Abstract
Background:
Liver is enriched in several innate-like unconventional T cells but their role in alcohol-related liver disease (ALD) is not fully understood. Studies in several acute alcohol feeding models but not in chronic alcoholic steatohepatitis (ASH) model have shown that invariant natural killer T (iNKT) cells play a pathogenic role in ALD. Here, we investigated the activation of iNKT cells in an intragastric (iG) infusion model of chronic ASH as well as the frequency and cytokine phenotype of three different unconventional T cells: iNKT, mucosal-associated invariant T (MAIT) and CD8+CD161hiVα7.2− cells in peripheral blood of ALD patients.
Methods:
Hepatic iNKT cells were investigated using the iG model of chronic ASH that combine feeding of High-Cholesterol/High-Fat diet (HCFD) with intragastric feeding of ethanol diet (HCFD+iG Alc). Human iNKT, MAIT and CD8+CD161hiVα7.2− cells were examined by flow cytometry in peripheral blood of patients with severe alcoholic hepatitis (SAH) and chronic alcoholics (ChA) and compared with healthy controls.
Results:
In the iG model of chronic ASH, IFNγ+ iNKT cells accumulate in their livers compared with pair-fed control mice and activated hepatic iNKT cells show high expression of Fas and FasL. Notably, IFNγ+ iNKT cells are also significantly increased in peripheral blood of ChA patients compared with SAH patients. MAIT cells are significantly reduced in all ALD patients, but CD8+CD161hiVα7.2− cells are increased in SAH patients. Although, MAIT and CD8+CD161hiVα7.2− cells displayed a similar cytokine production profile, IFNγ and TNFα production is significantly increased in SAH patients while significant IL-17A production is found in ChA patients.
Conclusions:
We found that the three unconventional T cells are activated in ALD patients showing interesting differences in their frequency and cytokine production profile between SAH and ChA patients. In the iG murine model of chronic ASH, iNKT cells are also activated secreting pro-inflammatory cytokines suggesting their involvement in liver disease.
Keywords: ALD, iG ASH model, CD8, chronic alcoholic, IFNγ
Introduction
Alcohol-related liver disease (ALD) is a major public health concern and leading cause of liver-related mortality worldwide (Garcia-Saenz-de-Sicilia et al., 2017, Louvet, 2017, Serste et al., 2018). ALD results from chronic alcohol overconsumption and the disease progress from hepatic steatosis (fatty liver) to alcoholic steatohepatitis (ASH) with liver inflammation and to alcoholic liver fibrosis and cirrhosis and in some cases hepatocellular carcinoma (HCC). In addition, severe ASH (with or without cirrhosis) can progress in 8 to 20% of patients to severe alcoholic hepatitis (SAH) that is an acute‐on‐chronic liver disease associated with liver failure, sepsis, multiorgan dysfunction and high mortality (Singal et al., 2018). It is becoming clear that complex interactions between innate and adaptive immunity in the gut-liver axis are crucial in mediating ALD (Szabo and Bala, 2010, Seitz et al., 2018). In fact, loss of gut integrity with enhanced translocation of bacteria and bacterial products leading to immune cell activation plays an important role in ALD (Rao, 2009, Leclercq et al., 2014, Tuomisto et al., 2014, Chen et al., 2015b).
The liver is enriched in several innate cells, including NK cells as well as unconventional or innate-like T cells that can potentially play crucial role in ALD. Unconventional T cells are a diverse population, comprising natural killer T (NKT) cells, γδ T cells, mucosal-associated invariant T (MAIT) cells, and MHC class Ib-restricted CD8 T cells (Crispe, 2011, Godfrey et al., 2018). Unconventional T cells recognize antigens presented via non-classical MHC class Ib (e.g., Qa-1b/HLA-E, H2-M3) and MHC class-I like (e.g., CD1, MR1) molecules and respond by rapidly secreting large amounts of cytokines (Godfrey et al., 2018).
NKT cells are comprised of two main subsets, invariant NKT (iNKT) and type II NKT cells. Both NKT cell subsets are predominantly NK1.1+ (mouse) or CD161+/CD56+ (human), and share common features in both mice and humans (Exley et al., 1997, Exley et al., 2003, Shaulov et al., 2008). iNKT cells express a semi-invariant TCR encoded predominantly by a germline Vα gene (Vα14/Jα18 in mice and Vα24/Jα18 in humans) and more diverse non-germline Vβ genes (Vβ8.2/7/2 in mice and Vβ11 in humans). In contrast, type II NKT cells use a relatively diverse TCR repertoire, are less abundant in mice though predominant over iNKT in humans and appear to be regulatory in nature (Jahng et al., 2004, Halder et al., 2007, Arrenberg et al., 2010, Arrenberg et al., 2011, Patel et al., 2012).
Experiments in murine models of alcohol feeding have shown that iNKT cells play a pathogenic role in mediating ALD (Bertola et al., 2013, Kumar, 2013, Maricic et al., 2015, Cui et al., 2015, Mathews et al., 2016, Bandyopadhyay et al., 2016, Yang et al., 2017, Khanova et al., 2018, Lee et al., 2019). We have shown that activated pro-inflammatory iNKT cells, but not type II NKT cells, accumulate in the liver of mice following chronic plus binge ethanol feeding leading to hepatic recruitment of inflammatory CD11b+Gr-1hiLy6G+ neutrophils that results in liver damage (Maricic et al., 2015). However, mice deficient in iNKT cells (Jα18−/−) or deficient in both NKT cell subsets (CD1d−/−) are protected against liver injury after alcohol feeding (Maricic et al., 2015, Mathews et al., 2016). The recruitment of iNKT cells following alcohol feeding is associated with up‐regulation of several hepatic proinflammatory genes, including IL‐1β, IL‐6, and CXCR1 (Maricic et al., 2015). Notably, direct inactivation of iNKT cells by all-trans-retinoic acid or a retinoid-receptor γ agonist or by indirect mechanisms involving sulfatide-mediated activation of type II NKT cells inhibited ALD (Kumar, 2013, Maricic et al., 2015, Marrero et al., 2015, Mathews et al., 2016). More recently, it has been shown that chronic alcohol feeding also activates iNKT cells in the intestine to migrate to liver contributing together with resident hepatic iNKT cells to hepatocyte apoptosis (Lee et al., 2019). In humans, the role of iNKT cells in ALD has not been completely investigated. However, comparative analysis of hepatic gene expression between human ALD and animal models suggest the involvement of common pathways in liver damage. Consistent with the data in murine models, pro-inflammatory cytokines, including OPN, IL-1, IL-6 and TNFα, are increased in the serum and liver biopsies of patients with alcoholic hepatitis (AH) and may correlate with disease severity/mortality (Colmenero et al., 2007). Moreover, in patients with AH, reduced NKG2D expression in CTLs, NK cells and iNKT cells has been found to correlate with disease severity, which suggests that these cells may be involved in promoting liver damage (Stoy et al., 2015). In contrast, increased frequencies of IL-22-producing cells and increased IL-17 plasma levels are associated with improved prognoses in patients with AH (Stoy et al., 2015, Gao and Shah, 2015, Ki et al., 2010).
One of the leading cause of alcohol-related death is an increased susceptibility to bacterial infections and sepsis (Louvet et al., 2009). MAIT cells, other relevant unconventional T cells, are identified in humans as predominantly CD8+ T cells expressing an invariant TCR α chain (Vα7.2-Jα33/20/12 in humans, Vα19-Jα33 in mice) together with high levels of CD161 and recognize bacterial-derived metabolites presented by the MHC-like molecule MR-1 (Dusseaux et al., 2011, Walker et al., 2012). MAIT cells are preferentially localized in anatomical site highly affected by alcohol consumption such as gut and liver, but also present in the peripheral blood (Martin et al., 2009, Dusseaux et al., 2011, Kjer-Nielsen et al., 2012). They are key players against bacterial infections and in the control of inflammatory diseases and gut microbiota (Kurioka et al., 2015, Kurioka et al., 2016, Jeffery et al., 2016).
In this study, we follow two major populations of unconventional T cells, iNKT and MAIT cells as well as another one referred to as CD8+CD161hiVα7.2− cells, in peripheral blood of SAH and chronic alcoholic (ChA) patients. We found that all these unconventional T cells are altered in ALD patients and differ in their frequencies and/or functional profiles in SAH and ChA patients. Furthermore, consistent with the activation of iNKT cells in ALD, we found an increased frequency of hepatic Fas/FasL-expressing, IFNγ+ iNKT cells in the murine intragastric (iG) infusion model of chronic ASH.
MATERIALS AND METHODS
Intragastric (iG) alcohol infusion murine model of chronic ASH
Eight week old male C57BL/6 (B6) mice were purchased from The Jackson Laboratory. All mice were maintained in specific pathogen-free conditions. Animal studies were carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of California San Diego and the University of Southern California.
Intragastric (iG) alcohol-fed mice and control mice were provided by the Animal Core of the NIAAA-funded Southern California Research Center for ALPD and Cirrhosis. Male B6 mice were implanted with a long term iG catheter for alcohol infusion as previously described (Ueno et al., 2012, Lazaro et al., 2015). Briefly, B6 mice were subjected to 2 weeks of ad libitum feeding of chow or diet high in cholesterol and saturated fat (high‐cholesterol/high‐fat diet (HCFD); #180529; Dyets Inc., Bethlehem, PA), surgical implantation of iG catheter, 1 week of acclimatization with infusion of a controlled high‐fat diet (corn oil, 36%Cal) for 60% of daily caloric requirement and ad libitum feeding of HCFD or chow for the remaining 40%. At this point, for ethanol‐fed mice, they were switched to an ethanol high‐fat diet with incrementally increasing ethanol concentration over an 8‐week period. Mice fed HCFD+iG alcohol developed chronic ASH with mononuclear cell inflammation and pericellular and perisinusoidal fibrosis.
Human subjects
Human studies were approved by the respective local ethics committee from UCSD, VA San Diego Healthcare System, University of Southern California and VA Long Beach Healthcare System. Written informed consent was obtained from all patients.
Severe alcoholic hepatitis patients (SAH, n= 14) were included according to modified Maddrey’s discriminant function (mDF) ≥ 32 and the Model for End‐Stage Liver Disease (MELD) score >20 (Maddrey et al., 1978, Kamath et al., 2001, Dunn et al., 2005). SAH patients were enrolled from University of Southern California and VA Long Beach Healthcare System (IRB# HS-14–00385).
Chronic alcoholic patients (ChA, n= 7): Patients who have alcohol use disorder with active alcohol consumption (self-reported > 60 g/day) and without clinical and/or by imaging evidence of cirrhosis. ChA patients were enrolled from UCSD (IRB# 160763X) and VA San Diego Healthcare System (IRB# H120108). The sample size was based on the availability of biological samples rather than a pre-specified effect size.
Healthy controls (HC, n= 22): frozen human peripheral blood mononuclear cells (PBMC) from individual healthy donors with no history of liver pathologic condition were obtained from iXCells Biotechnologies (San Diego, CA). These cells were obtained through an IRB approved donor program.
Isolation of human PBMC
Peripheral blood was collected from ChA patients and centrifuged over a Ficoll-Hypaque density gradient (MilliporeSigma, St. Louis, MO) at 2000 rpm at room temperature for 30 min on the same day to isolate PBMC for flow cytometry experiments. Cryopreserved PBMC from AH patients and healthy controls were defrosted the same day of experiments.
Isolation of murine hepatic mononuclear cells (MNCs)
Hepatic MNCs were isolated using a 35% isotonic Percoll solution (GE Healthcare Life Sciences, Marlborough, MA) containing 100 U/ml of heparin, as described before (Maricic et al., 2018). After isolation, the cells were washed with High Glucose DMEM medium (Thermo Fisher Scientific, Waltham, MA) supplemented with 2% FBS (HyClone, Thermo Fisher Scientific) and resuspended in FACS buffer (0.02% sodium azide, 2% FBS in PBS).
Flow cytometry analysis and intracellular staining
Data were collected on a FACSCanto flow cytometer (BD Biosciences [BD], San Diego, CA) at the Flow Cytometry Research Core Facility (VA San Diego Healthcare System, San Diego, CA) and analyzed with FlowJo v10 software (TreeStar, Ashland, OR). Mouse mAbs (clones) were as follows: anti-TCRβ (H57–597), anti-NK1.1 (PK136), anti-CD95 (Jo2) and anti-CD178 (FasL) (MFL3) (all from BD). PE-conjugated αGalCer/mCD1d tetramers were generated in our laboratory, as previously described (Halder et al., 2007). To minimize nonspecific Ab binding, cells were previously incubated with anti-CD16/CD32 (Fc Block, 2.4G2) (BD). For intracellular cytokine staining, 1 × 106 /well of liver MNCs were cultured for 6 h at 37°C in completed RPMI 1640 media (Hyclone) supplemented with 10% FBS, sodium pyruvate, L-Glutamine, Pen/Strep, Hepes, non-essential amino acids and 2-βME in the presence of PMA 10 ng/ml (MilliporeSigma), ionomycin 500 ng/ml (MilliporeSigma) and 1μl/ml of GolgiPlug protein transport inhibitor containing brefeldin A (BD). At the end of the incubation, cells were first stained for cell surface markers, then fixed and permeabilized using BD Cytofix/Cytoperm Plus kit and stained with the following fluorochrome-labeled mAbs: anti-IFNγ (XMG1.2), anti-IL-4 (BVD6–24G2), anti-IL-17 (eBio17B7) and anti-IL-22 (IL22JOP) from Thermo Fisher Scientific. Human Abs were as follows: anti-CD3 (UCHT1), anti-TCRαβ (T10B9.1A-31), anti-CD19 (SJ25-C1), anti-CD8α (RPA-T8), anti-CD8β (2ST8.5H7) from BD; anti-CXCR3 (49801) from R&D Systems; anti-CD161 (HP-3G10), anti-TCR Vα7.2-Jα33 (3C10) from BioLegend; anti-Vα24-Ja18 TCR (6B11) and LIVE/DEAD Fixable Aqua Dead Cell Stain Kit from Thermo Fisher Scientific. For intracellular cytokines and transcription factors staining, PBMC were not in vitro stimulated, instead PBMC were first stained directly for cell surface markers, then, fixed and permeabilized using BD Pharmingen™ Transcription Factor Buffer Set. Briefly, cells were permeabilized for 45 min with the Fixation/permeabilization buffer on ice, washed twice in perm/wash buffer, and stained for 45 min on ice with the following mAbs: anti-IFNγ (4S.B3), anti-IL-17A (eBio64DEC17), anti-IL-22 (22URTI), anti-T-bet (4B10) from Thermo Fisher Scientific; anti-IL-4 (MP4–25D2), anti-TNFα (MAb11) and anti-PLZF (R17–809) from BD. Samples were further resuspended in 1x BD Stabilizing Fixative and store at 4°C until acquisition.
Statistical analyses
Data were analyzed using GraphPad Prism v7 software (GraphPad Software). Data are reported as mean ± SEM. For comparison between two groups, two-tailed Mann-Whitney test was applied. For comparison between three groups, one-way ANOVA with Bonferroni multiple comparisons test or Kruskal-Wallis test with Dunn’s multiple comparison test were applied. Correlations were performed using nonparametric Spearman test. A Wilcoxon matched-pairs signed rank test was used when comparing two groups of paired data. For all analyses, significance was indicated as follow: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Results
A significant increase in IFNγ and Fas/FasL expression in hepatic iNKT cells in an intragastric (iG) model of chronic ASH.
To investigate the activation of iNKT cells in chronic ASH, we first used the iG infusion model that combines ad libitum feeding of High-Cholesterol/High-Fat Diet (HCFD) with intragastric feeding of ethanol diet (HCFD+iG Alc) (Lazaro et al., 2015). H&E staining of liver sections from mice fed control diet (Chow+Cont) show relatively normal liver histology with occasional hepatocytes with lipid droplets (Fig. 1A, panels A and C) whereas HCFD+iG Alc-fed mice show macro- and micro-vesicular steatosis and accumulation of inflammatory cells, predominantly mononuclear cells with occasional PMN infiltration (Fig. 1A, panels B and D). Accordingly, the total number of hepatic MNCs was significantly higher in HCFD+iG Alc-fed mice than Chow+Cont-fed mice (Fig. 1B). To identify iNKT cells and to differentiate them from type II NKT cells and NK1.1-expressing conventional T cells, we have utilized αGalCer/mCD1d tetramer staining (Fig. 1C). We found that the numbers of iNKT cells were significantly increased in HCFD+iG Alc-fed mice in comparison to Chow+Cont-fed mice (Fig. 1D) while no significant differences were found in the percentage tetramer+ cells. A slight decrease in TCR staining is likely due to down-regulation of the cell surface TCR following chronic stimulation as shown recently (Maricic et al., 2018, Lee et al., 2019).
Figure 1. Hepatic iNKT cells in an intragastric model of chronic ASH.
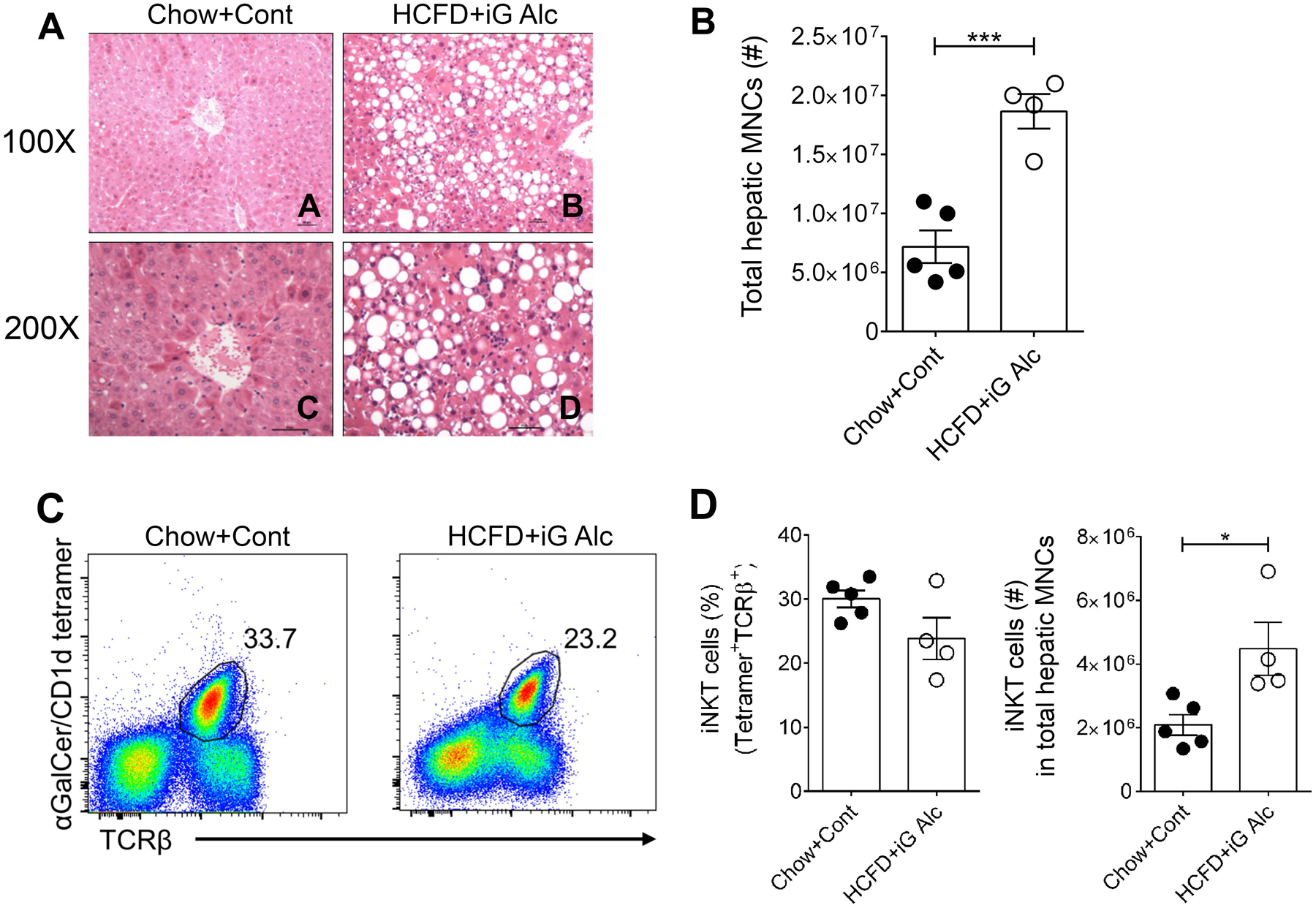
(A) H&E stained liver sections from a mouse subjected to hybrid feeding of High-Cholesterol/High-Fat Diet (HFCD) ad lib and iG control diet (Chow+Cont) (Panels A, C) or HFCD ad lib and iG ethanol diet (HCFD+iG Alc) (Panels B, D). (B) Summary data of the total number of hepatic MNCs in HCFD+iG Alc- (n= 4) and Chow+Cont-fed (n= 5) B6 mice. ***p≤ 0.001, unpaired t test. (C) Representative dot plots of iNKT cells (αGalCer-Tetramer+TCRβ+) in liver lymphocytes from mice in Fig. 1B. Numbers on dot plots indicate the percentage of iNKT cells within the lymphocyte gate. (D) Summary data of the percentage (left) and numbers (right) of iNKT cells from mice in Fig. 1B. The numbers of iNKT cells were calculated using the percentages obtained from the total hepatic MNCs. *p≤ 0.05, unpaired t test. All data are presented as mean ± SEM and are representative of two iG-feeding experiments. iNKT, invariant Natural Killer T cells; iG, intragastric; ASH, alcoholic steatohepatitis; HCFD, High-Cholesterol /High-Fat Diet; MNCs, Mononuclear cells.
Next, since iNKT cells can also up-regulate Fas (CD95) and/or Fas ligand (FasL) expression in response to their chronic activation (Leite-de-Moraes et al., 2000, Lee et al., 2019), we determined Fas and FasL expression in iNKT cells from these mice. As shown in Fig. 2A, Fas and FasL expression also increased significantly in hepatic iNKT cells from HCFD+iG Alc-fed mice in comparison to control fed mice, indicating that HCFD+iG Alc feeding results in activation of iNKT cells leading to expression of Fas and FasL.
Figure 2. A significant increase in IFNγ and Fas/FasL in hepatic iNKT cells in an intragastric model of chronic ASH.
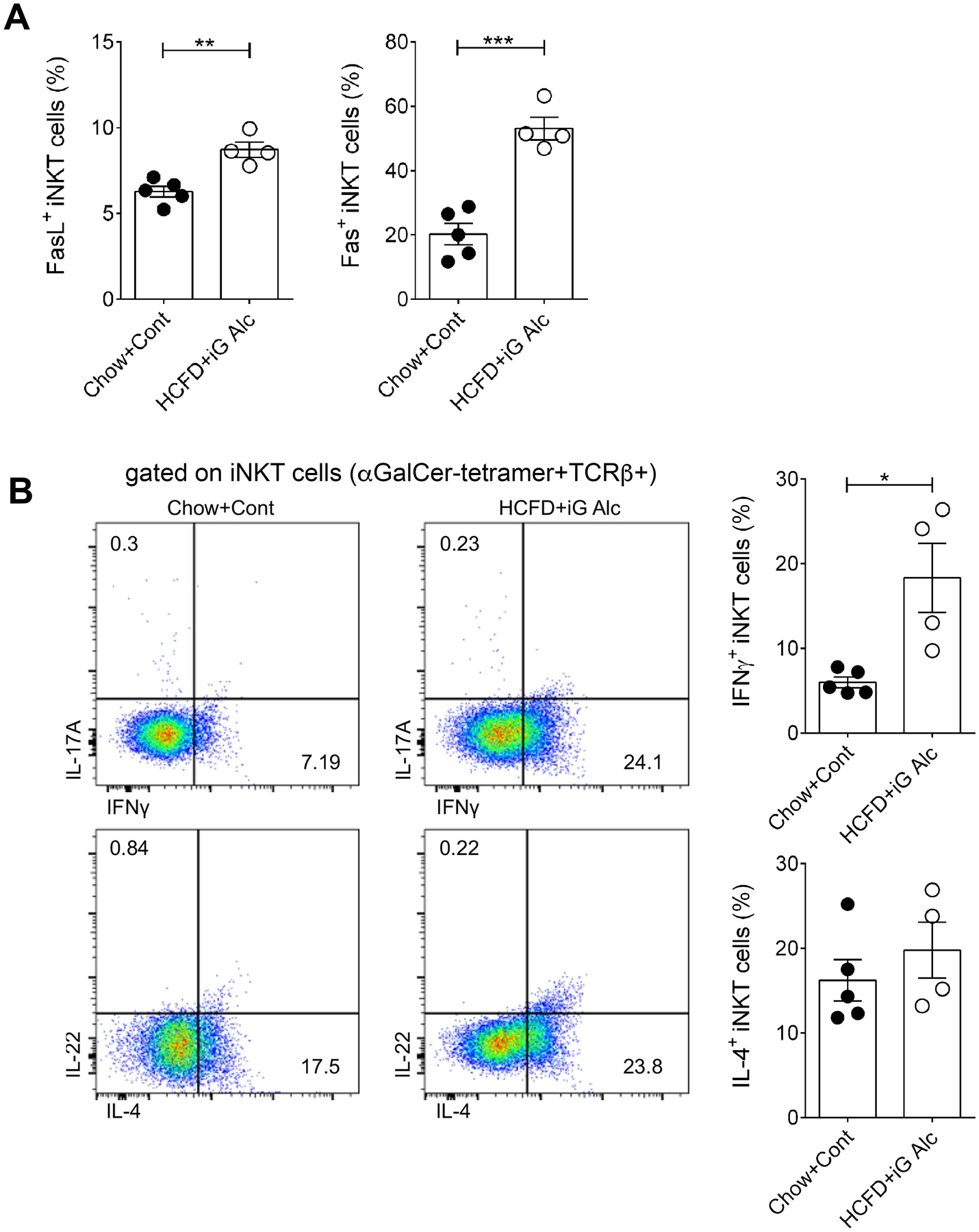
(A) Percentage of FasL+- and Fas+-hepatic iNKT cells present within the iNKT cells gate (αGalCer-Tetramer+TCRβ+) in HCFD+iG Alc- (n= 4) and Chow+Cont-fed (n= 5) B6 mice. (B) Representative dot plots and summary data of cytokine production by hepatic iNKT cells from mice in Fig. 2A. Cytokine producing cells were identified within the iNKT cells gate (αGalCer-Tetramer+TCRβ+). All data are presented as mean ± SEM and are representative of two iG-feeding experiments. *p≤ 0.05, **p≤ 0.01, ***p≤ 0.001, Mann Whitney test. iNKT, invariant Natural Killer T cells; iG, intragastric; ASH, alcoholic steatohepatitis; HCFD, High-Cholesterol /High-Fat Diet.
Because the iG model results in liver damage resembling ASH in humans (Lazaro et al., 2015, Khanova et al., 2018), we investigated the cytokine production profile of hepatic iNKT cells in HCFD+iG Alc-fed mice. Consistent with other ALD models, IFNγ+ iNKT cells but not IL-4+ iNKT cells were significantly increased in HCFD+iG Alc-fed mice in comparison to mice in the control group (Fig. 2B). IL-17A and IL-22 production by iNKT cells was not detected in HCFD+iG Alc-fed mice despite a significant increased production of both cytokines by conventional T cells in these mice (Xu et al., 2020, accepted for publication in JCI insight).
Peripheral blood iNKT cells are increased in AH patients but not in chronic alcoholics.
We first evaluated the frequency of TCRαβ+, CD3+ and CD8+ T cells in PBMC from 21 ALD patients (SAH, n= 14 and ChA, n= 7) and compared to that in healthy controls (HC, n= 22). The baseline characteristics of the patients are presented in Table 1. There was a slight decrease in CD3+ and CD8+ T cells in SAH patients but not in ChA patients (Fig. 3). To investigate the frequency of iNKT cells in human PBMC, we have used a mAb that recognizes the invariant CDR3 loop of the human canonical Vα24Jα18 TCR α-chain (clone 6B11) (Montoya et al., 2006) expressed by iNKT cells using a multiparameter flow cytometry approach. Thus, human iNKT cells were identified as TCR Vα24Jα18+/CD3+ in the CD3 gate following strategy in Fig. 4A. The numbers of iNKT cells were calculated using the percentages obtained from the gating strategy in total live cells. As shown in Fig. 4B, the frequency and numbers of iNKT cells were significantly increased in blood of SAH patients in comparison to healthy controls (0.24% ± SEM 0.05 vs. 0.10% ± 0.02 and 2.2 × 103 ± 3.3 × 102 vs. 1.4 × 103 ± 4.1 × 102) and even more increased when compared to ChA patients (0.04% ± 0.02 and 5.5 × 102 ± 1.7 × 102), which had reduced frequency and numbers in comparison to healthy controls. We also investigated whether clinical parameters (see Table 1) associate with blood iNKT cell frequency. We found only a significant positive correlation between blood iNKT cell frequency and total bilirubin in SAH patients (p= 0.0223 by Spearman correlation test, Fig. 4F).
Table 1.
Baseline clinical characteristics of the patients
| SAH | ChA | |
|---|---|---|
| n= 14 | n= 7 | |
| Sex (n) - M/F | 14/0 | 7/0 |
| Age | 48.79 ± 3.84 | 49.20 ± 4.59 |
| Child-Pugh Score (value) | 14.86 ± 2.09 | – |
| mDF | 59.76 ± 6.26 | – |
| MELD Score | 23.14 ± 1.79 | – |
| BMI | 29.43 ± 1.70 | 28.80 ± 3.12 |
| Bilirubin (mg/dL) | 15.91 ± 2.36 | – |
| ALT (IU/L) | 53.29 ± 6.94 | 93.20 ± 19.46 |
| AST (IU/L) | 130.86 ± 17.83 | 75.40 ± 21.08 |
| White cell count (x109/L) | 14.70 ± 3.63 | – |
All values represented as mean ± SEM.
SAH, severe alcoholic hepatitis; ChA, chronic alcoholics; mDF, Maddrey’s discriminant function; MELD, model for end-stage liver disease; BMI, body mass index; ALT, alanine transaminase; AST, aspartate transaminase.
Figure 3. No changes in T-cell subsets in PBMC.
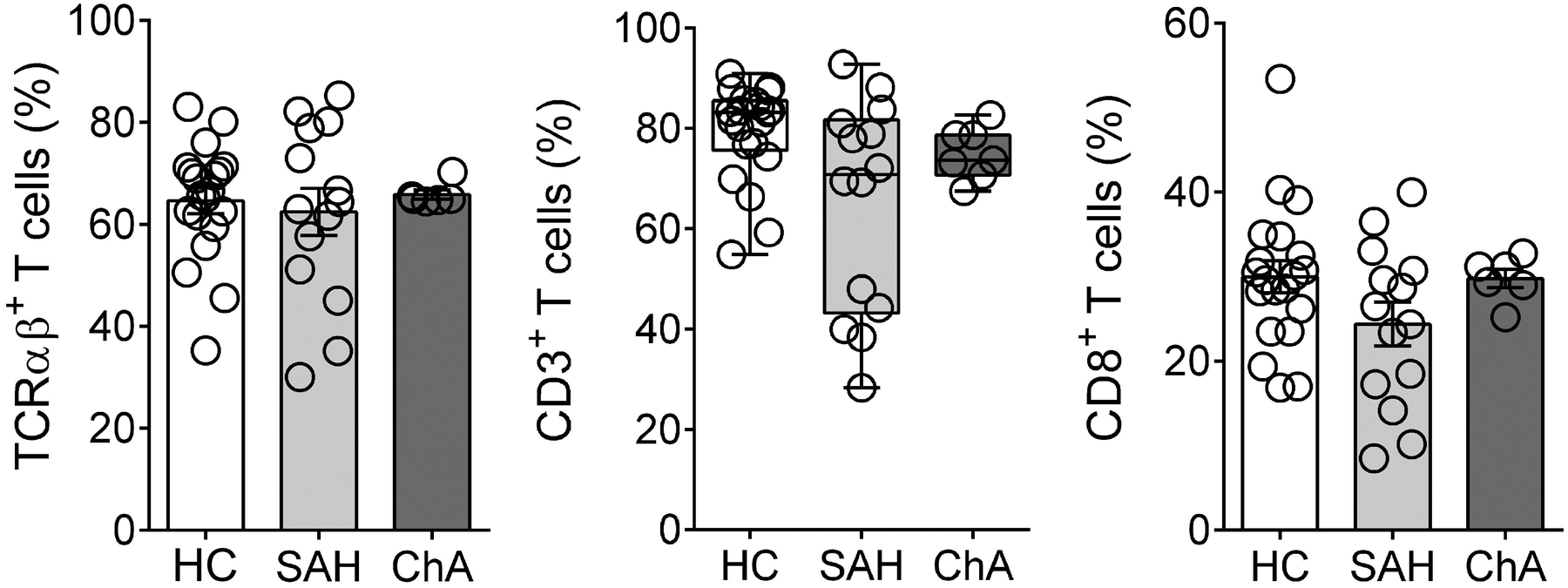
Summary data of percentage of TCRαβ+, CD3+ and CD8+ T cells in PBMC from SAH (n= 14) and ChA (n= 7) patients compared with HC (n= 22). All data are presented as mean ± SEM. PBMC, peripheral blood mononuclear cells; HC, healthy controls; SAH, severe alcoholic hepatitis; ChA, chronic alcoholics.
Figure 4. Peripheral blood iNKT cells are increased in SAH patients but reduced in chronic alcoholics and show different cytokine production profile.
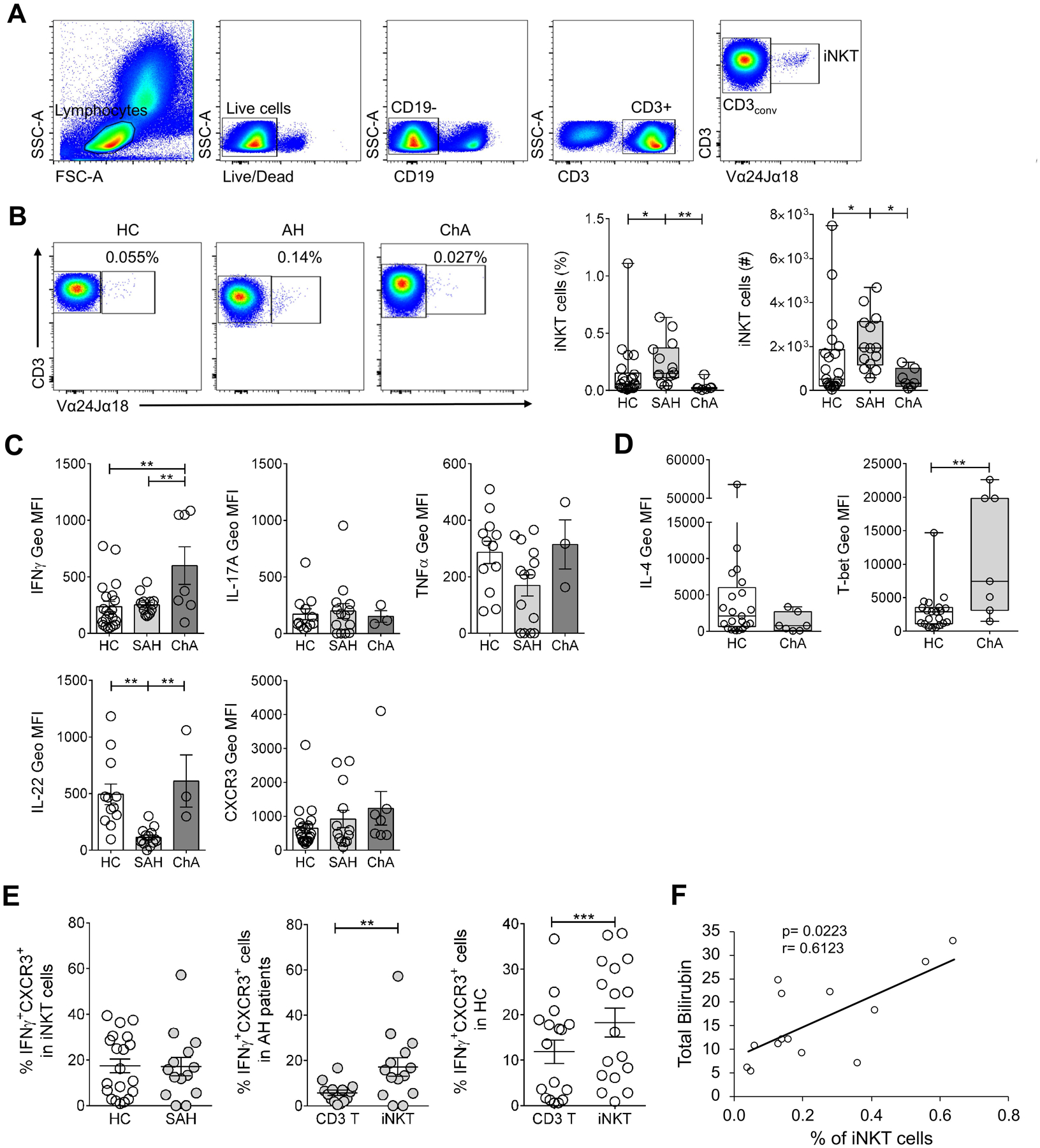
(A) Gating strategy to identify iNKT cells in human PBMC by flow cytometry. iNKT cells were defined as double positive cells for CD3 and Vα24-Jα18 TCR (CD3+Vα24Jα18TCR+) in the CD3+ gate and were expressed as percentage of CD3+ T cells. CD3conv were identified as CD3+ and, simultaneously, Vα24Jα18TCR− cells (CD3+Vα24Jα18TCR−) in the CD3+ gate. (B) Representative dot plots and summary data of percentage and numbers of iNKT cells in PBMC from SAH (n= 14) and ChA (n= 7) patients compared with HC (n= 22). Numbers on dot plots indicate percentage of iNKT cells following gating strategy as in (A). *p≤ 0.05; **p≤ 0.01, Kruskal-Wallis test with Dunn’s multiple comparisons test. (C) IFNγ, IL-17A, TNFα and IL-22 production as well as CXCR3 expression, expressed as Geo MFI values, by non-stimulated iNKT cells in PBMC of SAH (n= 13–14) and ChA (n= 3–7) patients compared with HC (n= 12–21). **p≤ 0.01, One way ANOVA with Bonferroni’s multiple comparisons test. (D) IL-4 production and T-bet expression, expressed as Geo MFI values, by non-stimulated iNKT cells in PBMC of ChA (n= 7) patients compared with HC (n= 21). **p≤ 0.01, Mann-Whitney test. (E) (Left) Percentage of IFNγ+CXCR3+ iNKT in PBMC from SAH patients (n= 14) compared with HC (n= 20). (Center) Percentage of IFNγ+CXCR3+ double-positive cells within the CD3conv and iNKT cells gates in SAH patients. (Right) Percentage of IFNγ+CXCR3+ double-positive cells within the CD3conv and iNKT cells gates in HC. **p≤ 0.01, ***p≤ 0.001, Wilcoxon matched-pairs signed rank test. (F) Correlation between peripheral blood iNKT cells frequency and total bilirubin in SAH patients (Spearman correlation test). All data are presented as mean ± SEM. iNKT, invariant Natural Killer T cells; CD3conv, conventional CD3 T cells; HC, healthy controls; SAH, severe alcoholic hepatitis; ChA, chronic alcoholics.
Differential cytokine production profile of iNKT cells in SAH and ChA patients.
Next, we determined the cytokine production (IFNγ, IL-17A, IL-22 and TNFα) and CXCR3 expression in blood iNKT cells from ALD patients. IFNγ production by iNKT cells was significantly increased in ChA patients, as measured by geometric mean fluorescence intensity, compared with controls (600 MFI ± 166 vs. 236 MFI ± 45.7 in HC) and vs. SAH patients (252 MFI ± 23) (Fig. 4C). Accordingly, T-bet expression by iNKT cells from ChA patients was also significantly higher in comparison to controls (11336 MFI ± 3415 vs. 2944 MFI ± 661 in HC) (Fig. 4D). Although, we observed a trend towards an increased CXCR3 expression in iNKT cells from ALD patients compared with controls (SAH: 922 MFI ± 254 and ChA: 1236 MFI ± 492 vs. 648 MFI ± 138 in HC), there was no significant difference between the groups (Fig. 4C). Consistent with this proinflammatory profile, IL-4 production by iNKT cells was reduced in ChA patients compared with controls (Fig. 4D). Interestingly, IL-22 production by iNKT cells was significantly reduced in SAH patients compared with both controls (113 MFI ± 20.7 vs. 494 MFI ± 91.2 in HC) and ChA patients (611 MFI ± 230) (Fig. 4C). Moreover, iNKT cells from SAH patients showed a trend toward reduced TNFα and increased IL-17 production compared with both ChA patients and controls (Fig. 4C).
Recently, we have identified a subset of iNKT cells co-expressing IFNγ and CXCR3 (IFNγ+CXCR3+ iNKT cells) that is significantly increased in nonalcoholic steatohepatitis patients compared with controls (Maricic et al., 2018). Here, we found comparable frequencies of IFNγ+CXCR3+ iNKT cells between SAH patients and controls (17.1% ± 4 vs. 17.4% ± 3) (Fig. 4E, left). Although, in comparison to IFNγ+CXCR3+ double positive cells within conventional CD3+ T cells (CD3conv), IFNγ+CXCR3+ iNKT cells were significantly increased in SAH patients (17.1% ± 4 vs. 5.7% ± 1.2) (Fig. 4E, center), they were also significantly increased in healthy controls (18.3% ± 3.2 vs. 11.9% ± 2.6) (Fig. 4E, right).
Altogether, these results show variations in the frequency of blood iNKT cells in ALD patients and a different pattern of cytokine production in iNKT cells between SAH and ChA patients, with an increased frequency of iNKT cells in SAH patients associated with low IL-22 production and a decreased frequency in ChA patients associated with high IFNγ production.
MAIT cells but not CD8+CD161hiVα7.2− cells are significantly reduced in peripheral blood of ALD patients.
Although, MAIT cells (CD8+CD161hiVα7.2+) represent the most abundant population of CD8+CD161hi T cells, there is also a population of CD8+CD161hi T cells that are Vα7.2− and share innate-like characteristics with MAIT cells (Fergusson et al., 2014). Thus, we investigated the frequency of MAIT and CD8+CD161hiVα7.2− cells in PBMC from SAH (n= 14) and ChA (n= 6) patients and compared these to that in healthy controls (n= 20) (Fig. 5A). The numbers of MAIT and CD8+CD161hiVα7.2− cells were calculated using the percentages obtained from the gating strategy in total live cells. Notably, MAIT cells were significantly reduced in SAH in comparison to controls (1.6% ± 0.3 vs. 12.9% ± 2.6 and 1.9 × 104 ± 4.5 × 103 vs. 2.4 × 105 ± 4.9 × 104) (Fig. 5B) as recently shown in ARC and SAH patients (Hegde et al., 2018, Riva et al., 2018). The frequency (3.6% ± 1) and numbers (8.3 × 104 ± 3.4 × 104) of MAIT cells were reduced in ChA patients compared with controls, but showed no significant difference between groups. In contrast, CD8+CD161hiVα7.2− cells were significantly increased in SAH patients in comparison to ChA patients (5.3% ± 1 vs. 0.9% ± 0.1 and 5.5 × 104 ± 1.1 × 104 vs. 1.9 × 104 ± 5.6 × 103), but significantly reduced in ChA patients compared with controls (3.3% ± 0.5 and 5.6 × 104 ± 7.1 × 103) (Fig. 5B). When the proportion of Vα7.2− versus Vα7.2+ cells within the CD8+CD161hi population was compared in the groups, we found that SAH patients have significantly higher frequency of Vα7.2− cells than Vα7.2+ cells (72.2% ± 6.6 vs. 27.6% ± 6.6) while in controls the frequency of Vα7.2+ cells was significantly higher than Vα7.2− cells (69.1% ± 5.1 vs. 30.9% ± 5.0) (Fig. 5C). In addition, ChA patients show a trend comparable to that in controls (66.9% ± 10.6 Vα7.2+ vs. 33.1% ± 10.6 Vα7.2−) (Fig. 5C). These results indicate that MAIT cells were depleted in peripheral blood of all ALD patients while CD8+CD161hiVα7.2− cells were increased in SAH patients but depleted in ChA patients, suggesting that their frequency could potentially distinguishes SAH patients from ChA patients.
Figure 5. MAIT cells but not CD8+CD161hiVα7.2− cells are significantly reduced in peripheral blood of ALD patients.
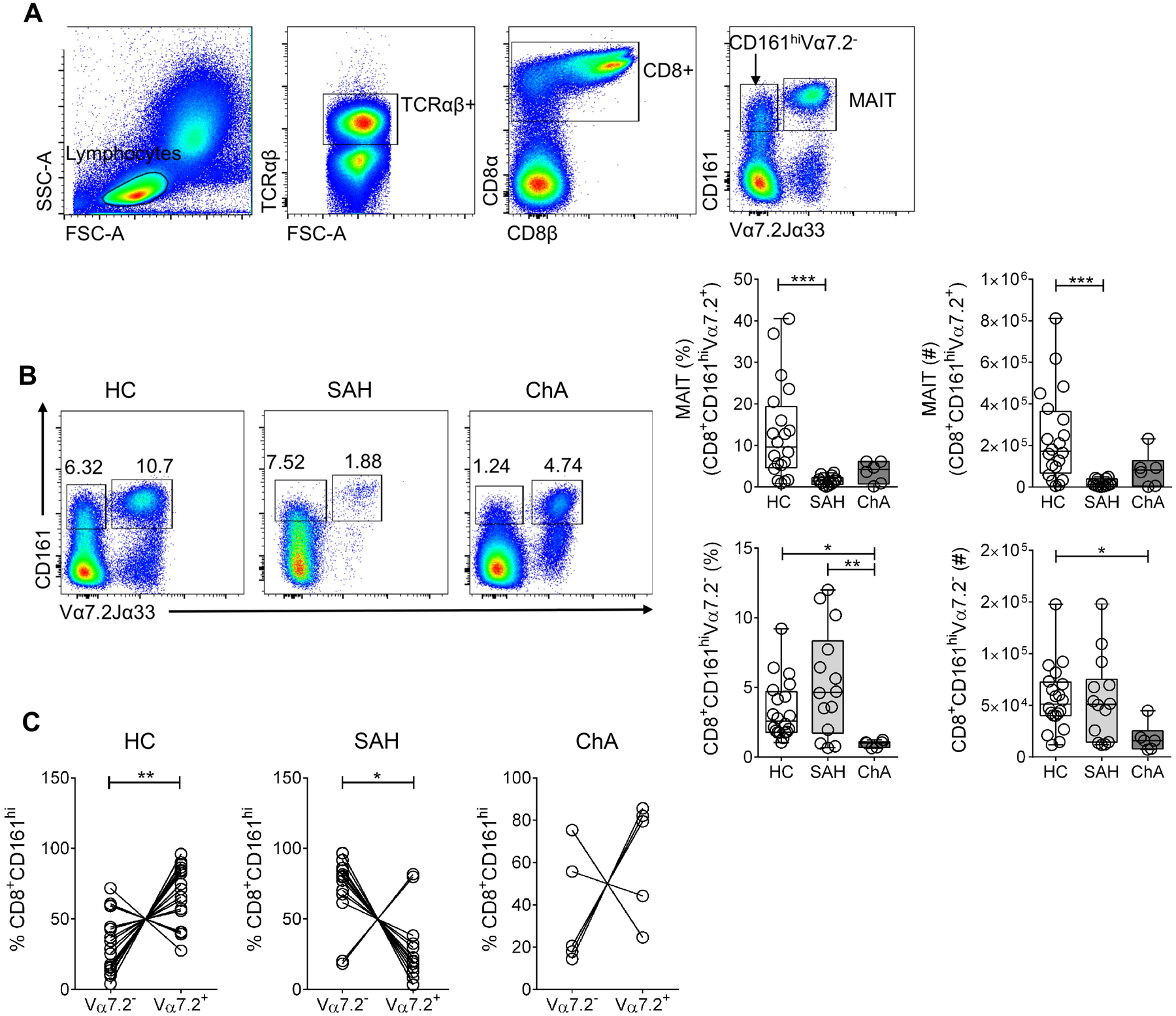
(A) Gating strategy to identify MAIT (CD8+CD161hiTCRVα7.2/Jα33+) and CD8+CD161hiVα7.2− in human PBMC. (B) Representative dot plot and summary data of percentage and numbers of MAIT and CD8+CD161hiVα7.2− cells in PBMC from SAH (n= 14) and ChA (n= 6) patients compared with HC (n= 20). Numbers on dot plots indicate the percentage of each population following gating strategy as in (A). *p≤ 0.05, **p≤ 0.01, ***p≤ 0.001, Kruskal-Wallis test with Dunn’s multiple comparisons test. (C) Proportion of Vα7.2+ and Vα7.2− cells within the CD8+CD161hi gate in SAH (n= 14) and ChA (n= 6) patients and HC (n= 18). *p≤ 0.05, **p≤ 0.01, Wilcoxon matched-pairs signed rank test. All data are presented as mean ± SEM. MAIT, mucosa-associated invariant T cells; ALD, alcohol-related liver disease; HC, healthy controls; SAH, severe alcoholic hepatitis; ChA, chronic alcoholics.
MAIT and CD8+CD161hiVα7.2− cells have different cytokine profile in SAH and ChA patients
Next, we investigated the cytokine profile of MAIT and CD8+CD161hiVα7.2− cells by measuring IFNγ, IL-17A and TNFα production in the absence of any ex vivo stimulation. MAIT cells from SAH patients significantly produced more IFNγ and TNFα than those from controls (IFNγ: 1152 MFI ± 115 vs. 665 MFI ± 99.6; TNFα: 476 MFI ± 63 vs. 194 MFI ± 28) (Fig. 6A). We found a significant positive correlation between IFNγ responses and frequency of MAIT cells in SAH patients (p= 0.0288 by Spearman correlation test, Fig. 6B). MAIT cells from ChA patients significantly produced more IL-17 than those from control (249 MFI ± 41 vs. 97 MFI ± 25) and SAH patients (114 MFI ± 15) (Fig. 6A).
Figure 6. MAIT and CD8+CD161hiVα7.2− cells have different cytokine profile in SAH and ChA patients.
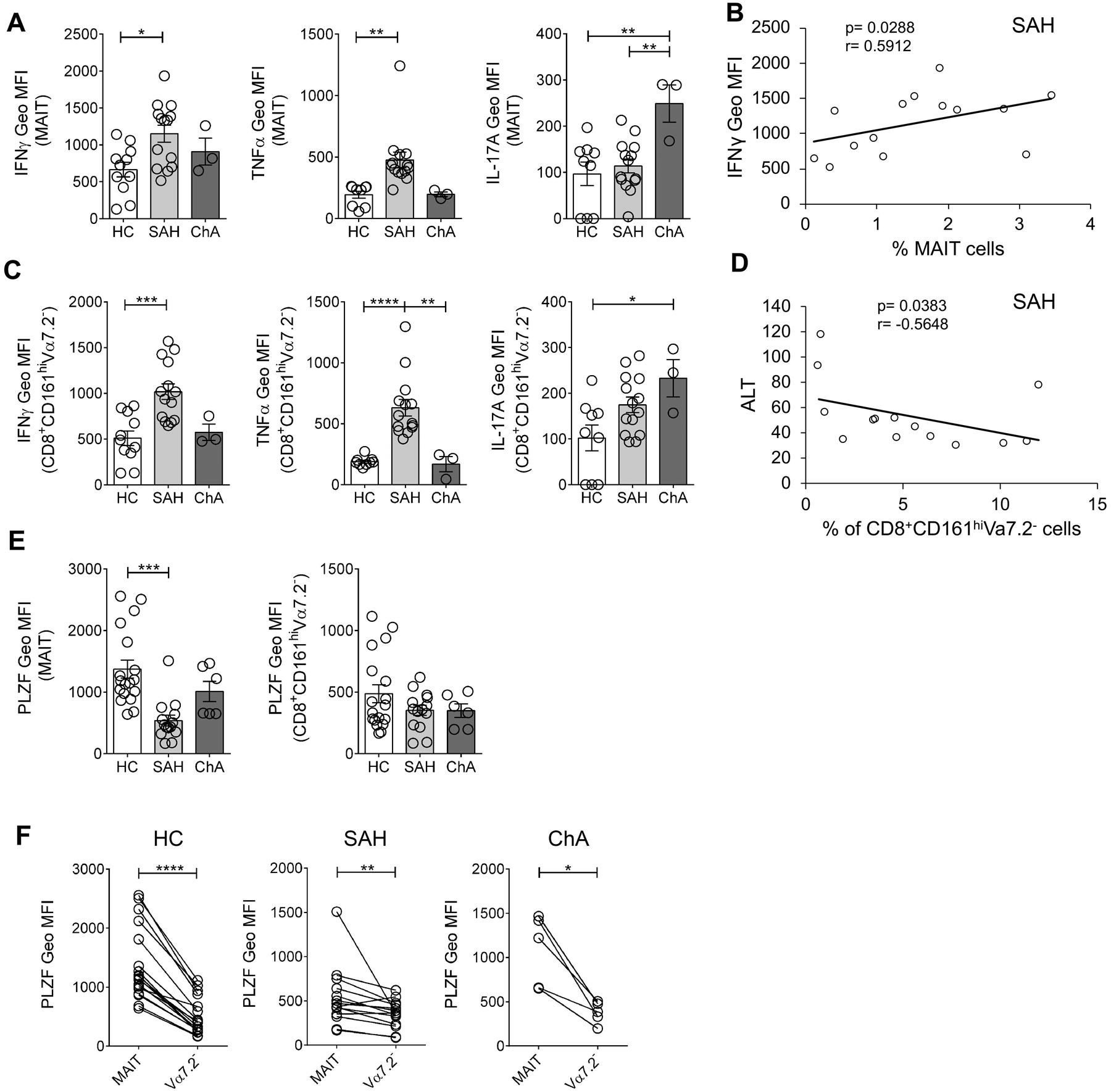
(A) IFNγ, TNFα and IL-17A production, expressed as Geo MFI values, by non-stimulated MAIT cells from SAH (n= 14) and ChA (n= 3) patients compared with HC (n= 9–11). *p≤ 0.05, **p≤ 0.01, One way ANOVA with Bonferroni’s multiple comparisons test. (B) Correlation between blood MAIT cells frequency and IFNγ production in SAH patients (Spearman correlation test). (C) IFNγ, TNFα and IL-17A production, expressed as Geo MFI values, by non-stimulated CD8+CD161hiVα7.2− cells from SAH (n= 14) and ChA (n= 3) patients compared with HC (n= 9–11). *p≤ 0.05, **p≤ 0.01, ***p≤ 0.001, ****p≤ 0.0001, One way ANOVA with Bonferroni’s multiple comparisons test. (D) Correlation between blood CD8+CD161hiVα7.2− cells frequency and ALT levels in SAH patients (Spearman correlation test). (E) PLZF expression, expressed as Geo MFI values, by MAIT cells (left) and CD8+CD161hiVα7.2− cells (right) in PBMC from SAH (n= 14) and ChA (n= 6) patients compared with HC (n= 18). ***p≤ 0.001, One way ANOVA with Bonferroni’s multiple comparisons test. (F) Comparison of PLZF expression between MAIT and CD8+CD161hiVα7.2− cells in PBMC from HC (n= 18), SAH (n= 14) and ChA (n= 6) patients. *p≤ 0.05, **p≤ 0.01, ****p≤ 0.0001, Wilcoxon matched-pairs signed rank test. All data are presented as mean ± SEM. MAIT, mucosa-associated invariant T cells; HC, healthy controls; SAH, severe alcoholic hepatitis; ChA, chronic alcoholics; ALT, alanine transaminase; PLZF, promyelocytic leukaemia zinc finger protein.
Interestingly, CD8+CD161hiVα7.2− cells exhibited a cytokine profile very similar to MAIT cells. Therefore, CD8+CD161hiVα7.2− cells from SAH patients significantly produced more IFNγ (1018 MFI ± 86.1 vs. 510 MFI ± 79.3) and more TNFα (631 MFI ± 68 vs. 189 MFI ± 12.6) than controls and more TNFα than ChA patients (169 MFI ± 62.3) (Fig. 6C). While IL-17 production by CD8+CD161hiVα7.2− cells was significantly increased in ChA patients compared with controls (233 MFI ± 41 vs. 102 MFI ± 28) (Fig. 6C).
We also investigated whether clinical parameters (see Table 1) may associate with blood MAIT and CD8+CD161hiVα7.2− cells frequencies and we found a significant negative correlation between CD8+CD161hiVα7.2− cells frequency and levels of ALT in SAH patients (p= 0.0383 by Spearman correlation test, Fig. 6D).
Since innate-like features and functions of unconventional T cells are driven by the expression of the transcription factor PLZF (Savage et al., 2008, Rahimpour et al., 2015), we determined its expression in MAIT and CD8+CD161hiVα7.2− cells. PLZF expression in MAIT cells was significantly reduced in SAH patients compared with controls (538 MFI ± 89.3 vs. 1373 MFI ± 145) (Fig. 6E) as shown before in patients with ALD (Riva et al., 2018), but also lower than in ChA patients (1011 MFI ± 163). In contrast, CD8+CD161hiVα7.2− cells from ALD patients and controls have similar levels of PLZF expression (Fig. 6E). However, in all groups, MAIT cells significantly expressed more PLZF than CD8+CD161hiVα7.2− cells (Fig. 6F).
Overall, these data suggest that blood MAIT and CD8+CD161hiVα7.2− cells displayed a similar cytokine production profile in ALD patients. However, their cytokine profile differ between SAH and ChA patients: high production of IFNγ and TNFα in SAH patients and high production of IL-17 in ChA patients. Although both MAIT and CD8+CD161hiVα7.2− cells have a similar cytokine profile in SAH patients, CD8+CD161hiVα7.2− cells significantly produced more IL-17 and TNFα than MAIT cells while, in controls, CD8+CD161hiVα7.2− cells significantly produced more Granzyme B and MAIT cells produced more IFNγ (data not shown).
Discussion
The role of innate-like unconventional T cell populations in the progression of ALD in both mice and humans is incompletely understood. In this study, we have found that three different unconventional T cells, namely iNKT, MAIT and CD8+CD161hiVα7.2− cells, are differentially activated and have distinct proinflammatory cytokine profile in peripheral blood of SAH and ChA patients. Furthermore, consistent with their activation in human ALD, iNKT cells are also activated and secrete pro-inflammatory cytokines in the iG model of chronic ASH, similar to that shown earlier in acute oral alcohol feeding models.
We have previously shown in a chronic-plus-binge ethanol feeding murine model that iNKT, but not type II NKT cells, are chronically activated promoting liver inflammation and macrophage and neutrophil infiltration and inducing early alcoholic liver injury (Maricic et al., 2015, Mathews et al., 2016). Also, in the absence of iNKT cells (Jα18−/− mice) or following their inactivation or inhibition, CD11b+Gr-1highLy6G+ neutrophils do not accumulate in the liver of alcohol-fed mice and they are protected from ALD (Kumar, 2013, Maricic et al., 2015, Marrero et al., 2015, Mathews et al., 2016). Consistent with the chronic-plus-binge feeding model, we have found in the iG model of chronic ASH that IFNγ+ iNKT cells are significantly increased in HCFD+iG Alc-fed mice. The tetramer staining of iNKT cells during chronic stimulation induces down-modulation of the TCR (Maricic et al., 2018, Lee et al., 2019), and, accordingly, tetramer+ cells are decreased in this model. Additionally, up-regulation of both Fas and FasL in iNKT cells in this model indicate chronic activation that may also lead to their elimination by apoptosis. Furthermore, FasL-expressing iNKT cells can also directly kill Fas-expressing hepatocytes sensitized by alcohol-consumption leading to more liver damage as shown before (Lee et al., 2019). Consistent with the role of Fas-FasL interaction, Fas-deficient mice have been shown to be significantly less susceptible to ALD (Minagawa et al., 2004). These results indicate that iNKT cells are activated and produced IFNγ and become FasL+ in HCFD+iG Alc-fed mice, therefore, they can contribute to liver damage by two mechanism: (i) directly killing hepatocytes using FasL/Fas pathway or (ii) indirectly by promoting the recruitment of macrophages and neutrophils via IFNγ production as shown earlier (Maricic et al., 2015, Mathews et al., 2016).
In this study, we found that iNKT cells are significantly increased in peripheral blood of SAH patients whereas ChA patients had reduced frequency of iNKT as recently reported in ARC patients (Hegde et al., 2018). Previous studies have found no differences in iNKT cells frequency among SAH patients, stable cirrhosis patients, heavy drinking controls and healthy controls (Stoy et al., 2015, Li et al., 2019). Despite the reduced frequencies of iNKT cells in peripheral blood, they are important immunoregulatory cells that play an important role in ameliorating or exacerbating a variety of diseases from autoimmunity to cancer (Godfrey et al., 2010, Tupin et al., 2007, Van Kaer et al., 2011).
Chronic alcohol consumption causes enteric dysbiosis and further trigger gut barrier dysfunction, promoting translocation of bacterial products into liver where they exhibit toxic effects and immune activation (Szabo and Bala, 2010, Chen et al., 2015b, Chen et al., 2015a, Samuelson et al., 2019). Accordingly, gut barrier defects have been shown in patients with ALD (Rao, 2009, Leclercq et al., 2014). Also, it has been shown that gut microbiota derived from chronic alcoholic hepatitis patients can induce liver disease in mice after their transplantation into recipients (Llopis et al., 2016) and that fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice (Ferrere et al., 2017). Thus, in SAH patients, the loss of gut integrity and heavy exposure to gut bacteria, bacterial antigens and metabolites may result in systemic increase of iNKT cells in blood while, in ChA patients, chronic alcohol consumption may results in the relocation of iNKT cells into liver where these cells are involved in promoting liver damage (Lee et al., 2019), resulting in a reduced frequency in peripheral blood.
Consistent with murine data, IFNγ production as well as T-bet expression by iNKT cells is significantly increased while IL-4 production is significantly reduced in peripheral blood of ChA patients in comparison to that in healthy donors. Furthermore, a significant increase in IL-22 production by iNKT cells is also found in ChA patients. Despite their increased frequency in SAH patients, IFNγ and IL-22 production by iNKT cells is significantly reduced. It is possible that iNKT cells are chronically activated in vivo in SAH patients and consequently, they become hyporesponsive as shown before in NASH patients (Maricic et al., 2018). Interestingly, increased frequency of IL-22-secreting cells has been associated with improved prognoses in SAH patients (Ki et al., 2010, Stoy et al., 2015, Gao and Shah, 2015). Furthermore, endogenous IL-22 was not detected in the liver of patients with alcoholic hepatitis (Ki et al., 2010). Collectively, iNKT cells from ALD patients are activated and are proinflammatory, but exhibit different cytokine production profile in SAH vs. ChA patients.
Recent studies have shown that blood MAIT cells are reduced, activated and functionally defective in patients with alcohol-related liver cirrhosis (ARC) or SAH (Hegde et al., 2018, Riva et al., 2018, Li et al., 2019), while, in the liver, MAIT cells accumulated and are functional. Consistently, we found that blood MAIT cells are significantly depleted in both SAH and ChA patients. The selective depletion of blood MAIT cells suggest an association with disease severity or higher exposition to bacterial antigens due to increased intestinal permeability as shown before using fecal extracts from ALD patients (Riva et al., 2018). However, CD8+CD161hiVα7.2− cells are increased in SAH patients, but decreased in ChA patients. To our knowledge this is the first study that has investigated CD8+CD161hiVα7.2− cells in peripheral blood of ALD patients. Notably, a recent study has shown the presence of Vα7.2− cells in the sinusoidal space of human cirrhotic livers (Hegde et al., 2018). Additionally, MAIT cells accumulated within the fibrotic septa are able to stimulate the mitogenic and proinflammatory properties of hepatic myofibroblasts and macrophages promoting the fibrogenic process (Hegde et al., 2018). Also, the recruitment of MAIT cells in the periportal regions of liver of ALD patients as well as increased MR1 hepatic expression suggest that MAIT cells can mediate liver damage by killing bacterially infected MR1+ cells (Riva et al., 2018). Whether MAIT cells or CD8+CD161hiVα7.2− cells migrate to the target tissue or are eliminated following apoptosis remain to be elucidated. Based on our data it is not possible to determine whether the recruitment of these cells is exclusive for ALD patients. However, our results suggest that the frequency of CD8+CD161hiVα7.2− cells in peripheral blood can discriminate SAH patients from ChA patients. Though, additional studies with large number of cohorts are needed to verify this.
Notably, functional analysis of MAIT and CD8+CD161hiVα7.2− cells in SAH and ChA patients reveal that both populations have a similar pro-inflammatory cytokine profile when compared to controls. Thus, MAIT and CD8+CD161hiVα7.2− cells produce more IFNγ and TNFα in SAH patients, but more IL-17 in ChA patients. Since IL-17 production by MAIT cells has been implicated in chronic inflammatory diseases (Gracey et al., 2016, Willing et al., 2018), our results suggest that MAIT cells from SAH patients are less functional than those in ChA patients and this is consistent with reduced PLZF expression in MAIT cells from SAH patients. Accordingly, CD8+CD161hiVα7.2− cells produce more TNFα and IL-17 than MAIT cells in SAH patients, but similar levels in ChA patients and healthy controls. These results indicate that MAIT and CD8+CD161hiVα7.2− cells are activated and may play different roles in the pathogenesis of ALD.
In conclusion, our results show that the three populations of unconventional T cells, iNKT, MAIT and CD8+CD161hiVα7.2− cells, are activated and are pro-inflammatory in peripheral blood of SAH and ChA patients, suggesting their involvement in ALD. It is likely that their differential frequency or cytokine production profile in peripheral blood may also reflect different stages of alcoholic liver disease. Since these cells are proinflammatory and potentially pathogenic, selective strategies resulting in their inhibition may protect the liver from alcohol-induced injury. Thus, understanding the functional diversity within the unconventional T cells underlying liver damage in ALD is essential for their manipulation leading to the potential novel approaches for disease intervention.
Sources of support:
This work was supported by NIAAA AA020864 to V.K.; NIDDK DK120515 to B.S.; NIAAA U01AA021857 to Z.X.L.; Southern California Research Center for ALPD & Cirrhosis P50AA011999 to H.T.; NIAAA U01AA021884 and U01AA021886 to T.M.
Footnotes
Conflict of Interest: V.K. is a scientific cofounder of and a consultant for GRI-Bio, La Jolla, CA. B.S. is a consulting for Ferring Research Institute.
References
- ARRENBERG P, HALDER R, DAI Y, MARICIC I & KUMAR V 2010. Oligoclonality and innate-like features in the TCR repertoire of type II NKT cells reactive to a beta-linked self-glycolipid. Proc Natl Acad Sci U S A, 107, 10984–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARRENBERG P, MARICIC I & KUMAR V 2011. Sulfatide-mediated activation of type II natural killer T cells prevents hepatic ischemic reperfusion injury in mice. Gastroenterology, 140, 646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANDYOPADHYAY K, MARRERO I & KUMAR V 2016. NKT cell subsets as key participants in liver physiology and pathology. Cell Mol Immunol, 13, 337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTOLA A, PARK O & GAO B 2013. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Hepatology, 58, 1814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN P, MIYAMOTO Y, MAZAGOVA M, LEE KC, ECKMANN L & SCHNABL B 2015a. Microbiota Protects Mice Against Acute Alcohol-Induced Liver Injury. Alcohol Clin Exp Res, 39, 2313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN P, STARKEL P, TURNER JR, HO SB & SCHNABL B 2015b. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology, 61, 883–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLMENERO J, BATALLER R, SANCHO-BRU P, BELLOT P, MIQUEL R, MORENO M, JARES P, BOSCH J, ARROYO V, CABALLERIA J & GINES P 2007. Hepatic expression of candidate genes in patients with alcoholic hepatitis: correlation with disease severity. Gastroenterology, 132, 687–97. [DOI] [PubMed] [Google Scholar]
- CRISPE IN 2011. Liver antigen-presenting cells. J Hepatol, 54, 357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUI K, YAN G, XU C, CHEN Y, WANG J, ZHOU R, BAI L, LIAN Z, WEI H, SUN R & TIAN Z 2015. Invariant NKT cells promote alcohol-induced steatohepatitis through interleukin-1beta in mice. J Hepatol, 62, 1311–8. [DOI] [PubMed] [Google Scholar]
- DUNN W, JAMIL LH, BROWN LS, WIESNER RH, KIM WR, MENON KV, MALINCHOC M, KAMATH PS & SHAH V 2005. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology, 41, 353–8. [DOI] [PubMed] [Google Scholar]
- DUSSEAUX M, MARTIN E, SERRIARI N, PEGUILLET I, PREMEL V, LOUIS D, MILDER M, LE BOURHIS L, SOUDAIS C, TREINER E & LANTZ O 2011. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood, 117, 1250–9. [DOI] [PubMed] [Google Scholar]
- EXLEY M, GARCIA J, BALK SP & PORCELLI S 1997. Requirements for CD1d recognition by human invariant Valpha24+ CD4-CD8- T cells. J Exp Med, 186, 109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EXLEY MA, BIGLEY NJ, CHENG O, SHAULOV A, TAHIR SM, CARTER QL, GARCIA J, WANG C, PATTEN K, STILLS HF, ALT FW, SNAPPER SB & BALK SP 2003. Innate immune response to encephalomyocarditis virus infection mediated by CD1d. Immunology, 110, 519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERGUSSON JR, SMITH KE, FLEMING VM, RAJORIYA N, NEWELL EW, SIMMONS R, MARCHI E, BJORKANDER S, KANG YH, SWADLING L, KURIOKA A, SAHGAL N, LOCKSTONE H, BABAN D, FREEMAN GJ, SVERREMARK-EKSTROM E, DAVIS MM, DAVENPORT MP, VENTURI V, USSHER JE, WILLBERG CB & KLENERMAN P 2014. CD161 defines a transcriptional and functional phenotype across distinct human T cell lineages. Cell Rep, 9, 1075–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRERE G, WRZOSEK L, CAILLEUX F, TURPIN W, PUCHOIS V, SPATZ M, CIOCAN D, RAINTEAU D, HUMBERT L, HUGOT C, GAUDIN F, NOORDINE ML, ROBERT V, BERREBI D, THOMAS M, NAVEAU S, PERLEMUTER G & CASSARD AM 2017. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J Hepatol, 66, 806–815. [DOI] [PubMed] [Google Scholar]
- GAO B & SHAH VH 2015. Combination therapy: New hope for alcoholic hepatitis? Clin Res Hepatol Gastroenterol, 39 Suppl 1, S7–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA-SAENZ-DE-SICILIA M, DUVOOR C, ALTAMIRANO J, CHAVEZ-ARAUJO R, PRADO V, DE LOURDES CANDOLO-MARTINELLI A, HOLANDA-ALMEIDA P, BECERRA-MARTINS-DE-OLIVEIRA B, FERNANDEZ-DE-ALMEIDA S, BATALLER R, CABALLERIA J & DUARTE-ROJO A 2017. A Day-4 Lille Model Predicts Response to Corticosteroids and Mortality in Severe Alcoholic Hepatitis. Am J Gastroenterol, 112, 306–315. [DOI] [PubMed] [Google Scholar]
- GODFREY DI, LE NOURS J, ANDREWS DM, ULDRICH AP & ROSSJOHN J 2018. Unconventional T Cell Targets for Cancer Immunotherapy. Immunity, 48, 453–473. [DOI] [PubMed] [Google Scholar]
- GODFREY DI, STANKOVIC S & BAXTER AG 2010. Raising the NKT cell family. Nat Immunol, 11, 197–206. [DOI] [PubMed] [Google Scholar]
- GRACEY E, QAIYUM Z, ALMAGHLOUTH I, LAWSON D, KARKI S, AVVARU N, ZHANG Z, YAO Y, RANGANATHAN V, BAGLAENKO Y & INMAN RD 2016. IL-7 primes IL-17 in mucosal-associated invariant T (MAIT) cells, which contribute to the Th17-axis in ankylosing spondylitis. Ann Rheum Dis, 75, 2124–2132. [DOI] [PubMed] [Google Scholar]
- HALDER RC, AGUILERA C, MARICIC I & KUMAR V 2007. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest, 117, 2302–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEGDE P, WEISS E, PARADIS V, WAN J, MABIRE M, SUKRITI S, RAUTOU PE, ALBUQUERQUE M, PICQ O, GUPTA AC, FERRERE G, GILGENKRANTZ H, KIAF B, TOUBAL A, BEAUDOIN L, LETTERON P, MOREAU R, LEHUEN A & LOTERSZTAJN S 2018. Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nat Commun, 9, 2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAHNG A, MARICIC I, AGUILERA C, CARDELL S, HALDER RC & KUMAR V 2004. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med, 199, 947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEFFERY HC, VAN WILGENBURG B, KURIOKA A, PAREKH K, STIRLING K, ROBERTS S, DUTTON EE, HUNTER S, GEH D, BRAITCH MK, RAJANAYAGAM J, IQBAL T, PINKNEY T, BROWN R, WITHERS DR, ADAMS DH, KLENERMAN P & OO YH 2016. Biliary epithelium and liver B cells exposed to bacteria activate intrahepatic MAIT cells through MR1. J Hepatol, 64, 1118–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMATH PS, WIESNER RH, MALINCHOC M, KREMERS W, THERNEAU TM, KOSBERG CL, D’AMICO G, DICKSON ER & KIM WR 2001. A model to predict survival in patients with end-stage liver disease. Hepatology, 33, 464–70. [DOI] [PubMed] [Google Scholar]
- KHANOVA E, WU R, WANG W, YAN R, CHEN Y, FRENCH SW, LLORENTE C, PAN SQ, YANG Q, LI Y, LAZARO R, ANSONG C, SMITH RD, BATALLER R, MORGAN T, SCHNABL B & TSUKAMOTO H 2018. Pyroptosis by caspase11/4-gasdermin-D pathway in alcoholic hepatitis in mice and patients. Hepatology, 67, 1737–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KI SH, PARK O, ZHENG M, MORALES-IBANEZ O, KOLLS JK, BATALLER R & GAO B 2010. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology, 52, 1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KJER-NIELSEN L, PATEL O, CORBETT AJ, LE NOURS J, MEEHAN B, LIU L, BHATI M, CHEN Z, KOSTENKO L, REANTRAGOON R, WILLIAMSON NA, PURCELL AW, DUDEK NL, MCCONVILLE MJ, O’HAIR RA, KHAIRALLAH GN, GODFREY DI, FAIRLIE DP, ROSSJOHN J & MCCLUSKEY J 2012. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature, 491, 717–23. [DOI] [PubMed] [Google Scholar]
- KUMAR V 2013. NKT-cell subsets: promoters and protectors in inflammatory liver disease. J Hepatol, 59, 618–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURIOKA A, USSHER JE, COSGROVE C, CLOUGH C, FERGUSSON JR, SMITH K, KANG YH, WALKER LJ, HANSEN TH, WILLBERG CB & KLENERMAN P 2015. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol, 8, 429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURIOKA A, WALKER LJ, KLENERMAN P & WILLBERG CB 2016. MAIT cells: new guardians of the liver. Clin Transl Immunology, 5, e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZARO R, WU R, LEE S, ZHU NL, CHEN CL, FRENCH SW, XU J, MACHIDA K & TSUKAMOTO H 2015. Osteopontin deficiency does not prevent but promotes alcoholic neutrophilic hepatitis in mice. Hepatology, 61, 129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LECLERCQ S, MATAMOROS S, CANI PD, NEYRINCK AM, JAMAR F, STARKEL P, WINDEY K, TREMAROLI V, BACKHED F, VERBEKE K, DE TIMARY P & DELZENNE NM 2014. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A, 111, E4485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE KC, CHEN P, MARICIC I, INAMINE T, HU J, GONG S, SUN JC, DASGUPTA S, LIN HC, LIN YT, LOOMBA R, STARKEL P, KUMAR V & SCHNABL B 2019. Intestinal iNKT cells migrate to liver and contribute to hepatocyte apoptosis during alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol, 316, G585–G597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEITE-DE-MORAES MC, HERBELIN A, GOUARIN C, KOEZUKA Y, SCHNEIDER E & DY M 2000. Fas/Fas ligand interactions promote activation-induced cell death of NK T lymphocytes. J Immunol, 165, 4367–71. [DOI] [PubMed] [Google Scholar]
- LI W, LIN EL, LIANGPUNSAKUL S, LAN J, CHALASANI S, RANE S, PURI P, KAMATH PS, SANYAL AJ, SHAH VH, RADAEVA S, CRABB DW, CHALASANI N & YU Q 2019. Alcohol Abstinence Does Not Fully Reverse Abnormalities of Mucosal-Associated Invariant T Cells in the Blood of Patients With Alcoholic Hepatitis. Clin Transl Gastroenterol, 10, e00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLOPIS M, CASSARD AM, WRZOSEK L, BOSCHAT L, BRUNEAU A, FERRERE G, PUCHOIS V, MARTIN JC, LEPAGE P, LE ROY T, LEFEVRE L, LANGELIER B, CAILLEUX F, GONZALEZ-CASTRO AM, RABOT S, GAUDIN F, AGOSTINI H, PREVOT S, BERREBI D, CIOCAN D, JOUSSE C, NAVEAU S, GERARD P & PERLEMUTER G 2016. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut, 65, 830–9. [DOI] [PubMed] [Google Scholar]
- LOUVET A 2017. Circulating levels of bacterial DNA and risk of infections in severe alcoholic hepatitis. Clin Res Hepatol Gastroenterol, 41, 354–356. [DOI] [PubMed] [Google Scholar]
- LOUVET A, WARTEL F, CASTEL H, DHARANCY S, HOLLEBECQUE A, CANVA-DELCAMBRE V, DELTENRE P & MATHURIN P 2009. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology, 137, 541–8. [DOI] [PubMed] [Google Scholar]
- MADDREY WC, BOITNOTT JK, BEDINE MS, WEBER FL JR., MEZEY E & WHITE RI JR. 1978. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology, 75, 193–9. [PubMed] [Google Scholar]
- MARICIC I, MARRERO I, EGUCHI A, NAKAMURA R, JOHNSON CD, DASGUPTA S, HERNANDEZ CD, NGUYEN PS, SWAFFORD AD, KNIGHT R, FELDSTEIN AE, LOOMBA R & KUMAR V 2018. Differential Activation of Hepatic Invariant NKT Cell Subsets Plays a Key Role in Progression of Nonalcoholic Steatohepatitis. J Immunol, 201, 3017–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARICIC I, SHENG H, MARRERO I, SEKI E, KISSELEVA T, CHATURVEDI S, MOLLE N, MATHEWS SA, GAO B & KUMAR V 2015. Inhibition of type I natural killer T cells by retinoids or following sulfatide-mediated activation of type II natural killer T cells attenuates alcoholic liver disease in mice. Hepatology, 61, 1357–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARRERO I, WARE R & KUMAR V 2015. Type II NKT Cells in Inflammation, Autoimmunity, Microbial Immunity, and Cancer. Front Immunol, 6, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN E, TREINER E, DUBAN L, GUERRI L, LAUDE H, TOLY C, PREMEL V, DEVYS A, MOURA IC, TILLOY F, CHERIF S, VERA G, LATOUR S, SOUDAIS C & LANTZ O 2009. Stepwise development of MAIT cells in mouse and human. PLoS Biol, 7, e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHEWS S, FENG D, MARICIC I, JU C, KUMAR V & GAO B 2016. Invariant natural killer T cells contribute to chronic-plus-binge ethanol-mediated liver injury by promoting hepatic neutrophil infiltration. Cell Mol Immunol, 13, 206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINAGAWA M, DENG Q, LIU ZX, TSUKAMOTO H & DENNERT G 2004. Activated natural killer T cells induce liver injury by Fas and tumor necrosis factor-alpha during alcohol consumption. Gastroenterology, 126, 1387–99. [DOI] [PubMed] [Google Scholar]
- MONTOYA CJ, JIE HB, AL-HARTHI L, MULDER C, PATINO PJ, RUGELES MT, KRIEG AM, LANDAY AL & WILSON SB 2006. Activation of plasmacytoid dendritic cells with TLR9 agonists initiates invariant NKT cell-mediated cross-talk with myeloid dendritic cells. J Immunol, 177, 1028–39. [DOI] [PubMed] [Google Scholar]
- PATEL O, PELLICCI DG, GRAS S, SANDOVAL-ROMERO ML, ULDRICH AP, MALLEVAEY T, CLARKE AJ, LE NOURS J, THEODOSSIS A, CARDELL SL, GAPIN L, GODFREY DI & ROSSJOHN J 2012. Recognition of CD1d-sulfatide mediated by a type II natural killer T cell antigen receptor. Nat Immunol, 13, 857–63. [DOI] [PubMed] [Google Scholar]
- RAHIMPOUR A, KOAY HF, ENDERS A, CLANCHY R, ECKLE SB, MEEHAN B, CHEN Z, WHITTLE B, LIU L, FAIRLIE DP, GOODNOW CC, MCCLUSKEY J, ROSSJOHN J, ULDRICH AP, PELLICCI DG & GODFREY DI 2015. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J Exp Med, 212, 1095–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAO R 2009. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology, 50, 638–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIVA A, PATEL V, KURIOKA A, JEFFERY HC, WRIGHT G, TARFF S, SHAWCROSS D, RYAN JM, EVANS A, AZARIAN S, BAJAJ JS, FAGAN A, PATEL V, MEHTA K, LOPEZ C, SIMONOVA M, KATZAROV K, HADZHIOLOVA T, PAVLOVA S, WENDON JA, OO YH, KLENERMAN P, WILLIAMS R & CHOKSHI S 2018. Mucosa-associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease. Gut, 67, 918–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMUELSON DR, GU M, SHELLITO JE, MOLINA PE, TAYLOR CM, LUO M & WELSH DA 2019. Intestinal microbial products from alcohol-fed mice contribute to intestinal permeability and peripheral immune activation. Alcohol Clin Exp Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAVAGE AK, CONSTANTINIDES MG, HAN J, PICARD D, MARTIN E, LI B, LANTZ O & BENDELAC A 2008. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity, 29, 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEITZ HK, BATALLER R, CORTEZ-PINTO H, GAO B, GUAL A, LACKNER C, MATHURIN P, MUELLER S, SZABO G & TSUKAMOTO H 2018. Alcoholic liver disease. Nat Rev Dis Primers, 4, 16. [DOI] [PubMed] [Google Scholar]
- SERSTE T, CORNILLIE A, NJIMI H, PAVESI M, ARROYO V, PUTIGNANO A, WEICHSELBAUM L, DELTENRE P, DEGRE D, TREPO E, MORENO C & GUSTOT T 2018. The prognostic value of acute-on-chronic liver failure during the course of severe alcoholic hepatitis. J Hepatol, 69, 318–324. [DOI] [PubMed] [Google Scholar]
- SHAULOV A, YUE S, WANG R, JOYCE RM, BALK SP, KIM HT, AVIGAN DE, UHL L, SACKSTEIN R & EXLEY MA 2008. Peripheral blood progenitor cell product contains Th1-biased noninvariant CD1d-reactive natural killer T cells: implications for posttransplant survival. Exp Hematol, 36, 464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGAL AK, BATALLER R, AHN J, KAMATH PS & SHAH VH 2018. ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol, 113, 175–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOY S, DIGE A, SANDAHL TD, LAURSEN TL, BUUS C, HOKLAND M & VILSTRUP H 2015. Cytotoxic T lymphocytes and natural killer cells display impaired cytotoxic functions and reduced activation in patients with alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol, 308, G269–76. [DOI] [PubMed] [Google Scholar]
- SZABO G & BALA S 2010. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol, 16, 1321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUOMISTO S, PESSI T, COLLIN P, VUENTO R, AITTONIEMI J & KARHUNEN PJ 2014. Changes in gut bacterial populations and their translocation into liver and ascites in alcoholic liver cirrhotics. BMC Gastroenterol, 14, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUPIN E, KINJO Y & KRONENBERG M 2007. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol, 5, 405–17. [DOI] [PubMed] [Google Scholar]
- UENO A, LAZARO R, WANG PY, HIGASHIYAMA R, MACHIDA K & TSUKAMOTO H 2012. Mouse intragastric infusion (iG) model. Nat Protoc, 7, 771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN KAER L, PAREKH VV & WU L 2011. Invariant natural killer T cells: bridging innate and adaptive immunity. Cell Tissue Res, 343, 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER LJ, KANG YH, SMITH MO, THARMALINGHAM H, RAMAMURTHY N, FLEMING VM, SAHGAL N, LESLIE A, OO Y, GEREMIA A, SCRIBA TJ, HANEKOM WA, LAUER GM, LANTZ O, ADAMS DH, POWRIE F, BARNES E & KLENERMAN P 2012. Human MAIT and CD8alphaalpha cells develop from a pool of type-17 precommitted CD8+ T cells. Blood, 119, 422–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLING A, JAGER J, REINHARDT S, KURSAWE N & FRIESE MA 2018. Production of IL-17 by MAIT Cells Is Increased in Multiple Sclerosis and Is Associated with IL-7 Receptor Expression. J Immunol, 200, 974–982. [DOI] [PubMed] [Google Scholar]
- YANG AM, INAMINE T, HOCHRATH K, CHEN P, WANG L, LLORENTE C, BLUEMEL S, HARTMANN P, XU J, KOYAMA Y, KISSELEVA T, TORRALBA MG, MONCERA K, BEERI K, CHEN CS, FREESE K, HELLERBRAND C, LEE SM, HOFFMAN HM, MEHAL WZ, GARCIA-TSAO G, MUTLU EA, KESHAVARZIAN A, BROWN GD, HO SB, BATALLER R, STARKEL P, FOUTS DE & SCHNABL B 2017. Intestinal fungi contribute to development of alcoholic liver disease. J Clin Invest, 127, 2829–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]


