Abstract
Disrupted operation of the reward circuitry underlies many aspects of affective disorders. Such disruption may manifest as aberrant behavior including risk taking, depression, anhedonia and addiction. Early life adversity is a common antecedent of adolescent and adult affective disorders involving the reward circuitry. However, whether early life adversity influences the maturation and operations of the reward circuitry, and the potential underlying mechanisms, remain unclear.
Here we present novel information using cutting-edge technologies in animal models to dissect out the mechanisms by which early life adversity provokes dysregulation of the complex interactions of stress and reward circuitries. We propose that certain molecularly defined pathways within the reward circuitry are particularly susceptible to early life adversity. We examine regions and pathways expressing the stress sensitive peptide corticotropin releasing hormone (CRH), which has been identified in critical components of the reward circuitry and interacting stress circuits. Notably, CRH is strongly modulated by early life adversity in several of these brain regions. Focusing on amygdala nuclei and their projections, we provide evidence suggesting that aberrant CRH expression and function may underlie augmented connectivity of the nucleus accumbens with fear/anxiety regions, disrupting the function of this critical locus of pleasure and reward.
Keywords: Anhedonia, CRH, Stress, Amygdala, Nucleus accumbens, Addiction
Introduction
Early life adversity is a common antecedent of adolescent and adult affective disorders involving disrupted operation of the reward circuitry. These include anhedonia, depression, excessive risk-taking (gambling) and drug and alcohol addiction. However, whether early life adversity influences the maturation and operations of the reward circuitry, and the potential underlying mechanisms, remain unclear.
The vulnerability of the developing (prenatal and early postnatal) brain to adversity derives from the fact that the mesolimbic reward circuitry undergoes significant growth, maturation and plasticity during this epoch (see Table 1). The nature of the eventual psychopathology engendered by early life adversity may depend on the nature or type of the insults and the developmental period in which they are experienced, as well as clear and well-established genetic and epigenetic factors that confer vulnerability to the insults. Indeed, genetics and early life adversity interact to modulate development of the reward circuitry, thus influencing its eventual functions[1,2]. In this review, we discuss reward circuit development and the mechanistic role of adversity in disrupting the normal maturation of this circuitry, conferring susceptibility to mental illness.
Table 1.
The development of the reward circuitry across species
| Human | Rodent | Developmental milestone | Reference(s) |
|---|---|---|---|
| Ventral tegmental area | |||
| 4 wk gestation (1st trimester) | Rat: E14 | Medial forebrain bundle appears | [120,121] |
| 5.5 wk gestation (1st trimester) | Mouse: E8.5 Rat: E12.5 | TH detectable in ventral mesencephalon | [122,123] |
| 19 wk gestation (2nd trimester) | Mouse: E16 Rat: E18 | VTA DA neurons distinguishable from neighboring groups | [124,125] |
| Nucleus accumbens | |||
| 10 wk gestation (1st trimester) | Rat: E15 | Nucleus accumbens appears* | [126,127] |
| 12 wk gestation (1st trimester) | Rat: E15 | D1R detectable in striatum | [128,129] |
| 3.5 postnatal months | Rat: P11 | Loss of AChE striosomes in NAc | [130,131] |
| Amygdala | |||
| 4 wk gestation (1st trimester) | Mouse: E11 Rat: E13 | Amygdala appears* | [132,133] |
| 6 wk gestation (1st trimester) | Rat: E17 | Basolateral nuclear group is identifiable | [134,135] |
| 12.5–16 wk gestation (2nd trimester) | Mouse: E11-E15 Rat: E15-E19 | Lateral amygdala generation | [133,136] |
| 30 wk gestation (3rd trimester) | Rat: E13 | Pyramidal neurons identifiable in basolateral amygdala | [137,138] |
| Prefrontal cortex | |||
| 3.5 years | Rat: P35 | Peak PFC synaptic density | [139,140] |
| 17–25 years | Rat: P90 | Synaptogenesis and myelination complete | [141] |
| Connectivity | |||
| 8 wk gestation (1st trimester) | Mouse: E10 Rat: E14 | Dorsal thalamocortical radiations appear | [142,143] |
| 26–32 wk gestation (3rd trimester) | Mouse: E15 Rat: E16 | Thalamocortical afferents reach the cortical plate | [144,145] |
| Functions | |||
| 2 months | Mouse: P3 Rat: P1 | Emergence of appetitive learning | [146,147] |
| Newborn | Rat: P6 | Emergence of sucrose preference | [148,149] |
| 9 months-1 year | Rat: P14-P28 | Emergence of social play | [150] |
Causality of early life adversity and psychopathology: a conundrum in humans that requires experimental paradigms
Whereas early life adversity, including poverty and chaotic environment, is associated with poor emotional outcomes and aberrant functional development of the reward system[3,4], the origins and mechanisms that underlie these observations are not fully understood. Specifically, it is not possible in human studies to dissociate genetics and environment. For example, poor parental care may predict anxiety and depression, yet the parent endows the child with both his/her behavior and DNA. Therefore, while well designed longitudinal human studies offer important clues and insights, they cannot conclusively establish causality and mechanisms[5]. Thus, the use of animal models of early life adversity is required[6]. Indeed, animal models for early life adversity (or stress) have been developed to probe the causal and mechanistic nature of these important observations in humans.
New experimental paradigms enable identifying causality and mechanisms of the role of early life adversity in aberrant maturation and operations of the reward circuitry
The development of preclinical models for early life adversity offers scientists the ability to understand complex neural mechanisms using techniques and approaches that are not possible in humans. Indeed, numerous approaches have been used to generate stress or adversity early in life, including the prenatal and / or postnatal epochs considered sensitive[7]. Maternal separation has been used for decades to study the effects of such adversity/stress, and several variants exist including daily short (3–4 hour) separation or a single prolonged deprivation[8,9]. These models have generally yielded deficits in cognitive abilities[10–12] as well as anxiety-like and depression-like behaviors[13,14] and addiction-like behaviors[15,16]. Aiming to generate a naturalistic, highly reproducible model for early life adversity, a paradigm of simulated poverty, using cages with limited bedding and nesting material (LBN) in rodents, has been devised and used extensively around the world [7,17,18]. This environment strongly disrupts caring behaviors in rodent dams and thus the sensory signals received by the developing pups. Whereas the overall duration and quality of maternal care remain unaltered, the pattern of caregiving is fragmented and unpredictable[17,19,20]. The fragmented, unpredictable sequences of maternal care cause chronic stress in the pups, which dissipates upon returning dams to normal bedded cages at the end of the one-week exposure. However, aberrant brain circuit maturation is generated in the pups, evident on magnetic resonance imaging (MRI) [27] and manifesting as impaired memory[21,22] as well as specific deficits in emotional-like behaviors[20,23]. Here we focus on alterations of the reward circuitry and their behavioral manifestations.
The reward circuitry and its development
A. overview
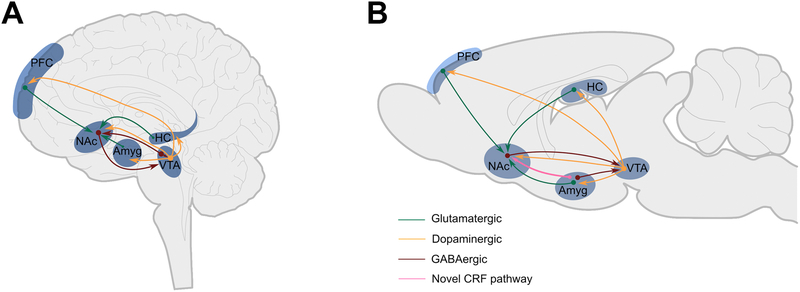
Reward processing encompasses the biological and behavioral functions to drive the acquisition of rewarding stimuli[24,25]. The hypothalamus is central to processing basic rewards, whereas higher cortical and subcortical forebrain structures are engaged when complex choices about these fundamental needs are required. The reward circuitry is a complex entity that includes the prefrontal cortex (PFC), nucleus accumbens (NAc), ventral tegmental area (VTA), amygdala (Amyg) and hippocampus (HC) acting as a neural network to effectively assess the likely outcomes of different choices. Studies have focused on the glutamatergic and dopaminergic input pathways to the NAc, a key brain region that integrates excitatory and inhibitory input to signal the salience of rewarding stimuli[26–31]. The primary function of the NAc is to modulate the response to reward-related cues, as well as the value of deviations of expected versus actual reward outcomes, which are encoded via projections to and from the amygdala, thalamic nuclei and prefrontal cortex[32–34] (Figure 1).
Figure 1. The reward circuitry in the human and rodent brain.
A schematic of the known major dopaminergic, glutamatergic and GABAergic connections between the ventral tegmental area (VTA), amygdala (Amyg) nucleus accumbens (NAc), hippocampus (HC) and prefrontal cortex (PFC) in human (A) and rodent (B) brain. The sine qua non of pleasure/reward in this system is a release of dopamine in the NAc from terminals of VTA-origin neurons. The NAc is further innervated by glutamatergic projections from the PFC, Amyg and HC. A CRH+ projection from the BLA to the NAc has recently been identified.
B. development
A tremendous body of work has elucidated the connectivity, operation and function of the mature reward circuitry, yet much remains unknown about the early development of this system and of its functionality in both humans and experimental models. This information is required in order to assess the nature of the influence on the circuitry by early life adverse events, and the potential impact.
In addition, although a large majority of mechanistic studies involve rodent models, there is a striking dearth of information regarding the comparative early development and maturation of the reward circuitry across species. Because the timing of adversity critically influences the outcome, this lack of information might result in imprecise inferences and difficulties in translating major preclinical studies to the human. It is important to note that because the development of distinct circuits occurs at different time-points and velocities across species[35,36], it is not optimal to consider global ‘brain development’ across species. Hence, milestones such as neurogenesis, synaptogenesis, connectivity and specific functions of a given circuit should be compared across species (Table 1). For example, studies examining over twenty distinct milestones across species suggest that, for the hippocampal circuit, the state of maturation of a 5–7 day old Sprague Dawley rat seems to approximate that of a human full-term neonate[35]. As shown in Table 1, such a comparison is far more difficult and complex for milestones within the reward circuitry. This is partially a result of very few studies as well as the different methods used across species and the different sensitivities of methods employed in historical and current work. Yet, in the aggregate, it can be gleaned that reward circuitry development during the first postnatal week in the rodent may approximate that of a full-term human neonate.
Neurotransmitter pathways of the reward circuitry
The role of dopamine in reward and motivated behaviors has been extensively studied and reviewed[37–39]. The ventral striatum and dopaminergic neurons of the substantia nigra are vital for processing reward. However, differential roles of dopamine in motivational and hedonic components of reward have been reported. For example, dopamine receptor antagonism in the NAc reduced the amount of effort an animal will expend to obtain a reward, whereas consumption and positive hedonic responses remained intact[40,41]. In addition, increased D2/D3 receptor availability in the ventral pallidum, nucleus accumbens, right ventral caudate and putamen correlated with the severity of anhedonia in clinically assessed patients with depression[42]. In rodents, incentive salience and instrumental behaviors from rewarding cues were also driven by dopaminergic control[43,44]. Together, these data support the notion that dopamine in the NAc is required for motivation of reward but not for hedonic experience and responsiveness to reward. Instead, opioids and endocannabinoids act as major neurochemical mediators of reward responsiveness[45–47].
The excitatory neurotransmitter glutamate plays a major role in the function of the reward circuit[48]. In the rodent, glutamate projections to the NAc originate from cortical, thalamic, hippocampal and amygdalar regions and function via AMPA, NMDA and mGluR receptors[49]. Further, blocking NMDA and AMPA receptors impaired the conditioned rewarding effects of drugs of abuse[50]. In humans, reward processing-driven ventral striatal activation correlated with hippocampal glutamate levels[51], and in rodents, glutamatergic ventral pallidal neurons increased activity in the lateral habenula, rostromedial tegmental nucleus and GABA VTA neurons, which resulted in constrained reward seeking[52].
The involvement of dorsal raphe serotonin transporter (SERT) terminals, which synapse onto VTA dopaminergic neurons has also been implicated in driving rewarding behaviors. In rodents, dorsal raphe serotonin fibers synapse on VTA dopaminergic neurons that co-express vesicular glutamate transporter 3 (VGlut3) and target the NAc to initiate a rapid release of dopamine via dual serotonin-glutamate input [53], yet optogenetic activation of dorsal raphe serotonin neurons prolonged the waiting time for future reward[54,55].
Neuromodulators contribute to molecular-defined pathways within the reward circuitry
In addition to classical neurotransmitters, several peptides and neuromodulators are expressed in structures involved with the reward circuitry. As noted above, opioids and endocannabinoids act as major neurochemical mediators of reward responsiveness[46,47]. Several neuropeptides are co-expressed in neurons within the reward circuitry[56,57] and specifically within the NAc. These include orexin[58], neuropeptide Y[59], and CRH and its receptors CRHR1 and CRHR2 [60–63]. More recently, Itoga et al. 2019, using viral genetic mapping and anterograde and retrograde tracing, mapped CRH expressing projection sources to the NAc in mice[64]. Intriguingly, the authors identified an enrichment of CRH-expressing inputs to the NAc from brain regions involved in aspects of sensing, processing and retrieval of emotionally salient events. These findings are intriguing because CRH, a stress-regulated peptide and a mediator of stress, is poised to execute the effects of adversity, including early-life adversity, on the reward circuitry[65–67].
The role of CRH in the reward circuitry
CRH is an essential, evolutionarily conserved stress neuropeptide that is expressed in specific neuronal populations throughout the brain to crucially modulate the functions of several circuits including those involved in processing of emotion and cognition[68,69]. CRH and its cognate receptors have been shown to exhibit experience-dependent plasticity in different nodes of the reward and stress circuitries such as the amygdala, locus coeruleus, dorsal raphe and hippocampus[21,70–73]. For instance, CRH in the NAc increases dopamine release promoting appetitive behavior, via CRH receptors that have been identified in rodent[74], and primate[75] NAc. However following prior stress exposure, CRH-mediated dopamine release was abolished and the behavioral consequence of CRH release in the NAc switched from appetitive to aversive [61]. Further, CRH in the NAc increased cholinergic interneuron firing and acetylcholine tone[76,77], as well as cFos activity[62] and phosphorylation of CREB in NAc medium spiny neurons[78].
Whereas CRH-expressing fibers have been identified in the NAc that originate from the basolateral amygdala, the function of this BLA-NAc pathway remains unclear (Figure 2). Better information is available for other CRH-expressing pathways: Dopaminergic neurons co-expressing CRH in the ventral tegmental area (VTA) drive the aversive effects of nicotine withdrawal, activating CRHR1 to block the GABAergic input to these neurons[79]. CRH-expressing projections between the amygdala and VTA modulate dopamine release[65]. A CRH-expressing projection between the VTA and the hypothalamic paraventricular nucleus (PVN) has been identified[80], which is interesting because CRH-expressing cells in the PVN fire during aversive events and their activity is decreased in response to appetitive stimuli [81,82]. Thus, reward, such as palatable food might relieve stress by specifically targeting the CRH-expressing PVN neurons. Recently, an additional role for CRH within the reward circuitry has been identified. Following early life adversity, CRH mRNA and protein expression were augmented in several nodes of the reward and stress circuitries including amygdala and hippocampus[21,83]. Concomitantly, adult rats that experienced early-life adversity were rendered anhedonic in several measures (Figure 3). Partial silencing of CRH in the central amygdala resulted in reversal of this anhedonia [23], further supporting a complex role for CRH-dependent modulation of reward and motivational behaviors.. Whereas the evidence presented above is derived from animal models, analogous functions of CRH in humans is supported by the finding that genetic variations in the CRH receptor CRHR1 are linked to stress related psychiatric disorders [84–88].
Figure 2. A CRH expressing pathway between the basolateral amygdala (BLA) and the nucleus accumbens (NAc).
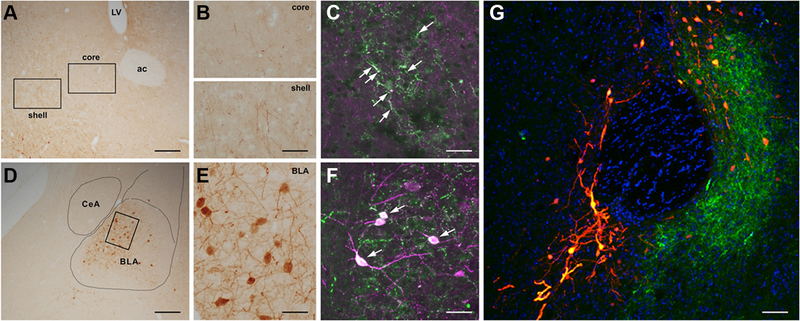
A Cre-driven retrograde adeno-associated virus (AAV2-retro-CAG-FLEX-tdTomato-WPRE) was injected into the NAc of CRH-IRES-Cre mice. (A) Low and (B) high magnification images of CRH+ fiber terminals in the NAc core and shell. (C) High magnification image of antibody-immunolabeled CRH+ fiber terminals colocalized with virus-labeled CRH+ fiber terminals in the NAc. (D-F) The virus retrogradely labels CRH+ cells in the BLA. (G) A low magnification image of the NAc. The tdTomato reporter is shown in orange, immunostaining to confirm CRH localization is shown in green. The section was counter stained with DAPI (blue). Bar = 200um in (A, D), 80um in (B), 35um in (C, F), 40um in (E) and 60um in (G).
Figure 3. Early life adversity induces anhedonia.
Rearing mice and rats in a model of simulated poverty results in adolescent and adult anhedonia. This is apparent as measured by reduced sucrose and M&M consumption, as well as diminished social play and hedonic set point for cocaine.
Functional output of the reward circuitry: Anhedonia as a readout.
Anhedonia, defined as the reduced ability to experience pleasure, is a prominent symptom of several neuropsychiatric disorders and is considered a trans-diagnostic marker for disrupted function of the reward circuitry [93]. In U.S. Marine Corps recruits, anhedonia was identified as a predictor for post-combat PTSD[89] and a potent harbinger of suicide[90]. Notably, anhedonia is a predictor of treatment outcome of cocaine dependence [91], chronic pain, and prescription opioid use[92]. Further support for altered reward circuitry in anhedonia comes from imaging studies: Structural MRI revealed that smaller right nucleus accumbens correlated with anhedonic symptoms, and that left and right putamen volume could predict the severity of present and future anhedonic states [93].
Early-life adversity induces anhedonia
Early life adversity induced by simulated poverty and unpredictable maternal behaviors resulted in decreased preference for sweets[20,94] a reduction in social play [23,95], and a reduced hedonic set point for cocaine[96] (Figure 3). All these behaviors are considered manifestations of anhedonia in rodents[20,97]. Notably, maternal separation stress alone did not result in anhedonia measured by sucrose preference; rather, a second stressor during adulthood was necessary to induce it[98,99]. Because both paradigms result in evidence of stress in the pups, these studies suggest that aberrant patterns of maternal-derived sensory signals rather than stress alone influence the development of the reward circuitry. Human studies using fMRI have probed the functional activation of components of the reward circuitry in individuals that had experienced early life adversity and identified several deficits. For examples, decreased activity was observed in the basal ganglia [100,101], and the development of ventral striatum activation in adolescents exposed to early life adversity was attenuated [102]. These authors identified a more robust effect when the stress was experienced earlier in life, indicating the importance of the timing of the insult[3].
How does early life adversity modify the reward circuitry?
Reward circuit function requires the integration and coordination of molecular, cellular, synaptic and network signaling. Failure to mature during sensitive developmental periods may result in neuropsychiatric disorders. The visual and auditory networks require patterned sensory signals of light and sound tones, respectively, to strengthen and prune synapses to form functional circuits[103,104]. In parallel, patterns of sensory signals from the mother early in life may influence the sculpting of the reward circuitry. There is evidence suggesting that predictable maternal signals enhance circuit maturation across species[22]. Conversely, unpredictable fragmented maternal care in rats and mice resulted in manifestations of anhedonia and in altered amygdala-PFC connectivity on MRI[20,23,96,105]. Thus, it is tempting to speculate that early-life adversity alters the maturation and function of the reward circuitry via several overlapping mechanisms. First, it leads to upregulation of CRH expression and neurotransmission in several nodes such as BLA-NAc and perhaps others. This aberrant CRH neurotransmission may disrupt the critically balanced combinatorial signaling within the circuit (Figure 4). In addition, aberrant sensory signal patterns during sensitive periods may promote inappropriate synaptic strengthening and pruning within the reward circuit (in analogy to visual and auditory circuits[106,107]) leading to aberrant functional signaling of the reward circuitry later in life (Figure 4).
Figure 4. Proposed changes to CRH+ connectivity of the reward circuitry following early life adversity.
(A) Connectivity between nodes of the reward circuitry following normal early life experiences. (B) Early life adversity results in aberrant connectivity of key nodes of the reward circuitry. Black arrows = known connectivity, pink arrows = known CRH+ connectivity. NAc -Nucleus accumbens, Hippo = Hippocampus, VTA = Ventral tegmental area, Amyg = Amygdala, PVT = Paraventricular thalamus, PFC = Prefrontal cortex.
Identifying predictive markers of early life adversity
The risk of early life adversity resulting in susceptibility to mental illness has led researchers to seek either genetic or epigenetic predictive markers to enable preventative or intervention approaches. For instance, meta-analysis supported an association between telomere length and early life adversity in humans, and further identified that adversity earlier in development resulted in greater negative effects compared with exposure later[108]. Genetic susceptibility might be conferred by variants in molecules involved in the functions of stress-related hormones. Thus, interactions between FKBP5 and early life adversity have been identified as markers for stress related disorders including post-traumatic stress disorder[109], and as mentioned above, polymorphisms in the CRHR1 gene were associated with greater depressive reactivity to chronic stress in those previously exposed to early life adversity[110].
A key goal in addressing the consequences of early life adversity and especially those that predict vulnerability or resilience to subsequent mental illness is identifying predictive ‘signatures’ of these consequences. In rat, distinct patterns of maternal care resulted in differences in histone acetylation and DNA methylation in stress-regulating targets[111] and BDNF methylation has been identified as a marker of early life adversity[112]. In human neonates, the glucocorticoid receptor promoter was more methylated in newborns exposed to prenatal maternal depression[113,114]. Peripheral indicators of early life adversity via DNA methylation have been identified in numerous studies[111,115,116], and more recently, repeated measurement in the same individual was successful in delineating an epigenetic ‘scar’ of early life adversity[117]. To date, the relevance of such markers for predicting early life adversity-provoked alterations of the reward circuitry is unclear, and longitudinal prospective imaging studies in humans[118,119] might uncover imaging changes that predict pathology associated with dysregulated reward circuity following this insult.
Conclusions
There is a strong association between early life adversity throughout infancy and early childhood and the subsequent development of mental illnesses associated with reward circuitry dysfunction. The key challenge is disentangling the preexisting genetic factors from the causal role of adversity and the mechanisms by which it might modify the normal functional and structural maturation of the reward circuitry. This goal is important, because it is required for identifying biomarkers and targets for prevention and intervention.
Experimental animal models and novel circuit technologies are enabling both hypothesis- driven and data driven investigations of these issues. Because adversity activates and influences the brain’s ‘stress system’, focusing on stress-related molecules is reasonable, and is supported by human genomic analyses [84]. The current review focused on aspects of these questions and investigations, highlighting areas of knowledge gaps. Notably, a key challenge is discovering sufficient information about the comparative development of the reward circuitry across species, which will allow for true translation of clinical questions to lab-based mechanistic studies, and to the translation of discoveries in experimental models back to the clinic.
Acknowledgements
We thank Sophie Levis, MD/PhD candidate and Dr. Steve Mahler for excellent discussions. The Authors’ are supported by NIH grants MH73136, MH096889 (TZB), K99 MH120327 (JLB), T32 GM008620 (CLK) as well as by the BBRF NARSAD award (JLB) and the Hewitt Foundation for Biomedical Research (MTB, JLB).
Footnotes
Financial disclosure statement: Drs. Birnie, Kooiker, Short, Bolton and Chen report no biomedical financial interests or potential conflicts of interest. Dr. Baram has received royalties for a book, reimbursements for her travel expenses from the Gordon Research Conferences and consultant fees from Amzell.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Di Segni M, Andolina D, Luchetti A, Babicola L, D’Apolito LI, Pascucci T, et al. Unstable Maternal Environment Affects Stress Response in Adult Mice in a Genotype-Dependent Manner. Cereb Cortex. 2016;26:4370–4380. [DOI] [PubMed] [Google Scholar]
- 2.Klengel T, Binder EB. Gene—Environment Interactions in Major Depressive Disorder. Can J Psychiatry. 2013;58:76–83. [DOI] [PubMed] [Google Scholar]
- 3.Hanson JL, Hariri AR, Williamson DE. Blunted Ventral Striatum Development in Adolescence Reflects Emotional Neglect and Predicts Depressive Symptoms. Biol Psychiatry. 2015;78:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaffrey MS, Barch DM, Bogdan R, Farris K, Petersen SE, Luby JL. Amygdala Reward Reactivity Mediates the Association Between Preschool Stress Response and Depression Severity. Biol Psychiatry. 2018;83:128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Short A, Baram TZ. Adverse early-life experiences and neurological disease: Age-old questions and novel answers. Nat Rev Neurol. 2019. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bale TL, Abel T, Akil H, Carlezon WA, Moghaddam B, Nestler EJ, et al. The critical importance of basic animal research for neuropsychiatric disorders. Neuropsychopharmacology. 2019;44:1349–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molet J, Maras PM, Avishai-Eliner S, Baram TZ. Naturalistic rodent models of chronic early-life stress. Dev Psychobiol. 2014;56:1675–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Bodegom M, Homberg JR, Henckens MJAG Modulation of the Hypothalamic-Pituitary-Adrenal Axis by Early Life Stress Exposure. Front Cell Neurosci. 2017;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khoury JE, Pechtel P, Andersen CM, Teicher MH, Lyons-Ruth K. Relations among maternal withdrawal in infancy, borderline features, suicidality/self-injury, and adult hippocampal volume: A 30-year longitudinal study. Behav Brain Res. 2019;374:112139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002;950:52–63. [DOI] [PubMed] [Google Scholar]
- 11.Hulshof HJ, Novati A, Sgoifo A, Luiten PGM, Den Boer JA, Meerlo P. Maternal separation decreases adult hippocampal cell proliferation and impairs cognitive performance but has little effect on stress sensitivity and anxiety in adult Wistar rats. Behav Brain Res. 2011;216:552–560. [DOI] [PubMed] [Google Scholar]
- 12.Grassi-Oliveira R, Honeycutt JA, Holland FH, Ganguly P, Brenhouse HC. Cognitive impairment effects of early life stress in adolescents can be predicted with early biomarkers: Impacts of sex, experience, and cytokines. Psychoneuroendocrinology. 2016;71:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leussis MP, Freund N, Brenhouse HC, Thompson BS, Andersen SL. Depressive-Like Behavior in Adolescents after Maternal Separation: Sex Differences, Controllability, and GABA. Dev Neurosci. 2012;34:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniels WMU, Pietersen CY, Carstens ME, Stein DJ. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab. Brain Dis, vol. 19, 2004. p. 3–14. [DOI] [PubMed] [Google Scholar]
- 15.Vazquez V, Penit-Soria J, Durand C, Besson MJ, Giros B, Daugé V. Maternal deprivation increases vulnerability to morphine dependence and disturbs the enkephalinergic system in adulthood. J Neurosci. 2005;25:4453–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levis SC, Bentzley BS, Molet J, Bolton JL, Perrone CR, Baram TZ, et al. On the early-life origins of vulnerability to opioid addiction. Mol Psychiatry. 2019;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker C-D, Bath KG, Joels M, Korosi A, Larauche M, Lucassen PJ, et al. Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress. 2017;20:421–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naninck EFG, Hoeijmakers L, Kakava-Georgiadou N, Meesters A, Lazic SE, Lucassen PJ, et al. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus. 2015;25:309–328. [DOI] [PubMed] [Google Scholar]
- 19.Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: A clinically relevant model for early-life stress. Neuroscience. 2008;154:1132–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molet J, Heins K, Zhuo X, Mei YT, Regev L, Baram TZ, et al. Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Transl Psychiatry. 2016;6:e702–e702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivy AS, Rex CS, Chen Y, Dube C, Maras PM, Grigoriadis DE, et al. Hippocampal Dysfunction and Cognitive Impairments Provoked by Chronic Early-Life Stress Involve Excessive Activation of CRH Receptors. J Neurosci. 2010;30:13005–13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis EP, Stout SA, Molet J, Vegetabile B, Glynn LM, Sandman CA, et al. Exposure to unpredictable maternal sensory signals influences cognitive development across species. Proc Natl Acad Sci. 2017;114:10390–10395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolton JL, Molet J, Regev L, Chen Y, Rismanchi N, Haddad E, et al. Anhedonia Following Early-Life Adversity Involves Aberrant Interaction of Reward and Anxiety Circuits and Is Reversed by Partial Silencing of Amygdala Corticotropin-Releasing Hormone Gene. Biol Psychiatry. 2018;83:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. [DOI] [PubMed] [Google Scholar]
- 25.Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH. Assessing anhedonia in depression: Potentials and pitfalls. Neurosci Biobehav Rev. 2016;65:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christoffel DJ, Golden SA, Walsh JJ, Guise KG, Heshmati M, Friedman AK, et al. Excitatory transmission at thalamo-striatal synapses mediates susceptibility to social stress. Nat Neurosci. 2015;18:962–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballard IC, Murty VP, Carter RM, MacInnes JJ, Huettel SA, Adcock RA. Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. J Neurosci. 2011;31:10340–10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and Behavioral Profile of Multiple Glutamatergic Inputs to the Nucleus Accumbens. Neuron. 2012;76:790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagot RC, Parise EM, Peña CJ, Zhang H-X, Maze I, Chaudhury D, et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun. 2015;6:7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown MTC, Tan KR, O’Connor EC, Nikonenko I, Muller D, Lüscher C. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature. 2012;492:452–456. [DOI] [PubMed] [Google Scholar]
- 32.Lichtenberg NT, Wassum KM. Amygdala mu-opioid receptors mediate the motivating influence of cue-triggered reward expectations. Eur J Neurosci. 2017;45:381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung BK, Balleine BW. Ventral pallidal projections to mediodorsal thalamus and ventral tegmental area play distinct roles in outcome-specific Pavlovian-instrumental transfer. J Neurosci. 2015;35:4953–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurley MM, Robble MR, Callan G, Choi S, Wheeler RA. Pituitary adenylate cyclase-activating polypeptide (PACAP) acts in the nucleus accumbens to reduce hedonic drive. Int J Obes. 2019;43:928–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25:518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clancy B, Finlay BL, Darlington RB, Anand KJS. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelley AE, Berridge KC. The Neuroscience of Natural Rewards: Relevance to Addictive Drugs. J Neurosci. 2002;22:3306–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. [DOI] [PubMed] [Google Scholar]
- 39.Mahler S V, Aston-Jones G. CNO Evil? Considerations for the Use of DREADDs in Behavioral Neuroscience. Neuropsychopharmacology. 2018;43:934–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berridge KC. Affective valence in the brain: modules or modes? Nat Rev Neurosci. 2019;20:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. [DOI] [PubMed] [Google Scholar]
- 42.Pecina M, Sikora M, Avery ET, Heffernan J, Pecina S, Mickey BJ, et al. Striatal dopamine D2/3 receptor-mediated neurotransmission in major depression: Implications for anhedonia, anxiety and treatment response. Eur Neuropsychopharmacol. 2017;27:977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher wanting but not liking for sweet rewards. J Neurosci. 2003;23:9395–9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soares-Cunha C, Coimbra B, David-Pereira A, Borges S, Pinto L, Costa P, et al. Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat Commun. 2016;7:11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ironside M, Kumar P, Kang M, Pizzagalli D. Brain mechanisms mediating effects of stress on reward sensitivity. Behav Sci (Basel). 2018;22:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pecina S, Berridge KC. Hedonic Hot Spot in Nucleus Accumbens Shell: Where Do -Opioids Cause Increased Hedonic Impact of Sweetness? J Neurosci. 2005;25:11777–11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahler S V, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32:2267–2278. [DOI] [PubMed] [Google Scholar]
- 48.Krystal J, Petrakis I, Mason G, Trevisan L, D’Souza D. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, depedence, treatment, and vulnerability. Pharmacol Ther. 2003;99:79–94. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Hamida SB, Darcq E, Zhu W, Gibb SL, Lanfranco MF, et al. Ethanol-Mediated Facilitation of AMPA Receptor Function in the Dorsomedial Striatum: Implications for Alcohol Drinking Behavior. J Neurosci. 2012;32:15124–15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garcia-Pardo MP, Miñarro J, Llansola M, Felipo V, Aguilar MA. Role of NMDA and AMPA glutamatergic receptors in the effects of social defeat on the rewarding properties of MDMA in mice. Eur J Neurosci. 2018 24 October 2018. 10.1111/ejn.14190. [DOI] [PubMed] [Google Scholar]
- 51.Bossong MG, Wilson R, Appiah-Kusi E, McGuire P, Bhattacharyya S. Human Striatal Response to Reward Anticipation Linked to Hippocampal Glutamate Levels. Int J Neuropsychopharmacol. 2018;21:623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tooley J, Marconi L, Alipio JB, Matikainen-Ankney B, Georgiou P, Kravitz A V., et al. Glutamatergic Ventral Pallidal Neurons Modulate Activity of the Habenula-Tegmental Circuitry and Constrain Reward Seeking. Biol Psychiatry. 2018;83:1012–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H-L, Zhang S, Qi J, Wang H, Cachope R, Mejias-Aponte CA, et al. Dorsal Raphe Dual Serotonin-Glutamate Neurons Drive Reward by Establishing Excitatory Synapses on VTA Mesoaccumbens Dopamine Neurons. Cell Rep. 2019;26:1128–1142.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fonseca M, Murakami M, ZF M. Activation of Dorsal Raphe Serotonergic Neurons Promotes Waiting but Is Not Reinforcing. Cell. 2015;25:306–315. [DOI] [PubMed] [Google Scholar]
- 55.Miyazaki KW, Miyazaki K, Tanaka KF, Yamanaka A, Takahashi A, Tabuchi S, et al. Optogenetic Activation of Dorsal Raphe Serotonin Neurons Enhances Patience for Future Rewards. Curr Biol. 2014;24:2033–2040. [DOI] [PubMed] [Google Scholar]
- 56.DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73:759–768. [DOI] [PubMed] [Google Scholar]
- 57.Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, et al. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castro DC, Terry RA, Berridge KC. Orexin in Rostral Hotspot of Nucleus Accumbens Enhances Sucrose ‘Liking’ and Intake but Scopolamine in Caudal Shell Shifts ‘Liking’ Toward ‘Disgust’ and ‘Fear’. Neuropsychopharmacology. 2016;41:2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Den Heuvel JK, Furman K, Gumbs MCR, Eggels L, Opland DM, Land BB, et al. Neuropeptide Y activity in the nucleus accumbens modulates feeding behavior and neuronal activity. Biol Psychiatry. 2015;77:633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peng J, Long B, Yuan J, Peng X, Ni H, Li X, et al. A Quantitative Analysis of the Distribution of CRH Neurons in Whole Mouse Brain. Front Neuroanat. 2017;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lemos JC, Wanat MJ, Smith JS, Reyes BAS, Hollon NG, Van Bockstaele EJ, et al. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012;490:402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peciña S, Schulkin J, Berridge KC. Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: Paradoxical positive incentive effects in stress? BMC Biol. 2006;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B, et al. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci. 2014; 17:27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Itoga CA, Chen Y, Fateri C, Echeverry PA, Lai JM, Delgado J, et al. New viral-genetic mapping uncovers an enrichment of corticotropin-releasing hormone-expressing neuronal inputs to the nucleus accumbens from stress-related brain regions. J Comp Neurol. 2019;527:2474–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dedic N, Kühne C, Jakovcevski M, Hartmann J, Genewsky AJ, Gomes KS, et al. Chronic CRH depletion from GABAergic, long-range projection neurons in the extended amygdala reduces dopamine release and increases anxiety. Nat Neurosci. 2018;21:803–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pleil KE, Rinker JA, Lowery-Gionta EG, Mazzone CM, McCall NM, Kendra AM, et al. NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat Neurosci. 2015;18:545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maras PM, Baram TZ. Sculpting the hippocampus from within: stress, spines, and CRH. Trends Neurosci. 2012;35:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. [DOI] [PubMed] [Google Scholar]
- 69.Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kalin N, Takahashi L, Research FC-B, 1994 U. Restraint stress increases corticotropin-releasing hormone mRNA content in the amygdala and paraventricular nucleus. Brain Res. 1994;656:182–186. [DOI] [PubMed] [Google Scholar]
- 71.Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, et al. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003;23:1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gunn B, Cox C, Chen Y, Frotscher M, Gall CM, Baram TZ, et al. The endogenous stress hormone CRH modulates excitatory transmission and network physiology in hippocampus. Cereb Cortex. 2017;27:4182–4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Short A, Maras P, Pham A, Ivy A, Baram T. Short-term block of CRH receptor in adults mitigates age-related memory impairments provoked by early-life adversity. Neuropsychopharmacology. 2019; I n press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Y, Brunson KL, Müller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF(1))-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. J Comp Neurol. 2000;420:305–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Millan MA, Jacobowitz DM, Hauger RL, Catt KJ, Aguilera G. Distribution of corticotropin-releasing factor receptors in primate brain. Proc Natl Acad Sci. 1986;83:1921–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen YW, Rada P V., Bützler BP, Leibowitz SF, Hoebel BG Corticotropin-releasing factor in the nucleus accumbens shell induces swim depression, anxiety, and anhedonia along with changes in local dopamine/acetylcholine balance. Neuroscience. 2012;29:155–166. [DOI] [PubMed] [Google Scholar]
- 77.Lemos JC, Shin JH, Alvarez VA. Striatal Cholinergic Interneurons Are a Novel Target of Corticotropin Releasing Factor. J Neurosci. 2019;39:5647–5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stern CM, Luoma JI, Meitzen J, Mermelstein PG. Corticotropin Releasing Factor-Induced CREB Activation in Striatal Neurons Occurs via a Novel Gβγ Signaling Pathway. PLoS One. 2011;6:e18114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grieder TE, Herman MA, Contet C, Tan LA, Vargas-Perez H, Cohen A, et al. VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation. Nat Neurosci. 2014;17:1751–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodaros D, Caruana D, Amir S, Neuroscience JS-, 2007 U. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. [DOI] [PubMed] [Google Scholar]
- 81.Yuan Y, Wu W, Chen M, Cai F, Fan C, Shen W, et al. Reward Inhibits Paraventricular CRH Neurons to Relieve Stress. Curr Biol. 2019;29:1243–1251.e4. [DOI] [PubMed] [Google Scholar]
- 82.Kim J, Lee S, Fang YY, Shin A, Park S, Hashikawa K, et al. Rapid, biphasic CRF neuronal responses encode positive and negative valence. Nat Neurosci. 2019;22:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dube CM, Molet J, Singh-Taylor A, Ivy A, Maras PM, Baram TZ. Hyper-excitability and epilepsy generated by chronic early-life stress. Neurobiol Stress. 2015;2:10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gelernter J, Sun N, Polimanti R, Pietrzak R, Levey DF, Bryois J, et al. Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in 165,000 US veterans. Nat Neurosci. 2019;22:1394–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. [DOI] [PubMed] [Google Scholar]
- 86.Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, Nemeroff CB, et al. Effect of Childhood Trauma on Adult Depression and Neuroendocrine Function: Sex-Specific Moderation by CRH Receptor 1 Gene. Front Behav Neurosci. 2009;3:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ressler KJ, Bradley B, Mercer KB, Deveau TC, Smith AK, Gillespie CF, et al. Polymorphisms in CRHR1 and the serotonin transporter loci: Gene x Gene x Environment interactions on depressive symptoms. Am J Med Genet Part B Neuropsychiatr Genet. 2009;153:812–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, et al. Influence of Child Abuse on Adult Depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Risbrough VB, Glynn LM, Davis EP, Sandman CA, Obenaus A, Stern HS, et al. Does Anhedonia Presage Increased Risk of Posttraumatic Stress Disorder? Curr. Top. Behav. Neurosci., vol. 38, 2018. p. 249–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ducasse D, Loas G, Dassa D, Gramaglia C, Zeppegno P, Guillaume S, et al. Anhedonia is associated with suicidal ideation independently of depression: A meta-analysis. Depress Anxiety. 2018;35:382–392. [DOI] [PubMed] [Google Scholar]
- 91.Crits-Christoph P, Wadden S, Gaines A, Rieger A, Gallop R, McKay JR, et al. Symptoms of anhedonia, not depression, predict the outcome of treatment of cocaine dependence. J Subst Abuse Treat. 2018;92:46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garland EL, Trøstheim M, Eikemo M, Ernst G, Leknes S. Anhedonia in chronic pain and prescription opioid misuse. Psychol Med. 2019:1–12. [DOI] [PubMed] [Google Scholar]
- 93.Auerbach RP, Pisoni A, Bondy E, Kumar P, Stewart JG, Yendiki A, et al. Neuroanatomical Prediction of Anhedonia in Adolescents. Neuropsychopharmacology. 2017;42:2087–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ventura A JW, Early Influences on the Development of Food Preferences. Cell. 2013;23:401–408. [DOI] [PubMed] [Google Scholar]
- 95.Shu C, Xiao L, Tang J, Wang G, Zhang X, Wang X. Blunted Behavioral and Molecular Responses to Chronic Mild Stress in Adult Rats with Experience of Infancy Maternal Separation. Tohoku J Exp Med. 2015;235:81–87. [DOI] [PubMed] [Google Scholar]
- 96.Bolton JL, Ruiz CM, Rismanchi N, Sanchez GA, Castillo E, Huang J, et al. Early-life adversity facilitates acquisition of cocaine self-administration and induces persistent anhedonia. Neurobiol Stress. 2018;8:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wright JS, Panksepp J. Toward affective circuit-based preclinical models of depression: Sensitizing dorsal PAG arousal leads to sustained suppression of positive affect in rats. Neurosci Biobehav Rev. 2011;35:1902–1915. [DOI] [PubMed] [Google Scholar]
- 98.Hill RA, Klug M, Kiss Von Soly S, Binder MD, Hannan AJ, van den Buuse M. Sex-specific disruptions in spatial memory and anhedonia in a “two hit” rat model correspond with alterations in hippocampal brain-derived neurotrophic factor expression and signaling. Hippocampus. 2014;24:1197–1211. [DOI] [PubMed] [Google Scholar]
- 99.Uchida S, Hara K, Kobayashi A, Funato H, Hobara T, Otsuki K, et al. Early Life Stress Enhances Behavioral Vulnerability to Stress through the Activation of REST4-Mediated Gene Transcription in the Medial Prefrontal Cortex of Rodents. J Neurosci. 2010;30:15007–15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boecker R, Holz NE, Buchmann AF, Blomeyer D, Plichta MM, Wolf I, et al. Impact of Early Life Adversity on Reward Processing in Young Adults: EEG-fMRI Results from a Prospective Study over 25 Years. PLoS One. 2014;9:e104185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mehta MA, Gore-Langton E, Golembo N, Colvert E, Williams SCR, Sonuga-Barke E. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. J Cogn Neurosci. 2010;22:2316–2325. [DOI] [PubMed] [Google Scholar]
- 102.Corral-Frías NS, Nikolova YS, Michalski LJ, Baranger DAA, Hariri AR, Bogdan R. Stress-related anhedonia is associated with ventral striatum reactivity to reward and transdiagnostic psychiatric symptomatology. Psychol Med. 2015;45:2605–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y, Fitzpatrick D, White L. The development of direction selectivity in ferret visual cortex requires early visual experience. Nat Neurosci. 2006:676–681. [DOI] [PubMed] [Google Scholar]
- 104.Zhang L, Bao S, Merzenich M. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat Neurosci. 2001:1123–1130. [DOI] [PubMed] [Google Scholar]
- 105.Chen Y, Baram TZ. Toward Understanding How Early-Life Stress Reprograms Cognitive and Emotional Brain Networks. Neuropsychopharmacology. 2016;41:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barkat TR, Polley DB, Hensch TK. A critical period for auditory thalamocortical connectivity. Nat Neurosci. 2011;14:1189–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. [DOI] [PubMed] [Google Scholar]
- 108.Ridout KK, Levandowski M, Ridout SJ, Gantz L, Goonan K, Palermo D, et al. Early life adversity and telomere length: a meta-analysis. Mol Psychiatry. 2018;23:858–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang Q, Shelton RC, Dwivedi Y. Interaction between early-life stress and FKBP5 gene variants in major depressive disorder and post-traumatic stress disorder: A systematic review and meta-analysis. J Affect Disord. 2018;225:422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Starr LR, Hammen C, Conway CC, Raposa E, Brennan PA. Sensitizing effect of early adversity on depressive reactions to later proximal stress: Moderation by polymorphisms in serotonin transporter and corticotropin releasing hormone receptor genes in a 20-year longitudinal study. Dev Psychopathol. 2014;26:1241–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. [DOI] [PubMed] [Google Scholar]
- 112.Kundakovic M, Gudsnuk K, Herbstman JB, Tang D, Perera FP, Champagne FA. DNA methylation of BDNF as a biomarker of early-life adversity. Proc Natl Acad Sci. 2015;112:6807–6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. [DOI] [PubMed] [Google Scholar]
- 114.Monk C, Feng T, Lee S, Krupska I, Champagne FA, Tycko B. Distress During Pregnancy: Epigenetic Regulation of Placenta Glucocorticoid-Related Genes and Fetal Neurobehavior. Am J Psychiatry. 2016;173:705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Klengel T, Binder E. Epigenetics of stress-related psychiatric disorders and gene x environment interactions. Neuron. 2015;86:1343–1357. [DOI] [PubMed] [Google Scholar]
- 116.Peter C, Fischer L, Kundakovic M, Garg P, Jakovcevski M, Dincer A, et al. DNA methylation signatures of early childhood malnutrition associated with impairments in attention and cognition. Biol Psychiatry. 2016;80:765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jiang S, Kamei N, Bolton JL, Ma X, Stern HS, Baram TZ, et al. Intra-individual methylomics detects the impact of early-life adversity. Life Sci Alliance. 2019;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bjork JM, Straub LK, Provost RG, Neale MC. The ABCD Study of Neurodevelopment: Identifying Neurocircuit Targets for Prevention and Treatment of Adolescent Substance Abuse. Curr Treat Options Psychiatry. 2017;4:196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018;32:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kallén B. The Embryology of the Telencephalic Fibre Systems in the Mouse. Development. 1954;2. [Google Scholar]
- 121.Altman J, Bayer SA. Development of the diencephalon in the rat. IV. Quantitative study of the time of origin of neurons and the internuclear chronological gradients in the thalamus. J Comp Neurol. 1979;188:455–471. [DOI] [PubMed] [Google Scholar]
- 122.Silani V, Mariani D, Donato FM, Ghezzi C, Mazzucchelli F, Buscaglia M, et al. Development of dopaminergic neurons in the human mesencephalon and in vitro effects of basic fibroblast growth factor treatment. Exp Neurol. 1994;128:59–76. [DOI] [PubMed] [Google Scholar]
- 123.Specht LA, Pickel VM, Joh TH, Reis DJ. Light-microscopic immunocytochemical localization of tyrosine hydroxylase in prenatal rat brain. II. Late ontogeny. J Comp Neurol. 1981;199:255–276. [DOI] [PubMed] [Google Scholar]
- 124.Aubert I, Brana C, Pellevoisin C, Giros B, Caille I, Carles D, et al. Molecular anatomy of the development of the human substantia nigra. J Comp Neurol. 1997;379:72–87. [DOI] [PubMed] [Google Scholar]
- 125.Hu Z, Cooper M, Crockett DP, Zhou R. Differentiation of the midbrain dopaminergic pathways during mouse development. J Comp Neurol. 2004;476:301–311. [DOI] [PubMed] [Google Scholar]
- 126.Macchi G. The ontogenic development of the olfactory telencephalon in man. J Comp Neurol. 1951;95:245–305. [DOI] [PubMed] [Google Scholar]
- 127.Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- 128.Brana C, Caille I, Pellevoisin C, Charron G, Aubert I, Caron MG, et al. Ontogeny of the striatal neurons expressing the D1 dopamine receptor in humans. J Comp Neurol. 1996;370:23–34. [DOI] [PubMed] [Google Scholar]
- 129.Caille I, Dumartin B, Le Moine C, Begueret J, Bloch B. Ontogeny of the D1 dopamine receptor in the rat striatonigral system: an immunohistochemical study. Eur J Neurosci. 1995;7:714–722. [DOI] [PubMed] [Google Scholar]
- 130.Graybiel AM, Ragsdale CW. Clumping of acetylcholinesterase activity in the developing striatum of the human fetus and young infant. Proc Natl Acad Sci U S A. 1980;77:1214–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Murrin LC, Ferrer JR. Ontogeny of the rat striatum: Correspondence of dopamine terminals, opiate receptors and acetylcholinesterase. Neurosci Lett. 1984;47:155–160. [DOI] [PubMed] [Google Scholar]
- 132.Bayer SA. Quantitative 3H-thymidine radiographic analyses of neurogenesis in the rat amygdala. J Comp Neurol. 1980;194:845–875. [DOI] [PubMed] [Google Scholar]
- 133.McConnell J, Angevine JB. Time of neuron origin in the amygdaloid complex of the mouse. Brain Res. 1983;272:150–156. [DOI] [PubMed] [Google Scholar]
- 134.Humphrey T. The development of the human amygdala during early embryonic life. J Comp Neurol. 1968;132:135–165. [DOI] [PubMed] [Google Scholar]
- 135.Berdel B, Moryś J, Maciejewska B, Dziewiatkowski J. Volume and topographical changes of the basolateral complex during the development of the rat’s amygdaloid body. Folia Morphol (Warsz). 1997;56:1–11. [PubMed] [Google Scholar]
- 136.Nikolic I, Kostović I. Development of the lateral amygdaloid nucleus in the human fetus: transient presence of discrete cytoarchitectonic units. Anat Embryol (Berl). 1986;174:355–360. [DOI] [PubMed] [Google Scholar]
- 137.Ulfig N, Setzer M, Bohl J. Ontogeny of the human amygdala. Ann N Y Acad Sci. 2003;985:22–33. [DOI] [PubMed] [Google Scholar]
- 138.Legaz I, Olmos L, Real MÁ, Guirado S, Davila JC, Medina L. Development of neurons and fibers containing calcium binding proteins in the pallial amygdala of mouse, with special emphasis on those of the basolateral amygdalar complex. J Comp Neurol. 2005;488:492–513. [DOI] [PubMed] [Google Scholar]
- 139.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. [DOI] [PubMed] [Google Scholar]
- 140.Koss WA, Belden CE, Hristov AD, Juraska JM. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse. 2014;68:61–72. [DOI] [PubMed] [Google Scholar]
- 141.Watson RE, DeSesso JM, Hurtt ME, Cappon GD. Postnatal growth and morphological development of the brain: a species comparison. Birth Defects Res Part B Dev Reprod Toxicol. 2006;77:471–484. [DOI] [PubMed] [Google Scholar]
- 142.Yakovlev P. The development of the nuclei of the dorsal thalamus and of the cerebral cortex: Morphogenetic and tectogenetic correlation. Mod Neurol. 1969 1969. [Google Scholar]
- 143.Angevine J. Time of neuron origin in the diencephalon of the mouse: An autoradiographic study. J Comp Neurol. 1970:129–187. [DOI] [PubMed] [Google Scholar]
- 144.Mrzljak L, Uylings HBM, Kostovic I, van Eden CG. Prenatal development of neurons in the human prefrontal cortex: I. A qualitative Golgi study. J Comp Neurol. 1988;271:355–386. [DOI] [PubMed] [Google Scholar]
- 145.Auladell C, Perez-Sust P, Supèr H, Soriano E. The early development of thalamocortical and corticothalamic projections in the mouse. Anat Embryol (Berl). 2000;201:169–179. [DOI] [PubMed] [Google Scholar]
- 146.Greco C, Rovee-Collier C, Hayne H, Griesler P, Earley L. Ontogeny of early event memory: I. Forgetting and retrieval by 2- and 3-month-olds. Infant Behav Dev. 1986;9:441–460. [Google Scholar]
- 147.Johanson IB, Hall WG. Appetitive learning in 1-day-old rat pups. Science. 1979;205:419–421. [DOI] [PubMed] [Google Scholar]
- 148.Desor JA, Maller O, Turner RE. Taste in acceptance of sugars by human infants. J Comp Physiol Psychol. 1973;84:496–501. [DOI] [PubMed] [Google Scholar]
- 149.Vigorito M, Sclafani A. Ontogeny of polycose and sucrose appetite in neonatal rats. Dev Psychobiol. 1988;21:457–465. [DOI] [PubMed] [Google Scholar]
- 150.Wood SL, Beyer BK, Cappon GD. Species comparison of postnatal CNS development: Functional measures. Birth Defects Res Part B Dev Reprod Toxicol. 2003;68:391–407. [DOI] [PubMed] [Google Scholar]