Abstract
Clostridium difficile is a leading cause of morbidity and mortality particularly in hospital settings. In addition, treatment is very challenging due to the scarcity of effective therapeutic options. Thus, there remains an unmet need to identify new therapeutic agents capable of treating C. difficile infections. In the current study, we screened two FDA-approved drug libraries against C. difficile. Out of almost 3,200 drugs screened, 50 drugs were capable of inhibiting the growth of C. difficile. Remarkably, some of the potent inhibitors have never been reported before and showed activity in a clinically achievable range. Structure-activity relationship (SAR) analysis of the active hits clustered the potent inhibitors into four chemical groups; nitroimidazoles (MIC50= 0.06 − 2.7 μM), salicylanilides (MIC50= 0.2 − 0.6 μM), imidazole antifungals (MIC50= 4.8 − 11.6 μM) and miscellaneous group (MIC50= 0.4 − 22.2 μM). The most potent drugs from the initial screening were further evaluated against additional clinically-relevant strains of C. difficile. Moreover, we tested the activity of potent inhibitors against representative strains of human normal gut microbiota to investigate the selectivity of the inhibitors towards C. difficile. Overall, this study provides a platform that could be used for further development of potent and selective anticlostridial antibiotics.
Keywords: Clostridium difficile, Imidazole anticlostridials, salicylanilides anticlostridials, Gut microbiota, Drug library screening
1. Introduction
Clostridium difficile infection (CDI) has recently drawn a significant worldwide attention. In 2011, CDI afflicted nearly half a million people and was a direct cause of death of over 29,000 patients in the United States alone (1). In Europe, the European Centre for Disease Prevention and Control estimated there were nearly 124,000 cases of healthcare-associated CDIs in acute care hospitals alone between 2011 ‒ 2012 (2). Further, CDIs are not restricted to the healthcare setting only; community-acquired infections represent about 41% of all CDIs while 9% transpired in the residents of long-term care services such as retirement homes (3).
The United States Centers for Disease Control and Prevention (CDC) has classified C. difficile and CDIs as an urgent public health threat that necessitates immediate and rigorous action (4). However, despite numerous calls for effective preventive measures and potent treatments, only three drugs are used for the treatment of CDI, vancomycin, metronidazole and fidaxomicin. A challenge with treating CDI with either vancomycin or metronidazole is that both agents harm the gut microflora. A second limitation with metronidazole is this drug is completely absorbed from the intestinal tract leaving a very minute concentration at the site of infection. These drawbacks contribute to a high percentage of treatment failure and relapse (5). Fidaxomicin, FDA-approved for the treatment of CDI in 2011, has a better profile than both vancomycin and metronidazole as pertaining to bacterial specificity and oral bioavailability (6). Nonetheless, the clinical outcome is still unsatisfactory regarding treatment failure and relapse especially against the more virulent NAP1 strains of C. difficile. Furthermore, treatment with fidaxomicin costs 150 times more than metronidazole thus compounding the cost to treat CDI. This highlights the need to identify and develop new, safe, and effective anticlostridial drugs.
In spite of the massive advances in drug discovery technologies, developing a de novo drug takes up to 15 years and can cost up to 2 billion US dollars. Drug repositioning, or finding a novel indication for a known drug, is a way to lessen the time and cost of drug discovery since these drugs have well-characterized toxicity and pharmacokinetic profiles. Many successful drugs being used now are repurposed from their original indication (7–14). We used this approach in the current study to conduct a screening of about 3,200 drugs and clinical molecules to identify drugs or lead molecules with potent anticlostridial activity and limited effect against important bacterial species that comprise the gut microflora.
2. Materials and Methods
2.1. Bacterial strains and reagents
C. difficile and human gut microbiota strains used in this study (Supplementary Table 1) were acquired from the Biodefense and Emerging Infections Research Resources Repository (BEI Resources, Manassas, VA) and the American Type Culture Collection (ATCC, Manassas, VA). Strains were cultured in brain heart infusion supplemented broth (BHIS, Brain heart infusion medium from Becton, Dickinson and Company, Cockeysville, MD), supplemented with yeast extract, L-cysteine, vitamin K1 and hemin (Sigma-Aldrich, St. Louis, MO). Phosphate buffered saline (PBS) was purchased from Corning (Corning, NY).
2.2. Compounds and libraries
The Pharmakon 1600 repositioning compound library was purchased from MicroSource Discovery Systems, Inc. (Gaylordsville, CT) and the Johns Hopkins library was provided by Johns Hopkins University (Baltimore, MD). Both libraries were supplied in 96-well plates of 10 mM stocks of the compounds in either dimethyl sulfoxide or water and stored in −80° C. Ornidazole, miconazole nitrate, econazole nitrate, tioconazole, butoconazole, clotrimazole, metoprolol tartrate, metoclopramide hydrochloride, chloroquine diphosphate, miconazole, dimetridazole, nithiamide and methylthiouracil (Alfa Aesar, Ward Hill, MA), dichlorophen, triclabendazole, closantel, nitazoxanide (Ark Pharm Inc, Libertyville, IL), ronidazole, oxyclozanide (Sigma-Aldrich, St. Louis, MO), tinidazole (TCI, Portland, OR) and niclosamide (Cayman Chemical, Ann Arbor, MI) were all purchased separately to confirm the results of both libraries. Vancomycin hydrochloride (Gold Biotechnology, Olivette, MO) and metronidazole (BTC, Hudson, NH) were used as positive controls.
2.3. Screening assay
Libraries were screened at a fixed concentration of 16 μM against one strain of C. difficile (C. difficile NAP07) to identify active compounds (Table 1). Briefly, bacteria were streaked on BHIS agar plates and incubated anaerobically at 37 °C for 48 hours. Colonies were scraped off from the agar plates, suspended in PBS and diluted in BHIS broth at a concentration of about 5 × 105 CFU/mL. Compounds, at a concentration of 16 μM, were mixed with the bacterial suspension in 96-well plates and incubated anaerobically for 48 hours at 37 °C. Drugs that inhibited bacterial growth visually were considered as “hits”. Active drugs hits were purchased from commercial vendors and their activity was confirmed against C. difficile NAP07. Commercial drugs that did not show activity were excluded from the study.
Table 1:
Active drugs “hits” identified from initial screening against C. difficile NAP07
| Compound name | Chemical structure | MIC (μM) | Main use | |
|---|---|---|---|---|
| 1 | Ronidazole | 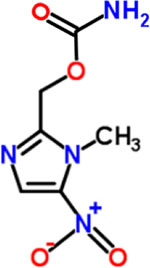 |
0.06 | Anthelmintic |
| 2 | Phenylmercuric acetate | 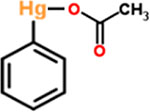 |
0.25 | Antifungal, antimicrobial |
| 3 | Ornidazole | 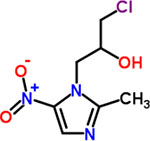 |
0.25 | Anthelmintic |
| 4 | Dimetridazole | 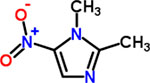 |
0.25 | Anthelmintic |
| 5 | Nithiamide | 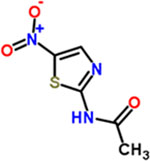 |
0.25 | Antiprotozoal (trichomonas). |
| 6 | Closantel | 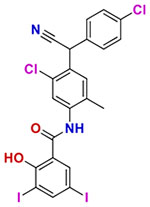 |
0.25 | Anthelmintic. |
| 7 | Bithionate sodium | 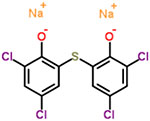 |
0.50 | Anthelmintic, antiseptic |
| 8 | Secnidazole | 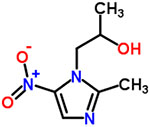 |
0.50 | Anthelmintic, antitrichomonas |
| 9 | Oxyclozanide | 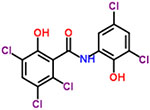 |
0.50 | Anthelmintic |
| 10 | Tinidazole | 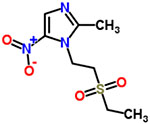 |
0.50 | Antiprotozoal |
| 11 | Metoprolol tartrate | 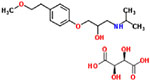 |
1 | Antihypertensive, antianginal |
| 12 | Miconazole nitrate | 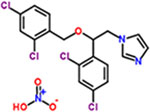 |
1 | Antifungal (topical) |
| 13 | Metoclopramide hydrochloride | 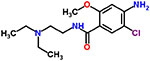 |
2 | Antiemetic |
| 14 | Chloroquine diphosphate | 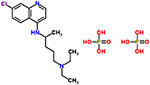 |
2 | Anthelmintic, antirheumatic, intercalating agent |
| 15 | Nitazoxanide | 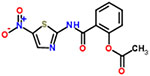 |
2 | Anthelmintic |
| 16 | Benznidazole (n-benzyl-2-nitro-1h-imidazole-1-acetamide) | 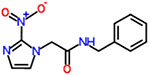 |
2 | Antiprotozoal (trypanosoma) |
| 17 | Bithionol oxide (2,2′-sulfinyl-bis(4,6-dichlorophenol)) | 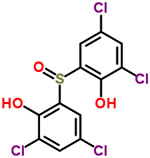 |
2 | Anthelmintic |
| 18 | Nifursol | 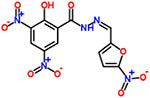 |
4 | Anthelmintic |
| 19 | Butoconazole | 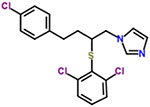 |
4 | Antifungal |
| 20 | Broxaldine | 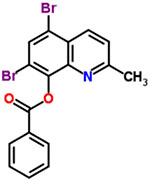 |
4 | Anthelmintic, antifungal |
| 21 | Oxiconazole nitrate | 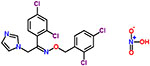 |
4 | Antifungal |
| 22 | Clotrimazole | 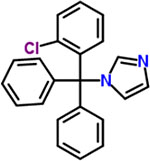 |
8 | Antifungal |
| 23 | Diethylstilbestrol | 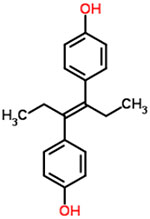 |
8 | Estrogen |
| 24 | Methylthiouracil | 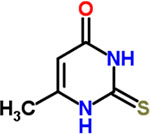 |
8 | Antithyroid agent |
| 25 | Econazole nitrate | 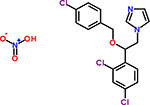 |
8 | Antifungal |
| 26 | Diclazuril | 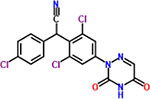 |
8 | Coccidiostat |
| 27 | Niclosamide | 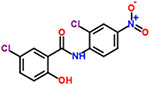 |
8 | Anthelmintic, teniacide |
| 28 | Triclabendazole | 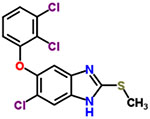 |
8 | Anthelmintic |
| 29 | Dichlorophene | 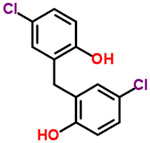 |
8 | Anthelmintic (cestodes) |
| 30 | Sulconazole | 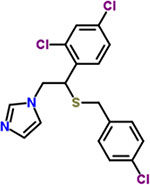 |
8 | Antifungal |
| 31 | Tioconazole | 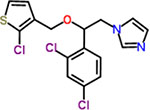 |
8 | Antifungal (topical) |
| 32 | Puromycin | 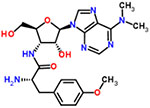 |
8 | Antineoplastic |
| 33 | Sanguinarium chloride | 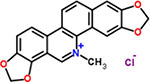 |
16 | Antineoplastic, antiplaque agent |
| 34 | Norgestimate | 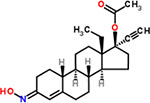 |
16 | Progestin |
| 35 | Estradiol valerate | 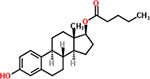 |
16 | Estrogen |
| 36 | Methylprednisolone | 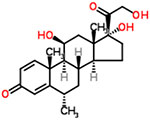 |
16 | Anti-inflammatory, glucocorticoid |
| 37 | Proglumide | 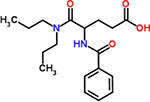 |
16 | Anticholinergic and cholecystokinin antagonist |
| 38 | Sulconazole nitrate | 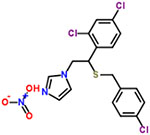 |
16 | Antifungal |
| 39 | Tretinoin | 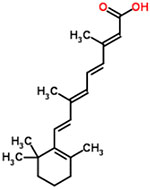 |
16 | Keratolytic, antiacne, antineoplastic |
| 40 | Chloroxine | 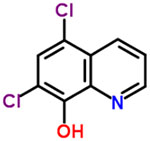 |
16 | Chelating agent, antiseborrheic |
| 41 | Benzalkonium chloride | 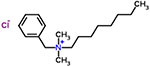 |
16 | Preservative |
| 42 | Prednicarbate | 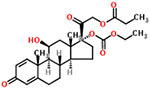 |
16 | Anti-inflammatory, glucocorticoid |
| 43 | Teniposide | 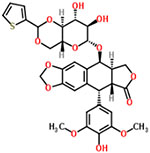 |
16 | Antineoplastic |
| 44 | Bifonazole | 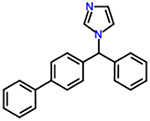 |
16 | Antifungal, calmodulin antagonist |
| 45 | Benzbromarone | 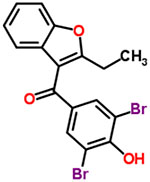 |
16 | Uricosuric |
| 46 | Toremifene citrate | 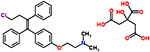 |
16 | Antineoplastic, antiestrogen |
| 47 | Quinaldine blue (pinacyanol chloride) | 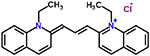 |
16 | Antineoplastic |
| 48 | Dithiazanine iodide | 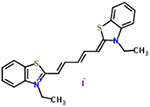 |
16 | Anthelmintic (nematodes) |
| 49 | Rose bengal | 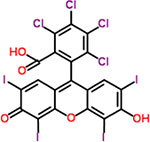 |
16 | Diagnostic aid (corneal trauma indicator). |
| 50 | Alprenolol hydrochloride | 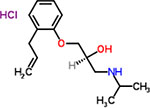 |
16 | Antihypertensive |
2.4. Microdilution assay against C. difficile strains
The most potent hits were divided into four groups based on their chemical structure (Table 2) and tested for their minimum inhibitory concentrations (MICs) against a panel of ten C. difficile strains according to the Clinical and Laboratory Standards Institute guidelines (CLSI, M11-A8) (15). Drugs, at the required concentrations, were anaerobically incubated with bacterial suspensions (5 × 105 CFU/mL) at 37 °C for 48 hours in 96-well plates. After incubation, plates were examined for bacterial turbidity. MIC was defined as the lowest concentration where bacterial growth was halted and turbidity was unnoticeable.
Table 2:
Classification of the most potent anticlostridial hits
| Class | Drug | Use(s) | ||
|---|---|---|---|---|
| A | Nitroimidazole | 1 | Ronidazole | Antiprotozoal in veterinary medicine |
| 2 | Dimetridazole | Antiprotozoal in veterinary medicine | ||
| 3 | Tinidazole | Antiparasitic | ||
| 4 | Ornidazole | Antiparasitic | ||
| 5 | Secnidazole | Antiparasitic | ||
| B | Salicylanilides | 5 | Closantel | Anthelmintic and pesticide |
| 6 | Oxyclozanide | Anthelmintic in veterinary medicine | ||
| 7 | Niclosamide | Anthelmintic in humans and animals | ||
| C | Imidazole antifungal | 8 | Miconazole nitrate | Antifungal |
| 9 | Econazole | Antifungal | ||
| 10 | Tioconazole | Antifungal | ||
| 11 | Butoconazole | Antifungal | ||
| 12 | Clotrimazole | Antifungal | ||
| D | Miscellaneous | 13 | Dichlorophene | Antiparasitic in veterinary medicine |
| 14 | Triclabendazole | Antiparasitic in human and animal medicine | ||
| 15 | Nitazoxanide | Antiparasitic and antiviral | ||
| 16 | Nithiamide | Antiprotozoal | ||
2.5. Activity against human microbiota
Active compounds were evaluated for antibacterial activity against representative strains of human normal gut flora previously described (16). Different types of bacteria were used in this experiment; for anaerobic bacteria (Bifidobacterium and Bacteroides) and Lactobacillus, bacteria were first streaked on agar plates and incubated for 48 hours at 37 °C (anaerobically using BHIS agar for anaerobes and in 5% CO2 using MRS agar plate for Lactobacillus). Bacterial colonies were suspended in BHIS broth (for anaerobes) or in MRS broth (for Lactobacillus) to achieve a bacterial concentration of approximately 105 CFU/ml. Bacteria were then added to 96-well plates containing serial dilutions of the compounds and incubated as mentioned above for 48 hours. Regarding Escherichia coli and Enterococcus faecalis, bacteria were scraped off tryptic soy agar plates and suspended in tryptic soy broth to achieve a bacterial concentration of 5 × 105 CFU/mL. The bacterial suspensions were aerobically incubated with serial dilutions of the drugs at 37 °C for 16 − 20 hours. Reported MICs are the minimum concentration of the compounds that could inhibit visual growth of bacteria.
3. RESULTS AND DISCUSSION
3.1. Screening assay and Structure-activity relationship (SAR) analysis:
Two drug libraries consisting of approximately 3,200 FDA-approved drugs and clinical molecules were evaluated against one strain of C. difficile (NAP07, CDC#2007054, a reference strain in the human microbiota project). All molecules were initially tested at a single concentration, 16 μM, in order to pinpoint active compounds or “hits”. The initial screening revealed 116 compounds from Johns Hopkins library and 111 compounds from Pharmakon library that inhibited C. difficile at 16 μM (Supplementary Figure 1, Supplementary Tables 2 and 3). After excluding antiseptic and antibacterial agents and combining drugs from both libraries, 50 compounds were identified (Table 1). To confirm the screening results, the minimum inhibitory concentrations (MICs) of these 50 hits were determined against C. difficile NAP07. As depicted in Table 1, the MIC values for the active hits ranged from 0.06 μM to 16 μM.
At first glance, the active compounds seem to be highly scattered structurally. However, the vast majority of the active molecules are imidazole-containing structures. Among the imidazole-containing structures, the nitroimidazoles seem to be the most efficient as six nitroimidazoles (dimetridazole, secnidazole, ronidazole, ornidazole, tinidazole and benznidazole) possessed MIC values below 2 μM against C. difficile NAP07 (Table 1). Additionally, the anticlostridial activity was impacted by the position of the nitro group on the imidazole ring. In this vein, the 2-nitroimidazole derivative benznidazole was remarkably less active than all 5-nitromidazole analogs. This is also in accordance with the potent activity of the 5-nitrimidazole metronidazole which was used for a long time as a first-line therapy for CDIs (17). Moreover, the alkyl substitution seems to have less effect on the anticlostridial activity as dimetridazole (with only two methyl groups at positions 1 and 2) was nearly equipotent to the 5-nitroimidazole derivatives carrying more complex and bulkier substituents at positions 1 and 2, such as ornidazole and secnidazole (Figure 1). Apart from the nitroimidazole ring system, 5-nitrothiazole, a 5-nitroimidazole close bioisostere heterocyclic system, revealed very promising anticlostridial activity. In particular, nithiamide inhibited the growth of C. difficile NAP07 at a sub-micromolar concentration (the MIC value was 0.25 μM, Table 1). Increasing the bulkiness at thiazole position-2 (like in nitazoxanide) decreased the anticlostridial activity by a factor of 8 in where the MIC value of the antiprotozoal nitazoxanide was 2 μM (Figure 1).
Figure 1: Effect of the nitro group position and alkyl substitution on the anticlostridial activity of nitroim idazoles and nitrothiazoles.
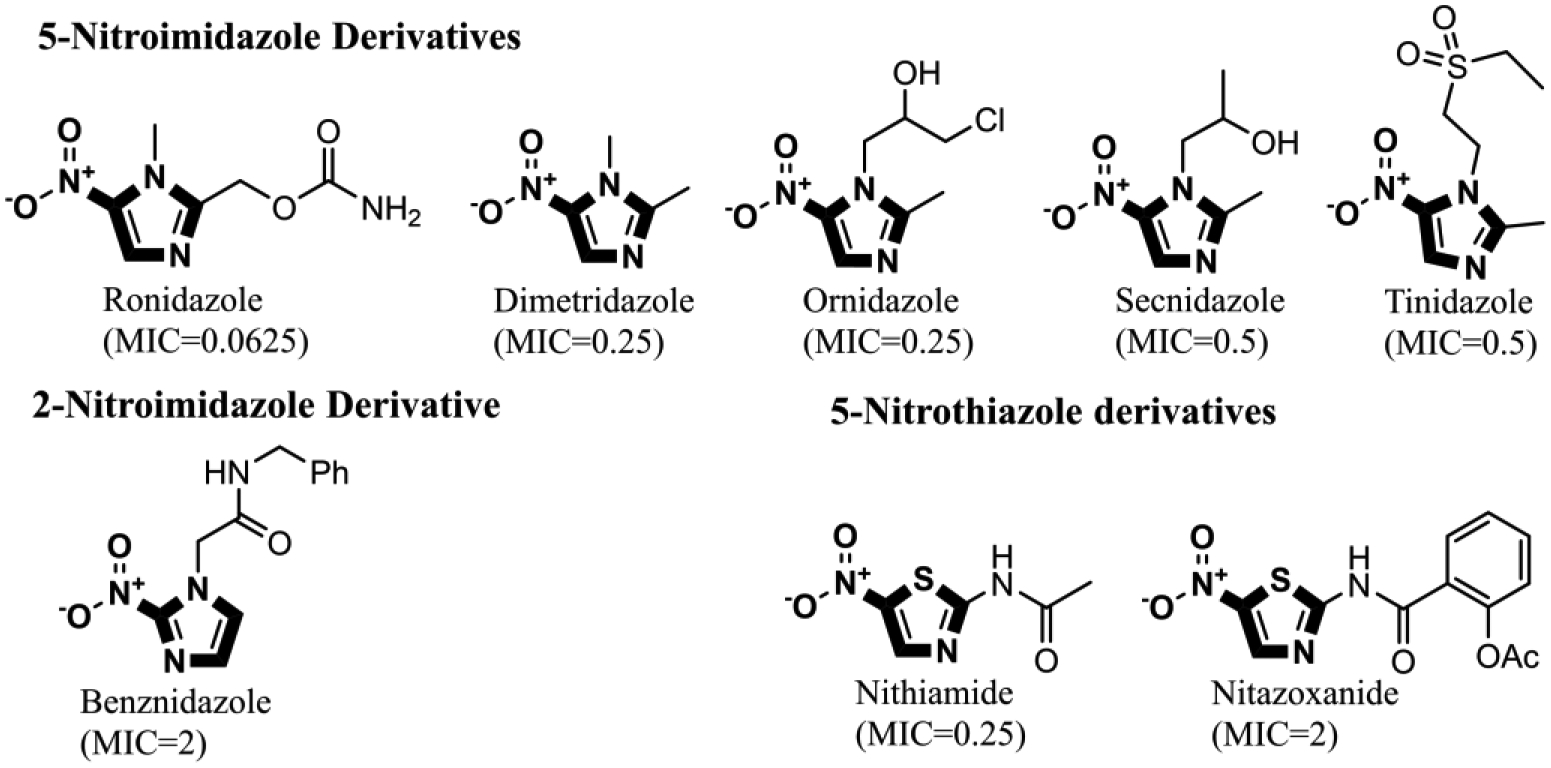
5-nitroimidazoles were found to be more potent than 2-nitroimdazle. Contrarily, variation in the alkyl substitution of 5-nitroimdazole did not significantly affect anticlostridial activity. Additionally, Increasing the size of the substitution at position 2 of the nitrothiazole ring increased the MIC from 0.25 μM in the case of nithiamide to 2 μM in case of nitazoxanide. MIC values are against C. difficile NAP07 and are expressed in μM.
The last group of compounds with a similar scaffold was the imidazole antifungals as seven of them were active against C. difficile NAP07 with MIC values ranging between 1 and 8 μM (Table 1). This set of compounds shares more than the imidazole ring since most of them are 1-(2,4-dichlorophenylethyl)imidazole derivatives. From a structure-activity relationship position, the second chlorination at the side chain, benzyloxy moiety, drastically improves anticlostridial activity as observed with the dichlorinated miconazole (MIC value is 1 μM) and its monochlorinated analog econazole (MIC value is 8 μM). On the other hand, the type of linker seems to have less effect on anticlostridial activity. In this regard, econazole with an ether linker possessed the same MIC value against C. difficile NAP07 as its thioether analog sulconazole (MIC = 8 μM). This value is identical to the MIC for the first-generation imidazole-antifungal clotrimazole (Table 1). Additionally, the oxime linker seems to reduce the anticlostridial activity as observed with oxiconazole (MIC = 4 μM) whereas the ether analog miconazole had a MIC of 1 μM (Figure 2).
Figure 2: Effect of chlorination and linker type on the anticlostridial activity of imidazole antifungals.
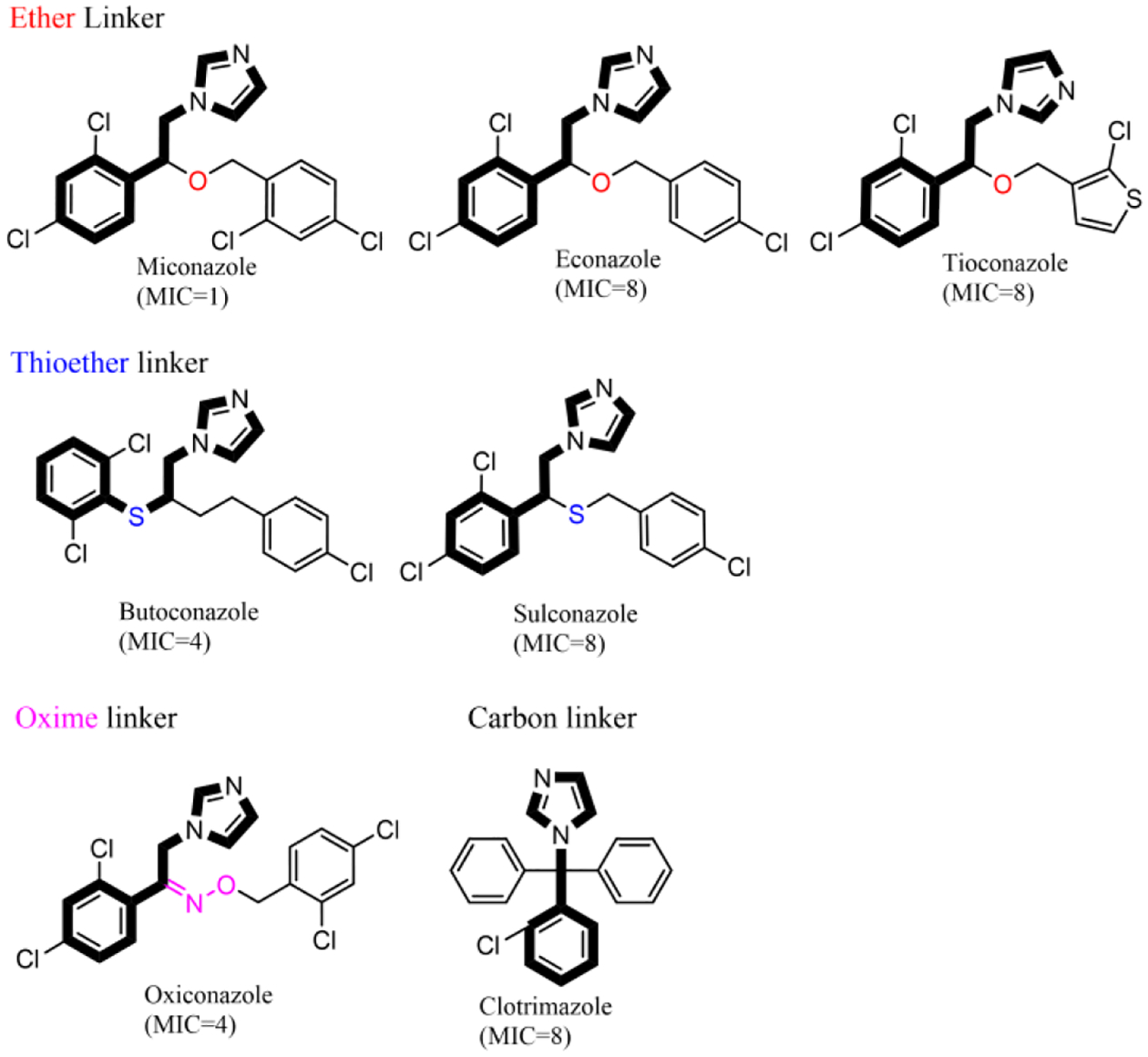
Dichlorinated miconazole is more potent than monochlorinated econazole. Additionally, the type of linker between the imidazole and phenyl rings does not affect anticlostridial activity. MIC values are against C. difficile NAP07 and are expressed in μM.
3.2. Activity against clinical C. difficile strains
The most potent drugs from the initial screening were further evaluated against additional clinically-relevant strains of C. difficile. These compounds were grouped based on their chemical structure and tested against additional ten C. difficile strains. For each compound, we calculated the minimum concentration that inhibited the growth of 50% of the tested strains, MIC50, in order to use it to compare the activities of different drugs. Grouping of the hits yielded four distinct structural classes of molecules, namely nitroimidazole, imidazole antifungals, salicylanilide and a fourth group of potent inhibitors that did not belong to a specific chemical class (Table 2).
The first group we investigated was nitroimidazole-containing compounds. Members of this group included ronidazole, dimetridazole, ornidazole, secnidazole and tinidazole in addition to metronidazole, the positive control. Nitroimidazoles inhibited the growth of all tested C. difficile strains at low concentrations (MIC50s ranged from 0.3 to 2.7 μM, Table 3).Nitroimidazole-containing compounds, and nitroheterocyclic drugs in general, are known to exert potent inhibitory activity against anaerobic bacteria (18). Although nitroimidazoles can diffuse into both aerobic and anaerobic bacterial cells, reductive activation occurs only in obligate anaerobes by pyruvate:ferredoxin oxidoreductase system. As a result, nitro group reduction produces imidazole radical and nitrite, both of which damage bacterial DNA leading to cell death. In addition, reduction reserves the concentration gradient around the bacterial cell envelop and allows more diffusion of the drug into the bacterial cells (19). Metronidazole was previously used as a first-line treatment for mild to moderated CDIs and is still recommended when vancomycin and fidaxomicin are not attainable (17, 20). However, the activity of metronidazole is limited by the high bioavailability of the drug, leaving a minute concentration of drug in the gut lumen where the infection is localized (21). As a result, the treatment outcome is not satisfactory. In addition, several cases of metronidazole-resistant CDIs have been reported (22). Still, nitroimidazole represents an attractive scaffold that could be modified in order to obtain a better anticlostridial drug (with decreased oral bioavailability). The activities of 5-nitroimidazole-containing compounds were thoroughly studied against parasites in comparison to metronidazole. In most cases, several nitroimidazoles (whether FDA-approved or not) were found to be more effective than metronidazole and some of them possessed activity against metronidazole-resistant strains (23). On the contrary, fewer comparative studies have been conducted to evaluate the activity of 5-nitroimidazoles against anaerobic bacteria (only a handful of these studies involved C. difficile) (24). Tinidazole was previously shown to possess excellent in vitro activity against C. difficile and was found to be more effective than metronidazole against metronidazole-resistant strains (25). However a limitation with this study is that tinidazole was not assessed in an in vivo model of CDI (26). Ornidazole is an alternative therapy to metronidazole in the treatment of giardiasis and bacterial vaginosis. (27). Though ornidazole is not recommended for treatment of CDIs, it is reported to be used as a treatment for CDI in certain parts of the world (28). The most potent anticlostridial nitroimidazole was ronidazole, a veterinary antiprotozoal drug (29). Ronidazole inhibited the growth of all the tested C. difficile strains at a concentration of 0.6 μM or less. Indeed, ten out of the eleven tested strains were inhibited at 0.3 μM. Although ronidazole is anecdotally reported to have carcinogenic and embryotoxic effects, it was shown to be safe in albino rats and pigs at very high concentrations and for prolonged periods of time (30). Overall, nitroimidazoles warrant further investigation as more potent anticlostridial alternatives to metronidazole.
Table 3:
MICs (μM ) of the active compounds against clinical strains of C. difficile
| C. difficile Strain | NR number | Nitroimidazoles | Imidazole antifungals | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ronidazole | Dimetridazole | Ornidazole | Tinidazole | Secnidazole | Miconazole | Econazole | Tioconazole | Butoconazole | Clotrimazole | ||
| P 3 | 32884 | 0.3 | 1.8 | 0.6 | 2 | 2.7 | 4.8 | 10.5 | 10.3 | 4.9 | 23.2 |
| P 5 | 32885 | 0.3 | 1.8 | 0.6 | 2 | 2.7 | 4.8 | 5.2 | 5.2 | 4.9 | 5.8 |
| P 6 | 32886 | 0.3 | 1.8 | 0.3 | 2 | 1.4 | 4.8 | 10.5 | 10.3 | 4.9 | 11.6 |
| P 7 | 32887 | 0.3 | 1.8 | 0.6 | 16.2 | 5.4 | 4.8 | 10.5 | 10.3 | 4.9 | 11.6 |
| P 19 | 32895 | 0.3 | 1.8 | 0.6 | 4 | 5.4 | 4.8 | 10.5 | 10.3 | 4.9 | 11.6 |
| P 30 | 32904 | 0.3 | 1.8 | 0.6 | 8.1 | 5.4 | 2.4 | 10.5 | 10.3 | 4.9 | 23.2 |
| Isolate 7 | 13433 | 0.3 | 1.8 | 0.6 | 4 | 2.7 | 2.4 | 10.5 | 5.2 | 4.9 | 11.6 |
| Isolate 11 | 13437 | 0.3 | 1.8 | 0.3 | 2 | 2.7 | 4.8 | 10.5 | 10.3 | 9.7 | 11.6 |
| Isolate 13 | 13553 | 0.6 | 1.8 | 0.6 | 16.2 | 2.7 | 9.6 | 5.2 | 20.6 | 4.9 | 11.6 |
| ATCC BAA 1801 | 0.3 | 1.8 | 0.6 | 2 | 1.35 | 9.6 | 21 | 20.6 | 19.4 | 23.2 | |
| C. difficile Strain | NR number | Salicylanilides | Miscellaneous | Vancomycin | Metronidazole | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Closantel | Oxyclozanide | Niclosamide | Nitazoxanide | Dichlorophene | Triclabendazole | Nithiamide | ||||
| P 3 | 32884 | 0.4 | 1.2 | 0.8 | 0.8 | 7.4 | 22.2 | 0.7 | 0.7 | 1.5 |
| P 5 | 32885 | 0.2 | 0.3 | 0.2 | 0.8 | 7.4 | 22.2 | 0.7 | 0.7 | 0.7 |
| P 6 | 32886 | 0.2 | 1.2 | 0.8 | 0.8 | 3.7 | 11.1 | 0.3 | 0.7 | 0.7 |
| P 7 | 32887 | 0.4 | 1.2 | 0.8 | 1.6 | 7.4 | 22.2 | 0.7 | 0.7 | 0.7 |
| P 19 | 32895 | 0.2 | 0.6 | 0.4 | 0.2 | 14.9 | 11.1 | 0.7 | 1.4 | 1.5 |
| P 30 | 32904 | 0.2 | 0.6 | 0.4 | 0.2 | 7.4 | 22.2 | 0.3 | 0.3 | 1.5 |
| Isolate 7 | 13433 | 0.2 | 0.3 | 0.4 | 0.4 | 7.4 | 22.2 | 0.7 | 0.3 | 1.5 |
| Isolate 11 | 13437 | 0.4 | 1.2 | 0.4 | 0.4 | 14.9 | 22.2 | 0.7 | 0.7 | 1.5 |
| Isolate 13 | 13553 | 0.8 | 0.6 | 0.4 | 0.8 | 14.9 | 22.2 | 0.7 | 0.7 | 1.5 |
| ATCC BAA 1801 | 0.2 | 0.3 | 0.4 | 0.2 | 7.4 | 11.1 | 0.3 | 0.3 | 0.7 | |
| MIC50 | 0.2 | 0.6 | 0.4 | 0.4 | 7.4 | 22.2 | 0.7 | 0.7 | 1.5 | |
The second group to possess a potent anticlostridial activity was salicylanilide-related drugs/clinical molecules. Three drugs were included in this group, namely niclosamide, oxyclozanide and closantel. Salicylanilides inhibited the growth of C. difficile at concentrations that ranged from 0.2 to 1.2 μM, while their MIC50s were 0.2 μM for closantel, 0.4 μM for niclosamide and 0.6 μM for oxyclozanide (Table 3). Salicylanilides were recently reported to exert potent activities against several Gram-positive bacterial pathogens including C. difficile and vancomycin-resistant Enterococci (VRE) (7, 31). Niclosamide and oxyclozanide exhibited activity against methicillin resistant Staphylococcus aureus through compromising the integrity of the bacterial cell envelope without causing cell lysis (32). Against Helicobacter pylori, niclosamide disrupted the bacterial proton motive force resulting in growth inhibition (33). Similar activity was observed against C. difficile, whereby salicylanilides were found to inhibit the growth of both logarithmic and stationary phase bacteria via dissipation of their membrane potential (31). However, the in vivo activity of this group of compounds in an animal model of CDI is yet to be tested. On the other hand, salicylanilides exhibited potent in vitro activity against VRE, while niclosamide was very effective in reducing the bacterial burden in a VRE colonization reduction mouse model (7). Knowing that VRE overgrowth is a major side effect of vancomycin and metronidazole when used to treat CDI (5, 34), the activity of salicylanilides against VRE can be exploited in the treatment of CDI.
The third group of C. difficile inhibitors included imidazole antifungal compounds, miconazole, econazole, tioconazole, butoconazole and clotrimazole. The MICs for these drugs varied from 2.4 to 23.2 μM and the MIC50 was 4.8 μM for miconazole, 4.9 μM for butoconazole, 10.3 μM for tioconazole, 10.5 μM for econazole and 11.6 μM for clotrimazole (Table 3). Azoles in general, and imidazoles in particular, represent an attractive scaffold for drug discovery. They can easily interact with enzymes through a wide array of noncovalent interactions. Imidazole compounds exert their antifungal activity through inhibition of ergosterol biosynthesis. Ergosterol depletion is primarily due to inhibition of cytochrome P-450-dependant 14α- demethylase activity and results in mitigating membrane integrity and fungal inhibition (35). On the contrary, two mechanisms have been proposed for imidazoles activity as antibacterial agents. The first one is through the inhibition of enoyl acyl carrier protein reductase (FabI) with a resultant inhibition of bacterial fatty acid synthesis (36). Although this mechanism of action applies to several bacterial pathogens, e.g. S. aureus and E. coli, it cannot be expected in C.difficile due to the absence of FabI as a catalyst in fatty acid biosynthesis (37). The second proposed mechanism of bacterial inhibition by imidazoles is blocking of flavohaemoglobins-mediated metabolism of nitric oxide leading to bacterial cell death (38). Nevertheless, more investigation is required to confirm this activity in C. difficile. Although the antibacterial activity of imidazoles has been reported against other bacteria, this is the first report, to our knowledge, of the anticlostridial activity of imidazole antifungals.
The last group of C. difficile inhibitors contained compounds from scattered chemical classes. The most potent two compounds among this group were nitazoxanide and nithiamide. Both drugs contain a nitrothiazole ring structure. The MICs for nitazoxanide ranged from 0.2 to 1.6 μM and the MIC50 was 0.4 μM. On the other hand, nithiamide’s MICs were between 0.3 and 0.7 μM against all the tested C. difficile strains and its MIC50 was 0.7 μM (Table 3). Nitazoxanide is an antiprotozoal drug that possesses, along with its active metabolite tizoxanide, a potent antibacterial activity against both aerobes and anaerobes. Although it is not included in CDI treatment guidelines, nitazoxanide has been reported to possess potent anticlostridial activity both in vitro and in vivo (39). In addition, nitazoxanide proved to be noninferior to both vancomycin and metronidazole in clinical studies against CDI (40). Despite the similarity between the spectrum of activity of metronidazole and nitazoxanide, studies have shown that nitazoxanide is mechanistically distinct from metronidazole. The anticlostridial activity of nitazoxanide is attributed to its noncompetitive inhibition of pyruvate:ferredoxin/flavodoxin oxidoreductases which in turn blocks the oxidative decarboxylation of pyruvate to acetyl coenzyme A and lead subsequently to bacterial killing (41). Nithiamide, on the other hand, has been used as an antiparasitic agent in both human and animal medicine (42). However nithiamide has never been tested against C. difficile and the data about its antibacterial activity is very erratic (43). In addition, nithiamide is structurally similar to nitazoxanide, hence it is expected that they might share the same mechanism of action, although this hypothesis will need to be confirmed experimentally. The last two drugs in the miscellaneous group (dichlorophene and triclabendazole) had less potent activity than nithiamide and nitazoxanide in vitro. Dichlorophene and triclabendazole are both antiparasitic drugs with no significant known antibacterial activity (44, 45). Dichlorophene inhibited the growth of C. difficile strains at concentrations ranging between 3.7 and 14.9 μM while triclabendazole inhibited the same strains at concentrations between 11.1 and 22.2 μM. The MIC50 values for dichlorophene and triclabendazole were 7.4 and 22.2 μM, respectively (Table 3).
3.3. Activity of the potent hits against hum an microbiota
Intestinal microbiota protects against C. difficile colonization through the production of short chain fatty acids (SCFAs). SCFAs stimulate the growth of gut epithelium, reduce inflammation through the induction of regulatory T cells (Tregs), induce antimicrobial peptides like thuricin CD and augment the mucus barrier through increasing production of mucin (46). Furthermore, resident bacteria compete with invading C. difficile cells for intestinal niches and nutrients. In addition, the microbiota is involved in the transformation of primary bile acids, a germinant for C. difficile spores, into secondary bile acids resulting in a reduction of spore germination and inhibition of vegetative growth (46). Based on that, we sought to test the selectivity of the active anticlostridial hits by assessing their activity against representative strains of commensal bacteria present in the human gastrointestinal tract. Evaluated strains included anaerobic bacteria (Bifidobacteria and Bacteroides), microaerophilic bacteria (Lactobacilli), Gram-positive (Enterococci) and Gram-negative (Escherichia coli) bacteria.
In accordance with their reported activity against anaerobes, nitroimidazoles inhibited the growth of Bifidobacteria and Bacteroides (24, 47). Only one strain of Bifidobacterium, B. breve HM-856, was not inhibited by nitroimidazoles including metronidazole, the positive control (Table 4). Nitroimidazoles had minimal or no activity against the rest of the tested bacterial strains. Imidazole antifungals had a similar pattern of activity against Bifidobacteria and Bacteroides, in addition, they inhibited the growth of both enterococcal strains tested. The activity of the imidazole antifungal miconazole was reported previously against several Gram-positive bacteria e.g. Staphylococcus, Streptococcus and Enterococcus (48). Similarly, salicylanilides inhibited both anaerobes and had some activity against Enterococcus. Additionally, they inhibited growth of several Lactobacillus strains (Table 4). Closantel was the most potent inhibitor amongst salicylanilides potentially due to the difference in its physicochemical properties relative to other salicylanilides (7). All four drugs in the miscellaneous group inhibited the growth of anaerobes and some Gram-positive strains. The most selective drug in this group was nitazoxanide; it inhibited the growth of the microbiota at concentrations that were several folds higher than the drug’s MIC50 against C. difficile. Nithiamide also exhibited a good selectivity profile against most of the tested strains of microbiota. The MIC of nithiamide against most of the inhibited microbiota strains was much higher than its MIC50 against C. difficile (Table 4).
Table 4:
MICs (μg/mL) of the active compounds against human normal gut flora
| Bacterial Strain | Strain ID | Nitroimidazole | Imidazole antifungals | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ronidazole | Dimetridazole | Ornidazole | Tinidazole | Miconazole | Econazole | Tioconazole | Butoconazole | Clotrimazole | ||
| Lactobacillus casei | ATCC-334 | >640 | >907 | >583 | >518 | 308 | 335 | 330 | 311 | 371 |
| Lactobacillus acidophilus | ATCC-314 | 640 | >907 | >583 | >518 | 308 | 335 | >330 | >311 | 371 |
| Bifidobacterium bifidum | ATCC-11863 | ≤5 | ≤7 | ≤5 | 32 | 10 | 21 | 21 | 19 | 23 |
| Bifidobacterium breve | ATCC-15700 | ≤5 | 227 | 146 | 518 | 38 | 42 | 41 | 39 | 46 |
| Bifidobacterium longum | HM-845 | ≤5 | ≤7 | 36 | 16 | 38 | 42 | 41 | 5 | 46 |
| Bifidobacterium breve | HM-856 | >640 | >907 | >583 | >518 | 38 | 42 | >330 | 10 | 46 |
| Bacteroides fragilis | HM-711 | ≤5 | 28 | ≤5 | ≤4 | 38 | 42 | 5 | 10 | 46 |
| Bacteroides fragilis | HM-709 | ≤5 | 28 | ≤5 | ≤4 | 19 | 21 | ≤3 | 10 | 46 |
| Lactobacillus crispatus | HM-371 | 640 | 907 | >583 | >518 | 308 | 335 | >330 | >311 | 371 |
| Lactobacillus gasseri | HM-407 | 320 | 907 | >583 | >518 | 308 | 335 | >330 | >311 | 371 |
| Escherichia coli | ATCC-35150 | 640 | 113 | >583 | >518 | >308 | >335 | >330 | 78 | >371 |
| Escherichia coli | 1411 | 640 | 113 | >583 | >518 | >308 | >335 | >330 | 78 | >371 |
| Enterococcus faecalis -- TX0104 | HM-201 | 640 | 454 | >583 | >518 | 38 | 42 | >330 | 19 | >371 |
| Strain | Strain ID | Salicylanilides | Miscellaneous | Vancomycin | Metronidazole | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Closantel | Oxyclozanide | Niclosamide | Nitazoxanide | Dichlorophene | Triclabendazole | Nithiamide | ||||
| Lactobacillus casei | ATCC-334 | 193 | 80 | 196 | >417 | >476 | >356 | >684 | >88 | >748 |
| Lactobacillus acidophilus | ATCC-314 | 97 | 40 | >391 | >417 | >476 | >356 | >684 | 0.7 | >47 |
| Bifidobacterium bifidum | ATCC-11863 | ≤1.5 | 40 | ≤3 | ≤3 | 30 | 11 | ≤5 | 0.7 | 5.8 |
| Bifidobacterium breve | ATCC-15700 | ≤1.5 | 10 | ≤3 | ≤3 | 30 | 22 | ≤5 | 2.8 | 47 |
| Bifidobacterium longum | HM-845 | ≤1.5 | ≤2.5 | ≤3 | 52 | 30 | 5.5 | ≤5 | 0.3 | 23 |
| Bifidobacterium breve | HM-856 | 3 | 40 | ≤3 | 26 | 30 | 22 | ≤5 | 0.3 | >748 |
| Bacteroides fragilis | HM-711 | 3 | 5 | 6 | 26 | 30 | 44 | 43 | 88 | ≤5.8 |
| Bacteroides fragilis | HM-709 | 3 | 5 | ≤3 | 26 | 30 | 22 | 11 | 44 | ≤5.8 |
| Lactobacillus crispatus | HM-371 | 12 | >319 | 49 | 417 | 476 | >356 | 342 | 1.4 | >748 |
| Lactobacillus gasseri | HM-407 | 12 | >319 | 49 | 417 | 476 | >356 | 342 | 1.4 | >748 |
| Escherichia coli | ATCC-35150 | >193 | >319 | >391 | >417 | >476 | >356 | >684 | ND | ND |
| Escherichia coli | 1411 | 193 | >319 | >391 | >417 | >476 | >356 | >684 | ND | ND |
| Enterococcus faecalis -- TX0104 | HM-201 | 24 | >319 | >391 | 26 | 59 | 22 | 11 | >88 | ND |
| Enterococcus faecalis -- TX1322 | HM-202 | 24 | >319 | >391 | 26 | 59 | 11 | 11 | 0.7 | ND |
ND= Not detected
To summarize, we screened two libraries consisting of FDA-approved drugs and clinical molecules against C. difficile in order to identify potent and selective inhibitors. We identified three distinct chemical classes of molecules that have potent inhibitory activity against C. difficile, nitroimidazoles, salicylanilides and imidazole antifungals. Additionally, we identified four drugs that do not belong to any of the previous chemical categories, nitazoxanide, nithiamide, dichlorophene and triclabendazole. All the active compounds were tested against a panel of C. difficile strains and were found to exhibit potent inhibitory activity. In addition, they were tested against normal intestinal microflora strains to investigate their selectivity for C. difficile over other beneficial bacteria. Overall, the current study can serve as a reference for anti-C. difficile drug developers and can provide leads for further development for the treatment of CDIs.
Supplementary Material
4. Funding:
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI130186.
Footnotes
Competing financial interests: The authors declare no competing financial interests. Supplementary information is available at the journal website.
5. References
- 1.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, et al. Burden of Clostridium difficile infection in the United States. The New England journal of medicine. 2015;372(9):825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ECDC. European surveillance of Clostridium difficile infections Surveillance protocol version 2.2. European Centre for Disease Prevention and Control, 2015. [Google Scholar]
- 3.Khanna S, Pardi DS, Aronson SL, Kammer PP, Orenstein R, St Sauver JL, et al. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. The American journal of gastroenterology. 2012;107(1):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barra-Carrasco J, Paredes-Sabja D. Clostridium difficile spores: a major threat to the hospital environment. Future microbiology. 2014;9(4):475–86. [DOI] [PubMed] [Google Scholar]
- 5.Kelly CP, LaMont JT. Clostridium difficile--more difficult than ever. The New England journal of medicine. 2008;359(18):1932–40. [DOI] [PubMed] [Google Scholar]
- 6.Cruz MP. Fidaxomicin (Dificid), a Novel Oral Macrocyclic Antibacterial Agent For the Treatment of Clostridium difficile-Associated Diarrhea in Adults. P & T : a peer-reviewed journal for formulary management. 2012;37(5):278–81. [PMC free article] [PubMed] [Google Scholar]
- 7.Mohammad H, AbdelKhalek A, Abutaleb NS, Seleem MN. Repurposing niclosamide for intestinal decolonization of vancomycin-resistant enterococci. International journal of antimicrobial agents. 2018;51(6):897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AbdelKhalek A, Abutaleb NS, Mohammad H, Seleem MN. Repurposing ebselen for decolonization of vancomycin-resistant enterococci (VRE). PloS one. 2018;13(6):e0199710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younis W, AbdelKhalek A, Mayhoub AS, Seleem MN. In Vitro Screening of an FDA-Approved Library Against ESKAPE Pathogens. Curr Pharm Des. 2017;23(14):2147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thangamani S, Eldesouky HE, Mohammad H, Pascuzzi PE, Avramova L, Hazbun TR, et al. Ebselen exerts antifungal activity by regulating glutathione (GSH) and reactive oxygen species (ROS) production in fungal cells. Biochim Biophys Acta. 2016;1861(1 Pt A):3002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Younis W, Thangamani S, Seleem MN. Repurposing Non-Antimicrobial Drugs and Clinical Molecules to Treat Bacterial Infections. Curr Pharm Des. 2015;21(28):4106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thangamani S, Younis W, Seleem MN. Repurposing celecoxib as a topical antimicrobial agent. Front Microbiol. 2015;6:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thangamani S, Younis W, Seleem MN. Repurposing ebselen for treatment of multidrug-resistant staphylococcal infections. Sci Rep. 2015;5:11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thangamani S, Mohammad H, Younis W, Seleem MN. Drug repurposing for the treatment of staphylococcal infections. Curr Pharm Des. 2015;21(16):2089–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute (CLSI). Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria, 8th Edition M11–A8. 2012. [Google Scholar]
- 16.Shao X, AbdelKhalek A, Abutaleb NS, Velagapudi UK, Yoganathan S, Seleem MN, et al. Chemical Space Exploration Around Thieno[3,2-d]pyrimidin-4(3H)-one Scaffold led to a Novel Class of Highly Active Clostridium difficile Inhibitors. Journal of medicinal chemistry. 2019. [DOI] [PubMed] [Google Scholar]
- 17.McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018;66(7):e1–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar M, Adhikari S, Hurdle JG. Action of nitroheterocyclic drugs against Clostridium difficile. International journal of antimicrobial agents. 2014;44(4):314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarrad AM, Karoli T, Blaskovich MA, Lyras D, Cooper MA. Clostridium difficile drug pipeline: challenges in discovery and development of new agents. Journal of medicinal chemistry. 2015;58(13):5164–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. The American journal of gastroenterology. 2013;108(4):478–98; quiz 99. [DOI] [PubMed] [Google Scholar]
- 21.Bolton RP, Culshaw MA. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut. 1986;27(10):1169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ang CW, Jarrad AM, Cooper MA, Blaskovich MAT. Nitroimidazoles: Molecular Fireworks That Combat a Broad Spectrum of Infectious Diseases. Journal of medicinal chemistry. 2017;60(18):7636–57. [DOI] [PubMed] [Google Scholar]
- 23.Upcroft JA, Dunn LA, Wright JM, Benakli K, Upcroft P, Vanelle P. 5-Nitroimidazole drugs effective against metronidazole-resistant Trichomonas vaginalis and Giardia duodenalis. Antimicrobial agents and chemotherapy. 2006;50(1):344–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jokipii L, Jokipii AM. Comparative evaluation of the 2-methyl-5-nitroimidazole compounds dimetridazole, metronidazole, secnidazole, ornidazole, tinidazole, carnidazole, and panidazole against Bacteroides fragilis and other bacteria of the Bacteroides fragilis group. Antimicrobial agents and chemotherapy. 1985;28(4):561–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jokipii AM, Jokipii L. Comparative activity of metronidazole and tinidazole against Clostridium difficile and Peptostreptococcus anaerobius. Antimicrobial agents and chemotherapy. 1987;31(2):183–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedge DD, Strain JD, Heins JR, Farver DK. New advances in the treatment of Clostridium difficile infection (CDI). Therapeutics and clinical risk management. 2008;4(5):949–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erkkola R, Jarvinen H. Single dose of ornidazole in the treatment of bacterial vaginosis. Annales chirurgiae et gynaecologiae Supplementum. 1987;202:94–6. [PubMed] [Google Scholar]
- 28.Gorenek L, Dizer U, Besirbellioglu B, Eyigun CP, Hacibektasoglu A, Van Thiel DH. The diagnosis and treatment of Clostridium difficile in antibiotic-associated diarrhea. Hepato-gastroenterology. 1999;46(25):343–8. [PubMed] [Google Scholar]
- 29.Gookin JL, Copple CN, Papich MG, Poore MF, Stauffer SH, Birkenheuer AJ, et al. Efficacy of ronidazole for treatment of feline Tritrichomonas foetus infection. Journal o f veterinary internal medicine. 2006;20(3):536–43. [DOI] [PubMed] [Google Scholar]
- 30.Steiner JM, Schwamberger S, Pantchev N, Balzer HJ, Vrhovec MG, Lesina M, et al. Use of Ronidazole and Limited Culling To Eliminate Tritrichomonas muris from Laboratory Mice. Journal of the American Association for Laboratory Animal Science : JAALAS. 2016;55(4):480–3. [PMC free article] [PubMed] [Google Scholar]
- 31.Gooyit M, Janda KD. Reprofiled anthelmintics abate hypervirulent stationary-phase Clostridium difficile. Scientific reports. 2016;6:33642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajamuthiah R, Fuchs BB, Conery AL, Kim W, Jayamani E, Kwon B, et al. Repurposing salicylanilide anthelmintic drugs to combat drug resistant Staphylococcus aureus. PloS one. 2015;10(4):e0124595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tharmalingam N, Port J, Castillo D, Mylonakis E. Repurposing the anthelmintic drug niclosamide to combat Helicobacter pylori. Scientific reports. 2018;8(1):3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.AbdelKhalek A, Abutaleb NS, Mohammad H, Seleem MN. Antibacterial and antivirulence activities of auranofin against Clostridium difficile. International journal of antimicrobial agents. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clinical microbiology reviews. 1999;12(4):501–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heerding DA, Chan G, DeWolf WE, Fosberry AP, Janson CA, Jaworski DD, et al. 1,4-Disubstituted imidazoles are potential antibacterial agents functioning as inhibitors of enoylacyl carrier protein reductase (FabI). Bioorganic & medicinal chemistry letters. 2001;11(16):2061–5. [DOI] [PubMed] [Google Scholar]
- 37.Marreddy RKR, Wu X, Sapkota M, Prior AM, Jones JA, Sun D, et al. The Fatty Acid Synthesis Protein Enoyl-ACP Reductase II (FabK) is a Target for Narrow-Spectrum Antibacterials for Clostridium difficile Infection. ACS infectious diseases. 2019;5(2):208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rani N, Sharma A, Singh R. Imidazoles as promising scaffolds for antibacterial activity: a review. Mini reviews in medicinal chemistry. 2013;13(12):1812–35. [DOI] [PubMed] [Google Scholar]
- 39.McVay CS, Rolfe RD. In vitro and in vivo activities of nitazoxanide against Clostridium difficile. Antimicrobial agents and chemotherapy. 2000;44(9):2254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musher DM, Logan N, Bressler AM, Johnson DP, Rossignol JF. Nitazoxanide versus vancomycin in Clostridium difficile infection: a randomized, double-blind study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;48(4):e41–6. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman PS, Sisson G, Croxen MA, Welch K, Harman WD, Cremades N, et al. Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and Campylobacter jejuni. Antimicrobial agents and chemotherapy. 2007;51(3):868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willcox RR. Treatment of vaginal trichomoniasis with 2-acetylamino-5-nitrothiazole (aminitrozole) given orally. The British journal of venereal diseases. 1957;33(2):115–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalinichenko NF. [Nitazole--an antimicrobial substance]. Mikrobiolohichnyi zhurnal. 1998;60(1):83–91. [PubMed] [Google Scholar]
- 44.Villegas F, Angles R, Barrientos R, Barrios G, Valero MA, Hamed K, et al. Administration of triclabendazole is safe and effective in controlling fascioliasis in an endemic community of the Bolivian Altiplano. PLoS neglected tropical diseases. 2012;6(8):e1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andrews P B G. Chemistry of Anticestodal Agents. Campbell WC R RS, editor. Boston, MA: Springer; 1986. [Google Scholar]
- 46.Perez-Cobas AE, Moya A, Gosalbes MJ, Latorre A. Colonization Resistance of the Gut Microbiota against Clostridium difficile. Antibiotics. 2015;4(3):337–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wust J Susceptibility of anaerobic bacteria to metronidazole, ornidazole, and tinidazole and routine susceptibility testing by standardized methods. Antimicrobial agents and chemotherapy. 1977;11(4):631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nenoff P, Koch D, Kruger C, Drechsel C, Mayser P. New insights on the antibacterial efficacy of miconazole in vitro. Mycoses. 2017;60(8):552–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


