Abstract
The development of the adrenal cortex varies considerably across primates, being most conspicuous in humans, where a functional zona reticularis–the site of dehydroepiandrosterone-sulfate (DHEA/S) production–does not develop until middle childhood (5–8 years). Prior reports suggest that a human-like adrenarche, associated with a sharp prepubertal increase in DHEA/S, may only occur in the genus Pan. However, the timing and variability in adrenarche in chimpanzees remain poorly described, owing to the lack of longitudinal data, or data from wild populations. Here, we use urine samples from East African chimpanzees (Pan troglodytes schweinfurthii) collected over 20 years at Kanyawara in Kibale National Park, Uganda, to trace the developmental trajectories of DHEAS (n = 1,385 samples, 53 individuals) and cortisol (n = 12,726 samples, 68 individuals). We used generalized additive models (GAM) to investigate the relationship between age, sex, and hormone levels. Adrenarffche began earlier in chimpanzees (~2–3 years) compared with what has been reported in humans (6–8 years) and, unlike humans, male and female chimpanzees did not differ significantly in the timing of adrenarche nor in DHEAS concentrations overall. Similar to what has been reported in humans, cortisol production decreased through early life, reaching a nadir around puberty (8–11 years), and a sex difference emerged with males exhibiting higher urinary cortisol levels compared with females by early adulthood (15–16 years). Our study establishes that wild chimpanzees exhibit a human-like pattern of cortisol production during development and corroborates prior reports from captive chimpanzees of a human-like adrenarche, accompanied by significant developmental increases in DHEAS. While the role of these developmental hormone shifts are as yet unclear, they have been implicated in stages of rapid behavioral development once thought unique to humans, especially in regard to explaining the divergence of female and male social behavior before pubertal increases in gonadal hormones.
Keywords: adrenarche, hormonal development, juvenile period, life history, sex differences
1 |. INTRODUCTION
Steroid hormones produced by the adrenal gland mediate individual responses to external/environmental conditions through their direct effects on metabolism, immune function, and behavior. Cortisol, the principal glucocorticoid produced by primates, helps to organize the stress response and regulate metabolism (Katsu & Iguchi, 2016). The adrenal androgens dehydroepiandrosterone-sulfate (DHEAS), and its unconjugated form, DHEA (denoted jointly as DHEA/S), play important roles in neurological (Compagnone & Mellon, 1998; Maninger, Wolkowitz, Reus, Epel, & Mellon, 2009; Pluchino et al., 2015), immunological (Hechter, Grossman, & Chatterton, 1997; Prall & Muehlenbein, 2018), and socio-cognitive functioning (Campbell, 2006, 2011; Del Giudice, 2018; Del Giudice, Angeleri, & Manera, 2009). During development, adrenal hormones are thought to organize critical stages of behavioral maturation through their effects on growth and development (Bernstein, 2016; Bernstein, Sterner, & Wildman, 2012; Del Giudice, 2018).
While many features of adrenal structure and function are conserved across mammals, humans exhibit some unusual characteristics. In humans the mature adrenal cortex–specifically the innermost layer called the zona reticularis (ZR)–serves as the major source of DHEA/S (Endoh, Kristiansen, Casson, Buster, & Hornsby, 1996), but in most other species, DHEA/S derives primarily from the gonads and few have the ability to synthesize it in the adrenals (Cutler et al., 1978; its expression across development. Notably, humans and some other primates exhibit a unique fetal zone (FZ) of the adrenal cortex which produces very high levels of DHEA/S that serve as an essential precursor for placental estrogen production (Mesiano & Jaffe, 1997; Xing, Lerario, Rainey, & Hammer, 2015). The FZ then recedes early in postnatal life, thus postnatal adrenal production of DHEA/S depends on the eventual development of the ZR, a process known as adrenarche (Mesiano & Jaffe, 1997; Nguyen & Conley, 2008; Vinson, 2016; Xing et al., 2015).
Among primates, adrenarche appears to occur reliably only among some catarrhines, as evidenced by both histological (e.g., Levine, Wolfe, Schiebinger, Loriaux, & Cutler, 1982; Nguyen, Mapes, Corbin, & Conley, 2008) and genetic (Bernstein et al., 2012) studies (n.b., a functional ZR has been reported in marmosets, but only selectively in some females, Pattison, Abbott, Saltzman, Conley, & Bird, 2009; and in mouse lemurs, but only seasonally, Perret & Aujard, 2005). In most primates, this process is complete during the first few months of development, meaning that DHEA/S levels peak early in life and then depreciate during development (Castracane, Cutler, & Loriaux, 1981; Conley et al., 2011; Conley, Pattison, & Bird, 2004; Cutler et al., 1978; Ducsay, Hess, McClellan, & Novy, 1991; Levine et al., 1982; Nguyen et al., 2008). However, in humans, adrenarche is delayed until middle childhood (approx. ages 6–8 years in western populations). This results in a dramatic postnatal decline in DHEA/S as the FZ recedes followed by a prepubertal surge as the ZR matures (Dhom, 1973; Havelock, Auchus, & Rainey, 2004; Hopper & Yen, 1975; Ibáñez, DiMartino-Nardi, Potau, & Saenger, 2000; Korth-Schutz, Levine, & New, 1976; Palmert et al., 2001; Sperling, 2014). Girls generally reach adrenarche about a year earlier than boys but increases in DHEAS are both relatively and absolutely larger in boys, and adult men have higher circulating DHEAS than women (Campbell, 2006; Hopper & Yen, 1975; Labrie, 2010; Orentreich, Brind, Rizer, & Vogelman, 1984; Šulcová, Hill, Hampl, & Starka, 1997; Zumoff & Bradlow, 1980). The unique developmental pattern of adrenal hormone production is thought to be linked both with the extended period of childhood in humans (Bogin, 1997) and with the specific neurological and behavioral shifts that occur before puberty (Campbell, 2006, 2011), suggesting a pivotal role in calibration of life history strategies (Del Giudice et al., 2009; Del Giudice, 2018).
Only great apes have been proposed to exhibit a pattern of adrenarche that resembles that of humans, featuring delayed ZR development and a prepubertal “spurt” in DHEA/S. However, in contrast to the human pattern, captive gorillas exhibit only transient developmental increases in DHEA/S (Bernstein et al., 2012). Small but significant increases in DHEAS were detected with age among rehabilitant orangutans in Malaysia (Prall et al., 2015), though other studies of orangutans are equivocal (Bernstein et al., 2012; Cutler et al., 1978). Several reports propose that developmental shifts in chimpanzee and bonobo DHEA/S most closely resemble human adrenarche in both timing and degree (Behringer, Hohmann, Stevens, Weltring, & Deschner, 2012; Bernstein et al., 2012; Cutler et al., 1978; Smail, Faiman, Hobson, Fuller, & Winter, 1982). However, these studies are all based on cross-sectional samples from captive colonies, and most distinguish only between broad developmental age groups (e.g., “juvenile” vs. “adolescent”). Histological evidence is similarly imprecise, confirming only that precursors to the ZR were detectable in the adrenal tissue of a captive chimpanzee at 3 years, and that this layer was fully developed in the next oldest individual, a 12-year-old (Parker, Grizzle, Blevins, & Hawkes, 2014). Thus, there is still considerable uncertainty about the timing of adrenal maturation in chimpanzees, particularly in the wild, where many developmental processes are delayed compared with captivity (e.g., Kimura & Hamada, 1996; Zihlman, Bolter, & Boesch, 2007; Emery Thompson & Sabbi, 2019).
Glucocorticoids are produced in a different layer of the adrenal cortex, the zona fasciculata (ZF), that is functional soon after birth (Mesiano & Jaffe, 1997; Xing et al., 2015). Though the ZF does undergo further maturation between infancy and adulthood, cortisol production was long assumed to be static throughout early development in humans, after controlling for body size (e.g., Aranoff & Rosier, 1980; Honour, Kelnar, & Brook, 1991). However, a large investigation of adrenal hormone production in 400 children reported that the sum of cortisol metabolites in 24-hr urine samples declined through early development, reaching a nadir between 7 and 8 year, coincident with increases in DHEA/S (Wudy, Hartmann, & Remer, 2007). Cortisol subsequently increased in both sexes and, in line with sex differences reported for adults (Shamim, Yousufuddin, Bakhai, Coats, & Honour, 2000; Vierhapper, Nowotny, & Waldhäusl, 1998), males produced more cortisol than females following puberty (11–12 year; Wudy et al., 2007). Though few studies have investigated developmental changes in cortisol under conditions that have not been experimentally altered to increase stress (e.g., ring-tailed lemurs: Tennenhouse, Putman, Boisseau, & Brown, 2017; baboons: Castracane et al., 1981; marmosets: Pryce, Palme, & Feldon, 2002; orangutans: Carlitz, Kirschbaum, Stalder, & van Schaik, 2014; lowland gorillas: Stoinski, Czekala, Lukas, & Maple, 2002), and even fewer do so in wild populations (baboons: Fourie & Bernstein, 2011; Fourie, Jolly, Phillips-Conroy, Brown, & Bernstein, 2015; Gesquiere et al., 2005; vervets: Laudenslager, Jorgensen, & Fairbanks, 2012; mountain gorillas: Robbins & Czekala, 1997), a general pattern of infant hypercortisolism followed by a juvenile lull in cortisol has been reported across taxa. The significance of the lull during late juvenility is poorly understood, but there is some evidence to suggest that high cortisol during early life has deleterious effects on cognitive development (e.g., Arnsten, 2009; Lupien, McEwen, Gunnar, & Heim, 2009; Pechtel & Pizzagalli, 2011), especially in experimental studies of extreme early life stress (e.g., Clarke & Schneider, 1993; Harlow & Harlow, 1962; Sanchez, 2006). High cortisol may also suppress gonadal function and maturation, and alter the timing of puberty (Ellis, 2004; Ellis & Essex, 2007; Shi et al., 2011). Though the deleterious effects of high cortisol and high stress during development have received much attention, its pattern of normative development, and potential integration with DHEA/S during development is less well-understood and warrants further investigation.
In this study, we use long-term data from wild East African chimpanzees (Pan troglodytes schweinfurthii) to track adrenal hormone production throughout development. We aim to test the hypothesis, based on prior studies in captivity, that chimpanzees experience human-like patterns in the timing of adrenal maturation, and extend previous work by providing increased resolution on the timing of adrenal development. First, we describe developmental increases in DHEAS in wild male and female chimpanzees from infancy through early adulthood (20 year). In line with cross-sectional studies, we predicted that DHEAS concentrations would begin to rise before puberty and continue to rise through early adulthood. As chimpanzees generally mature faster than humans, we expected adrenarche to occur earlier in chimpanzees compared with humans. If sex differences in adrenarche are evolutionarily conserved, we expect that increases in DHEAS should occur earlier among female chimpanzees compared with males, but that those increases should be larger among males. Second, we aimed to establish developmental patterns in cortisol production during the same developmental period and determine whether this pattern differs by sex. While we had no a priori prediction specific to chimpanzees, we aimed to determine whether these patterns parallel those reported for humans by Wudy et al. (2007). If both adrenal hormones follow a common developmental blueprint in chimpanzees and humans, this would be strong evidence to suggest an integrated pattern of adrenal development present in the last common ancestor and implicate a role for adrenal maturation in the evolution of extended development in humans and great apes.
2 |. METHODS
2.1 |. Study site and sampling methods
Data were collected between 1998 and 2017 from the Kanyawara community of chimpanzees in Kibale National Park, Uganda. This Kanyawara community resides in primarily moist deciduous forest punctuated by secondary forest and small swamps. Kanyawara chimpanzees have been followed continuously by researchers and local field assistants with the Kibale Chimpanzee Project since 1987 (Muller & Wrangham, 2014; Wrangham, Chapman, Clark-Arcadi, & Isabirye-Basuta, 1996). All chimpanzees included in this study were well-habituated and individually recognized. Community size ranged from 41 to 54 chimpanzees over the study including 8–11 adult males (age 15+), 13–18 adult females (age 10–12, after first fully tumescent swelling), 11–17 immature males, and 6–16 immature females. Immigrant females are assigned an age of 12.5 years from the first day that they are seen in the community based on the known age of emigration from this community. Birthdates for those individuals born after the study began were estimated based on the time since the mother had last been observed without the infant and the infant’s appearance compared to infants of known age. Birthdates for all individuals born during our sampling period are known to within 1 month.
As the developmental patterns that we were interested in examining have been corroborated across multiple matrices (e.g., serum, urine, feces, and hair) in humans and nonhuman primates, we assayed urine samples for adrenal hormones because they can be collected noninvasively, which allowed us to collect samples more frequently and without disturbing the chimpanzees. Further, noninvasive collection does not cause undue stress that may affect adrenal hormone levels (Castracane et al., 1981). Urine samples were collected throughout the day via one of two methods. Under ideal conditions, researchers and field assistants collect fresh, uncontaminated urine by catching it in clean plastic sheeting or plastic bags spread beneath subjects. Alternatively, when researchers and field assistants could confirm that the sample was uncontaminated by other, previous deposits, urine was pipetted from surrounding vegetation (Emery Thompson, 2005; Kahlenberg, Thompson, Muller, & Wrangham, 2008). Urine was cataloged and frozen on the day of collection. Samples were stored frozen and then transported to the US, where analyses took place at Boston University (2003–2008) and the University of New Mexico (after 2008). All urine samples with a specific gravity higher than 1.003 are assayed for cortisol as a part of the core endocrine program of the Kibale Chimpanzee Project and a subset of the sample collection were assayed for DHEAS.
2.2 |. Ethics statement
In accordance with guidelines and best practices for field primatology put forth by the American Society of Primatologists, all data collected for this study were noninvasively collected. Protocols were reviewed and approved by the University of New Mexico (IACUC 19–200862-MC). All data were collected with explicit permission and appropriate permits from the Uganda Wildlife Authority, Uganda National Council for Science and Technology, and Makerere University Biological Field Station.
2.3 |. DHEAS assay procedure
We assayed for urinary DHEAS, as opposed to DHEA, because it is more stable than its unconjugated form, is known to circulate in higher concentrations in chimpanzees (Cutler et al., 1978), and prior studies indicate that approximately 74% of administered DHEA is excreted as DHEA-S by chimpanzees (Hauser, Deschner, & Boesch, 2008). Both chimpanzee and bonobo urine have previously been validated for use in commercially available immunoassays of DHEA-S (Anestis, Blanco, & Bribiescas, 2009; Behringer, Deschner, Deimel, Stevens, & Hohmann, 2014). We aimed to sample individuals under 12 years at least once per month and those over 12 years quarterly using the following criteria. We excluded dilute samples (specific gravity < 1.003), as such samples generally contain very low and often undetectable amounts of steroid hormones, unless those samples were the only ones available for assay (4% of samples). Second, as DHEAS is known to degrade with multiple freeze-thaw cycles (Behringer et al., 2012), we excluded samples that had been stored for more than 5 years and those that had been previously thawed for other assays. Previous studies did not detect any significant diurnal pattern in urinary DHEAS among bonobos (Behringer et al., 2014) or chimpanzees (Anestis et al., 2009), and we neither could we detect a clear pattern in our chimpanzee pilot sample. However, we prioritized mid-day samples to minimize the potential for any confounding effects of time of day.
We used a liquid–liquid extraction technique following Behringer et al. (2012) using tert-butyl methyl ether (TMBE) to separate free steroids from conjugated ones and, thus, isolate DHEAS from DHEA. We then dried down each sample and stored them frozen at −40°F until the day of assay. To assay samples for DHEAS, we brought samples to room temperature and reconstituted in Dulbecco’s phosphate buffered saline. After reconstituting, we vortexed samples for 45 min to ensure that each was thoroughly mixed. Reconstituted samples were assayed for DHEAS in duplicate using a commercially available RIA kit (MP Biomedicals, catalog number 07230105, Santa Ana, CA) with a minimum sensitivity of 9 ng/ml. We followed the manufacturer’s instructions included in the kit with one adjustment: to minimize potential for matrix effects, we added 25 μl of assay buffer to all standards and samples. Interassay coefficients of variation were 15.2% for high controls and 15.6% for low controls, respectively. We confirmed accuracy using a recovery test of standard concentrations suspended in a urine sample. Percent recovery was between 94.1% and 99.7% of expected values. Serial dilution of a chimpanzee urine sample resulted in a response (y = 0.53 × −40.77) that was parallel with the standard curve (y = 0.50 × +0.49; f = 0, df = 1, p = 1.0). MP Biomedicals reports 100% cross reactivity of this assay with DHEA; however, the extraction technique accounts for this by isolating DHEA in the organic layer thus after decanting, only DHEAS remains in the sample (Behringer et al., 2012). Cross reactivity with androsterone was 20%, androstenedione was 6%, and all others (estrone, progesterone, testosterone, and 17β-estradiol) were < 1%.
Because we could not repeat samples due to decay in recovery associated with multiple thaws (Behringer et al., 2012), results were only obtained on initial run. Therefore, for samples near the minimum limit of detection (8–40 ng/ml), we excluded all samples with a cortisol values (CV) over 30%, leading to an intra-assay CV of 12.5% based on duplicates of low concentration samples (< 40 ng/ml). For the remaining samples (>40 ng/ml), we excluded samples with a CV higher than 20% and the intra-assay CV was 7.4%. Our final data set included 1,385 urine samples from 56 individuals (f = 34, m = 22; Table 1). As one specific goal of this investigation is to compare hormone levels across age groups and standardizing for creatinine would introduce error due to body size (Emery Thompson, Muller, & Wrangham, 2012), we corrected samples for urine concentration using specific gravity.
TABLE 1.
Samples sizes and summary statistics for DHEAS and cortisol
| Hormone | Urine samples | Unique IDs | Chimp half-years | Half-years per ID | Samples per ID per half-year ave. (min-max) | Ave. hormone values per half-year‡ (min-max) | CV | |
|---|---|---|---|---|---|---|---|---|
| DHEAS | F | 756 | 31 | 203 | 6.55 (1–12) | 3.61 (1–20) | 185.98 ng/ml (0.84–2,635.10) | 7.4%, 12.5%† |
| M | 629 | 22 | 181 | 8.23 (2–16) | 3.60 (1–17) | 170.43 ng/ml (0.90–1,401.63) | ||
| CORTISOL | F | 6,476 | 45 | 363 | 9.37 (1–33) | 17.84 (3–111) | 34.93 ng/ml (0.08–1,562.27) | 6.0% |
| M | 6,250 | 23 | 302 | 13.36 (1–28) | 20.74 (3–95) | 43.60 ng/ml (0.13–1,087.36) |
Abbreviations: CV, cortisol value; DHEAS, dehydroepiandrosterone-sulfate.
CV for high concentration samples (>40 ng/ml) and low concentration samples (<40 ng/ml), respectively.
Average untransformed half-year values of each hormone corrected for specific gravity, cortisol values have not been corrected for time of day.
DHEAS levels were undetectable in 296 of 1,385 samples assayed. Given that overly dilute samples were already excluded, the remaining undetectable results likely represent true low values, indicating that DHEAS is circulating at minimal levels. Undetectable values should be expected before adrenarche, and, indeed, most undetectable samples were concentrated among chimpanzees aged 1–5 years (n = 218, 74%). To adequately characterize developmental shifts, it was necessary to assign these undetectable samples to the minimum detectable value for the assay: 9 ng/ml.
2.4 |. Cortisol assay procedure
Enzyme-immunoassay procedures were described in detail in several previous publications (see Emery Thompson, 2005; Kahlenberg et al., 2008; Muller, Kahlenberg, Emery Thompson, & Wrangham, 2007). Cortisol was assayed at various points between 2005 and 2018. While we used a consistent protocol across the entire time period, this involved two different laboratories (Boston University 2005–2007; University of New Mexico 2008–2018). Upon the initial transition, we used the same stocks of reagents and internal controls which yielded consistent performance. Interassay CVs for the full set of assays across both labs were 13.4% for the low control and 12.5% for the high control, and the CV between the average control values from the two labs was 7.3% for the low control and 5.0% for the high control. In 2016, we exhausted our reagent stocks and calibrated new stocks against the old assay. To verify that the standard curve performed equivalently, we ran six assays with the old controls and verified that they performed nearly identically to the previous assay (low control: 536 vs. 548 pg/ml, CV = 1.6%; high control: 1,096 vs. 1,087 pg/ml, CV = 0.1%). Interassay CVs for assays conducted between 2016 and 2018 were 17.3% for the low control and 10.3% for the high control. While these data generally indicate that the assay performed consistently over time, we entered a random factor in our models for the year of assay to minimize noise that may have resulted from unavoidable change in reagents, equipment, and software across the study.
We selected results from individuals 20 years old and younger, excluding results with CVs higher than 15%. Our data set included 12,726 samples from 68 individuals (f = 45, m = 23, Table 1). Intraassay precision, calculated as mean CV of duplicate determinations, was 6.0%. Accuracy, measured as the recovery of standard concentrations suspended in a urine sample, was 105 ± 23%. Serial dilution of a chimpanzee urine sample resulted in a response (y = −0.38 × +1.23) that was parallel with the standard curve (y = −0.34 × +1.06; t = −0.69, df = 12, p = .50).
Cortisol values were corrected for urine concentration using specific gravity and log-transformed to satisfy assumptions of normality. As cortisol is produced (Chung, Son, & Kim, 2011; Young, Carlson, & Brown, 2001) and excreted (Muller & Lipson, 2003) according to a strong circadian pattern, we adjusted values for time of day before analysis. Preliminary analyses indicated that urinary cortisol levels followed the expected sigmoidal pattern with time of day, starting high and ending low. Thus, we used generalized linear mixed-modeling, or GLMM (R package lme4), to fit a predicted third-order polynomial equation for cortisol against time of day. To account for uneven sampling, we included random effects for assay year and individual identity in generating the global curve, but then calculated the residual value of each sample from the fixed effects of the model only (i.e., preserving individual variation in intercepts).
2.5 |. Data analysis
Our primary goal for this investigation was to describe developmental patterns in urinary DHEAS and cortisol as opposed to identifying the sources of daily variation in hormone levels. Thus, to minimize noise and also help to mitigate any potential problems that may arise from unequal sampling across individual chimpanzees which is unavoidable due to the noninvasive, opportunistic nature of this study, we calculated median hormone values for each individual for each half-year age interval that they were included in the study. We believe that, despite the smaller sample size, this approach should lead to a better model for our parameters of interest (age and sex) and provides less risk of violating model assumptions. Regardless of the number of total urine samples, our limiting factors in this analysis are number of individual chimpanzees that contribute to the model and the amount of developmental time that we each individual is captured in the sample. Our final data set for DHEAS was calculated from 1,385 samples and represented 384 chimpanzee half-years (female = 203, male = 181) from 53 unique individuals (females = 31, males = 22, Table 1). For cortisol, we calculated median time-corrected values for each individual during each half-year age interval with a minimum inclusion criteria of three samples. Our final data set used 12,726 urine samples and represented 664 chimpanzee half-years (female = 363, male = 301) from 68 unique individuals (females = 45, males = 23, Table 1).
We anticipated nonlinear relationships between these variables and that the data would be relatively noisy. Accordingly, we used generalized additive models, (GAM; R package mgcv version 1.8–27; Wood, 2017), to investigate age-related shifts in DHEAS and cortisol production from infancy through early adulthood. Visual inspection indicated that the distribution of both DHEAS and cortisol half-year data-points were skewed so we log-transformed each to satisfy assumptions of normality. We used GAMs to model the developmental trajectory of each hormone including sex and age as fixed effects. Sex was a factor of two levels (male and female) and was entered as a parametric effect similar to an intercept-only effect in generalized linear modeling (GLM). To model changes in hormone levels with age, we included age as a smooth term, a mathematical function that describes a nonlinear relationship between a given continuous predictor and a dependent variable. To allow this relationship to vary by sex, we used the “by” function to fit separate smooth for each sex. This approach is similar to an age by sex interaction in a generalized linear model where the slope of a line may vary between levels of a second predictor. Individual identity and mother’s identity were included as random variables to account for unequal sampling of individuals across age categories and maternal effects, including multiple sets of siblings, respectively.
To more precisely examine the average age of adrenarche, we extracted the first derivatives and associated confidence intervals of the age smooth from each GAM using the gratia package in R (version 0.2–1; Simpson, 2019). We identified average age of adrenarche for each sex as the point at which the derivative of age on DHEAS crossed the zero-point line, representing a shift from a negative slope to a positive slope. We included simultaneous confidence intervals to estimate uncertainty around age of adrenarche.
3 |. RESULTS
3.1 |. DHEAS and adrenarche
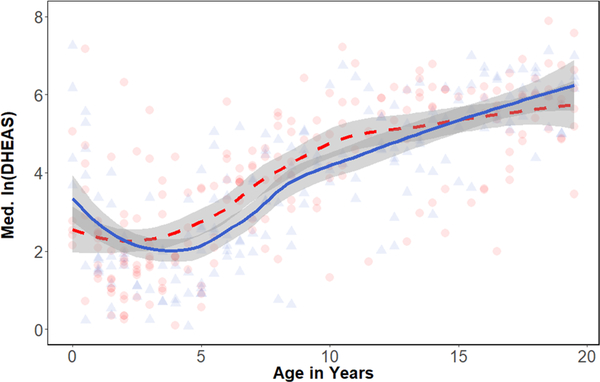
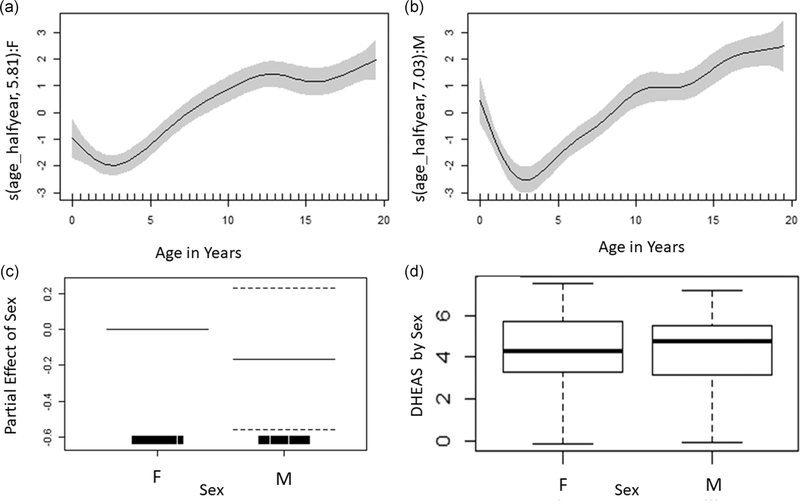
Across sexes, median DHEAS (specific gravity-corrected half-year averages) exhibited an initial decrease after early infancy, reaching a nadir between 2 and 4 years (16.96 ± 16.13 ng/ml) and then steadily increasing through early adulthood (488.39 ± 187.45 ng/ml; Figure 1). Male and female DHEAS levels largely overlapped and the GAM (Table 2; Figure 2, R2 = 0.65, deviance explained = 68.6%) indicated no overall sex difference in DHEAS (p = .40). The initial GAM indicated that age splines were significant in both sexes and the female spline was less wiggly, having relatively fewer effective degrees of freedom compared with the males (Table 2). To explicitly test whether the spline for males was significantly different from the spline for females, we ran the GAM a second time considering sex as an ordered factor and found no significant difference in the spline of age on DHEAS for males with respect to females (p = .12).
FIGURE 1.
Scatterplot and loess regression of DHEAS concentrations with age (in years) in females (n = 31) and males (n = 21). Each point represents an individual during a given 6-month age bin. Females are shown in red, circles, and dashed line. Males are shown in blue, triangles, and solid line. DHEAS, dehydroepiandrosterone-sulfate
TABLE 2.
Results for estimates of parametric coefficients and effective degrees of freedom of smooth terms for DHEAS (GAM, R2 = 0.65, deviance explained = 68.6%)
| Parametric coefficients | Estimate | p-value |
|---|---|---|
| Intercept | 3.88 | < .001 |
| Sex (m) | −0.17 | 0.404 |
| Smooth terms | edf | p-value |
| Age × female | 5.81 | < .001 |
| Age × male | 7.03 | < .001 |
Abbreviation: DHEAS, dehydroepiandrosterone-sulfate; GAM, generalized additive model.
FIGURE 2.
Partial effects plots for DHEAS showing the smooth of DHEAS concentrations on age for females (a, n = 31, edf = 5.81, p < .001) and males (b, n = 22, edf = 7.03, p < .001), and the partial parametric effect of sex on DHEAS (c, −1.17, p = .40). Overall comparison of DHEAS concentrations between males and females also shown for reference (d). DHEAS, dehydroepiandrosterone-sulfate
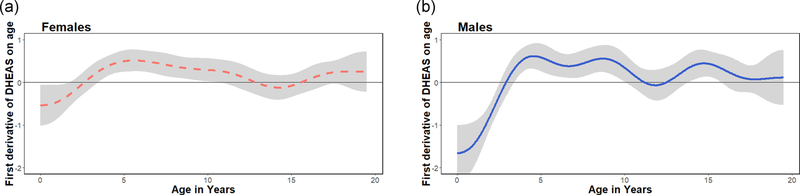
To identify take-off points in DHEAS production that would indicate the onset of adrenarche, we extracted the first derivatives and their confidence intervals from each smooth on age (Figure 3). The slope of the male smooth switched from negative to positive at approximately 2.85 years (95% CI, 2.46–3.32). Females exhibited a shift in velocity around the same age, as the slope became positive around 2.61 years (95% CI, 1.75–3.44). After crossing the zero-point, first derivatives remained elevated from about 4–10 years before slowing down and neither males nor female derivatives crossed back into negative values. Thus, these crossover points marked the start of sustained increases in DHEAS consistent with adrenarche.
FIGURE 3.
First derivatives of DHEAS on age for females (a, n = 31) and males (b, n = 22). Horizontal line at zero added to highlight adrenarche (females = 2.61 [1.75–3.44] years, males = 2.85 [2.34–3.32 years]), defined as the earliest age at which the smooth of DHEAS on age changes from negative to positive. DHEAS, dehydroepiandrosterone-sulfate
3.2 |. Cortisol
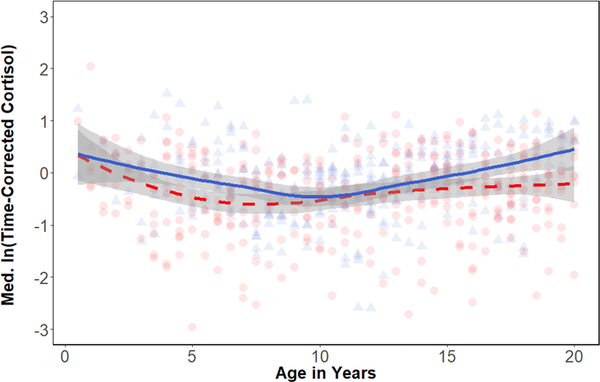
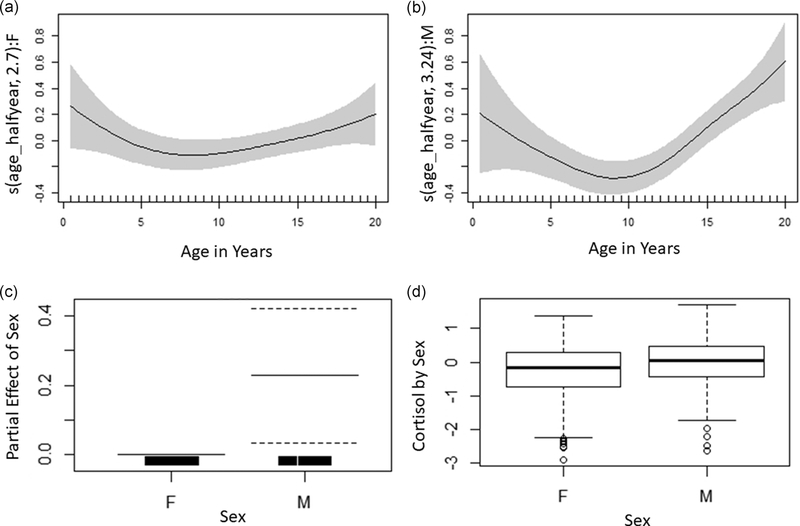
After correcting for time of day, median residual cortisol values ranged from −3.21 to 2.04. Cortisol levels generally declined through infancy and juvenility, reaching a nadir between 6–7 years in females (−0.71 ± 0.08) and 11–12 year in males (−0.50 ± 0.21; Figure 4). After this nadir, concentrations increased slightly in both sexes (Table 3, R2= 0.19, deviance explained = 24.2%). At approximately 15–16 years old a sex difference emerged as male concentrations continued to increase, while females levelled off. The male spline of age on cortisol was not significantly different than the spline for females (p = .09), however, despite the broad overlap in males’ and females’ cortisol early in life, males had higher than females in early adulthood (m = 0.39 ± 0.38, f = −0.23 ± 0.15) and overall (p = .03; Figure 5).
FIGURE 4.
Scatterplot and loess regression of time-corrected cortisol concentrations with age (in years) in females (n = 45) and males (n = 23). Each point represents an individual during a given 6-month age bin. Females are shown in red, circles, and dashed line. Males are shown in blue, triangles, and solid line
TABLE 3.
Results for estimates of parametric coefficients and effective degrees of freedom of smooth terms for cortisol (GAM, R2 = 0.19, deviance explained = 24.2%)
| Parametric coefficients | Estimate | p-value |
|---|---|---|
| Intercept | −0.28 | < .001 |
| Sex (m) | 0.21 | .023 |
| Smooth terms | edf | p-value |
| Age × female | 2.70 | .013 |
| Age × male | 3.24 | < .001 |
Abbreviation: GAM, generalized additive model.
FIGURE 5.
Partial effects plots showing the smooth of time-corrected cortisol and age for females (a, edf = 2.70, p = .13) and males (b, edf = 3.24, p < .001) and partial parametric effect of male sex compared with female as the reference sex on time-corrected cortisol (c, m = 0.21, p = .03). Overall comparison of time-corrected cortisol between males and females (d) also shown for reference
4 |. DISCUSSION
While prior reports have pointed to a human-like pattern of adrenal maturation in chimpanzees, they have provided limited resolution on the timing and variability of this developmental pattern. In this first report on adrenal development in wild chimpanzees, we used an extensive longitudinal data set to quantify developmental shifts in urinary DHEAS and cortisol, allowing for the determination of the ages of adrenarchal onset and potential sex differences in prepubertal steroid production. A clear signature of human-like delayed adrenarche was observed. In chimpanzees of both sexes, levels of urinary DHEAS were very low after 6 months and began increasing around 2.5–3 years, well before puberty (which occurs at approximately 8–12 years; Emery Thompson and Sabbi, 2019). Our results are consistent with the earliest cross-sectional reports of increased DHEAS among captive chimpanzees (3 years; Cutler et al., 1978) and histological examination of chimpanzee adrenal tissue that detected islands of the ZR in a 3 years chimpanzee (Parker et al., 2014). Both sexes exhibited a significant pattern of increase that continued through adolescence leading to levels of DHEAS in early adulthood that were over 20× as high as those as those measured at the takeoff point. This pattern contrasts with what has been observed in Old World monkeys, where maturation of the ZR occurs in the early postnatal period and DHEA/S declines across development (Castracane et al., 1981; Conley et al., 2004; Cutler et al., 1978; Ducsay et al., 1991; Levine et al., 1982; Nguyen et al., 2008).
Unlike humans, chimpanzee females did not reach adrenarche at earlier ages than males, and there was no significant sex difference in urinary DHEAS concentrations during development or in early adulthood. Previous reports from great apes have similarly failed to find a sex difference in DHEAS concentrations (orangutans: Prall et al., 2015; western lowland gorillas: Bernstein et al., 2012; Cutler et al., 1978; bonobos: Behringer et al., 2012; and captive chimpanzees: Anestis et al., 2009; Copeland, Eichberg, Parker, & Bartke, 1985; Cutler et al., 1978; Smail et al., 1982). In humans, higher DHEAS among men is detectable by 11–15 years (Šulcová et al., 1997), and this difference persists throughout life (Hopper & Yen, 1975; Orentreich et al., 1984; Šulcová et al., 1997). While the adrenal glands produce the majority of circulating DHEA/S in humans (Endoh et al., 1996; Ibáñez, DiMartino-Nardi, Potau, & Saenger, 2000; Parker, 1991a, 1991b), DHEA/S is also synthesized in the human gonads, which may contribute to sex differences in DHEA/S after puberty (Forest, 1978; Remer, Boye, Hartmann, & Wudy, 2005). Although it is impossible to determine the tissue of origin for DHEAS detected in urine, the lack of a detectable sex difference in DHEAS concentrations among great apes, could reflect species differences in adrenal or gonadal production.
In humans, adrenarche coincides with a period of significant morphological and behavioral change, including the development of complex social behavior, and associated cognitive competencies (Campbell, 2011; Del Giudice, 2018; Del Giudice et al., 2009; Haith & Sameroff, 1996). While its secondary conversion to other steroids in the gonads and other peripheral tissues (Labrie, Belanger, Simard, Luu-The, & Labrie, 1995) may contribute to emerging phenotypic sex differences during adrenarche, DHEA/S is suggested to play only a minor, supporting role in physical aspects of development (Del Giudice, 2018; Palmert et al., 2001). However DHEAS has been implicated more directly in neurocognitive development (reviewed in Majewska, 1995; Ritsner, 2010) and is thought to play a pivotal role in the prepubertal activation of prenatally organized sexually differentiated neural pathways (Del Giudice, 2018; Del Giudice et al., 2009). Sex differences in behavior also diverge around the time of adrenarche in humans, including differences in play style (Smith, 2005), increases in boys’ aggression (Pellegrini & Archer, 2005), and, in turn, increasingly sex-segregated play (Benenson, 1994; Benenson, Apostoleris, & Parnass, 1997; Maccoby, 1990; Smith, 2005). While DHEA/S increases sharply in both sexes, it can nonetheless influence sexually differentiated behavior by interacting with sex steroid receptors in the brain, activating sexually differentiated pathways that were organized prenatally (Del Giudice et al., 2009; Soma, Rendon, Boonstra, Albers, & Demas, 2015). It is important to note that DHEA/S is also produced locally in the brain and, as with other steroids, links between measurable peripheral circulation and action on the brain are uncertain (Zwain & Yen, 1999). However, the comparative data across primate taxa provide strong circumstantial evidence to suggest that differences in the timing and pace of adrenal development correspond with different paces of brain development (Conley, Bernstein, & Nguyen, 2012).
Although relationships between DHEAS and chimpanzee behavior have not yet been directly investigated, several milestones in behavioral and cognitive development generally overlap with the timing of adrenarche. In populations where tool-assisted foraging is prevalent, chimpanzees become more exploratory and begin interacting with tools by about 3 years, achieving their first tool-use successes around 4 years (Inoue-Nakamura & Matsuzawa, 1997; Lonsdorf, 2005). While play is a prominent feature of chimpanzee behavior throughout development, the amount of time that young chimpanzees spent in solitary play trades off for social play around 3 years (Lonsdorf et al., 2014b). Some sex differences in chimpanzee sociality also can also be detected around the time of adrenarche. At Gombe, males were more independent from their mothers and also directly interacted with more social partners than females by 3–4 years (Lonsdorf et al., 2014a, 2014b). Infant and juvenile males at Mahale had higher centrality in play networks compared to females, who were more peripheral (Shimada & Sueur, 2014). These early-emerging sex differences among chimpanzees foreshadow sex differences in adult social strategies and, as they become detectable up to 5 years before puberty, they cannot be easily explained by differences in gonadal steroid concentrations. Future work should delve further into the possible roles of DHEAS in behavioral development, especially in regard to the relationships between rising DHEAS concentrations and diverging social strategies in prepubertal great apes.
In addition to similarities in DHEAS, developmental shifts in chimpanzees’ cortisol concentrations also resembled recent findings from humans (Wudy et al., 2007). Cortisol decreased through infancy and early juvenility, reaching a mid-juvenile nadir (between 6 and 12 years) before increasing into adulthood. This general age-related pattern has been reported in the few available studies of normative cortisol in other primates (e.g., baboon spp: Fourie & Bernstein, 2011; Fourie et al., 2015; Gesquiere et al., 2005; Laudenslager et al., 2012; marmosets: Pryce et al., 2002; ring-tailed lemurs: Tennenhouse, Putman, Boisseau, & Brown, 2017); however, sex differences are less consistent across taxa. In this study, chimpanzee male cortisol concentrations increased to higher levels than females, leading adult males to have higher cortisol than females. A similar sex difference has not been reported in other nonhuman primates (Fourie & Bernstein, 2011; Fourie et al., 2015; Gesquiere et al., 2005; Laudenslager et al., 2012; Pryce et al., 2002; Tennenhouse, Putman, Boisseau, & Brown, 2017), but has been observed in humans (e.g., Shamim et al., 2000; Vierhapper et al., 1998; Wudy et al., 2007). Increased male cortisol emerged later in chimpanzees (15–16 years) compared with humans (11–12 years), which was unexpected given that chimpanzees generally mature and achieve developmental milestones more quickly than humans (Emery Thompson & Sabbi, 2019). The causes and consequences of these changes in cortisol levels remain relatively unexplained. Wudy et al. (2007) detected a sex difference in 5α-reductase activity that also emerged around puberty. While this may implicate that broader sex differences in steroid hormone metabolism contribute to sex differences in glucocorticoid levels among humans, it has not been directly tested in humans or other primates.
Several authors have suggested that, in humans, the ultimate explanation for dipping cortisol levels during the years that DHEA/S is rapidly increasing relates to the anti-glucocorticoid actions of DHEAS as elevated cortisol in early life can be detrimental to normal development (Campbell, 2006, 2011; Del Giudice et al., 2009, 2011; Del Giudice, 2018). However, whether these aspects of adrenal development are functionally coordinated remains a matter of speculation. The normative development of HPA activity has rarely been examined in humans, much less in other primates, perhaps because early studies suggested that there was little developmental change. However, as similar developmental shifts in cortisol have been documented in humans, chimpanzees, and the few other primate species that have been studied, while reported shifts in DHEAS differ so markedly between the apes and other primates, broader comparative data are needed to understand the functional relevance of these patterns and how they evolved.
Our results take an important step forward by establishing developmental shifts in cortisol concentration in a wild great ape. Establishing this normative pattern of cortisol production during early life is critical in any attempt to understand how variation in early life stress might impact development, stress physiology, and behavior later in life. While adding enhanced resolution to the prior captive reports from chimpanzees, these data also confirm that a strong adrenarchal signal is present under natural developmental conditions. In doing so, our study supports the conclusion that the unusual pattern of prolonged adrenal development in humans is not a novel evolutionary trait but an elaboration on a pre-existing derived pattern in our last common ancestor with great apes, or perhaps more recently, with chimpanzees and bonobos. Given that the human pattern of adrenarche is hypothetically implicated in the extension of brain development (Campbell, 2011; Conley et al., 2012), and particularly to the significant neurological and behavioral development of middle “childhood” (Bogin, 1997; Campbell, 2006, 2011; Del Giudice, Ellis, & Shirtcliff, 2011), these similarities in pattern may suggest functional similarities for the as-yet understudied postweaning developmental period of apes.
ACKNOWLEDGMENTS
The authors thank Dr. Karen Bales and two anonymous reviewers for their valuable feedback on this manuscript. Funding for fieldwork by the Kibale Chimpanzee Project and for hormonal assays has been provided by the U.S. National Science Foundation (grant no. 1355014, 9807448, and 0416125), the U.S. National Institute on Aging and Office for Research on Women’s Health (NIH grant no. R01-AG045395), the L.S.B. Leakey Foundation, the Wenner-Gren Foundation, the Nacey Maggioncalda Foundation, the American Philosophical Society, Harvard University, and the University of New Mexico. The Uganda Wildlife Authority and the Uganda National Council of Science and Technology, and Makerere Biological Field Station granted research permissions for this project. Long-term data collection, including urine sampling, was performed by a team of Ugandan field assistants including the late John Barwogeza, Sunday John, Christopher Katongole, J. Kyomuhando, Francis Mugurusi, the late Donor Muhangyi, the late Christopher Muruuli, Solomon Musana, Japan Musunguzi, Dennis Sebugwawo, Peter Tuhairwe, James Kyomuhendo, Wilberforce Tweheyo, Richard Karamagi, Seezi Atweijuze, Daniel Akaruhanga, Fred Baguma, Steven Alio, and Bashil Musabe. Maggy Kobusingye, Christine Abbe, and Jovia Mahoro assisted with data collection and management in the field.
Funding information
National Science Foundation, Grant/Award, Number: 0416125, 1355014, 9807448; American Philosophical Society; Wenner-Gren Foundation; National Institutes of Health, Grant/Award Number: R01-AG045395; Nacey Maggioncalda Foundation; U.S. National, Institute on Aging and Office for Research on Women’s Health; L.S.B. Leakey Foundation; Harvard University; University of New Mexico
Footnotes
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Anestis SF, Blanco M, & Bribiescas RG (2009). Changes in urinary dehydroepiandrosterone sulfate (DHEA-S) levels with age in juvenile captive chimpanzees (Pan troglodytes), American Journal of Physical Anthropology (pp. 78–78). Div. John Wiley & Sons Inc., 111 River St., Hoboken, NJ 07030 USA: Wiley-Liss. [Google Scholar]
- Aranoff G, & Rosier A (1980). Urinary tetrahydrocortisone and tetrahydrocortisol glucosiduronates in normal newborns, children and adults. European Journal of Endocrinology, 94(3), 371–375. 10.1530/acta.0.0940371 [DOI] [PubMed] [Google Scholar]
- Arnsten AF (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience, 10(6), 410 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer V, Hohmann G, Stevens JM, Weltring A, & Deschner T (2012). Adrenarche in bonobos (Pan paniscus): Evidence from ontogenetic changes in urinary dehydroepiandrosterone-sulfate levels. Journal of Endocrinology, 214(1), 55–65. 10.1530/JOE-12-0103 [DOI] [PubMed] [Google Scholar]
- Behringer V, Deschner T, Deimel C, Stevens JM, & Hohmann G (2014). Age-related changes in urinary testosterone levels suggest differences in puberty onset and divergent life history strategies in bonobos and chimpanzees. Hormones and Behavior, 66(3), 525–533. 10.1016/j.yhbeh.2014.07.011 [DOI] [PubMed] [Google Scholar]
- Benenson J (1994). Ages four to six years: Changes in the structures of play networks of girls and boys. Merrill-Palmer Quarterly, 40(4), 478–487. [Google Scholar]
- Benenson JF, Apostoleris NH, & Parnass J (1997). Age and sex differences in dyadic and group interaction. Developmental Psychology, 33(3), 538 10.1037/0012-1649.33.3.538 [DOI] [PubMed] [Google Scholar]
- Bernstein R (2016). Hormones and the evolution of childhood in human and nonhuman primates In Meehan CL, & Crittenden AN (Eds.), Childhood: Origins, evolution, and implications (pp. 103–119). Albuquerque: University of New Mexico Press. [Google Scholar]
- Bernstein RM, Sterner KN, & Wildman DE (2012). Adrenal androgen production in catarrhine primates and the evolution of adrenarche. American Journal of Physical Anthropology, 147(3), 389–400. 10.1002/ajpa.22001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogin B (1997). Evolutionary hypotheses for human childhood. American Journal of Physical Anthropology: The Official Publication of the American Association of Physical Anthropologists, 104(S25), 63–89. [Google Scholar]
- Campbell B (2006). Adrenarche and the evolution of human life history. American Journal of Human Biology: The Official Journal of the Human Biology Association, 18(5), 569–589. 10.1002/ajhb.20528 [DOI] [PubMed] [Google Scholar]
- Campbell B (2011). Adrenarche in comparative perspective. American Journal of Human Biology, 23(1), 44–52. 10.1002/ajhb.21111 [DOI] [PubMed] [Google Scholar]
- Campbell BC (2011). Adrenarche and middle childhood. Human Nature, 22(3), 327 10.1007/s12110-011-9120-x [DOI] [PubMed] [Google Scholar]
- Carlitz EH, Kirschbaum C, Stalder T, & van Schaik CP (2014). Hair as a long-term retrospective cortisol calendar in orangutans (Pongo spp.): New perspectives for stress monitoring in captive management and conservation. General and Comparative Endocrinology, 195, 151–156. 10.1016/j.ygcen.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Castracane VD, Cutler GB Jr, & Loriaux DL (1981). Pubertal endocrinology of the baboon: Adrenarche. American Journal of Physiology-Endocrinology And Metabolism, 241(4), E305–E309. 10.1152/ajpendo.1981.241.4.E305 [DOI] [PubMed] [Google Scholar]
- Chung S, Son GH, & Kim K (2011). Circadian rhythm of adrenal glucocorticoid: Its regulation and clinical implications. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1812(5), 581–591. 10.1016/j.bbadis.2011.02.003 [DOI] [PubMed] [Google Scholar]
- Clarke A, & Schneider ML (1993). Prenatal stress has long-term effects on behavioral responses to stress in juvenile rhesus monkeys. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology, 26(5), 293–304. 10.1002/dev.420260506 [DOI] [PubMed] [Google Scholar]
- Compagnone NA, & Mellon SH (1998). Dehydroepiandrosterone: A potential signalling molecule for neocortical organization during development. Proceedings of the National Academy of Sciences, 95(8), 4678–4683. 10.1073/pnas.95.8.4678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley AJ, Pattison JC, & Bird IM (2004). Variations in adrenal androgen production among (nonhuman) primates. Seminars in Reproductive Medicine, 22(4), 311–326. 10.1055/s-2004-861548. November. In. [DOI] [PubMed] [Google Scholar]
- Conley AJ, Bernstein RM, & Nguyen AD (2012). Adrenarche in nonhuman primates: The evidence for it and the need to redefine it. Journal of Endocrinology, 214(2), 121–131. 10.1530/JOE-11-0467 [DOI] [PubMed] [Google Scholar]
- Conley AJ, Moeller BC, Nguyen AD, Stanley SD, Plant TM, & Abbott DH (2011). Defining adrenarche in the rhesus macaque (Macaca mulatta), a nonhuman primate model for adrenal androgen secretion. Molecular and Cellular Endocrinology, 336(1–2), 110–116. 10.1016/j.mce.2010.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland KC, Eichberg JW, Parker CR Jr, & Bartke A (1985). Puberty in the chimpanzee: Somatomedin-C and its relationship to somatic growth and steroid hormone concentrations. The Journal of Clinical Endocrinology and Metabolism, 60(6), 1154 10.1210/jcem-60-6-1154 [DOI] [PubMed] [Google Scholar]
- Cutler GB Jr, Glenn M, Bush M, Hodgen GD, Graham CE, & Loriaux DL (1978). Adrenarche: A survey of rodents, domestic animals, and primates. Endocrinology, 103(6), 2112–2118. 10.1210/endo-103-6-2112 [DOI] [PubMed] [Google Scholar]
- Del Giudice M (2018). Middle childhood: An evolutionary-developmental synthesis, Handbook of life course health development (pp. 95–107). Cham: Springer; 10.1007/978-3-319-47143-3 [DOI] [Google Scholar]
- Del Giudice M, Angeleri R, & Manera V (2009). The juvenile transition: A developmental switch point in human life history. Developmental Review, 29(1), 1–31. 10.1016/j.dr.2008.09.001 [DOI] [Google Scholar]
- Del Giudice M, Ellis BJ, & Shirtcliff EA (2011). The adaptive calibration model of stress responsivity. Neuroscience & Biobehavioral Reviews, 35(7), 1562–1592. 10.1016/j.neubiorev.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhom G (1973). The prepuberal and puberal growth of the adrenal (adrenarche). Beiträge zur Pathologie, 150(4), 357–377. 10.1016/S0005-8165(73)80086-1 [DOI] [PubMed] [Google Scholar]
- Ducsay CA, Hess DL, McClellan MC, & Novy MJ (1991). Endocrine and morphological maturation of the fetal and neonatal adrenal cortex in baboons. The Journal of Clinical Endocrinology and Metabolism, 73(2), 385–395. 10.1210/jcem-73-2-385 [DOI] [PubMed] [Google Scholar]
- Ellis BJ (2004). Timing of pubertal maturation in girls: An integrated life history approach. Psychological Bulletin, 130(6), 920 10.1037/0033-2909.130.6.920 [DOI] [PubMed] [Google Scholar]
- Ellis BJ, & Essex MJ (2007). Family environments, adrenarche, and sexual maturation: A longitudinal test of a life history model. Child Development, 78(6), 1799–1817. 10.1111/j.1467-8624.2007.01092.x [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, & Sabbi K (2019). Evolutionary demography of the great apes In Sear R, & Burger O (Eds.), Human evolutionary demography volume (pp. 1–75). Cambridge, UK: Open Book Publishers; https://osf.io/p59eu/ [Google Scholar]
- Emery Thompson M (2005). Reproductive endocrinology of wild female chimpanzees (Pan troglodytes schweinfurthii): Methodological considerations and the role of hormones in sex and conception. American Journal of Primatology: Official Journal of the American Society of Primatologists, 67(1), 137–158. 10.1002/ajp.20174 [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN, & Wrangham RW (2012). Variation in muscle mass in wild chimpanzees: Application of a modified urinary creatinine method. American Journal of Physical Anthropology, 149(4), 622–627. 10.1002/ajpa.22157 [DOI] [PubMed] [Google Scholar]
- Endoh AKIRA, Kristiansen SB, Casson PR, Buster JE, & Hornsby PJ (1996). The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3 beta-hydroxysteroid dehydrogenase. The Journal of Clinical Endocrinology & Metabolism, 81(10), 3558–3565. 10.1210/jcem.81.10.8855801 [DOI] [PubMed] [Google Scholar]
- Forest MG (1978). Pattern of plasma dehydroepiandrosterone sulfate levels in humans from birth to adulthood: Evidence for testicular production. The Journal of Clinical Endocrinology and Metabolism, 47(3), 572–577. 10.1210/jcem-47-3-572 [DOI] [PubMed] [Google Scholar]
- Fourie NH, & Bernstein RM (2011). Hair cortisol levels track phylogenetic and age related differences in hypothalamic–pituitary–adrenal (HPA) axis activity in nonhuman primates. General and Comparative Endocrinology, 174(2), 150–155. 10.1016/j.ygcen.2011.08.013 [DOI] [PubMed] [Google Scholar]
- Fourie NH, Jolly CJ, Phillips-Conroy JE, Brown JL, & Bernstein RM (2015). Variation of hair cortisol concentrations among wild populations of two baboon species (Papio anubis, P. hamadryas) and a population of their natural hybrids. Primates, 56(3), 259–272. 10.1007/s10329-015-0469-z [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Altmann J, Khan MZ, Couret J, Yu JC, Endres CS, … Wango EO (2005). Coming of age: Steroid hormones of wild immature baboons (Papio cynocephalus). American Journal of Primatology: Official Journal of the American Society of Primatologists, 67(1), 83–100. 10.1002/ajp.20171 [DOI] [PubMed] [Google Scholar]
- Harlow HF, & Harlow MK (1962). Social deprivation in monkeys. Scientific American, 207(5), 136–150. 10.1038/scientificamerican1162-136 [DOI] [PubMed] [Google Scholar]
- Hauser B, Deschner T, & Boesch C (2008). Development of a liquid chromatography–tandem mass spectrometry method for the determination of 23 endogenous steroids in small quantities of primate urine. Journal of Chromatography B, 862(1–2), 100–112. 10.1016/j.jchromb.2007.11.009 [DOI] [PubMed] [Google Scholar]
- Havelock JC, Auchus RJ, & Rainey WE (2004). The rise in adrenal androgen biosynthesis: Adrenarche. Seminars in Reproductive Medicine, 22(4), 337–347. 10.1055/s-2004-861550 [DOI] [PubMed] [Google Scholar]
- Hechter O, Grossman A, & Chatterton RT Jr (1997). Relationship of dehydroepiandrosterone and cortisol in disease. Medical Hypotheses, 49(1), 85–91. https://doi.org/0.1016/S0306-9877(97)90258-9 [DOI] [PubMed] [Google Scholar]
- Honour JW, Kelnar CJH, & Brook CGD (1991). Urine steroid excretion rates in childhood reflect growth and activity of the adrenal cortex. European Journal of Endocrinology, 124(2), 219–224. 10.1530/acta.0.1240219 [DOI] [PubMed] [Google Scholar]
- Hopper BR, & Yen SSC (1975). Circulating concentrations of dehydroepiandrosterone and dehydroepiandrosterone sulfate during puberty. The Journal of Clinical Endocrinology & Metabolism, 40(3), 458–461. 10.1210/jcem-40-3-458 [DOI] [PubMed] [Google Scholar]
- Ibáñez L, DiMartino-Nardi J, Potau N, & Saenger P (2000). Premature adrenarche—normal variant or forerunner of adult disease? Endocrine Reviews, 21(6), 671–696. 10.1210/edrv.21.6.0416 [DOI] [PubMed] [Google Scholar]
- Inoue-Nakamura N, & Matsuzawa T (1997). Development of stone tool use by wild chimpanzees (Pan troglodytes). Journal of Comparative Psychology, 111(2), 159 10.1037/0735-7036.111.2.159 [DOI] [PubMed] [Google Scholar]
- Kahlenberg SM, Thompson ME, Muller MN, & Wrangham RW (2008). Immigration costs for female chimpanzees and male protection as an immigrant counterstrategy to intrasexual aggression. Animal Behaviour, 76(5), 1497–1509. 10.1016/j.anbehav.2008.05.029 [DOI] [Google Scholar]
- Katsu Y, & Iguchi T (2016). Cortisol, Handbook of hormones (e95D) (First, p. 533). USA: Academic Press; 10.1016/B978-0-12-801028-0.00231-210.1016/B978-0-12-801028-0.00231-2 [DOI] [Google Scholar]
- Kimura T, & Hamada Y (1996). Growth of wild and laboratory born chimpanzees. Primates, 37(3), 237–251. 10.1007/BF02381856 [DOI] [Google Scholar]
- Korth-Schutz S, Levine LS, & New MI (1976). Serum androgens in normal prepubertal and pubertal children and in children with precocious adrenarche. The Journal of Clinical Endocrinology and Metabolism, 42(1), 117–124. 10.1210/jcem-42-1-117 [DOI] [PubMed] [Google Scholar]
- Labrie F (2010). DHEA, important source of sex steroids in men and even more in women, Progress in brain research (182, pp. 97–148. Elsevier; 10.1016/S0079-6123(10)82004-7 [DOI] [PubMed] [Google Scholar]
- Labrie F, Belanger A, Simard J, Luu-The VAN, & Labrie C (1995). DHEA and peripheral androgen and estrogen formation: Intracrinology. Annals of the New York Academy of Sciences, 774(1), 16–28. 10.1111/j.1749-6632.1995.tb17369.x [DOI] [PubMed] [Google Scholar]
- Laudenslager ML, Jorgensen MJ, & Fairbanks LA (2012). Developmental patterns of hair cortisol in male and female nonhuman primates: Lower hair cortisol levels in vervet monkey males emerge at puberty. Psychoneuroendocrinology, 37(10), 1736–1739. 10.1016/j.psyneuen.2012.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J, Wolfe LG, Schiebinger RJ, Loriaux DL, & Cutler GB, Jr (1982). Rapid regression of fetal adrenal zone and absence of adrenal reticular zone in the marmoset. Endocrinology, 111(6), 1797 10.1210/endo-111-6-1797 [DOI] [PubMed] [Google Scholar]
- Lonsdorf EV (2005). Sex differences in the development of termite-fishing skills in the wild chimpanzees, Pan troglodytes schweinfurthii, of Gombe National Park, Tanzania. Animal Behaviour, 70(3), 673–683. 10.1016/j.anbehav.2004.12.014 [DOI] [Google Scholar]
- Lonsdorf EV, Anderson KE, Stanton MA, Shender M, Heintz MR, Goodall J, & Murray CM (2014a). Boys will be boys: Sex differences in wild infant chimpanzee social interactions. Animal Behaviour, 88, 79–83. 10.1016/j.anbehav.2013.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf EV, Markham AC, Heintz MR, Anderson KE, Ciuk DJ, Goodall J, & Murray CM (2014b). Sex differences in wild chimpanzee behavior emerge during infancy. PLOS One, 9(6), e99099 10.1371/journal.pone.0099099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour, and cognition. Nature Reviews Neuroscience, 10(6), 434 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Maccoby EE (1990). Gender and relationships: A developmental account. American Psychologist, 45(4), 513 10.1037//0003-066x.45.4.513 [DOI] [PubMed] [Google Scholar]
- Majewska MD (1995). Neuronal actions of dehydroepiandrosterone possible roles in brain development, aging, memory, and affect. Annals of the New York Academy of Sciences, 774(1), 111–120. 10.1111/j.1749-6632.1995.tb17375.x [DOI] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, & Mellon SH (2009). Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Frontiers in Neuroendocrinology, 30(1), 65–91. 10.1016/j.yfrne.2008.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesiano S, & Jaffe RB (1997). Developmental and functional biology of the primate fetal adrenal cortex. Endocrine Reviews, 18(3), 378–403. 10.1210/er.18.3.378 [DOI] [PubMed] [Google Scholar]
- Muller MN, & Lipson SF (2003). Diurnal patterns of urinary steroid excretion in wild chimpanzees. American Journal of Primatology: Official Journal of the American Society of Primatologists, 60(4), 161–166. 10.1002/ajp.10103 [DOI] [PubMed] [Google Scholar]
- Muller MN, & Wrangham RW (2014). Mortality rates among Kanyawara chimpanzees. Journal of Human Evolution, 66, 107–114. 10.1016/j.jhevol.2013.10.004 [DOI] [PubMed] [Google Scholar]
- Muller MN, Kahlenberg SM, Emery Thompson M, & Wrangham RW (2007). Male coercion and the costs of promiscuous mating for female chimpanzees. Proceedings of the Royal Society B: Biological Sciences, 274(1612), 1009–1014. 10.1098/rspb.2006.0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AD, & Conley AJ (2008). Adrenal androgens in humans and nonhuman primates: Production, zonation and regulation, Disorders of the Human Adrenal Cortex (13, pp. 33–54. Karger Publishers; 10.1159/000134765 [DOI] [PubMed] [Google Scholar]
- Nguyen AD, Mapes SM, Corbin CJ, & Conley AJ (2008). Morphological adrenarche in rhesus macaques: Development of the zona reticularis is concurrent with fetal zone regression in the early neonatal period. Journal of Endocrinology, 199(3), 367 10.1677/JOE-08-0337 [DOI] [PubMed] [Google Scholar]
- Orentreich N, Brind JL, Rizer RL, & Vogelman JH (1984). Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. The Journal of Clinical Endocrinology & Metabolism, 59(3), 551–555. 10.1210/jcem-59-3-551 [DOI] [PubMed] [Google Scholar]
- Palmert MR, Hayden DL, Mansfield MJ, Crigler JF Jr, Crowley WF Jr, Chandler DW, & Boepple PA (2001). The longitudinal study of adrenal maturation during gonadal suppression: Evidence that adrenarche is a gradual process. The Journal of Clinical Endocrinology & Metabolism, 86(9), 4536–4542. 10.1210/jcem.86.9.7863 [DOI] [PubMed] [Google Scholar]
- Parker CR Jr, Grizzle WE, Blevins JK, & Hawkes K (2014). Development of adrenal cortical zonation and expression of key elements of adrenal androgen production in the chimpanzee (Pan troglodytes) from birth to adulthood. Molecular and Cellular Endocrinology, 387(1–2), 35–43. 10.1016/j.mce.2014.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LN (1991a). Adrenarche. Endocrinology and Metabolism Clinics of North America, 20(1), 71–83. 10.1016/S0889-8529(18)30282-2 [DOI] [PubMed] [Google Scholar]
- Parker LN (1991b). Control of adrenal androgen secretion. Endocrinology and Metabolism Clinics of North America, 20(2), 401–421. 10.1016/S0950-3579(96)80019-7 [DOI] [PubMed] [Google Scholar]
- Pattison JC, Abbott DH, Saltzman W, Conley AJ, & Bird IM (2009). Plasticity of the zona reticularis in the adult marmoset adrenal cortex: Voyages of discovery in the New World. Journal of Endocrinology, 203(3), 313 10.1677/JOE-08-0554 [DOI] [PubMed] [Google Scholar]
- Pechtel P, & Pizzagalli DA (2011). Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology, 214(1), 55–70. 10.1007/s00213-010-2009-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini AD, & Archer J (2005). Sex differences in competitive and aggressive behavior In Ellis BJ, & Bjorklund DJ (Eds.), Origins of the social mind: Evolutionary psychology and child development (pp. 219–244). New York: Guilford. [Google Scholar]
- Perret M, & Aujard F (2005). Aging and season affect plasma dehydroepiandrosterone sulfate (DHEA-S) levels in a primate. Experimental Gerontology, 40(7), 582–587. 10.1016/j.exger.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Pluchino N, Drakopoulos P, Bianchi-Demicheli F, Wenger JM, Petignat P, & Genazzani AR (2015). Neurobiology of DHEA and effects on sexuality, mood and cognition. The Journal of Steroid Biochemistry and Molecular Biology, 145, 273–280. 10.1016/j.jsbmb.2014.04.012 [DOI] [PubMed] [Google Scholar]
- Prall SP, & Muehlenbein MP (2018). DHEA modulates immune function: A review of evidence, Vitamins and Hormones (108, pp. 125–144. Academic Press; 10.1016/bs.vh.2018.01.023 [DOI] [PubMed] [Google Scholar]
- Prall SP, Ambu L, Nathan S, Alsisto S, Ramirez D, & Muehlenbein MP (2015). Androgens and innate immunity in rehabilitated semi-captive orangutans (Pongo pygmaeus morio) from Malaysian Borneo. American Journal of Primatology, 77(6), 642–650. 10.1002/ajp.22387 [DOI] [PubMed] [Google Scholar]
- Pryce CR, Palme R, & Feldon J (2002). Development of pituitaryadrenal endocrine function in the marmoset monkey: Infant hypercortisolism is the norm. The Journal of Clinical Endocrinology & Metabolism, 87(2), 691–699. 10.1210/jcem.87.2.8244 [DOI] [PubMed] [Google Scholar]
- Rege J, Turcu AF, Else T, Auchus RJ, & Rainey WE (2019). Steroid biomarkers in human adrenal disease. The Journal of Steroid Biochemistry and Molecular Biology, 190, 273–280. 10.1016/j.jsbmb.2019.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remer T, Boye KR, Hartmann MF, & Wudy SA (2005). Urinary markers of adrenarche: Reference values in healthy subjects, aged 3–18 years. The Journal of Clinical Endocrinology & Metabolism, 90(4), 2015–2021. 10.1210/jc.2004-1571 [DOI] [PubMed] [Google Scholar]
- Ritsner MS (2010). Pregnenolone, dehydroepiandrosterone, and schizophrenia: Alterations and clinical trials. CNS Neuroscience & Therapeutics, 16(1), 32–44. 10.1111/j.1755-5949.2009.00118.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins MM, & Czekala NM (1997). A preliminary investigation of urinary testosterone and cortisol levels in wild male mountain gorillas. American Journal of Primatology, 43(1), 51–64. [DOI] [PubMed] [Google Scholar]
- Sanchez MM (2006). The impact of early adverse care on HPA axis development: Nonhuman primate models. Hormones and Behavior, 50(4), 623–631. 10.1016/j.yhbeh.2006.06.012 [DOI] [PubMed] [Google Scholar]
- Shamim W, Yousufuddin M, Bakhai A, Coats AJ, & Honour JW (2000). Gender differences in the urinary excretion rates of cortisol and androgen metabolites. Annals of Clinical Biochemistry, 37(6), 770–774. 10.1258/0004563001900084 [DOI] [PubMed] [Google Scholar]
- Shi L, Wudy SA, Buyken AE, Maser-Gluth C, Hartmann MF, & Remer T (2011). Prepubertal glucocorticoid status and pubertal timing. The Journal of Clinical Endocrinology & Metabolism, 96(6), E891–E898. 10.1210/jc.2010-2935 [DOI] [PubMed] [Google Scholar]
- Shimada M, & Sueur C (2014). The importance of social play network for infant or juvenile wild chimpanzees at Mahale Mountains National Park, Tanzania. American Journal of Primatology, 76(11), 1025–1036. 10.1002/ajp.22289 [DOI] [PubMed] [Google Scholar]
- Simpson GL (2019). gratia: Graceful ‘ggplot’-based graphics and other functions for GAMs fitted using ‘mgcv’. R package version 0.2–1, https://CRAN.R-project.org/package=gratia [Google Scholar]
- Smail PJ, Faiman C, Hobson WC, Fuller GB, & Winter JS (1982). Further studies on adrenarche in nonhuman primates. Endocrinology, 111(3), 844 10.1210/endo-111-3-844 [DOI] [PubMed] [Google Scholar]
- Smith PK (2005). Play: Types and functions in human development In Ellis BJ & Bjorklund DF (Eds.), Origins of the social mind: Evolutionary psychology and child development (pp. 271–291). New York: Guilford. [Google Scholar]
- Haith MM, & Sameroff AJ (Eds.), 1996). The five to seven year shift: the age of reason and responsibility. Chicago, IL: University of Chicago Press. [Google Scholar]
- Soma KK, Rendon NM, Boonstra R, Albers HE, & Demas GE (2015). DHEA effects on brain and behavior: Insights from comparative studies of aggression. The Journal of Steroid Biochemistry and Molecular Biology, 145, 261–272. 10.1016/j.jsbmb.2014.05.011 [DOI] [PubMed] [Google Scholar]
- Sperling MA (2014). Pediatric endocrinology e-book: Expert consult-online and print. Elsevier Health Sciences. [Google Scholar]
- Stoinski TS, Czekala N, Lukas KE, & Maple TL (2002). Urinary androgen and corticoid levels in captive, male western lowland gorillas (Gorilla g. gorilla): Age-and social group-related differences. American Journal of Primatology: Official Journal of the American Society of Primatologists, 56(2), 73–87. 10.1002/ajp.1065 [DOI] [PubMed] [Google Scholar]
- Šulcová J, Hill M, Hampl R, & Starka L (1997). Age and sex related differences in serum levels of unconjugated dehydroepiandrosterone and its sulphate in normal subjects. Journal of Endocrinology, 154(1), 57–62. 10.1677/joe.0.1540057 [DOI] [PubMed] [Google Scholar]
- Tennenhouse EM, Putman S, Boisseau NP, & Brown JL (2017). Relationships between steroid hormones in hair and social behaviour in ring-tailed lemurs (Lemur catta). Primates, 58(1), 199–209. 10.1007/s10329-016-0566-7 [DOI] [PubMed] [Google Scholar]
- Vierhapper H, Nowotny P, & Waldhäusl W (1998). Sex-specific differences in cortisol production rates in humans. Metabolism: Clinical and Experimental, 47(8), 974–976. 10.1016/S0026-0495(98)90353-5 [DOI] [PubMed] [Google Scholar]
- Vinson GP (2016). Functional zonation of the adult mammalian adrenal cortex. Frontiers in Neuroscience, 10, 238 10.3389/fnins.2016.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SN (2017). Generalized additive models: An introduction with R (2nd edition). Chapman and Hall/CRC; [Google Scholar]
- Wrangham RW, Chapman CA, Clark-Arcadi AP, & Isabirye-Basuta G (1996). Social ecology of Kanyawara chimpanzees: Implications for understanding the costs of great ape groups In McGrew WC, Marchant LF, & Nishida T (Eds.), Great ape societies (pp. 45–57). Cambridge: Cambridge University Press. [Google Scholar]
- Wudy SA, Hartmann MF, & Remer T (2007). The sexual dimorphism in cortisol secretion starts after age 10 in healthy children: Urinary cortisol metabolite excretion rates during growth. American Journal of Physiology-Endocrinology and Metabolism, 293, E970–E976. 10.1152/ajpendo.00495.2006 [DOI] [PubMed] [Google Scholar]
- Xing Y, Lerario AM, Rainey W, & Hammer GD (2015). Development of adrenal cortex zonation. Endocrinology and Metabolism Clinics, 44(2), 243–274. 10.1016/j.ecl.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Carlson NE, & Brown MB (2001). 24-hour ACTH and cortisol pulsatility in depressed women. Neuropsychopharmacology, 25(2), 267–276. 10.1016/S0893-133X(00)00236-0 [DOI] [PubMed] [Google Scholar]
- Zihlman AL, Bolter DR, & Boesch C (2007). Skeletal and dental growth and development in chimpanzees of the Taï National Park, Côte D’Ivoire. Journal of Zoology, 273(1), 63–73. 10.1111/j.1469-7998.2007.00301.x [DOI] [Google Scholar]
- Zumoff BV, & Bradlow HL (1980). Sex difference in the metabolism of dehydroisoandrosterone sulfate. The Journal of Clinical Endocrinology and Metabolism, 51(2), 334 [DOI] [PubMed] [Google Scholar]
- Zwain IH, & Yen SS (1999). Dehydroepiandrosterone: Biosynthesis and metabolism in the brain. Endocrinology, 140(2), 880–887. [DOI] [PubMed] [Google Scholar]