Parlato R, Otto C, Tuckermann J, et al. Conditional inactivation of glucocorticoid receptor gene in dopamine-beta-hydroxylase cells impairs chromaffin cell survival. Endocrinology. 2009;150(4):1775-1781. [DOI] [PubMed] [Google Scholar]