Abstract
Background
Telerehabilitation, an emerging method, extends rehabilitative care beyond the hospital, and facilitates multifaceted, often psychotherapeutic approaches to modern management of patients using telecommunication technology at home or in the community. Although a wide range of telerehabilitation interventions are trialed in persons with multiple sclerosis (pwMS), evidence for their effectiveness is unclear.
Objectives
To investigate the effectiveness and safety of telerehabilitation intervention in pwMS for improved patient outcomes. Specifically, this review addresses the following questions: does telerehabilitation achieve better outcomes compared with traditional face‐to‐face intervention; and what types of telerehabilitation interventions are effective, in which setting and influence which specific outcomes (impairment, activity limitation and participation)?
Search methods
We performed a literature search using the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Review Group Specialised Register( 9 July, 2014.) We handsearched the relevant journals and screened the reference lists of identified studies, and contacted authors for additional data.
Selection criteria
Randomised controlled trials (RCTs) and controlled clinical trials (CCTs) that reported telerehabilitation intervention/s in pwMS and compared them with some form of control intervention (such as lower level or different types of intervention, minimal intervention, waiting‐list controls or no treatment (or usual care); interventions given in different settings) in adults with MS.
Data collection and analysis
Two review authors independently selected studies and extracted data. Three review authors assessed the methodological quality of studies using the GRADEpro software (GRADEpro 2008) for best‐evidence synthesis. A meta‐analysis was not possible due to marked methodological, clinical and statistical heterogeneity between included trials and between measurement tools used. Hence, we performed a best‐evidence synthesis using a qualitative analysis.
Main results
Nine RCTs, one with two reports, (N = 531 participants, 469 included in analyses) investigated a variety of telerehabilitation interventions in adults with MS. The mean age of participants varied from 41 to 52 years (mean 46.5 years) and mean years since diagnosis from 7.7 to 19.0 years (mean 12.3 years). The majority of the participants were women (proportion ranging from 56% to 87%, mean 74%) and with a relapsing‐remitting course of MS. These interventions were complex, with more than one rehabilitation component and included physical activity, educational, behavioural and symptom management programmes.
All studies scored 'low' on the methodological quality assessment. Overall, the review found 'low‐level' evidence for telerehabilitation interventions in reducing short‐term disability and symptoms such as fatigue. There was also 'low‐level' evidence supporting telerehabilitation in the longer term for improved functional activities, impairments (such as fatigue, pain, insomnia); and participation measured by quality of life and psychological outcomes. There were limited data on process evaluation (participants'/therapists' satisfaction) and no data available for cost effectiveness. There were no adverse events reported as a result of telerehabilitation interventions.
Authors' conclusions
There is currently limited evidence on the efficacy of telerehabilitation in improving functional activities, fatigue and quality of life in adults with MS. A range of telerehabilitation interventions might be an alternative method of delivering services in MS populations. There is insufficient evidence to support on what types of telerehabilitation interventions are effective, and in which setting. More robust trials are needed to build evidence for the clinical and cost effectiveness of these interventions.
Keywords: Adult, Humans, Middle Aged, Telemedicine, Multiple Sclerosis, Multiple Sclerosis/rehabilitation, Randomized Controlled Trials as Topic, Treatment Outcome
Plain language summary
Telerehabilitation for persons with multiple sclerosis
Review questions
Does telerehabilitation achieve better outcomes in persons with multiple sclerosis compared with traditional face‐to‐face intervention? What types of telerehabilitation interventions are effective, in which setting and influence which specific outcomes?
Background
Multiple sclerosis (MS) is a common disease of the nervous system among young adults, with no cure and causing long‐term disability. Rehabilitation provides treatments and therapies to lessen the impact of any disability and improve function. Despite recent advances in MS care including rehabilitation, many people with MS are unable to access these developments due to limited mobility, fatigue and related issues, and costs associated with travel.Telerehabilitation is a newer approach to delivering rehabilitation programmes at the patient’s home or in the community, using telecommunication technology such as phone lines, video technology, internet applications and others. A wide range of telerehabilitation interventions are trialed in persons with multiple sclerosis, however, evidence for their effectiveness is still unclear.
Study characteristics
This review looked for evidence on how telerehabilitation interventions work in adults with MS. We searched widely for randomised controlled trials (RCTs), a particular kind of study where participants are placed in treatment groups by chance (that is, randomly) because in most settings these provide the highest quality evidence. We were interested in studies that compared a telerehabilitation programme with standard or minimal care, or with different kinds of rehabilitation programmes.
Key results
We found nine relevant RCTs covering 531 participants (469 included in the analyses), evaluating a wide variety of telerehabilitation interventions in persons with MS. The telerehabilitation interventions evaluated were complex, with more than one rehabilitation component and included physical activity, educational, behavioural and symptom management programmes. These interventions had different purposes and used different technologies, so a single overall definite conclusion was not possible. The methodological quality of the included studies is low and varied among the studies.
Quality of evidence
There was 'low‐quality' evidence from the included RCTs to support the benefit of telerehabilitation in reducing short‐term disability and managing symptoms such as fatigue in adults with MS. We found limited evidence to support the benefit of telerehabilitation interventions in improving disability, reducing symptoms and improving quality of life in the longer term. Furthermore, the interventions and outcomes being investigated in the included studies were different to each other. No studies reported any serious harm from telerehabilitation and there was no information on the associated costs.
There is a need for further research to assess the effects of the range of telerehabilitation techniques and to establish the clinical and cost effectiveness of these interventions in people with MS. The evidence in this review is up to date to July 2014.
Summary of findings
for the main comparison.
| Telerehabilitation for persons with multiple sclerosis | |||
|
Patient or population: People with multiple sclerosis Settings: Participants' home, MS regional centres Intervention: Telerehabilitation Comparison: Standard care in rehabilitation centres, participants in wait‐list, other type/intensity of rehabilitation intervention |
|||
| Outcomes | No of Participants (studies) | Effect of telerehabilitation interventions for people with multiple sclerosis | Quality of the evidence (GRADE) # |
| Change in functional activity | |||
| Change in disability directly post‐intervention Measures: GLTEQ, DGI, BBS, ARAT, NHPT, 25FWT, CES, VPR Follow‐up: depended on the type of intervention; range from (1 month – 12 weeks) | 232 (intervention group = 122) (6 studies) | Two studies (Dlugonski 2012; Motl 2011, N = 99) with same cohort of participants showed significant improvement in physical activity in the treatment group at post‐intervention assessment as measured by GLTEQ (P < 0.01). Weekly step count (pedometer) increased significantly in the treatment group at post‐intervention assessment (P < 0.001) One study (Frevel 2014, N = 18) showed significant improvement in dynamic and static balance capacity compared to baseline values in both intervention group (e‐training) (DGI: P = 0.016, BBS: P = 0.011) and control (hippotherapy) group (DGI: P = 0.011, BBS: P = 0.011). There was no difference between groups One study (Huijgen 2008, N = 35) showed no statistically significant differences between intervention and control groups in arm function as measured by ARAT (mean change 1.26, 90% CI ‐1.90 to 4.42) and NHPT (mean change 7.24, 90% CI ‐6.55 to 23.25) One study (Paul 2014, N = 30) showed that gait speed measured using 25FWT increased in the intervention group compared to the control group but this was not statistically significant (P = 0.170); and the intervention group showed a statistically significant improvement in the physical subscale of the MSIS (P = 0.048) One study (Gutíerrez 2013a, N = 50) showed improvements in balance and postural control, with a significant increase in CES of the intervention group (mean change; 8.21 points, P < 0.001), but no significant improvement in the control group (mean change: 1.93, P = 0.123). Visual Preference Ratio (VPR) and the contribution of vestibular information (Vestibular Ratio) improved significantly in the intervention group (P < 0.001), but not in the control group (P > 0.05). There were significant post‐treatment differences between treatment and control groups in the CES (F = 37.873, P < 0.001) and the VPR (F = 12.156, P < 0.001). Significant post‐treatment differences between groups were also found for the ability to accept incorrect visual information expressed by the visual conflict parameter (F = 15.05, P < 0.000). There were no significant between‐group differences in the contribution of the visual system (F = 2.64, P = 0.11) or use of somatosensory information (F = 0.117, P = 0.734) in the maintenance of balance and stability |
⊕⊕⊝⊝ low1 |
| Change in short‐term disability 3 months or less after the start of the intervention Measures: GLTEQ Follow‐up: up to 3 months | 45 (intervention group = 22) (1 study) | One study (Dlugonski 2012, N = 45) reported that the treatment group showed a significant increase in physical activity at 3‐month follow‐up compared to the control group as measured by GLTEQ (P < 0.001). There was a non‐significant change in assessment scores from post‐intervention to 3‐month follow‐up (P = 0.61) | ⊕⊕⊝⊝ low2 |
|
Change in long‐term disability more than 3 months after the intervention Measure:6MWT Follow‐up: 6 months – 2 years |
82 (intervention group = 41) (1 study with 2 reports) | One study with 2 reports (Pilutti 2014, N = 82) showed a significant and positive effect of the intervention on increase in 6MWT distance relative to those in the control group (P = 0.07). Physical activity increased most in those with mild disability in the intervention group. | ⊕⊕⊝⊝ low2 |
| Change in symptoms or impairments | |||
|
Change in impairments directly post‐intervention
Measures: FIS, FSS, MFIS, MS Symptom Cheklist Follow‐up: depended on the type of intervention; range from (1 month – 12 weeks) |
265 (intervention group = 138) (4 studies) | One study (Finlayson 2011, N = 190) showed a significant reduction in fatigue in intervention group compared to a wait‐list control group immediately after intervention as measured by FIS sub‐scales (Mean (SD): Cognitive ‐3.12 (6.1), P = 0.001; Physical ‐2.53 (6.4), P = 0.014; Social ‐6.01 (12.1), P = 0.002) One study (Egner 2003, N = 27) reported similar fatigue scores (measured using FSS) for all 3 groups (video, telephone and standard care) at 9 weeks post‐intervention; however the video group had significantly lower scores than the other 2 groups at month 6 (P < 0.05; telephone: SE = 0.478; standard care: SE = 0.536) and month 18 (P < 0.05; telephone: SE = 0.569; standard care: SE = 0.624) One study (Frevel 2014, N = 18) reported that fatigue improved significantly in the control (hippotherapy) group (P < 0.05 for all MFIS subscales); while the e‐training group improved only on the MFIS cognitive subscale (P = 0.031). A significant difference between the groups was noted only in the cognitive subscale of the MFIS ( P = 0.012) One study (Paul 2014, N = 30) reported no improvements in symptoms as measured by MS Symptom Checklist. |
⊕⊕⊝⊝ low3 |
| Change in short‐term impairments 3 months or less after the start of the intervention Measures: FIS Follow‐up: up to 3 months | 190 (intervention group = 94) (1 study) | One study (Finlayson 2011, N = 190) showed a reduction in fatigue at 3 months with large effect size as measured by FIS subscales (ES (95% CI): Cognitive 0.58 (0.48 to 0.68); Physical 0.68 (0.55 to 0.82); Social 0.65 (0.53 to 0.77) and FSS scores: ‐0.38 (‐0.45 to ‐0.31)) | ⊕⊕⊝⊝ low4 |
|
Change in long‐term impairments more than 3 months after the intervention Measures: FIS, FSS Follow‐up: 6 months – 2 years |
299 (intervention group = 155) (3 studies) | One study (Egner 2003, N = 27) showed a reduction of fatigue measured by FSS in those using video telerehabilitation compared with those using telephone telerehabilitation or standard care groups at 6 months (P < 0.05; telephone: SE = 0.478; standard care: SE = 0.536) and 18 months (P < 0.05; telephone: SE = 0.569; standard care: SE = 0.624). At 12 months follow‐up, there was a significant difference in fatigue scores between the video and standard care groups (P < 0.05; SE = 0.471) One study with 2 reports (Pilutti 2014, N = 82) showed a significant and positive effect of the intervention on fatigue severity (FSS, P = 0.001) and its physical impact (FIS, P = 0.008) at 6‐month post‐intervention. The results also indicated a favourable effect of the intervention on symptoms of pain (MPQ, P =. 0.08) and sleep quality post‐trial (PSQI, P = 0.06), although the differences between groups did not reach statistical significance One study (Finlayson 2011, N = 190) showed reduction in fatigue at 6 months with a large effect size as measured by FIS subscales (ES (95% CI): Cognitive 0.55 (0.46 to 0.64); Physical 0.61 (0.50 to 0.72); Social 0.67 (0.58 to 0.76) and FSS score:: ‐0.33 (‐0.36 to ‐0.30)) |
⊕⊕⊝⊝ low5 |
| Change in participation | |||
|
Change in psychological outcomes Measures:CES‐D, HADS, SDMT Follow‐up: variable (range 1 month – 2 years) |
139 (intervention group = 76) (3 studies) |
One study (Egner 2003, N = 27) showed no significant difference in depressive symptoms measured by CES‐D at end of the intervention period (9 weeks). Mean depression scores were lower in those receiving telerehabilitation by video compared with telephone and standard care group symptoms decreased at 6, 8 and 24 months follow‐up. Being male was a significant predictor for an increased depression score at every measurement point except at 24 months (P < 0.05). Mean CES‐D scores fluctuated throughout each measurement point for all groups, but seemed to decrease at 24 months in all 3 groups, but not statistically significant. Mean depression scores were lower in those receiving telerehabilitation by video compared to telephone and standard care groups and depressive symptoms also decreased at the 6‐, 8‐ and 24‐month follow‐ups, but this was not significantly different between groups. One study (Paul 2014, N = 30) reported a small non‐significant improvement in anxiety measured by HADS in the control group compared with the treatment group at post‐treatment (8 ‐ 9 weeks) (P = 0.016) One study with two reports (Pilutti 2014, N = 82) showed a statistically significant group interaction in psychological outcomes on SDMT scores (F = 5.68, P = 0.02), which was moderate in magnitude (partial eta squared (ɳ₂) = 0.08). There was a clinically meaningful improvement in SDMT scores in the subgroup with mild disability in the intervention condition (∼ 6 points increase, moderate effect size (d) = 0.41), whereas those with moderate disability in the intervention condition demonstrated minimal change (∼ 1 point decrease, d = 0.12). There were minimal changes in SDMT scores for those with both mild or moderate disability (∼ 1 point increase, d = 0.10 for both) in the control group. There was also significant improvement in depression and anxiety in the intervention group (with large effect size (ɳ₂ = 0.10 for both) compared with the control group measured by the HADS (depression: F =7.90, P = 0.006; anxiety: F = 8.00, P = 0.006) |
⊕⊕⊝⊝ low6 |
|
Change in quality of life Measures: QWB, HAQUAMS, MSIS‐29, SF‐36, LMSQOLS, Follow‐up: variable (range 1 month – 2 years) |
392 (intervention group = 201) (6 studies, 1 with 2 reports) |
One study (Egner 2003, N = 27) reported no significant difference in QoL measured using QWB at the end of the intervention period (9 weeks). Mean QWB scores for each measurement point (6, 9, 12, 18 and 24 months) were higher (indicating higher QoL) for those in the video group than for the standard care and telephone groups, but were significantly better in the video group compared to the telephone group at month 12 only (P < 0.05; SE = 0.023). The telephone group and standard care groups reported similar mean QWB scores over the 2‐year follow‐up period. One study (Frevel 2014, N = 18) showed significant improvement in QoL measured by HAQUAMS (cognition: P = 0.026; function of lower limb: P = 0.008; mood: P = 0.045) in the control group (hippotherapy), but not in the intervention group (e‐training) One study (Dlugonski 2012, N = 45) showed non‐significant condition‐by‐time interactions for QoL measured by MSIS‐29. There was no significant correlation between changes in QoL from base line to post‐intervention in either the treatment or control groups One study (Finlayson 2011, N = 190) showed that significant improvement in HRQoL in the intervention group on the SF‐36 subscales except the physical functioning and bodily pain subscales: change score (95% CI): Vitality 6.99 (4.29 to 9.69); Role Emotion 10.08 (4.13 to 16.04); Mental Health 5.78 (3.89 to 7.67); Social Function 7.95 (4.09 to 11.82); General Health 3.61 (1.37 to 5.85); Role Physical 11.12 (6.22 to 16.02) One study (Paul 2014, N = 30) reported non‐significant improvement in HRQoL measured by LMSQOLS in the treatment group compared with control group post‐treatment (8 ‐ 9 weeks) (mean difference ‐0.07 vs 1.0) One study with 2 reports (Pilutti 2014, N = 82) reported that participants in the intervention group perceived a positive change in physical HRQoL measured by MSIS‐29 (P = 0.06) |
⊕⊕⊝⊝ low7 |
| Change in other outcomes | |||
| Cost effectiveness | 531 (intervention group = 277) (9 studies) | Not measured in any of the studies | See 'Impact' |
|
Process evaluation (user satisfaction) Measures: Self‐designed Likert scale, VAS scale Follow‐up: variable (range 1 ‐ 3 months) |
80 (intervention group =46) (2 studies) |
One study (Dlugonski 2012, N = 45) showed that participants were most satisfied with (mean ± SD): the overall programme: 4.8 ± 0.4, staff: 4.9 ± 0.2 and pedometer: 4.7 ± 0.6, but slightly less satisfied with the website itself: 4.1 ± 0.9 One study (Huijgen 2008, N = 35) reported that overall, both participants and therapists were satisfied with the intervention (over 55% in all 6 items). Both participants and therapists were less satisfied with the aesthetic aspect of the system and had difficulty completing tasks |
⊕⊝⊝⊝ very low8 |
| Serious adverse events | 531 (intervention group = 277) (9 studies) | No serious adverse events reported | See 'Impact' |
| Caregivers‐related outcomes | 531 (intervention group = 277) (9 studies) | Not measured in any of the studies | See 'Impact' |
| ARAT: Action Research Arm Test; CES: Composite Equilibrium Score; CES‐D: Center for Epidemiologic Studies Depression Scale; CI: Confidence interval;DGI: Dynamic Gait Index; EDSS: Expanded Disability Status Scale; ES: Effect size; FIS: Fatigue Impact Scale; FSS: Fatigue Severity Score; GLTEQ: Godin Leisure‐Time Exercise Questionnaire; HADS: Hospital Anxiety and Depression Scale; HAQUAMS: Hamburg QoL Questionnaire in MS; HRQoL: Health related quality of life; IQR: inter quartile range; LMSQOLS: Leeds MS Quality of Life Scale; MPQ: McGill Pain Questionnaire; MS: Multiple Sclerosis;MSIS‐29: MS Impact Scale; NHPT: Nine Hole Peg Test; PSQI: Pittsburg Sleep Quality Index;QoL: quality of life; QWB: Quality of Well‐ Being Scale; SD: Standard deviation; SDMT: Symbol Disit Modalities Test; SE: Standard Error; SF‐36: 36‐Item Short Form Health Survey; SOT: Sensory organisation Test; VPR: Visual Preference Ratio; 6MWT: 6 Meters Waltk Test;25FWT: 25 Feet Walk Test; 95% CI: 95 percent confidence interval | |||
| # GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
1Methods of randomisation not described or poorly described in 4 studies, only 1 study reported blinding of the assessor, and allocation concealment was described in only 1 study
2Unclear randomisation procedure, allocation concealment not reported, no blinding of the participants or assessors
3Methods of randomisation not described or poorly described in 1 study, none of the studies reported blinding of the participants or assessor, and allocation concealment was not described or unclear in 2 studies
4No blinding of the participants or assessors, high risk of attrition bias (> 20% drop‐out)
5Methods of randomisation and allocation concealment not described or poorly described in 2 studies, all 3 studies did not report blinding of the participants or assessor
6Methods of randomisation not described or poorly described in 2 studies, none of the studies reported blinding of the participants or assessor and allocation concealment procedure
7Methods of randomisation not described or poorly described in 3 studies, allocation concealment procedure described only in 2 studies, and none of the studies reported blinding of the participants or assessor
8Methods of randomisation and allocation concealment procedure not described or poorly described, and blinding of the participants or assessor not reported in both studies
Background
Description of the condition
Multiple sclerosis (MS) is a chronic neurological disease, characterised by patchy inflammation, gliosis and demyelination within the central nervous system (CNS), that affects approximately 1.3 million people worldwide (WHO 2008). The median estimated incidence of MS globally is 2.5 per 100,000 (with a range of 1.1 to 4) (WHO 2008), the prevalence is about 30 per 100,000 population (range 5 to 80), with a female preponderance (female to male ratio of 3:1) (Trisolini 2010; WHO 2008).
The patterns of presentation in MS are heterogeneous and include: ‘relapsing remitting’ (RR) MS (85%), characterised by exacerbations and remissions; ‘secondary progressive’ (SP) MS with progressive disability acquired between attacks (in 70% to 75% who start with RR, it is estimated more than 50% will develop SPMS within 10 years, and 90% within 25 years); ‘primary progressive’ (PP) MS (10%), where persons develop progressive disability from the onset; and ‘progressive relapsing’ (PR) MS (5%), where persons begin worsening gradually and subsequently start to experience discrete attacks (MS Australia 2012; Weinshenker 1989). The prognosis in MS is variable and difficult to predict, and depends on the type, severity and location of demyelinating lesions within the CNS (Hammond 2000; MS Australia 2012). Various factors such as older age at onset, progressive disease course, multiple onset symptoms, pyramidal or cerebellar symptoms and a short interval between onset and first relapse are associated with worse prognosis (Hammond 2000). Persons with MS (pwMS) have a prolonged median survival time from the time of diagnosis of approximately 40 years (Weinshenker 1989). Therefore, issues related to progressive disability (physical and cognitive), psychosocial adjustment and social re‐integration progress over time. These have implications for pwMS, their carers, treating clinicians and society as a whole, in terms of healthcare access, provision of services and financial burden (Pfleger 2010; Trisolini 2010).
The pwMS can present with various combinations of deficits such as physical (motor weakness, spasticity, sensory dysfunction, visual loss, ataxia), fatigue, pain (neurogenic, musculoskeletal and mixed patterns), incontinence (urinary urgency, frequency), cognitive (memory, attention), psychosocial, behavioural and environmental problems, which limit a person’s activity (function) and participation (Khan 2007). Cognitive and behavioural problems can be subtle and often precede physical disability requiring long‐term care (Beer 2012). The care needs in this population are complex due to cumulative effects of the impairments and disabilities, the ‘wear and tear’ and the impact of aging with a disability. Longer‐term multidisciplinary management is recommended, both in hospital and in community settings to maintain functional gains and social re‐integration (participation) over time (Khan 2007; Khan 2010a; WHO 2008). Despite recent advances in MS management, many pwMS are unable to access these developments due to limited mobility, fatigue and related issues, plus costs associated with travel. With increasing financial constraints on healthcare systems, alternative methods of service delivery in the community and over a longer term are now a priority. Telerehabilitation for pwMS has potential as a tool to improve health care with reduction in care costs (Zissman 2012). The emerging advances in information and communication technology (ICT) may present as an alternative efficient and cost‐effective method to deliver rehabilitation treatment in a setting convenient to the patient, such as their home.
Description of the intervention
The terminology used in ICT in health care is often used interchangeably and includes: ‘telemedicine’, ‘telehealth’, ‘telehealthcare’, ‘e‐Health’, ‘e‐medicine’, ‘telerehabilitation’ etc. (Currell 2000; McLean 2010; McLean 2011; Winters 2002). In this review we define the term ‘telerehabilitation’ as ‘the use of information and communication technologies as a medium for the provision of rehabilitation services to sites or patients that are at a distance from the provider' (Rogante 2010; Theodoros 2008). The applications to date encompass systems ranging from low‐bandwidth, low‐cost videophones to highly expensive, fully immersive virtual reality systems with haptic interfaces (Theodoros 2008).
Telerehabilitation extends rehabilitative care beyond the hospital process and facilitates multifaceted, often psychotherapeutic approaches to modern management of pwMS at home or in the community (Huijgen 2008). It provides equal access to individuals who are geographically remote and to those who are physically and economically disadvantaged (Hailey 2011; Rogante 2010) and can improve the quality of rehabilitation delivered (Hailey 2011; Kairy 2009; McCue 2010; Rogante 2010; Steel 2011). It can give healthcare providers an opportunity to evaluate the intervention previously prescribed, monitor adverse events and identify areas in need of improvement. The treating therapists can monitor patients’ progress and optimise the timing, intensity and duration of therapy as required, which may not always be possible within the constraints of face‐to‐face treatment protocols in the current health systems (Hailey 2011; Steel 2011).
How the intervention might work
Telerehabilitation is an emerging method of delivering rehabilitation that uses technology to serve patients, clinicians and systems by minimising the barriers of distance, time and cost. The driving force behind this has been the need for an alternative to face‐to‐face intervention, enabling service delivery in the natural environment – that is, in patients’ homes (Hailey 2011). This method of in vivo delivery of healthcare services can address associated issues of efficacy, problems of generalisation and increasing patient participation and satisfaction with treatment.
The benefits and advantages of telerehabilitation have been well documented (Bendixen 2009; Brennan 2009; Chumbler 2012; Constantinescu 2010; Johansson 2011; Kairy 2009; Lai 2004; Legg 2004; Russell 2011; Steel 2011). A home‐based physical telerehabilitation programme was considered feasible and effective in improving function in pwMS (Finkelstein 2008). Telemedicine in pwMS as a tool has the potential for improved health care with reduction in care costs (Zissman 2012). A systematic review that analysed rehabilitation therapies delivered at home in stroke survivors showed positive outcomes, with a reduction in the risk of deterioration, improved ability to perform activities of daily living, reduced costs and duration of rehabilitation in a frail elderly population (Legg 2004). Other reports used telerehabilitation to direct multidisciplinary co‐ordinated, goal‐directed treatment to monitor clinical progress for patients at a distance (Hailey 2011; Kairy 2009; McCue 2010; Rogante 2010; Steel 2011). In these cases, telerehabilitation offered an opportunity to provide an individualised rehabilitation intervention beyond the hospital setting, by regular monitoring and evaluation of the patients' needs and progress, with a range of services suited to the individual and their environment (Hailey 2011; Kairy 2009; McCue 2010; Rogante 2010; Steel 2011). Telerehabilitation also provides health outcomes comparable to traditional in‐person patient encounters, including improved patient satisfaction (Egner 2003; Finkelstein 2008; Hailey 2011; Huijgen 2008; Kairy 2009). It can encompass single or multiple interventions, or both, aimed at improving the patient experience at the level of impairment, activity or participation, and can educate patients (and carers) in their ongoing self management.
Why it is important to do this review
There is strong evidence to support the effectiveness of rehabilitation programmes for pwMS (Khan 2007; Khan 2010a). With increasing financial constraints on healthcare systems, alternative methods of service delivery in the community and over a longer term are now a priority. Telerehabilitation was reported to be effective in various neurological conditions including MS (Egner 2003; Finkelstein 2008; Huijgen 2008). However, there is as yet no systematic review of telerehabilitation interventions in pwMS to guide treating clinicians on evidence for its validity, reliability, effectiveness and efficiency in this population.
This review analyses published and unpublished clinical trials relating to MS and telerehabilitation, identifies the evidence base for its use, and discusses issues for future expansion of the evidence base by traditional research and other methods.
Objectives
To investigate the effectiveness and safety of telerehabilitation intervention in persons with multiple sclerosis (pwMS) for improved patient outcomes.
Specifically, the review addresses the following questions:
Does telerehabilitation achieve better outcomes compared with traditional face‐to‐face intervention?
What types of telerehabilitation interventions are effective, in which setting and influence which specific outcomes (impairment, activity limitation and participation)?
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) and controlled clinical trials (CCTs), including quasi‐randomised and quasi‐experimental designs with comparative controls (where the method of allocation is known but is not considered strictly random).
Types of participants
We included studies in pwMS (18 years and over) with a confirmed diagnosis of MS (Mc Donald 2001; Polman 2005; Poser 1983) and all disease subgroups (relapsing remitting, secondary progressive and progressive MS).
Types of interventions
We considered all modalities (type, duration, frequency and intensity) of telerehabilitation intervention, using telecommunication technology as the delivery medium, such as internet, videoconferencing, telephone and virtual reality, aimed at achieving patient‐centred goals or enhancing function and participation. These included: (a) individual (unidisciplinary) treatments, e.g. physical interventions: exercise, self‐management education, etc., and (b) multidisciplinary rehabilitation, i.e. delivered by two or more disciplines: occupational therapy, physiotherapy, exercise physiology, orthotics, other allied health and nursing, in conjunction with medical input.
The settings of telerehabilitation intervention included the following:
outpatient or day treatment settings in community rehabilitation centres;
home‐based settings, in the patients' own homes and local community.
Control conditions included the following:
no treatment;
placebo/sham;
any type of traditional face‐to face rehabilitation treatment in outpatient or day treatment settings.
We excluded studies if they investigated:
acute medical/surgical/pharmacological interventions for pwMS provided via telemedicine technology in isolation, unless it was administered as a concomitant intervention along with the telerehabilitation intervention, which was administered in the same way in both control and treatment groups;
studies on telerehabilitation targeting mental health conditions or substance abuse;
studies on home care (or tele‐home care) with no rehabilitation objectives;
studies on satisfaction with or acceptance of telerehabilitation technology;
studies on technical development or feasibility of telerehabilitation;
studies exploring telerehabilitation technology for intra‐professional communication (such as for second opinions) and for passive information provision, e.g. online education, where there is no direct interaction or involvement of a healthcare professional with the patient.
Types of outcome measures
We identified diverse outcomes, given the varied presentations of MS‐related problems and goals of treatment related to MS severity. The specific outcome measures per se were not part of the exclusion criteria for this review. We report and list all outcome measures used in studies in Table 2.
1. List of outcome measures used in the included studies*.
| Outcome Measures |
| Function |
| Action Research Arm Test (ARAT) |
| Berg Balance Scale (BBS) |
| Computerized Dynamic Posturography (CDP) |
| Composite Equilibrium Score (CES) |
| Dynamic gait Index (DGI) |
| Exercise Self‐Efficacy Scale (EXCE) |
| Godin Leisure‐Time Exercise Questionnaire (GLTEQ) |
| International Physical Activity Questionnaire (IPAQ) |
| Late‐Life Function and Disability Instrument (LL‐FDI) |
| Motor Control Test (MCT) |
| Multidimensional Outcomes Expectations for Exercise Scale (MOEES) |
| Multiple Sclerosis Walking Scale – 12 (MSWS‐12) |
| Nine‐Hole Peg Test (NHPT) |
| Physical Activity Readiness Questionnaire (PARQ) |
| Sensory organisation Test (SOT) |
| Six Meter Walk Test (6MWT) |
| Tineti Scale (TS) |
| Timed Up and Go (TUG) |
| Two Meter Walk Test (2MWT) |
| Twenty‐five Foot Walk Test (25‐FWT) |
| Visual Preference Ratio (VPR) |
| Impairment and symptoms |
| Fatigue impact scale (FIS) |
| Fatigue severity scale (FSS) |
| Modified Fatigue impact scale (MFIS) |
| McGill Pain Questionnaire (MPQ) |
| MS related symptom check list |
| Pittsburgh Sleep Quality Index (PSQI) |
| Participation |
| Quality of Life |
| Hamburg Quality of Life Questionnaire in Multiple Sclerosis (HAQUAMAS) |
| Leeds Multiple Sclerosis Quality of Life Scale (LMSQOL) |
| Multiple Sclerosis Impact Scale (MSIS‐29) |
| Quality of Well‐ Being Scale (QWB) |
| 36 item Short Form Health Survey Questionnaire (SF 36) |
| Psychological |
| Centre for Epidemiologic Studies Depression Scale (CES‐D) |
| Hospital Anxiety and Depression Scale (HADS) |
| Symbol Digit Modalities Test (SDMT) |
| Other |
| Expanded Disability Status Scale (EDSS) |
| Energy Conservation Questionnaire (ECQ) |
| Exercise Goal setting Scale (EGS) |
| Muscle strength |
| Patient Determined Disease Steps (PDDS) |
| Self‐Efficacy for Energy Conservation (SEEC) |
| Satisfaction with the intervention |
| Visual Analogue Scales (VAS) |
*Outcome measures are categorised according to the International Classification of Functioning, Disability and Health (ICF, WHO 2001)
Primary outcomes
We categorised primary outcomes according to the International Classification of Functioning, Disability and Health (ICF; WHO 2001), and included:
improvement in functional activity; such as activities of daily living (ADL), mobility, continence, etc.;
improvement in symptoms or impairments, e.g. pain, spasm frequency, joint range of movement, involuntary movements, spasticity, etc.;
improvement in participation and environmental or personal context, or both; e.g. quality of life (QoL), psychosocial function, employment, education, social and vocational activities, patient and carer mood, relationships, social integration, etc.
We included the measure of achievement of intended goals for treatment, e.g. goal attainment scaling or other measure of goal achievement.
It should be noted, however, that some outcome scales crossed boundaries between these ICF concepts, for example, items relating both to impairment (symptoms) and activity.
Secondary outcomes
These reflect compliance with the intervention, service utilisation, and cost effectiveness of telerehabilitation compared with traditional rehabilitation interventions.
We report all adverse events that may have resulted from the intervention. A serious adverse event is defined 'as an event that is life‐threatening or requires prolonged hospitalisation' (Khan 2007). We also explored carer‐related issues, such as carer strain.
Timing of outcome measures The time points for outcome assessments were: short‐term (immediately after intervention or up to three months) and long‐term (greater than three months) from the start of the intervention. We considered patient follow‐up assessments similarly as short‐term (up to three months) and long‐term follow‐up (greater than three months) after cessation of the intervention.
Search methods for identification of studies
We considered articles in all languages with a view to translation, if necessary. We extracted trials coded with the specific key words and considered them for inclusion in the review.
Electronic searches
The review authors, along with the Trials Search Co‐ordinator, searched the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group Specialised Register, last searched on 9 July 2014, which contains the following:
The Cochrane Central Register of Controlled Trials (CENTRAL) (2014 Issue 7).
MEDLINE (PubMed) (1966 to 9 July 2014).
EMBASE (EMBASE.com) (1974 to 9 July 2014).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO host) (1981 to 9 July 2014).
Latin American and Caribbean Health Science Information Database (LILACS) (Bireme) (1982 to 9 July 2014).
Clinical trial registries; clinicaltrials.gov.
World Health Organization (WHO) International Clinical Trials Registry Portal (apps.who.int/trialsearch/).
The keywords used to search for studies for this review are listed in Appendix 1.
Information on the Trial Register of the Review Group and details of search strategies used to identify trials can be found in the 'Specialised Register' section within the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group module.
Searching other resources
We performed an expanded search to identify articles potentially missed through the database searches and articles from ‘grey literature' from 1996 to latest date. This included the following:
handsearches of reference lists of all retrieved articles, texts and other reviews on the topic;
handsearches of the most relevant journals related to MS and spasticity research and treatment (such as, but not limited to: Archives of Physical Medicine and Rehabilitation, Journal of Rehabilitation Medicine, Journal of Neurology, Journal of Neurology, Neurosurgery and Psychiatry, Clinical Rehabilitation, Neurology, Physical Therapy, Multiple Sclerosis, Telemedicine Journal and e‐Health, Journal of Medical Internet Research and others);
searches using the 'Related articles' feature (via PubMed);
searches of ProQuest Dissertations and Theses;
searches of Web of Science for citation of key authors;
searches of System for Information on Grey Literature in Europe (SIGLE);
contacting local and foreign experts for further information, such as MS Groups/Associations, the Cochrane MS Group, key authors of publications in this review;
contacting authors and researchers active in this field.
We also searched the following websites for ongoing and unpublished trials:
Current Controlled Trials (www.controlled‐trials.com);
UK Clinical Research Network Portfolio Database (public.ukcrn.org.uk/search/).
Data collection and analysis
Selection of studies
Two review authors (BA, FK) independently screened and short‐listed all abstracts and titles of studies identified by the search strategy for appropriateness based on the selection criteria. We independently evaluated each study from the shortlist of potentially appropriate studies for inclusion or exclusion. We obtained the full text of the article for further assessment to determine if the trial met the inclusion criteria. If we could not reach a consensus about the inclusion or exclusion of any individual study, we made a final consensual decision by discussion amongst all the review authors. We had intended to submit the full article to the editorial board for arbitration when there was no consensus regarding the inclusion or exclusion of a study between the review authors; however, this was not necessary. We were not masked to the name(s) of the study author(s), institution(s) or publication source at any level of the review.
We had planned to seek further information, where necessary, about the method of randomisation or a complete description of the telerehabilitation interventions from the trialists, but this was not required.
Data extraction and management
Two review authors (BA, FK) independently extracted data from each study that met the inclusion criteria, using a standardised data collection form, with other review authors (JK, MG) making a final check. We had intended to contact the primary authors of eligible studies to provide data and clarification where adequate data were not reported, but this was not required. We summarise all studies that met the inclusion criteria in the 'Characteristics of included studies' table provided in Review Manager 5 software developed by Cochrane (Review Manager 2014), and include details on design, participants, interventions and outcomes.
We report the following information from individual studies:
publication details;
study design, study setting, inclusion and exclusion criteria, method of allocation, risk of bias;
participant population, e.g. age, type of MS, disease duration, disability (according to Kurtzke's Expanded Disability Status Scale (EDSS) score (Kurtzke 1982);
details of intervention;
outcome measures (primary and secondary);
withdrawals, compliance, length and method of follow‐up and number of participants followed up.
We extracted data for every participant assessed for each outcome measure, and for dichotomous data the number in each treatment group and the numbers experiencing the outcome of interest where possible. We extracted data for intention‐to‐treat (ITT) analysis from each study, and where ITT data were not available, we retrieved 'on‐treatment’ data or the data of those who completed the trial. We resolved any disagreement by recourse to other review authors (JK, MG) and through discussion, with reference to the original report. We had planned to contact study authors for additional information and data if necessary, but this was not required. We present the results in a tabulated format in the Table 1.
Assessment of risk of bias in included studies
Three review authors (BA, FK, MG) independently assessed the methodological quality of the included studies using the Cochrane 'Risk of bias' tool (Higgins 2011) for sequence generation, allocation concealment, blinding of participants, therapists and outcome assessors, incomplete outcome data and selective outcome reporting. Further, we also checked baseline data amongst the study groups for stability.
We considered a study to be of 'high' methodological quality if the risks of bias for all domains were low. We termed this a 'high‐quality study'. We rated a study as being of 'low' methodological quality where there was a lack of clarity or a high risk of bias for one or more domains, and termed this as a 'low‐quality study'. If we rated most domains at high risk of bias, we rated the study as a 'very low‐quality study'. We resolved any disagreements by consensus between the review authors. We present results using 'Risk of bias' summary figures.
Measures of treatment effect
A quantitative analysis was not possible due to clinical heterogeneity (see below), the use of diverse methodology, interventions and outcome measures, and insufficient data available. We entered and analysed all data in Review Manager 5 software (Review Manager 2014). We qualitatively summarised the studies in the Characteristics of included studies tables, presented the results of primary and secondary outcomes of included studies, categorised according to the ICF framework, in the Table 1. We describe the results in a narrative form in the Discussion section below. If studies had been available, and if meta analyses become feasible in future updates, we will analyse treatment effects as described in the protocol version of this review (Khan 2013).
Unit of analysis issues
For each study, we assessed the appropriate units of analysis, which included the level at which randomisation occurred (e.g. parallel‐group design, cluster‐randomised trials, cross‐over trials, etc.), type, duration, intensity and setting of telerehabilitation interventions.
Dealing with missing data
We provide information about missing data related to participants dropping out or lost to follow‐up in the Characteristics of included studies tables. We contacted the primary authors to obtain additional information and clarification by personal communication (email), to clarify possible overlapping of the data in the four eligible studies. We did not perform imputation of missing data as we were not able to perform meta‐analyses.
Assessment of heterogeneity
We assessed clinical heterogeneity by examining the characteristics of studies, the similarity between the types of participants, settings, interventions (frequency, intensity, duration) and outcomes, as specified in the Criteria for considering studies for this review section. Due to apparent clinical heterogeneity, a comprehensive quantitative analysis (meta‐analysis) was not possible. We did not assess statistical heterogeneity and presented the studies separately. We will consider both clinical and statistical heterogeneity, if data become available in future updates, as described in the protocol version of this review (Khan 2013).
Assessment of reporting biases
We used a comprehensive search strategy, which included searching for unpublished studies (grey literature), and searching trials registers (See Search methods for identification of studies) to avoid reporting biases and publication bias (Egger 1998). We did not analyse trial data using funnel plots to investigate the likelihood of publication bias, due to the small number of included studies.
Data synthesis
There was a wide variation in several variables of the included studies, such as MS course and severity, content; frequency, duration, mode of delivery and aim of the interventions; outcome measures used; presentation of results; and methodological quality. Because of the observed heterogeneity, we did not pool data for a quantitative analysis. If studies had been available and if data become available in future updates, we will attempt a quantitative analysis, as described in the protocol version of this review (Khan 2013). We have highlighted the strength of study findings, discussed gaps in the current literature and identified future research directions in the Discussion section.
Subgroup analysis and investigation of heterogeneity
We were unable to perform subgroup analysis for the following subgroups, owing to the lack of available data:
Type of telerehabilitation intervention (unidisciplinary or multidisciplinary, or both).
Type of MS (relapsing remitting, progressive)
Severity of MS (i.e. EDSS < 6; > 6)
Duration of follow‐up of participants (≤ 3 months; > 3 months)
Sensitivity analysis
We were not able to conduct sensitivity analyses due to our narrative presentation of the results of the included studies. If studies had been available, and heterogeneity existed across trials, we would have conducted sensitivity analyses by omitting trials with a high risk of bias as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If meta‐analyses become feasible in future updates, we will perform sensitivity analyses as described in the protocol version of this review (Khan 2013).
'Summary of findings' table
These outcomes are included in the Table 1:
Change in disability (post‐intervention, ≤ 3 months, > 3 months)
Change in impairments (post‐intervention, ≤ 3 months, > 3 months)
Change in participation (psychological outcomes, QoL)
Cost effectiveness
Process evaluation
Serious adverse events
Caregivers'‐related outcomes
We used the five GRADE considerations (risk of bias, inconsistency, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for prespecified outcomes. We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro software (GRADEpro 2008). We justified all decisions to downgrade or upgrade the quality of studies by using footnotes, and we made comments to aid readers' understanding of the review when necessary.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies
Results of the search
Electronic and manual searches identified 4030 references (MEDLINE = 79; EMBASE = 3799; CENTRAL = 136; CINAHL = 5; LILACS = 9; CRD database = 0; Cochrane Opportunity Fund Project = 0; Trial Registries via WHO Portal = 0; handsearching journals = 0; handsearching trial registries = 2) with our search criteria. After elimination of duplicates records, we screened the remaining 3842 for closer scrutiny. Of these, we retrieved the full text of 29 articles for further assessment to determine inclusion in the review. We did not identify any ongoing or unpublished studies awaiting classification. See: Figure 1 for Study flow chart.
1.
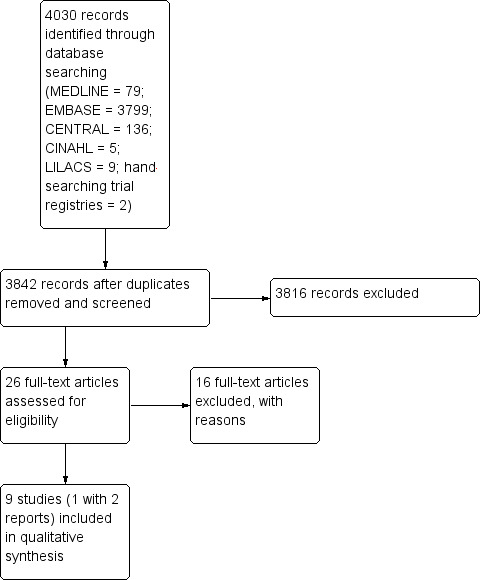
Study flow diagram.
Included studies
In total, nine RCTs, one with two reports (Pilutti 2014; Sandroff 2014), published between 2003 and 2014 (Dlugonski 2012; Egner 2003; Finlayson 2011; Frevel 2014; Gutíerrez 2013a; Huijgen 2008; Motl 2011; Paul 2014; Pilutti 2014) fulfilled the inclusion criteria for this review (see Characteristics of included studies table).
Five of the included studies were conducted in the United States (Dlugonski 2012; Egner 2003; Finlayson 2011; Motl 2011; Pilutti 2014); one each was conducted in Spain (Gutíerrez 2013a), Germany (Frevel 2014) and the United Kingdom (Paul 2014), while one was a multicentre study conducted in three different countries (Italy, Spain and Belgium; Huijgen 2008). Three studies were conducted by the same group of authors in the same setting and with the same cohort of participants recruited from a single database (Dlugonski 2012; Motl 2011; Pilutti 2014), of which one reported different outcomes in two different articles (Pilutti 2014).
Participants
Participants' detailed information, including inclusion/exclusion criteria and baseline demographics, are listed in the Characteristics of included studies table. The nine included studies involved a total of 531 participants (277 participants in the treatment groups and 254 in the control groups). The number of participants in the studies ranged from 27 to 190 (median 45). As expected, there were more women, with their proportion ranging from 56% to 87% (mean 74%). The mean age of participants varied from 41 to 52 years (mean 46.5 years) and mean years since diagnosis from 7.7 to 19.0 years (mean 12.3 years). The majority of participants had a relapsing‐remitting course of MS (RRMS), two studies involved only people with RRMS (Dlugonski 2012; Motl 2011) and two studies did not provide details of MS type (Egner 2003; Huijgen 2008). The study inclusion criteria varied between trials. All trials included participants with definite MS, although only two trials specified the commonly‐used McDonald's criteria (Mc Donald 2001) (Frevel 2014; Gutíerrez 2013a). One study reported secondary data regarding MS participants which were collected as part of a larger study of a telerehabilitation intervention in people with severe mobility impairment (Egner 2003).
Intervention
Detailed information about interventions in the included studies is presented in the Characteristics of included studies tables and is further summarised in Table 3. The various telerehabilitation interventions in the included studies consisted generally of physical activity and educational components.
2. Summary of telerehabilitation interventions in the included studies.
| Study | Telerehabilitation interventions | |||
| Contents | Settings | Technology | Duration/intensity | |
| Dlugonski 2012 | Same as Motl 2011 ( see below) | Participants' home | Internet‐delivered | 12 weeks Same as Motl 2011 ( see below) |
| Egner 2003 | Structured in‐home education and counselling session delivered by a rehabilitation nurse, which included individual rehabilitation education sessions | Participants' home | Telephone or video | 30 to 40 minutes, weekly for 5 weeks, then once every 2 weeks for 1 month. |
| Finlayson 2011 | Group‐based fatigue management programme, facilitated by a licensed Occupational Therapist | Rehab centre | Teleconference | 70‐minute weekly for 6 weeks |
| Frevel 2014 | Training programme: balance, postural control exercises and strength training with additional interactive sessions | Participants' home | Internet‐delivered | 2 training sessions/(45 minutes) weekly for 12 weeks |
| Gutíerrez 2013a | Monitored telerehabilitation programme, which included gaming protocol, proposing activities that involve integrating proprioceptive, visual, and vestibular sensory information. Experimental group attended at home | Participants' home | Virtual reality system via video‐conference using the Xbox 360 and Kinect console | 40 sessions, 4 sessions per week (20 minutes per session) |
| Huijgen 2008 | Home Care Activity Desk (HCAD) – a telerehabilitation intervention for arm/hand function and additional features for videoconferencing and recording. HCAD system | Participants' home | Virtual telerehabilitation programme and video‐conference, comprising a hospital‐based server and portable unit installed at participant’s home | 1 month of usual care followed by HCAD‐ 1 session (30 minutes)/day for 5 days per week for 1 month |
| Motl 2011 | Same as Dlugonski 2012 (see above) | Participants' home | Internet‐delivered | Same as Dlugonski 2012 (see above) |
| Paul 2014 | Individualised physiotherapy programme consisting of exercise page containing a video and text explaining the exercise, an audio description of the exercise and a timer | Participants' home | Internet‐delivered | Twice per week for 12 weeks |
| Pilutti 2014 | Same as in Motl 2011 (see above), in addition, participant wore a Yamax SW‐401 Digiwalker pedometer, completed a log book and used Goal Tracker software, and received a web‐cam, and website information | Participants' home | Internet‐delivered | 15 scheduled one‐on‐one video coaching sessions for 6 months |
| Sandroff 2014 | Same as in Motl 2011, Pilutti 2014 (see above). In addition, website materials were delivered in a titrated manner over the 6‐month period such that new content became available 7 times during the first 2‐month period, 4 times during the second 2‐month period, and twice during the final 2 months of the intervention. | Participants' home | Internet‐delivered | Weekly one‐on‐one behavioural coaching sessions via Skype (15 scheduled sessions) for 6 months |
Three studies used similar internet‐delivered, social cognitive theory‐based behavioural intervention to increase physical activity (Dlugonski 2012; Motl 2011; Pilutti 2014)
One study evaluated a structured in‐home education and counselling session delivered via telephone or video by a rehabilitation nurse (Egner 2003)
One study examined a group‐based, teleconference‐delivered fatigue management programme (Finlayson 2011)
One study evaluated a telerehabilitation intervention for arm/hand function at home ‐ the 'Home Care Activity Desk' (HCAD), which consists of a set of exercises for functional activity of the upper limb (Huijgen 2008)
One study evaluated the effectiveness of an individualised web‐based physiotherapy programme (Paul 2014)
One study published in two different journals by the same authors (Gutíerrez 2013a; Gutierrez 2013b) examined the effectiveness of an individualised virtual reality telerehabilitation programme for improvement in postural control
One study examined the effectiveness of an internet‐based home training programme (e‐Training) in comparison with hippotherapy to improve balance (Frevel 2014)
The duration and intensity of the telerehabilitation interventions varied significantly depending on the nature of the intervention, and ranged from one to six months (median 12 weeks). None of the studies reported the recruitment time period. The follow‐up periods varied between trials, but all studies assessed the participants immediately after intervention. Only one trial reported long‐term follow‐up of up to 24 months (Egner 2003). For details of assessment time points for each trial refer to the Characteristics of included studies tables.
Excluded studies
We excluded 16 studies after appraisals of the full reports (listed in the Characteristics of excluded studies tables). The primary reason for exclusion was:
10 studies addressed mental health care as a primary intervention (Amato 2014; Beckner 2010; Cerasa 2013; Fischer 2013; Mohr 2000; Mohr 2005; Mohr 2007; Moss‐Morris 2012; Solari 2004; Stuifbergen 2012)
One study had a medical‐care intervention only (Zissman 2012)
One study evaluated the effectiveness of an online fatigue self‐management programme for people with various chronic neurological conditions including MS, but did not provide a subgroup analysis for the MS cohort (Ghahari 2010)
Two studies assessed counselling interventions for health promotion and major depression (Bombardier 2008; Bombardier 2013)
Two studies assessed interventions with no rehabilitation objectives, such as education, self management (Miller 2011; Wiles 2003)
Risk of bias in included studies
See: ’Risk of bias’ tables in the Characteristics of included studies and Figure 2 and Figure 3.
Figure 2 and Figure 3 represent the review authors’ judgements about each methodological quality item, presented as percentages across all included studies and a summary of the risk of bias, respectively. Where studies failed to report sufficient methodological detail to assess the potential risk of bias, we graded them as being at 'unclear’ risk (presented as symbol '?' in Figure 3). The methodological quality of the nine included trials was 'low’, with substantial flaws in the methodological design and a high risk of bias related to their randomisation procedure; blinding of participants, therapists and outcome assessors, and outcome analysis.
2.
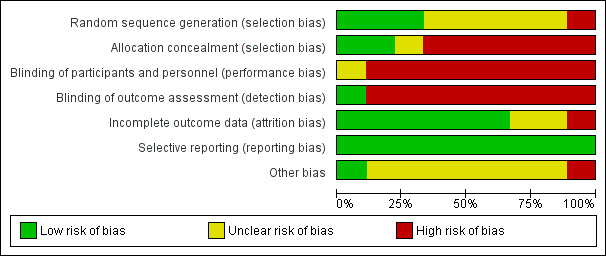
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
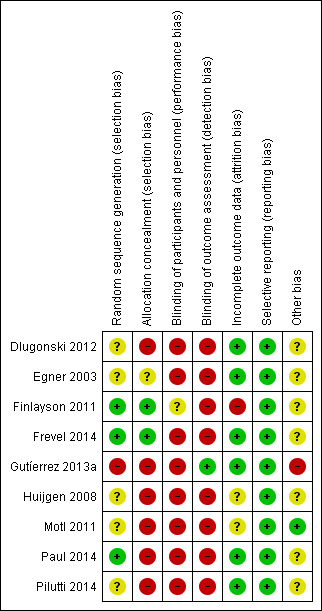
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Although all included studies stated that the procedure was randomised, the methods of randomisation were adequately reported in only six studies (one with two reports) (Dlugonski 2012; Finlayson 2011; Frevel 2014; Motl 2011; Paul 2014; Pilutti 2014).
Two studies used a random number generator for randomisation (Dlugonski 2012; Pilutti 2014)
One study used a random permutated block design (Finlayson 2011)
One randomly allocated the participants using simple allocation by drawing lots of preshuffled opaque envelopes (Frevel 2014)
One study used a series of random numbers generated in Microsoft Excel (consecutive numbers allocated, where even numbers represented the intervention group and odd numbers the control group) (Paul 2014)
Only three studies described in detail concealment of allocation prior to entry to the study (Finlayson 2011; Frevel 2014; Motl 2011). Other studies either gave little or no information about the randomisation procedure, or used non‐random components like alternation, assignment to comparable groups with respect to clinical and demographic factors, or allocation of participants to the intervention group after initial randomisation.
Blinding
Blinding of participants and treating personnel can be challenging in rehabilitation trials, because of the characteristics of interventions. However, blinding of outcome assessors is possible and highly desirable (Amatya 2013). The blinding of participants and personnel was insufficiently reported in most of the studies. Only one study took measures to blind participants to group allocation (Finlayson 2011). None of the studies attempted to blind the treating personnel. One study mentioned blinding of the outcome assessors, but provided no details (Gutíerrez 2013a).
Incomplete outcome data
The drop‐out rate of participants during the trial period ranged from 0% to 21%. In four studies, there were no or minimal losses to follow‐up (Dlugonski 2012; Egner 2003; Gutíerrez 2013a; Paul 2014). Drop‐outs and withdrawals were higher than 20% in only one study (Finlayson 2011), which recruited the highest number of participants. One study which included MS participants as one of the subgroups failed to report the attrition rate (Huijgen 2008). Most of the studies did not conduct intention‐to‐treat analysis.
Selective reporting
All the included studies reported prespecified (primary and secondary) outcomes (see Table 2 and Table 4 for a list of the outcome measures).
3. Summary of outcome assessed in the included studies.
|
Study |
Outcome assessed* | |||
| Function | Impairment | Participation | Others | |
| Dlugonski 2012 | GLTEQ, MSWS‐12 | MSIS‐29 | PDDS, SATISFACTION | |
| Egner 2003 | FSS | QWB, CES‐D | ||
| Finlayson 2011 | FIS, FSS | SF‐36 | ECQ, PDDS | |
| Frevel 2014 | BBS, DGI, TUG, 2MWT | MFIS | HAQUAMAS | Muscle strength |
| Gutíerrez 2013a | SOT, MCT, BBS, TS | |||
| Huijgen 2008 | ARAT, NHPT | VAS satisfaction survey | ||
| Motl 2011 | GLTEQ, LL‐FDI, EXCE, MOEES | EGS, PDSS | ||
| Paul 2014 | 25 FWT, BBS, TUG | MS related symptom check list | MSIS, LMSQOL, HADS | |
| Pilutti 2014 | GLTEQ | MFIS, FSS, MPQ, PSQI | MSIS‐29, HADS | PDDS |
| Sandroff 2014 | 6MWT, IPAQ | SDMT | PDDS | |
*Categorised according to the International Classification of Functioning, Disability and Health (ICF, WHO 2001)
ARAT: Action Research Arm Test; BBS: Berg Balance Scale;CDP: Computerized Dynamic Posturography; CES: Composite Equilibrium Score; CES‐D: Center for Epidemiologic Studies Depression Scale; DGI: Dynamic gait Index;ECQ: Energy Conservation Questionnaire; EDSS: Expanded Disability Status Scale; EGS: Exercise Goal setting Scale;EXSE: Exercise Self‐Efficacy Scale; FIS: Fatigue Impact Scale; FSS: Fatigue Severity Score; GLTEQ: Godin Leisure‐Time Exercise Questionnaire; HADS: Hospital Anxiety and Depression Scale; HAQUAMS: Hamburg Quality of Life Questionnaire in Multiple Sclerosis; IPAQ: International Physical Activity Questionnaire; LMSQOLS: Leeds Multiple Sclerosis Quality of Life Scale;LL‐FDI: Late‐Life Function and Disability Instrument; MCT: Motor Control Test; MOEES: Multidimensional Outcomes Expectations for Exercise Scale; MPQ: McGill Pain Questionnaire; MSIS‐29: Multiple Sclerosis Impact Scale; MSWS‐12: Multiple Sclerosis Walking Scale–12; NHPT: Nine Hole Peg Test; PARQ: Physical Activity Readiness Questionnaire; PDDS: Patient Determined Disease Steps; PSQI: Pittsburgh Sleep Quality Index; QWB: Quality of Well‐ Being Scale; SDMT: Symbol Digit Modalities Test; SF‐36: 36‐Item Short Form Health Survey; SOT: Sensory organisation Test; TS: Tineti Scale; TUG: Timed Up and Go;VAS: Visual Analogue Scale; 6MWT: 6 minute walk test; 25FWT: 25 Foot Walk Test
Other potential sources of bias
Sample sizes were small (< 40 participants) in four studies (Egner 2003; Frevel 2014; Huijgen 2008; Paul 2014). A series of three studies was conducted by the same group of authors, which recruited selective participants who volunteered for research through a single database for the same institutions (Dlugonski 2012; Motl 2011; Pilutti 2014). Although none of these studies mentioned overlapping of the recruited participants, we cannot rule out the possibility of inclusion of the same participants in different trials. Furthermore, this series of studies published one trial (Pilutti 2014) with different outcomes in another report (Sandroff 2014). Most included studies had short‐term follow‐up, and were restricted to immediate post‐treatment assessments. Most studies seemed to be underpowered and only one study performed a sample size calculation (Finlayson 2011). One study (Egner 2003 ) failed to report the participant recruitment process and methodology in detail, and allocation of participants to treatment and control groups was unbalanced in two studies (Egner 2003; Huijgen 2008).
Effects of interventions
See: Table 1
Meta‐analysis was not possible due to the heterogeneity of the included studies mentioned earlier. The included studies used a range of telerehabilitation approaches in pwMS (see Table 3 for the summary of telerehabilitation interventions) and a broad range of outcome measures (see Table 4 for a list of outcome measure used). A summary of the findings of the included trials is presented based on primary and secondary outcomes categorised according to the International Classification of Functioning, Disability and Health (ICF) framework in the Table 1. Pooling of data from the included studies was confounded by the differences between interventions and the use of different outcome measures, as highlighted above.
Primary outcomes
Improvement in functional activity
All studies except two (Egner 2003; Finlayson 2011) assessed the first prespecified primary endpoint to improve functional activity in pwMS (N = 314 participants,low quality evidence). All studies evaluated participants immediately after the intervention, using different instruments (see Table 4 and Table 1), with intervention periods ranging from one to six months. Overall six studies assessed the functional endpoint post‐intervention up to 12 weeks (Dlugonski 2012; Frevel 2014; Gutíerrez 2013a; Huijgen 2008; Motl 2011; Paul 2014).
Two studies (Dlugonski 2012; Motl 2011) conducted in different time periods with the same cohort of participants showed significant improvement in physical activity in the treatment group at the post‐intervention assessment, as measured by the Godin Leisure‐Time Exercise Questionnaire (GLTEQ) (P < 0.01). The authors' reported increase in physical activity was sustained at three‐month follow‐up compared with the control group (P < 0.001) (Motl 2011).
One study (Frevel 2014) comparing two interventions, e‐training and hippotherapy, showed significant improvement in dynamic and static balance capacity compared with baseline values in both the intervention (e‐training) (Dynamic Gait Index (DGI): P = 0.016, Berg Balance Scale (BBS): P = 0.011) and control (hippotherapy) groups (DGI: P = 0.011, BBS: P = 0.011). However, there was no difference between groups.
Huijgen 2008 showed no statistically significant differences between the intervention using telerehabilitation for arm functions (Home Care Activity Desk (HCAD)) and control groups in arm function as measured by Action Research Arm Test (ARAT) (mean change 1.26, 90% confidence interval (CI) ‐1.90 to 4.42) and Nine‐Hole Peg Test (NHPT) (mean change 7.24, 90% CI ‐6.55 to 23.25).
Paul 2014 reported an increase in gait speed using the 25 Foot Walk Test (25FWT) in the intervention group compared with the control group, but this was not statistically significant (P = 0.170). The intervention group had a statistically significant improvement in the physical subscale of the Multiple Sclerosis Impact Scale (MSIS) (P = 0.048).
Another study (Gutíerrez 2013a) showed improvements in balance and postural control, with a significant increase in Composite Equilibrium Score (CES) in the intervention group (mean change 8.21 points, P < 0.001), but not in the control group (mean change 1.93, P = 0.123). Visual Preference Ratio and contribution of vestibular information (VEST, Vestibular Ratio) improved significantly in the intervention group (P < 0.001), but not in the control group (P > 0.05). There were significant post‐treatment differences between treatment and control groups in the CES (F = 37.873, P < 0.001) and the VEST (F = 12.156, P < 0.001). Significant post‐treatment differences between groups were also found for the ability to accept incorrect visual information expressed by the visual conflict parameter (F = 15.05, P < 0.000), which demonstrates that the treatment group showed a greater ability to accept post‐treatment afferent inputs compared with the control groups. There were no significant between‐group differences in the contribution of the visual system (F = 2.64, P = 0.11) or use of somatosensory information (F = 0.117, P = 0.734) in the maintenance of balance and stability.
One study (Sandroff 2014) evaluating an internet‐delivered behavioural intervention, showed a significant positive effect of the intervention on the Six Minute Walk (6MW) test relative to the control group (P = 0.07). The authors also found physical activity increased most in those with mild disability.
Improvement in impairments
Five studies assessed the prespecified primary endpoint (improvement in impairments) using different measures (N = 347 participants;low quality evidence) (Egner 2003; Finlayson 2011; Frevel 2014; Paul 2014; Pilutti 2014).
Fatigue was the primary outcome in three studies (Egner 2003; Finlayson 2011; Pilutti 2014), all reporting significant differences between groups in favour of the intervention group. One study (Finlayson 2011) showed a significant reduction in fatigue in the intervention group immediately after intervention compared to a wait‐list control group as measured by the Fatigue Impact Scale (FIS) in all three subscales: mean difference (SD): Cognitive ‐3.12 (6.1), P = 0.001; Physical ‐2.53 (6.4), P = 0.014; Social ‐6.01 (12.1), P = 0.002. These changes were maintained with large effect sizes in all FIS subscales at three‐month follow‐up: Effect Size (95% CI): Cognitive 0.58 (0.48 to 0.68); Physical 0.68 (0.55 to 0.82); Social 0.65 (0.53 to 0.77), and at six‐month follow‐up: Cognition: 0.55 (0.46 to 0.64); Physical: 0.61 (0.5 to 0.72) and Social: (0.67 (0.58 to 0.76). There was also a significant reduction in the Fatigue Severity Scale (FSS) scores at all three time periods.
Egner 2003 analysed the impact of a telerehabilitation intervention (structured in‐home counselling and education) delivered via telephone or video, and reported similar fatigue scores (measured using FSS) for all three groups (video, telephone and standard care) at nine weeks post‐intervention; however, the participants in the video group had significantly lower scores than the other two groups at six months (P < 0.05) and at 18 months (P < 0.05).
One study (Pilutti 2014) showed a significant positive effect of the behavioural intervention on fatigue severity (FSS, P = 0.001) and its physical impact (FIS, P = 0.008) at six‐month post‐intervention. There was a favourable effect of the intervention on symptoms of pain (McGill Pain Questionnaire (MPQ), P = 0.08) and sleep quality post‐trial (Pittsburgh Sleep Quality Index (PSQI), P = 0.06), although the differences between groups did not reach statistical significance.
Frevel 2014 reported significant improvement in fatigue in the control group (hippotherapy) (P < 0.05) for all subscales of the Modified Fatigue Impact Scale (MFIS), while the intervention group (e‐training) improved only on the MFIS cognitive subscale (P = 0.031). A significant difference between the groups was noted only in the cognitive subscale of the MFIS ( P = 0.012).
One study (Paul 2014) reported no improvements in symptoms as measured by the MS Symptom Checklist.
Improvement in participation
Psychological outcomes
Overall three studies (one with two reports), assessed cognitive functions as one of the outcomes (N = 139 participants, low quality evidence) (Egner 2003; Paul 2014; Pilutti 2014).
Egner 2003 showed that a telerehabilitation intervention (structured in‐home counselling and education) delivered via telephone or video, improved depressive symptoms as measured by the Centre for Epidemiologic Studies Depression Scale (CES‐D) at the end of the intervention period (nine weeks) in both groups. Mean CES‐D scores fluctuated, but decreased at 24 months in all three groups. This was, however, not statistically significant. Mean depression scores were lower in those receiving telerehabilitation by video compared with telephone and standard‐care groups, and depressive symptoms also decreased at the six‐, eight‐ and 24‐months follow‐ups, but this was not significantly different between groups. The authors reported that being male was a significant predictor for increased depression score at every measurement point except at 24 months (P < 0.05) (Egner 2003).
Paul 2014 reported a small non‐significant improvement in anxiety measured by the Hospital Anxiety and Depression Scale (HADS) in the control group compared to the treatment group post‐treatment (eight to nine weeks) (P = 0.016).
One study with two reports (Pilutti 2014) showed a statistically significant group interaction in psychological outcomes on Symbol Digit Modalities Test (SDMT) scores (F = 5.68, P = 0.02), which was moderate in magnitude (partial eta squared (ɳ₂) = 0.08). There was a clinically meaningful improvement in SDMT scores in the subgroup with mild disability in the intervention condition (∼ 6 points increase, moderate effect size (d) = 0.41), whereas those with moderate disability in the intervention condition demonstrated minimal change (∼ 1 point decrease, d = 0.12). There were minimal changes in SDMT scores for those with mild or moderate disability (∼ 1 point increase, d = 0.10 for both) in the control group. There was also significant improvement in depression and anxiety in the intervention group (with large effect size (ɳ₂ = 0.10 for both) compared with the control group measured by the HADS (depression: F =7.90, P = 0.006; anxiety: F = 8.00, P = 0.006) (Pilutti 2014).
Quality of life
Six studies assessed quality of life (QoL), using different outcome measures (N = 392 participants;low quality evidence) (Dlugonski 2012; Egner 2003; Finlayson 2011; Frevel 2014; Paul 2014; Pilutti 2014).
Egner 2003 reported no significant difference in QoL between the treatment groups (video or telephone) and control group (standard care) measured using the Quality of Well‐Being Scale (QWB) at the end of the intervention period (nine weeks). However, mean QWB scores for each measurement point (6‐, 9‐, 12‐, 18‐ and 24‐months) were higher (indicating higher QoL) for participants in the video group than for those in the standard care and telephone groups. There were significantly higher QWB scores in the video compared with the telephone groups at 12 months follow‐up only (P < 0.05; standard error (SE) = 0.023). The telephone group and standard‐care groups reported similar mean QWB scores over the two‐year follow‐up period (Egner 2003).
One study (Frevel 2014) showed significant improvement in QoL measured by the Hamburg Quality of Life Questionnaire in Multiple Sclerosis (HAQUAMS) (in subscales ‐ Cognition: P = 0.026; Function of lower limb: P = 0.008; Mood: P = 0.045) in the control group (hippotherapy), but not in the intervention group (e‐training).
Finlayson 2011 reported that a fatigue management programme showed significant improvement in QoL in the intervention group on the 36‐item Short Form Health Survey Questionnaire (SF‐36) in all subscales except physical functioning and bodily pain (change score (95% CI)): Vitality 6.99 (4.29 to 9.69); Role Emotion 10.08 (4.13 to 16.04); Mental Health 5.78 (3.89 to 7.67); Social Function 7.95 (4.09 to 11.82); General Health 3.61 (1.37 to 5.85); Role Physical 11.12 (6.22 to 16.02).
Two studies (Dlugonski 2012; Pilutti 2014) assessed QoL using the Multiple Sclerosis Impact Scale (MSIS‐29) and found no significant correlation between changes in QoL from baseline to post‐intervention in either treatment or control groups.
Similar non‐significant improvement in QoL was reported in another study (Paul 2014), at post‐treatment (eight to nine weeks), in which authors used the Leeds Multiple Sclerosis Quality of Life Scale (LMSQOL) (mean difference in treatment group: ‐0.07 versus control group: 1.0).
Secondary outcomes
Two studies (Dlugonski 2012; Huijgen 2008) reported process evaluation (satisfaction and acceptance of the telerehabilitation).
Dlugonski 2012 used a five‐item Likert satisfaction scale and found that participants were most satisfied with the overall programme (mean ± SD):4.8 ± 0.4, staff: 4.9 ± 0.2 and pedometer: 4.7 ± 0.6, but slightly less satisfied with the website: 4.1 ± 0.9.
Huijgen 2008 used a six‐item Visual Analogue Scale (VAS) to evaluate users' and therapists' satisfaction with the upper limb telerehabilitation intervention. Overall, both participants and therapists were satisfied with the intervention (over 55% in all six items). The authors found that both participants and therapists were less satisfied with the aesthetic aspect of the intervention and had difficulty in completing prescribed tasks.
No studies reported data on cost effectiveness, investment costs or resource utilisation. None of the included studies reported any serious adverse effects attributable to telerehabilitation. carer burden or social integration (in the form of return to work, study etc.) were not evaluated in any of the studies.
Discussion
This review investigated the effectiveness of different forms of organised telerehabilitation in adults with multiple sclerosis (MS) on measures of activities, impairments and participation based on the International Classification of Functioning, Disability and Health (ICF) framework (WHO 2001), and also of the safety and cost effectiveness of these interventions. There was marked heterogeneity between the included trials in terms of characteristics, type and mode of delivery of the telerehabilitation interventions, measurement tools used (even for identical outcomes), treatment and control protocols and length of follow‐up. We therefore performed a best‐evidence synthesis using a qualitative analysis.
Summary of main results
This review of nine randomised controlled trials (RCTs) (one with two reports), involving 531 participants with MS (N = 277 participants in the intervention group) evaluated a wide variety of telerehabilitation interventions (see Table 3). All telerehabilitation interventions were complex, using more than one active rehabilitation component which differed in many aspects, including intervention goals, number and extent of the intervention components, duration and intensity, and mode of delivery. Control interventions also differed between studies ranging from 'usual care' or 'wait‐list' to active intervention (such as hippotherapy, Frevel 2014). Most interventions included physical activity as one of the main intervention components, followed by education and behavioural training. The included trials were heterogeneous in terms of outcome measures used and study quality. Quantitative synthesis was therefore not possible. A qualitative synthesis of 'best evidence’ for telerehabilitation interventions indicates low level evidence for:
Short‐term benefit in improving functional activities, such as physical activity, balance capacity and postural control compared with baseline, and some benefit in improving walking, physical activity;
Short‐term benefit in reducing and/or improving impairments, such as fatigue, and long‐term benefits in improving symptoms such as fatigue, pain and insomnia;
Longer‐term improvement in participation, such as improving psychological outcomes and quality of life (QoL)
There is a 'very low' level of evidence for participants' and therapists' satisfaction with the telerehabilitation interventions.
The quality of evidence is further compromised by the limited number of studies, heterogeneity and the methodological weaknesses identified (underpowered with small sample sizes, high risk of bias, short follow‐up periods, lack of rigorous methodology and different outcome measures) amongst the included trials.
Subgroup analysis for type of telerehabilitation intervention (unidisciplinary or multidisciplinary, or both), type of MS (relapsing remitting, progressive), severity of MS (Expanded Disability Status Scale (EDSS) < 6; > 6) and duration of follow‐up of participants (≤ 3 months; > 3 months) was not possible due to lack of data. There were no data for the cost effectiveness of telerehabilitation interventions, their impact on health service utilisation (hospitalisation or attendance/access to the health services) and carer burden or social integration (in the form of return to work, study etc.). There were limited data on process evaluation (satisfaction and acceptance of the telerehabilitation) and no reports of serious adverse effects attributable to telerehabilitation.
Overall completeness and applicability of evidence
Overall, this review indicates that telerehabilitation has some impact on improving function and symptoms (including cognitive function), but does not have an appreciable impact on disease‐specific QoL in persons with MS (pwMS). There are no cost data or data on hospitalisation or access to other services. As aforementioned, there was marked variation between studies concerning the content and mode of delivery of the interventions. This highlights the diversity of programmes currently offered to pwMS.
Pooling data for meta‐analyses to make meaningful statements for both primary and secondary outcomes was not possible. The generalisability and applicability of the results are limited, as most studies recruited participants from a single centre with strict inclusion and exclusion criteria. Moreover, generalisability of results to different countries and healthcare systems also seems limited, as the studies were conducted predominantly in the USA and Europe.
Quality of the evidence
In general, we rated the nine included studies (one with two reports) as of 'low' methodological quality due to substantial flaws in their methodological design with various biases observed. These included a lack of proper randomisation, problems with allocation concealment and a lack of blinding. Further, there was also insufficient information about these specific methodological issues, so that many domains of the 'Risk of bias' tables are rated as 'unclear’ (see Figure 2 and Figure 3). All studies except one were single‐centre trials, with fairly small participant numbers, with a concomitant risk of type I and II errors. The evidence is very heterogeneous, particularly in terms of interventions (technology employed, rehabilitation components within the intervention, duration and intensity of the intervention etc.), and diverse outcome measures used. The other methodological flaws include:
High risk of selection bias, as only three studies (Finlayson 2011; Frevel 2014; Sandroff 2014) described allocation concealment
Lack of description of the randomisation procedure, adequately reported in only three studies (Finlayson 2011; Frevel 2014; Paul 2014)
High risk of performance bias due to non‐blinding of the study participants and treating personnel; participants were blinded to group allocation in only one study (Finlayson 2011), but treating personnel were not blinded; and only one study took measures to blind outcome assessors (Gutíerrez 2013a)
Most studies were underpowered with small sample sizes
Lack of an intention‐to‐treat analysis protocol in most trials
Lack of longer‐term follow‐up to detect the long‐term effects of intervention; only three studies (one with two reports) followed the participants beyond three months (Egner 2003; Finlayson 2011; Pilutti 2014);
Lack of control for participants’ personal and other confounding factors, which influence patient‐therapist interaction, compliance, and delivery of therapy, thus impacting on outcomes such as participant motivation and self efficacy, comorbidity and activity level outside of therapy programmes (not assessed in any of the studies).
Potential biases in the review process
We conducted the search in conjunction with the Trials Search Co‐ordinator from the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Working Group in the Cochrane MS Group Specialised Register using a broad search strategy. In order to avoid publication bias, we performed literature searches at three different time points. This process would have captured both published and ongoing trials coded as MS by the Cochrane MS Group. Two review authors further selected relevant articles from this extensive list independently and agreed on a final list of included studies by consensus between all four review authors. We applied no language restriction, although all the included trials were published in English. Overall, the review methodology is comprehensive, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). However, we recognise a number of limitations in the methodology of the review itself, and the completeness of the retrieved literature:
Four of the included studies in this review (Dlugonski 2012; Motl 2011; Pilutti 2014; Sandroff 2014) were conducted by the same group of authors in the same cohort of participants recruited from a single database, and using the same behavioural intervention (modified in recent publications). Hence, we cannot rule out overlapping of the participants amongst the studies.
We categorised outcomes according to the World Health Organization (WHO) ICF, which might have posed some methodological problems, since many of the outcome measures used in the included trials crossed the boundaries between the different levels of the ICF model. However, this model is widely used worldwide and helpful in clarifying the experience of people who live with long‐term neurological conditions, such as MS (Khan 2007).
We cannot rule out some degree of selection bias from the literature search (Van Tulder 2003), given that the search strategy principally encompassed the cited literature, despite the extended range of terms for both MS and telerehabilitation that we used to capture the widest possible selection from the relevant literature.
We cannot rule out publication bias as we cannot exclude the possibility that there have been negative trials that have not reached the published literature (Egger 1998).
Reference bias (Gøetzsche 1987) is possible, as we searched the bibliography lists of only relevant papers for other possible articles missed in our electronic searches.
We therefore welcome contact from any readers who are aware of important studies that would meet the criteria for this review, but have not so far been included.
Agreements and disagreements with other studies or reviews
To date, there has been no systematic review assessing the effectiveness of telerehabilitation in pwMS to guide treating clinicians or policy makers. Positive effects and successful implementation of telerehabilitation were reported in various neurological conditions including stroke (Johansson 2011; Legg 2004), Parkinson’s disease (Giansanti 2008) and other non‐neurological conditions such as musculoskeletal conditions (Russell 2011; Tousignant 2011), injuries (Bendixen 2008; Forducey 2003; Houlihan 2011) and chronic diseases (Steel 2011). We found one systematic review (Hailey 2011) (also published earlier as a health technology assessment, Hailey 2010), with some overlap with our results. That review considered the evidence of benefit from the use of telerehabilitation for various conditions, including neurological ones. The authors conducted comprehensive searches in multiple databases up to November 2009 and included two studies (one observational and one RCT) on telerehabilitation in the management of people with MS. That review provided simply an overview of studies on telerehabilitation for certain groups of conditions in terms of feasibility of interventions, the clinical significance of results, and a requirement for further data to establish the application as suitable for routine use. Consistent with the results of our review, the authors found inconsistent or insufficient evidence of benefit for telerehabilitation interventions and their impact on routine rehabilitation programmes.
Authors' conclusions
Implications for practice.
Multiple sclerosis (MS) is a complex condition with different patterns of presentation and variable prognoses. The care needs in this population are complex due to cumulative effects of the impairments and disabilities, the ‘wear and tear’, and the impact of aging with a disability. Therefore, issues related to progressive disability, psychosocial adjustment and social re‐integration progress over time need to be considered. These have implications for persons with MS (pwMS), their family/carers, treating clinicians and society as a whole, in terms of healthcare access, provision of services and financial burden (Beer 2012; Khan 2010a). Multidisciplinary rehabilitation is recommended and proven to be effective for pwMS, both in hospital and in communities, to maintain functional gains and social re‐integration (participation) (Khan 2007). However, many pwMS are unable to access appropriate treatment due to limited mobility, fatigue and related issues, and limited access to services, and the costs and time associated with travel.
With advances in information and communication technology, new models of care such as telerehabilitation can be an alternative efficient and cost‐effective method to deliver rehabilitation. The MS population is likely to be receptive to and benefit from this type of care model as most are young and have high rates of internet use (Motl 2011; NMSS 2007). Telerehabilitation is an alternative to traditional face‐to‐face interventions, providing equal access for individuals who are geographically remote and for those who are physically and economically disadvantaged, and can improve the quality of rehabilitation delivered by addressing associated issues of efficacy, problems of generalisation and increasing patient participation and satisfaction with treatment (Hailey 2011; Kairy 2009; Rogante 2010). It can give healthcare providers an opportunity not only to evaluate the interventions previously prescribed, but also to monitor adverse events and identify areas in need of improvement by evaluating patients’ progress (McCue 2010). Moreover, it provides an opportunity to optimise the timing, intensity and duration of therapy as required, which may not always be possible within the constraints of face‐to‐face treatment protocols and scheduling in current health systems (Hailey 2011; Steel 2011). MS is a complex and challenging condition requiring individualised and integrated multidisciplinary care. The range of telerehabilitation interventions and their intensity requirements can vary from person to person and are difficult to standardise. Various factors such as the patient's personal characteristics, their comorbidities, functional and coping abilities, family dynamics, and the healthcare system may impact patient outcomes (Khan 2010b). There is a paucity of information on the interaction of these factors on patient outcomes and very little is understood about the 'black box' of rehabilitation in the MS population (Khan 2010b)
This review highlights the lack of robust, methodologically‐strong studies evaluating the effectiveness of telerehabilitation intervention in this population. Overall, the review found low quality evidence for a beneficial effect of telerehabilitation interventions on reducing short‐term disability and impairments, such as fatigue. There was also low‐quality of evidence suggesting some benefit in improving functional activities and impairments in the longer term, and improving psychological outcomes and quality of life (QoL). There are limited data on process evaluation (participants' and therapists' satisfaction) and, surprisingly, none of the studies addressed cost effectiveness.
Telerehabilitation has a major role in providing remote rehabilitation to people with chronic neurological conditions in future, and has potential to fill the existing service gap in the care of pwMS. However, the clinical applicability of the findings of this review and the effectiveness of telerehabilitation interventions need to be confirmed in future research.
Implications for research.
This review found various limitations and gaps in knowledge, which could suggest directions for future research. These include, but are not limited to:
More methodologically robust studies, e.g. randomised controlled trials (RCTs) comparing different models and intensity of telerehabilitation
Large‐scale systematic and 'practice‐based trials' in which data are routinely gathered without disrupting the natural milieu of treatment to provide valuable information about outcomes in real‐life clinical settings
Use of more sensitive and appropriate validated outcome measures that are important for patients and their representatives and that focus on impairments, activity limitations and restriction in participation
Longitudinal data in the MS population to ascertain long‐term care needs
More research about patient and carer perspectives and their involvement in telerehabilitation
Research about specific telerehabilitation modalities and interventions in MS to improve evidence‐based practices
Cost effectiveness of telerehabilitation
More emphasis on participatory domains (cognitive outcomes and quality of life (QoL)) in MS for impact on societal integration
Future studies in telerehabilitation should focus on improving the methodological and scientific rigour of clinical trials, with larger sample sizes and with longer‐term follow‐up. Further, active clinician involvement is needed to build evidence in this area for everyday clinical practice.
Acknowledgements
We thank the Editorial Board and Ms Liliana Coco, the Managing Editor, of the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Review Group for their support and assistance. We are grateful to Ms Andrea Fittipaldo, the Trials Search Co‐ordinator, for literature searches, and Dr. Paola Mosconi for reviewing the manuscript.
Appendices
Appendix 1. Keywords
{telecommunications*} OR {telemedicine} OR {telehealth} OR {telehealthcare} OR {telecoahing} OR {e‐health} OR {e‐medicine} OR {mobile health} OR {information technology} OR {information communication technology} OR {internet} OR {web‐based} OR {computer} OR {Software} OR {videoconferencing} OR {remote consultation} OR {remote sensing technology} OR {rehabilitation} OR {physiotherapy} OR {occupational therapy} OR {speech therapy} OR {dietician}
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dlugonski 2012.
| Methods | RCT, parallel group with wait‐list controls; USA Study period: one month period of July 2010 Funding source: not mentioned Declaration of interest: not mentioned |
|
| Participants | N = 45: treatment group = 22 and control = 23 Inclusion: Diagnosis of relapsing‐remitting MS (RRMS); relapse‐free in the past 30 days; Internet access; willingness to complete questionnaires; wear pedometer during intervention period; being non‐active, defined as engaging in regular activity (30 minutes accumulated/day) on ≤ 2 days of the week during previous 6 months; ability to ambulate with or without assistance (i.e., walking with or without a cane/walker, but not a wheelchair or scooter); free of contraindication for physical therapy (e.g., no underlying cardiovascular disease); physician approval for beginning a physical activity programme Exclusion: not specified Demographic characteristics: Mean age 46.6 years (SD: 9.7 years), 86.7% women, mean time since diagnosis 9.4 years (SD: 7.8 years), 64.4% had at least college degree, 95.6% white, 62.2% employed and 73.3% married |
|
| Interventions | (similar to Motl 2011) Treatment group: Internet‐delivered and social cognitive theory (self efficacy, outcome expectations, impediments, and goal setting) based behavioural intervention supplemented with video coaching for 12 weeks, which included text‐based content supplemented by video and portable document format (PDF) files (i.e. multimedia). The intervention consisted of 4 essential modules: Getting Started (benefits of physical activity and information for becoming more physically active), Planning for Success (goal setting and feedback, outcome expectations, and self efficacy), Beating the Odds (barriers and strategies of overcoming barriers, and social support), and Sticking with It (maintaining an active lifestyle and physical activity relapse prevention), with 10 total Chapters. This was further supported by automated e‐mail announcements about new information, updates, and changes on the web‐site Additionally, 7 one‐on‐one web‐based video coaching interactive sessions (5 ‐ 10 minutes) using web‐cam were conducted (4 in the first month, 2 in second month and 1 in third month), by an experienced doctoral student. The coaching sessions included discussions about progress towards goal achievements, content of website and adverse events For goal‐setting and self‐monitoring purposes a pedometer, log book to record steps and computer programme “Goal tracker” to upload weekly steps counts onto the website were provided Control group: wait‐list participants, who received the intervention materials after study completion |
|
| Outcomes |
Primary outcome: Physical activity: GLTEQ Secondary outcome: Walking mobility: MSWS‐12; QoL: MSIS‐29; disease severity: PDDS; participant satisfaction (Process evaluation questionnaire) Assessment time points: Baseline, post‐intervention (12 weeks) and 3 months |
|
| Notes | This study follows an earlier study (see below Motl 2011) and evaluated the same cohort of participants from a single database for similar intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were paired based on their baseline level of activity (GLTEQ) and neurologic disability (PDDS) score by the authors, then randomised using a random number table |
| Allocation concealment (selection bias) | High risk | Not reported, as randomisation was performed pairwise, allocation concealment was unlikely |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and treating personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall, only 1 participant from control group dropped out. ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | All prespecified (primary and secondary) outcomes reported |
| Other bias | Unclear risk | Selective participants: recruitment occurred through a database of self‐volunteering persons for research |
Egner 2003.
| Methods | RCT, 3 parallel groups; USA Study period: not mentioned Funding source: grant from the Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities, USA Declaration of interest: not mentioned |
|
| Participants | N = 27: Group 1 (video) = 9; Group 2 (telephone) = 11 and Group 3 (standard care) = 7 Inclusion: diagnosis of MS; experience of a recent functional setback in the disease process, such as a severe exacerbating episode or an increase or start of chemotherapy treatment; EDSS score ≥ 7 Exclusion: not specified Demographic characteristics: Mean age 46.0 years (SD: 9.0 years), 63% women, 44% married, 37% African –Americans and mean EDSS score of 7.8 (SD 0.6) |
|
| Interventions |
Treatment group (Groups 1 and 2): structured in‐home education and counselling session delivered via telephone or video by a rehabilitation nurse, which included individual rehabilitation education sessions (structured review of skin care, nutrition, bowel and bladder routines, psychosocial issues and any equipment needs, and referrals to mental health counsellors, physical therapists, or other health professionals as needed. The same protocol was followed for the video and telephone groups with video group trained in the use of the Plain Old Telephone System (POTS) units in their home which provided image and sound Sessions: 30 ‐ 40 minutes, weekly for a period of 5 weeks, then once every 2 weeks for 1 month. Control group: usual care with regular follow‐up offered by the rehabilitation facility |
|
| Outcomes |
Primary outcome: Fatigue: FSS; HRQOL: QWB; Depression: CES‐D Secondary outcome: none Assessment time points: Baseline, 5 weeks during intervention, post‐intervention (9 weeks) and every month for 24 months |
|
| Notes | This study was part of a larger study of the impact of a telerehabilitation intervention on people with severe mobility impairment, with people with spinal cord injuries and the prevention of pressure sores as the primary group of interest of the project | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to 1 of 3 intervention groups: video, telephone, or standard care. Further details not provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and treating personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs in either group |
| Selective reporting (reporting bias) | Low risk | All prespecified (primary and secondary) outcomes reported |
| Other bias | Unclear risk | Participant recruitment process and methodology not described in detail No power calculation for the study Small sample size with unbalanced allocation of participants to groups ITT analysis not performed |
Finlayson 2011.
| Methods | RCT, 2‐group time series design with a wait‐list control group; USA Study period: November 2007 to April 2009 Funding source: Field‐Initiated Research Grant, National Institute of Disability and Rehabilitation Research, USA Declaration of interest: authors declared no conflict of interest |
|
| Participants | N = 190: treatment group = 94 and control group = 96 Inclusion: living within the state of Illinois; diagnosis of MS; ≥ 18 years; functional English literacy; Fatigue Severity Scale (FSS) score ≥ 4 (i.e. moderate to severe fatigue); weighted score of at least 12 on the short version of the Blessed Orientation Memory Concentration test. Exclusion: not provided Demographic characteristics: Mean age 56 yrs (SD 9), 79% women, mean disease duration 15 yrs (SD 9 yrs), 88% white, 52% RRMS; 37% employed; 98% with education > 12 years |
|
| Interventions |
Treatment group: a 6‐week group‐based, teleconference‐delivered (70‐minute) fatigue management programme, facilitated by a licensed OT Control group: wait‐list control group receiving treatment after 8 ‐ 12 weeks |
|
| Outcomes |
Primary outcome: fatigue impact: FIS, fatigue severity: FSS; HRQoL:SF‐36 Secondary outcome: self efficacy: ECQ Assessment time points: Baseline, post‐intervention (6 weeks) 3 months and 6 months |
|
| Notes | No adverse events were identified during the trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants' randomisation completed by the statistician using a random permutated block design with each block consisting 4 people |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes were used and prepared in advance of recruitment. The envelopes were numbered sequentially and a statement indicating the allocation (immediate or wait‐list) was placed in each envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants blinded to group allocation only and treating personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall 39 participants (20.5%) drop‐out (17 in intervention, 22 in control group) |
| Selective reporting (reporting bias) | Low risk | All prespecified (primary and secondary) outcomes reported |
| Other bias | Unclear risk | ITT analysis performed for effectiveness analysis |
Frevel 2014.
| Methods | RCT, parallel group; Germany Study period: not mentioned Funding source: not mentioned Declaration of interest: not mentioned |
|
| Participants | N = 18: treatment group = 9 and control = 9 Inclusion: Definite MS diagnosis according to McDonald's criteria, EDSS 2‐6, ability to stand with or without an assistive device for 1 minute, age 18 ‐ 60 years, clinical stability for last 4 weeks Exclusion: clinically relevant internal or orthopaedic diseases unrelated to MS, an allergy or aversions to horses or previous experience with hippotherapy or therapeutic ridings (since diagnosis of MS) Demographic characteristics: Mean age 45.5 years (range 32 ‐ 57), mean EDSS 3.8 (range 2 – 6), mean disease duration 19.0 (range 1 ‐ 35), RRMS 67% |
|
| Interventions |
Treatment group; Internet‐based home training: balance, postural control exercises and strength training for main group of muscles of the lower extremities, trunk and shoulder griddle. Participant provided feedback (Borg scale) to the therapist, which provided further feedback after each sessions (duration 2 training sessions (45 minutes)/week for 12 weeks). Further, participants had an informative supervised meeting and received instructions and software prior Control group: hippotherapy twice per week/ 20 – 30 minutes under supervision of riding therapist for 12 weeks |
|
| Outcomes |
Primary Outcomes: Balance: BBS, DGI Secondary outcomes: Isometric muscle strength of knee and trunk; TUG; 2MWT; HAQUAMS, FSS, MFIS Assessment time points: Baseline and post intervention (12 weeks) |
|
| Notes | No report of adverse events | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by simple allocation by drawing lots of preshuffled opaque envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes containing an identifier were used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and treating personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall, 2 participants in treatment group dropped out (11%) |
| Selective reporting (reporting bias) | Low risk | All prespecified (primary and secondary) outcomes reported |
| Other bias | Unclear risk | No power calculations for the study No ITT analysis Small sample size |
Gutíerrez 2013a.
| Methods | RCT, parallel group; Spain Study period: not mentioned Funding source: not mentioned Declaration of interest: authors declared no conflict of interest |
|
| Participants | Spain. N = 50: treatment group = 25 and control group = 25 Inclusion: Confirmed diagnosis of MS for > 2 years based on McDonald's criteria; age 20 ‐ 60 years; medically stable during 6 months prior to baseline assessment; impaired balance demonstrated by MRI; EDSS score of 3 ‐ 5; Hauser ambulatory index > 4, absence of cognitive impairment (MMSE ≥ 24); no visual deficit; internet connection at home. Exclusion: diagnosis with other disease or pathological condition that affects balance; had a relapse in the month prior to baseline or during the intervention process; received intravenous or oral steroid cycle prior to beginning the evaluation protocol and within 4‐month duration of intervention Demographic characteristics: Treatment group: Mean age 39.7 years (SD 8.1), 54% women, mean disease duration 9.7 years (SD 6.8), EDSS score ≥ 4: 83.6%, RR MS: 71.9% Control group: mean age 42.8 years (SD 7.4), 61% women, mean disease duration 10.9 years (SD 5.4), EDSS score ≥ 4: 78.3%, RR MS: 65.2% |
|
| Interventions |
Treatment group: monitored virtual reality telerehabilitation programme via video‐conference using the Xbox 360® and Kinect console, which included gaming protocol consisted of 3 games (Kinect Sports, Kinect Joy Ride, and Kinect Adventures).proposing activities that involve integrating proprioceptive, visual, and vestibular sensory information. Responses directed to the maintenance of balance and postural stability are triggered by the visual feedback that participants continuously receive in real time with regard to their position, performance type, and the movement direction that the task requires. The protocol proposed tasks such as throwing and hitting objects with one’s hands and feet, hitting and receiving balls with different body parts, dodging objects, overcoming obstacles, imitating postures, or managing virtual elements that favour key aspects of postural control (e.g., girdle dissociation, alternating load distribution, changes in direction, multidirectional movement, reaction speed, hand‐eye co‐ordination, foot‐eye co‐ordination, and dexterity) in different positions across a stepwise gradient of difficulty. Experimental group attended 40 sessions, 4 sessions per week (20 minutes per session) at home Control group: Ambulatory PT twice/week for 10 weeks (40 minutes per session) at rehab centre |
|
| Outcomes |
Primary outcome: Postural control : CDP; SOT; motor function: MCT Secondary outcome: clinical outcomes: BBS, TS Assessment points: Baseline and post‐intervention (10 weeks) |
|
| Notes | No report of adverse events Short‐term follow‐up Same study published in different journals by the same authors (Gutierrez 2013b) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants allocated to treatment or control groups based on the specific criteria. Only after screening for the treatment group, remaining participants were randomly distributed into 2 groups using computer software. Further, 2 participants were added to the treatment group due to availability of the equipment |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and treating personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 drop‐outs ( 1 in treatment group and 2 in control group) |
| Selective reporting (reporting bias) | Low risk | All prespecified (primary and secondary) outcomes reported |
| Other bias | High risk | No power calculation No ITT analysis Small sample size |
Huijgen 2008.
| Methods | RCT, parallel group, multicentred; Italy, Spain and Belgium Study period: October 2005 to January 2007 Funding source: study was part of a project supported by European Union Declaration of interest: not mentioned |
|
| Participants | N = 81 (Stroke = 16, TBI = 30, MS = 35): treatment group = 55 (MS = 24) and control = 26 (MS = 11) Inclusion: age > 18 years; confirmed diagnosis of MS, stroke or TBI; Nine Hole Peg Test > 25 sec and ability to move at least 1 peg in 180 sec; sufficient autonomous functioning; Internet connection or telephone line and reachable Internet provider; stable clinical status and living at home Exclusion: disturbed upper limb function not related to MS, stroke or TBI; serious cognitive and/or behavioural problems; serious emotional problems; major visual problems; communication problems; medical complications; other problems possibly contraindicating autonomous exercise at home Demographic characteristics: Intervention group: mean age: 47 years (SD 18) (MS 48 years (SD 12)), 71% men (MS 46% men), mean disease duration 9.7 years (SD 7.8 years) (MS 15.1 years (SD 8.6)); Control group: mean age: 50 years (SD 18) (MS 51 years (SD 14)), 69% men (MS 64% men), mean disease duration 10.2 years (SD 7.6 years) (MS 15.6 years (SD 7.8)) |
|
| Interventions |
Treatment group: 1 month of usual care followed by the Home Care Activity Desk (HCAD) – a telerehabilitation intervention for arm/hand function at home which consisted a set of exercises for correct functional activity of the upper limb such as reaching, grasping, lateral pinch, pinch grip, holding, manipulation and finger dexterity; and additional features for videoconferencing and recording. HCAD system comprised a hospital‐based server and portable unit installed at participant’s home. At least 1 session (30 minutes)/day for 5 days per week for 1 month Control group: Usual care and generic exercises prescribed by their physicians |
|
| Outcomes |
Primary outcome: Upper limb function : ARAT; NHPT Secondary outcome: participant satisfaction (VAS) |
|
| Notes | No report of adverse events Heterogeneous in approach and intensity for control group activities Higher percentage of men in the control group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomly allocated to treatment or control group, in such way to fit the clinical practice in a 2:1 ratio |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and treating personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall 11 participants (14%) were lost to follow‐up (7 in intervention, 4 in control group). Percentage of drop‐outs reported but not time points |
| Selective reporting (reporting bias) | Low risk | All prespecified (primary and secondary) outcomes reported |
| Other bias | Unclear risk | Study was underpowered |
Motl 2011.
| Methods | RCT, parallel group, with wait‐list control; USA Study period: not mentioned Funding source: none Declaration of interest: authors declared no conflict of interest |
|
| Participants | N = 54: treatment group = 27 and control = 27 Inclusion: Definite diagnosis of RRMS; independently ambulatory or ambulatory with single‐point assistance (i.e. cane); relapse‐free in the past 30 days; Internet access; willingness to complete the questionnaires and undergo randomisations; being non‐active defined as not engaging in regular physical activity (30 minutes accumulated per day) on more than 2 days of the week during the previous 6 months; free of contraindications for physical activity (e.g. no underlying cardiovascular disease); and physician approval for beginning a physical activity programme Exclusion: not specified Demographic characteristics: Intervention group: mean age:46.1 years (SD 10.4), 90% women; mean disease duration: 8.1 years (SD 6.5); mean Determined Disease Steps Scale score (disease severity): 2.0 (SD 1.8) Control group: mean age 45.6 (SD 9.2), 88% women, mean disease duration: 7.3 (SD 6.2), mean Determined Disease Steps Scale score (disease severity): 2.1 (1.9) |
|
| Interventions |
Treatment group: Internet intervention based on social cognitive theory (self efficacy, outcome expectations, impediments, and goal setting), which included text‐based content supplemented by video and portable document format (PDF) files (i.e. multimedia). It consisted of 4 essential modules: Getting Started (benefits of physical activity and information for becoming more physically active), Planning for Success (goal setting and feedback, outcome expectations, and self efficacy), Beating the Odds (barriers and strategies of overcoming barriers, and social support), and Sticking with It (maintaining an active lifestyle and physical activity relapse prevention), with 10 total Chapters. Additionally, interactive sessions twice per week were conducted, which included an ongoing participant forum for discussions of physical activity behaviour change, and a toll‐free telephone line and a study e‐mail address for supporting the website. This was further supported by automated e‐mail announcements about new information, updates, and changes on the website Control group: wait‐list participants, who received the intervention materials after study completion |
|
| Outcomes | Measured at baseline, immediately post‐treatment (12 weeks after start of intervention) Primary outcome: Physical activity: GLTEQ; Self efficacy: EXSE; Outcome expectations: MOEES; Functional limitations: ‐ Functional Limitations component of the abbreviated LL‐FDI; Goal setting: EGS Secondary outcome: Disease severity: PDDS Assessment time points: Baseline and post‐intervention (1 month) |
|
| Notes | No report of adverse events | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were initially paired on physical activity and neurological disability levels by 2 authors and then members of the pairs were randomly assigned into intervention or wait‐list control conditions |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and treating personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall 10 participants (15%) dropped out (6 in intervention, 4 in control group). Percentage of drop‐outs reported but not time points |
| Selective reporting (reporting bias) | Low risk | All prespecified (primary and secondary) outcomes reported |
| Other bias | Low risk | None |
Paul 2014.
| Methods | RCT, parallel group; Scotland, UK Study period: not mentioned Funding source: grant, the Chief of Scientist Office, Scotland Declaration of interest: authors declared no conflict of interest |
|
| Participants | N = 30: treatment group = 5 and control = 15 Inclusion: Confirmed diagnosis of MS, EDSS: 5 ‐ 6, stable drug therapy for 30 days, no relapses in the previous 3 months, no significant comorbidities (such as co‐existing cardiac or pulmonary condition), have access to the Internet via personal or tablet computer Further inclusion in the treatment group if participants did not receive conventional physiotherapy treatment based on at least 1 the following criteria: (a) time on the waiting list; (b) limited geographic accessibility; (c) unable to reconcile working hours and therapy schedule; or d) dependent on others to arrive at the treatment centre Exclusion: not specified Demographic characteristics: Treatment group: Mean age 50.8 years (SD 7.4), 80% women; mean disease duration 12.5 years (SD 7.1), mean EDSS 6. 0 (SD 0.5) Control group: Mean age 52.5 years (SD 14.3), 80% women; mean disease duration 12.8 years (SD 10.9), mean EDSS 5.8 (SD 0.5) |
|
| Interventions |
Treatment group: 12 weeks of individualised web‐based physiotherapy completed twice per week. The website consisted of a home page, exercise pages and advice section. Each exercise page contained a video and text explaining the exercise, an audio description of the exercise and a timer. The catalogue of exercises consisted of: cardiovascular, strengthening and balance exercises, each at 4 levels of difficulty, as well as warm‐up and cool‐down exercises and stretches Control group: usual care |
|
| Outcomes |
Primary outcomes: 25 Foot Walk Test Secondary outcomes: BBS, TUG, MSIS, LMSQOLS, MS‐Related Symptom Checklist, HADS, feasibility and satisfaction with the programme Assessment points: Baseline and post‐intervention (12 weeks) |
|
| Notes | No report of adverse events Short‐term follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed using a series of random numbers, generated in Microsoft Excel. Recruited participants were allocated consecutive numbers, where even numbers represented the intervention group and odd numbers the control group |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and treating personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall, 1 participant dropped out from control group |
| Selective reporting (reporting bias) | Low risk | All prespecified (primary and secondary) outcomes reported |
| Other bias | Unclear risk | Underpowered study No ITT analysis Small sample size |
Pilutti 2014.
| Methods | RCT, parallel group with wait‐list controls, USA Study period: not mentioned Funding source: various grant from the National Multiple Sclerosis Society, USA; the Multiple Sclerosis Society of Canada and the Multiple Sclerosis International Federation Declaration of interest: authors declared no conflict of interest |
|
| Participants | N = 82: treatment group = 41 and control = 41 Inclusion: 18 – 64 years; definite diagnosis of MS based on physician verification; relapse‐free for the past 30 days; Internet access; and ability to walk with or without an assistive device; physician’s approval for participation; willing and able to travel to the research site; have minimal risk for engaging in physical activity (i.e. reported ‘yes’ to fewer than 2 questions on the PARQ) Exclusion: participants who self‐reported accumulating ≥ 30 minutes of moderate‐to‐vigorous physical activity per day on ≥ 2 days/week. Demographic characteristics: Treatment group: Mean age 48.4 years (SD: 9.1 years), 73.2% women, mean time since diagnosis 10.6 years (SD: 7.1 years), RRMS 75.6%, PDSS: median 2.0 (IQR 4, 0) Control group: Mean age 49.5 years (SD: 9.2 years), 78% women, mean time since diagnosis 13.0 years (SD: 9.1 years), RRMS 83%, PDSS: median 3.0 (IQR 3, 0) |
|
| Interventions |
Treatment group: same as in Dlugonski 2012, Motl 2011 (see above). In addition, participant wore a Yamax SW‐401 Digiwalker pedometer, completed a log book and used Goal Tracker software, and received a web‐cam, and website information. Participants participated in 15 scheduled one‐on‐one video coaching sessions for 6 months. Control group: wait‐list participants, who received the intervention materials after study completion. |
|
| Outcomes |
Primary outcome: Physical activity: GLTEQ; fatigue: FSS, MFIS; depression and anxiety: HADS; pain: MPQ; sleep: PSQI; HRQoL: MSIS‐29, Cognitive processing speed: SDMT Secondary outcome: disease severity:PDDS Assessment time points: baseline and post‐intervention (6 months) |
|
| Notes | This RCT was considered the primary study, whose results were described in 2 different articles reporting different outcomes (Sandroff 2014) . This study is part of a series of studies conducted earlier (Dlugonski 2012 and Motl 2011). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | After baseline testing, participants were grouped into matched pairs based on step counts from the accelerometer and level of disability, and then randomly assigned to either the intervention or wait‐list control condition using a random numbers sequence |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and treating personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall, 6 participants (7%) (4 from intervention and 2 from control group) dropped out |
| Selective reporting (reporting bias) | Low risk | All prespecified (primary and secondary) outcomes reported |
| Other bias | Unclear risk | Selective participants: recruitment occurred through a database of self‐volunteered persons for research No ITT analysis performed (analysis of completers only) USD 50 remuneration given to participants for completing each testing session |
ARAT: Action Research Arm Test; BBS: Berg Balance Scale;CCT: Controlled clinical trial; CDP: Computerized Dynamic Posturography; CES: Composite Equilibrium Score; CES‐D: Center for Epidemiologic Studies Depression Scale; CI: Confidence interval;DGI: Dynamic gait Index;ECQ: Energy Conservation Questionnaire; EDSS: Expanded Disability Status Scale; EGS: Exercise Goal setting Scale;ES: Effect size; EXSE: Exercise Self‐Efficacy Scale; FIS: Fatigue Impact Scale,), FSS: Fatigue Severity Score; GLTEQ: Godin Leisure‐Time Exercise Questionnaire; HADS: Hospital Anxiety and Depression Scale; HAQUAMS: Hamburg QoL Questionnaire in MS; HCAD: Home Care Activity Desk; HRQoL: Health related quality of life; IPAQ: International Physical Activity Questionnaire; IQR: inter quartile range;ITT: intention to treat; LMSQOLS: Leeds MS Quality of Life Scale;LL‐FDI: Late‐Life Function and Disability Instrument; MCT: Motor Control Test; MOEES: Multidimensional Outcomes Expectations for Exercise Scale; MPQ: McGill Pain Questionnaire; MRI: Magnetic Resonance Imaging: MS: Multiple Sclerosis;MSIS‐29: MS Impact Scale; MSWS‐12: MS Walking Scale – 12; NHPT: Nine Hole Peg Test; PARQ: Physical Activity Readiness Questionnaire; PDDS: Patient Determined Disease Steps; PSQI: Pittsburgh Sleep Quality Index; QoL: quality of life; QWB: Quality of Well‐ Being Scale; RCT: randomised controlled trial; RR: Risk Ratio; SD: Standard deviation; SDMT: Symbol Digit Modalities Test; SE: Standard Error; SF‐36: 36‐Item Short Form Health Survey; SOT: Sensory organisation Test; TBI: traumatic brain injury; TS: Tineti Scale; TUG: Timed Up and Go;UK: United Kingdom;USA: United States of America; VAS: Visual Analogue Scale; 2MWT: 2 minute walk test;6MWT: 6 minute walk test; 25FWT: 25 Foot Walk Test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amato 2014 | Not intervention of interest (mental health care only) |
| Beckner 2010 | Not intervention of interest (mental health care only) |
| Bombardier 2008 | Not Intervention of interest (telephone counselling for health promotion) |
| Bombardier 2013 | Not Intervention of interest (telephone counselling for major depression) |
| Cerasa 2013 | Not intervention of interest (mental health care only) |
| Fischer 2013 | Not intervention of interest (mental health care only) |
| Ghahari 2010 | No subgroup analysis for MS participants |
| Miller 2011 | Intervention with no rehabilitation objectives |
| Mohr 2000 | Not intervention of interest (mental health care only) |
| Mohr 2005 | Not intervention of interest (mental health care only) |
| Mohr 2007 | Not intervention of interest (mental health care only) |
| Moss‐Morris 2012 | Not intervention of interest (mental health care only) |
| Solari 2004 | Not intervention of interest (mental health care only) |
| Stuifbergen 2012 | Not intervention of interest (mental health care only) |
| Wiles 2003 | Intervention: no telerehabilitation |
| Zissman 2012 | Not intervention of interest (medical care only) |
Differences between protocol and review
We have included a 'Summary of findings' table in the review with the key outcomes identified categorised according to the WHO ICF framework, which the authors deemed to be the most relevant to decision‐makers including patients, clinicians and policy makers.
We have clarified ‘Types of interventions’ in this review to include control conditions: “any type of traditional face‐to face rehabilitation treatment in outpatient or day treatment settings”. We exclude studies if they investigated interventions related to: “telerehabilitation targeting mental health conditions or substance abuse”; “home care (or tele‐home care) with no rehabilitation objectives”; “satisfaction with or acceptance of telerehabilitation technology” and “technical development or feasibility of telerehabilitation”. We modified ‘Data extraction and management’ for the review and added the following statement: “Data were extracted for intention‐to‐treat (ITT) analysis from each study and where ITT data were not available, 'on‐treatment’ data or the data of those who completed the trial were retrieved.” Based on the findings, we did not implement the planned methods as described in the protocol related to assessment of heterogeneity, assessment of reporting bias, and data synthesis.
Contributions of authors
Fary Khan (FA), and Bhasker Amatya (BA) were involved in all aspects of the review. Jurg Kesselring (JK) provided valuable input into design of the review. Fary Khan, Bhasker Amatya, Mary Galea (MG) were responsible for all study selection, data extraction and methodological quality of included studies. M Galea and J Kessering also provided valuable assistance with the Discussion. All review authors critically reviewed the manuscript and discussed data collection, results and conclusions.
Sources of support
Internal sources
Department of Rehabilitation Medicine, Royal Melbourne Hospital, Australia.
External sources
None, Other.
Declarations of interest
The review authors are clinicians and researchers in the field of Physical and Medical Rehabilitation who wish to provide the best possible service to their patients.
Fary Khan: none known.
Bhasker Amatya: none known.
Jurg Kesselring: none known.
Mary Galea: none known.
New
References
References to studies included in this review
Dlugonski 2012 {published data only}
- Dlugonski D, Motl RW, Mohr DC, Sandroff BM. Internet‐delivered behavioral intervention to increase physical activity in persons with multiple sclerosis: sustainability and secondary outcomes. Psychology Health and Medicine 2012;17(6):636‐51. [DOI] [PubMed] [Google Scholar]
Egner 2003 {published data only}
- Egner A, Phillips VL, Vora R, Wiggers E. Depression, fatigue, and health‐related quality of life among people with advanced multiple sclerosis: results from an exploratory telerehabilitation study. NeuroRehabilitation 2003;18(2):125‐33. [PubMed] [Google Scholar]
Finlayson 2011 {published data only}
- Finlayson M, Preissner K, Cho C, Plow M. Randomized trial of a teleconference‐delivered fatigue management program for people with multiple sclerosis. Multiple Sclerosis 2011;17(9):1130‐40. [DOI] [PubMed] [Google Scholar]
Frevel 2014 {published data only}
- Frevel D, Mäurer M. Internet‐based home training is capable to improve balance in multiple sclerosis: a comparative trial with hippotherapy. European Journal of Physical and Rehabilitation Medicine 2015;51(1):23‐30. [PUBMED: 24755773] [PubMed] [Google Scholar]
Gutíerrez 2013a {published data only}
- Gutíerrez RO, Galán Del Río F, Cano de la Cuerda R, Alguacil Diego IM, González RA, Page JC. A telerehabilitation program by virtual reality‐video games improves balance and postural control in multiple sclerosis patients. NeuroRehabilitation 2013;33(4):545‐54. [DOI] [PubMed] [Google Scholar]
Huijgen 2008 {published data only}
- Huijgen BC, Vollenbroek‐Hutten MM, Zampolini M, Opisso E, Bernabeu M, Nieuwenhoven J, et al. Feasibility of a home‐based telerehabilitation system compared to usual care: arm/hand function in patients with stroke, traumatic brain injury and multiple sclerosis. Journal of Telemedicine and Telecare. 2008;14(5):249‐56. [DOI] [PubMed] [Google Scholar]
Motl 2011 {published data only}
- Motl RW, Dlugonski D, Wojcicki TR, McAuley E, Mohr DC. Internet intervention for increasing physical activity in persons with multiple sclerosis. Multiple Sclerosis 2011;17(1):116‐28. [DOI] [PubMed] [Google Scholar]
Paul 2014 {published data only}
- Paul L, Coulter EH, Miller L, McFadyen A, Dorfman J, George GMP. Web‐based physiotherapy for people moderately affected with multiple sclerosis; quantitative and qualitative data from a randomized, controlled pilot study. Clinical Rehabilitation 2014;28(9):924‐35. [DOI: 10.1177/0269215514527995] [DOI] [PubMed] [Google Scholar]
Pilutti 2014 {published data only}
- Pilutti L, Dlugonski D, Sandroff B, Klaren R, Motl R. Randomized controlled trial of a behavioral intervention targeting symptoms and physical activity in multiple sclerosis. Multiple Sclerosis 2014;20(5):594–601. [DOI] [PubMed] [Google Scholar]
- Sandroff BM, Klaren RE, Pilutti LA, Dlugonski D, Benedict RH, Motl RW. Randomized controlled trial of physical activity, cognition, and walking in multiple sclerosis. Journal of Neurology 2014;261(2):363‐72. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amato 2014 {published data only}
- Amato M, Goretti B, Viterbo R, Portaccio E, Niccolai C, Hakiki B, et al. Computer‐assisted rehabilitation of attention in patients with multiple sclerosis: results of a randomized, double‐blind trial. Multiple Sclerosis 2014;20(1):91‐8. [DOI] [PubMed] [Google Scholar]
Beckner 2010 {published data only}
- Beckner V, Howard I, Vella L, Mohr DC. Telephone‐administered psychotherapy for depression in MS patients: moderating role of social support. Journal of Behavioral Medicine 2010;33(1):47‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bombardier 2008 {published data only}
- Bombardier CH, Cunniffe M, Wadhwani R, Gibbons LE, Blake KD, Kraft GH. The efficacy of telephone counseling for health promotion in people with multiple sclerosis: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2008;89(10):1849‐56. [DOI] [PubMed] [Google Scholar]
Bombardier 2013 {published data only}
- Bombardier CH, Ehde DM, Gibbons LE, Wadhwani R, Sullivan MD, Rosenberg DE, et al. Telephone‐based physical activity counseling for major depression in people with multiple sclerosis. Journal of Consulting and Clinical Psychology. 2013;81(1):89‐99. [DOI] [PubMed] [Google Scholar]
Cerasa 2013 {published data only}
- Cerasa A, Gioia M C, Valentino P, Nistico R, Chiriaco C, Pirritano D, et al. Computer‐assisted cognitive rehabilitation of attention deficits for multiple sclerosis: a randomized trial with fMRI correlates. Neurorehabilitation and Neural Repair 2013;27(4):284‐95. [DOI] [PubMed] [Google Scholar]
Fischer 2013 {published data only}
- Fischer A, Schroder J, Pottgen J, Lau S, Heesen C, Moritz S, et al. Effectiveness of an internet‐based treatment programme for depression in multiple sclerosis: a randomized controlled trial. Multiple Sclerosis 2013;19(11):350‐1. [Google Scholar]
Ghahari 2010 {published data only}
- Ghahari S, Leigh Packer T, Passmore AE. Effectiveness of an online fatigue self‐management programme for people with chronic neurological conditions: a randomized controlled trial. Clinical Rehabilitation 2010;24(8):727‐44. [DOI] [PubMed] [Google Scholar]
Miller 2011 {published data only}
- Miller DM, Moore SM, Fox RJ, Atreja A, Fu AZ, Lee JC, et al. Web‐based self‐management for patients with multiple sclerosis: a practical, randomized trial. Telemedicine Journal and e‐Health 2011;17(1):5‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohr 2000 {published data only}
- Mohr DC, Likosky W, Bertagnolli A, Goodkin DE, Wende J, Dwyer P, et al. Telephone‐administered cognitive‐behavioral therapy for the treatment of depressive symptoms in multiple sclerosis. Journal of Consulting and Clinical Psychology 2000;68(2):356‐61. [DOI] [PubMed] [Google Scholar]
Mohr 2005 {published data only}
- Mohr DC, Hart SL, Julian L, Catledge C, Honos‐Webb L, Vella L, et al. Telephone‐administered psychotherapy for depression. Archives of General Psychiatry 2005;62(9):1007‐14. [DOI] [PubMed] [Google Scholar]
Mohr 2007 {published data only}
- Mohr DC, Hart S, Vella L. Reduction in disability in a randomized controlled trial of telephone‐administered cognitive‐behavioral therapy. Health Psychology 2007;26(5):554‐63. [DOI] [PubMed] [Google Scholar]
Moss‐Morris 2012 {published data only}
- Moss‐Morris R, McCrone P, Yardley L, Kessel K, Wills G, Dennison L. A pilot randomised controlled trial of an Internet‐based cognitive behavioural therapy self‐management programme (MS Invigor8) for multiple sclerosis fatigue. Behaviour Research and Therapy 2012;50(6):415‐21. [DOI] [PubMed] [Google Scholar]
Solari 2004 {published data only}
- Solari A, Motta A, Mendozzi L, Pucci E, Forni M, Mancardi G, et al. Computer‐aided retraining of memory and attention in people with multiple sclerosis: a randomized, double‐blind controlled trial. Journal of the Neurological Sciences 2004;222(1):99‐104. [DOI] [PubMed] [Google Scholar]
Stuifbergen 2012 {published data only}
- Stuifbergen AK, Becker H, Perez F, Morison J, Kullberg V, Todd A. A randomized controlled trial of a cognitive rehabilitation intervention for persons with multiple sclerosis. Clinical Rehabilitation 2012;26(10):882‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiles 2003 {published data only}
- Wiles CM, Newcombe RG, Fuller KJ, Jones A, Price M. Use of videotape to assess mobility in a controlled randomized crossover trial of physiotherapy in chronic multiple sclerosis. Clinical Rehabilitation 2003;17(3):256‐63. [DOI] [PubMed] [Google Scholar]
Zissman 2012 {published data only}
- Zissman K, Lejbkowicz I, Miller A. Telemedicine for multiple sclerosis patients: assessment using Health Value Compass. Multiple Sclerosis 2012;18(4):472‐80. [DOI] [PubMed] [Google Scholar]
Additional references
Amatya 2013
- Amatya B, Khan F, Mantia L, Demetrios M, Wade DT. Non pharmacological interventions for spasticity in multiple sclerosis. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD009974.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beer 2012
- Beer S, Khan F, Kesselring J. Rehabilitation interventions in multiple sclerosis: an overview. Journal of Neurology 2012;259(9):1994‐2008. [DOI] [PubMed] [Google Scholar]
Bendixen 2008
- Bendixen RM, Levy C, Lutz BJ, Horn KR, Chronister K, Mann WC. A telerehabilitation model for victims of polytrauma. Rehabilitation Nursing 2008;33(5):215‐20. [DOI] [PubMed] [Google Scholar]
Bendixen 2009
- Bendixen RM, Levy CE, Olive ES, Kobb RF, Mann WC. Cost effectiveness of a telerehabilitation program to support chronically ill and disabled elders in their homes. Telemedicine Journal and e‐Health 2009;15(1):31‐8. [DOI] [PubMed] [Google Scholar]
Brennan 2009
- Brennan DM, Mawson S, Brownsell S. Telerehabilitation: enabling the remote delivery of healthcare, rehabilitation, and self management. Studies in Health Technology and Informatics 2009;145:231‐48. [PubMed] [Google Scholar]
Chumbler 2012
- Chumbler NR, Quigley P, Li X, Morey M, Rose D, Sanford J, et al. Effects of telerehabilitation on physical function and disability for stroke patients: a randomized, controlled trial. Stroke 2012;43(8):2168‐74. [DOI] [PubMed] [Google Scholar]
Constantinescu 2010
- Constantinescu G, Theodoros D, Russell T, Ward E, Wilson S, Wootton R. Assessing disordered speech and voice in Parkinson's disease: a telerehabilitation application. International Journal of Language & Communication Disorders 2010;45(6):630‐44. [DOI] [PubMed] [Google Scholar]
Currell 2000
- Currell R, Urquhart C, Wainwright P, Lewis R. Telemedicine versus face to face patient care: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002098] [DOI] [PubMed] [Google Scholar]
Egger 1998
- Egger M, Smith GD. Bias in location and selection of studies. BMJ 1998;316(7124):61‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finkelstein 2008
- Finkelstein J, Lapshin O, Castro H, Cha E, Provance PG. Home‐based physical telerehabilitation in patients with multiple sclerosis: a pilot study. Journal of Rehabilitation Research and Development 2008;45(9):1361‐73. [PubMed] [Google Scholar]
Forducey 2003
- Forducey PG, Ruwe WD, Dawson SJ, Scheideman‐Miller C, McDonald NB, Hantla MR. Using telerehabilitation to promote TBI recovery and transfer of knowledge. NeuroRehabilitation 2003;18(2):103‐11. [PubMed] [Google Scholar]
Giansanti 2008
- Giansanti D, Macellari V, Maccioni G. Telemonitoring and telerehabilitation of patients with Parkinson's disease: health technology assessment of a novel wearable step counter. Telemedicine Journal and e‐Health 2008;14(1):76‐83. [DOI] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Brozek J, Oxman A, Schunemann H. GRADEpro. Version 3.2 for windows. GRADE working group, 2008.
Gutierrez 2013b
- Ortiz‐Gutierrez R, Cano‐de‐la‐Cuerda R, Galan‐del‐Rio F, Alguacil‐Diego IM, Palacios‐Cena D, Miangolarra‐Page JC. A telerehabilitation program improves postural control in multiple sclerosis patients: a Spanish preliminary study. International Journal of Environmental Research and Public Health 2013;10(11):5697‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gøetzsche 1987
- Gøetzsche PC. Reference bias in reports of drug trials. British Medical Journal (Clinical Research ed.) 1987;295(6599):654‐6. [PMC free article] [PubMed] [Google Scholar]
Hailey 2010
- Hailey D, Roine R, Ohinmaa A, Dennett L. Evidence on the effectiveness of telerehabilitation applications. Institute of Health Economics and Finnish Office for Health Technology Assessment 2010. [ISBN: 978‐1‐897443‐87‐3]
Hailey 2011
- Hailey D, Roine R, Ohinmaa A, Dennett L. Evidence of benefit from telerehabilitation in routine care: a systematic review. Journal of Telemedicine and Telecare 2011;17(6):281‐7. [DOI] [PubMed] [Google Scholar]
Hammond 2000
- Hammond SR, McLeod JG, Macaskill P, English DR. Multiple sclerosis in Australia: prognostic factors. Journal of Clinical Neuroscience 2000;7(1):16‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Houlihan 2011
- Houlihan BV, Jette A, Paasche‐Orlow M, Wierbicky J, Ducharme S, Zazula J, et al. A telerehabilitation intervention for persons with spinal cord dysfunction. American Journal of Physical Medicine & Rehabilitation 2011;90(9):756‐64. [DOI] [PubMed] [Google Scholar]
Johansson 2011
- Johansson T, Wild C. Telerehabilitation in stroke care, a systematic review. Journal of Telemedicine and Telecare 2011;17(1):1‐6. [DOI] [PubMed] [Google Scholar]
Kairy 2009
- Kairy D, Lehoux P, Vincent C, Visintin M. A systematic review of clinical outcomes, clinical process, healthcare utilization and costs associated with telerehabilitation. Disability and Rehabilitation 2009;31(6):427‐47. [DOI] [PubMed] [Google Scholar]
Khan 2007
- Khan F, Turner‐Stokes L, Ng L, Kilpatrick T, Amatya B. Multidisciplinary rehabilitation for adults with multiple sclerosis. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006036.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2010a
- Khan F, Gray O. Disability management and rehabilitation for persons with multiple sclerosis. Neural Regeneration Research 2010;5(4):301‐9. [Google Scholar]
Khan 2010b
- Khan F, Pallant JF, Zhang N, Turner‐Stokes L. Clinical practice improvement approach in multiple sclerosis rehabilitation: a pilot study. International Journal of Rehabilitation Research 2010;33(3):238‐47. [DOI] [PubMed] [Google Scholar]
Kurtzke 1982
- Kurtzke JF, Gundmunsson KR, Bergmann S. Multiple sclerosis in Iceland: evidence of post war epidemic. Neurology 1982;32(2):143‐50. [DOI] [PubMed] [Google Scholar]
Lai 2004
- Lai JC, Woo J, Hui E, Chan WM. Telerehabilitation ‐ a new model for community‐based stroke rehabilitation. Journal of Telemedicine and Telecare 2004;10(4):199‐205. [DOI] [PubMed] [Google Scholar]
Legg 2004
- Legg L, Langhorne P, Outpatient Service Trialists. Rehabilitation therapy services for stroke patients living at home: systematic review of randomised trials. Lancet 2004;363(9406):352‐6. [DOI] [PubMed] [Google Scholar]
Mc Donald 2001
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of Neurology 2001;50(1):121‐7. [DOI] [PubMed] [Google Scholar]
McCue 2010
- McCue M, Fairman A, Pramuka M. Enhancing quality of life through telerehabilitation. Physical Medicine and Rehabilitation Clinics of North America 2010;21(1):195‐205. [DOI] [PubMed] [Google Scholar]
McLean 2010
- McLean S, Chandler D, Nurmatov U, Liu JLY, Pagliari C, Car J, et al. Telehealthcare for asthma. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD007717.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McLean 2011
- McLean S, Nurmatov U, Liu JLY, Pagliari C, Car J, Sheikh A. Telehealthcare for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD007718.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
MS Australia 2012
- MS Australia. Practice for health professionals: Spasticity and multiple sclerosis (MS). www.msaustralia.org.au/sites/default/files/spasticity.pdf. [ISBN: 978‐0‐9806637‐4‐7]
NMSS 2007
- National Multiple Sclerosis Society. Technology and MS: new survey finds technology plays a critical role in lives of people with multiple sclerosis, yet many are not using It to overcome disease‐related challenges. www.prnewswire.com/news‐releases/video‐new‐survey‐finds‐technology‐plays‐a‐critical‐role‐in‐lives‐of‐people‐with‐multiple‐sclerosis‐yet‐many‐are‐not‐using‐it‐to‐overcome‐disease‐related‐challenges‐58929337.html (accessed 28th March 2015).
Pfleger 2010
- Pfleger CC, Flachs EM, Koch‐Henriksen N. Social consequences of multiple sclerosis (1): early pension and temporary unemployment, a historical prospective cohort study. Multiple Sclerosis 2010;16(1):121‐6. [DOI] [PubMed] [Google Scholar]
Polman 2005
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Annals of Neurology 2005;58(6):840‐6. [DOI] [PubMed] [Google Scholar]
Poser 1983
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;13(3):227‐31. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rogante 2010
- Rogante M, Grigioni M, Cordella D, Giacomozzi C. Ten years of telerehabilitation: a literature overview of technologies and clinical applications. NeuroRehabilitation 2010;27(4):287‐304. [DOI] [PubMed] [Google Scholar]
Russell 2011
- Russell TG, Buttrum P, Wootton R, Jull GA. Internet‐based outpatient telerehabilitation for patients following total knee arthroplasty: a randomized controlled trial. Journal of Bone and Joint Surgery. American Volume 2011;93(2):113‐20. [DOI] [PubMed] [Google Scholar]
Sandroff 2014
- Sandroff BM, Klaren RE, Pilutti LA, Dlugonski D, Benedict RH, Motl RW. Randomized controlled trial of physical activity, cognition, and walking in multiple sclerosis. Journal of Neurology 2014;261(2):363‐72. [DOI] [PubMed] [Google Scholar]
Steel 2011
- Steel K, Cox D, Garry H. Therapeutic videoconferencing interventions for the treatment of long‐term conditions. Journal of Telemedicine and Telecare 2011;17(3):109‐17. [DOI] [PubMed] [Google Scholar]
Theodoros 2008
- Theodoros D, Russell T. Telerehabilitation: current perspectives. Studies in Health Technology and Informatics 2008;131:191‐209. [PubMed] [Google Scholar]
Tousignant 2011
- Tousignant M, Moffet H, Boissy P, Corriveau H, Cabana F, Marquis F. A randomized controlled trial of home telerehabilitation for post‐knee arthroplasty. Journal of Telemedicine and Telecare 2011;17(4):195‐8. [DOI] [PubMed] [Google Scholar]
Trisolini 2010
- Trisolini M, Honeycutt A, Wiener J, Lesesne S. Global economic impact of multiple sclerosis: a literature review. www.msif.org/wp‐content/uploads/2014/09/ExecSummary_English.pdf (Accessed 28th March 2015).
Van Tulder 2003
- Tulder MW, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine 2003;28(12):1290‐9. [DOI] [PubMed] [Google Scholar]
Weinshenker 1989
- Weinshenker BG, Bass B, Rice GP, Noseworthy J, Carriere W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study I. Clinical course and disability. Brain 1989;112(Pt 1):133‐46. [DOI] [PubMed] [Google Scholar]
WHO 2001
- World Health Organization (WHO). International Classification of Functioning, Disability and Health (ICF). www.who.int/classifications/icf/en/ (Accessed May 2014).
WHO 2008
- World Health Organization (WHO). Atlas: Multiple Sclerosis Resources in the World 2008. WHO and Multiple Sclerosis International Federation, 2008. [ISBN 978 92 4 156375 8] [Google Scholar]
Winters 2002
- Winters JM. Telerehabilitation research: emerging opportunities. Annual Review of Biomedical Engineering 2002;4:287‐320. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Khan 2013
- Khan F, Amatya B, Kesselring J. Telerehabilitation for persons with multiple sclerosis. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD010508] [DOI] [PMC free article] [PubMed] [Google Scholar]


