The coronavirus disease 2019 (COVID-19) pandemic has, as of this writing, infected at least 200 countries and killed more than 33 000 people.1 Owing to frequent business dealings and being only 81 miles from mainland China, Taiwan was previously considered to be a hot spot for COVID-19. However, thus far, Taiwan has had 306 confirmed cases and ranked 73rd on the list of infected areas. With its previous experience of the severe acute respiratory syndrome epidemic in 2003, rapid response and quick implementation between government and private enterprise, Taiwan has been able to prevent a large-scale epidemic.2
On 20 January 2020, the Taiwan Centers for Disease Control (CDC) announced the activation of the Central Epidemic Command Center for COVID-19 and set up reporting criteria for real-time PCR tests.3 However, as the COVID-19 outbreak worsened, the criteria were rapidly updated to catch up with specialist recommendations. This demonstrates a fast response from the CDC to follow guidelines. However, clinicians have become exhausted and ordinary people overwhelmed. Dedicated phone lines for consultations about COVID-19 were jammed 24/7 with anxious people.
Hence, in early March, we developed a clinical website (https://1922.net.nsysu.edu.tw/) to assist clinicians and ordinary people in judging whether the person had matched the reporting criteria. It also provides instructions for quarantine regulations under selected circumstances, following Taiwan CDC guidelines. The system is designed to help both ordinary citizens and clinicians check the reporting criteria by themselves and decrease the need for consultation with the public health service. Users complete a questionnaire on the website regarding the patient’s clinical symptoms, occupation, travel history, contact history, cluster history and chest imaging results if available (figure 1) and receive recommendations and further quarantine instructions if needed. The risk classification algorithm was developed with the cooperation of the Emergency Department of Chang Gung Memorial Hospital and the Computer Science Department of Sun Yat-sen University.
Figure 1.
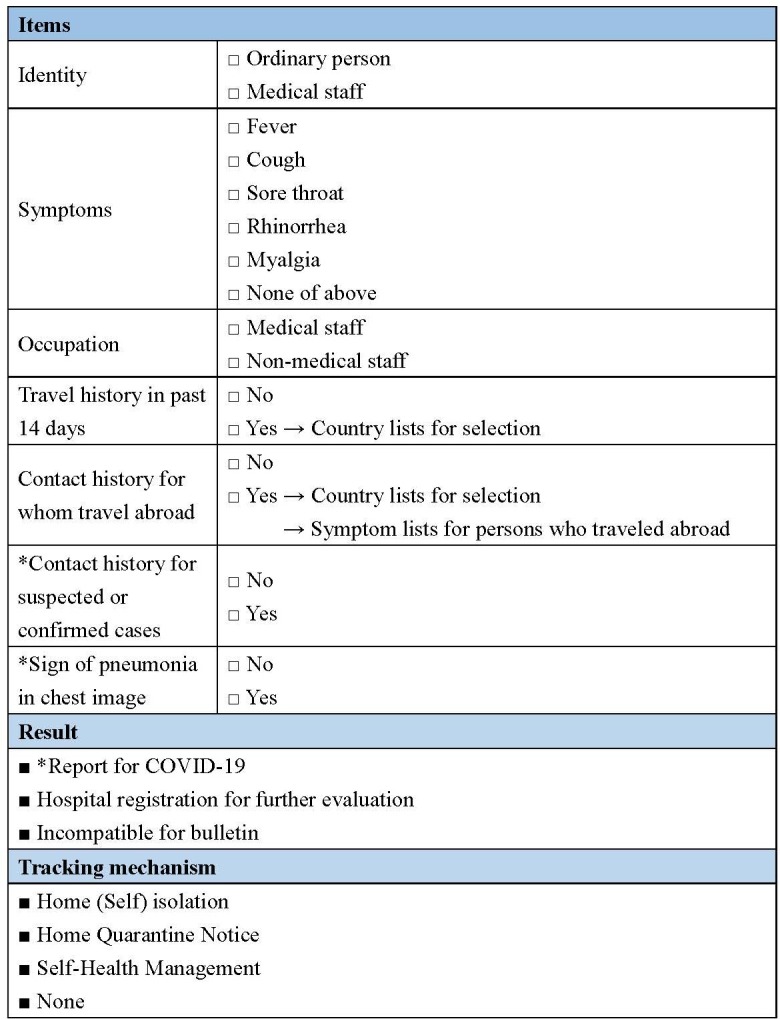
The content of the clinical website (https://1922.net.nsysu.edu.tw/). *For medical staff. The content of the clinical website was translated from Chinese to English in this figure.
From 8 March 2020 to 31 March 2020, the system has received over 3600 inquiries, 37.6% of which were filed by laypersons and 62.4% by clinicians. The average age of individuals who inquired was 39.2 years and 48.1% were men. Approximate 67.3% of the inquiries were regarding quarantine recommendations based on travel history and contact history. Among all inquiries, 64.8% have not met the reporting criteria.
This clinical website is expected to help clinical physicians report suspicious patients and assist the general population by updating them with CDC information regarding COVID-19. This website is continuously updated regarding reporting criteria from the CDC in Taiwan, and it is expected to be implemented in the government healthcare system in the near future. This experience shows how simple technology can help in setting up an epidemic prevention system and preventing widespread panic.
Footnotes
Contributors: I-MC and C-HRL conceived the study. I-MC and CYC managed the data, including quality control. HZ set up the clinical website. I-MC and CYC drafted the manuscript, and all of the authors contributed substantially to its revision. C-HRL assumed responsibility for the paper as a whole.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1. World Health Organization Novel coronavirus (COVID-19) situation. Available: https://experience.arcgis.com/experience/685d0ace521648f8a5beeeee1b9125cd
- 2. Wang CJ, Ng CY, Brook RH. Response to COVID-19 in Taiwan: big data analytics, new technology, and proactive testing. JAMA 2020. doi: 10.1001/jama.2020.3151. [Epub ahead of print: 03 Mar 2020]. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention Coronavirus disease (COVID-19). Available: https://www.cdc.gov.tw/En


