Abstract
Purpose:
We explored the Medicare database (1999–2014) to provide a comprehensive assessment of testosterone therapy (TT) patterns in the older US male population.
Materials and Methods:
We estimated annual age-standardized incidence (new users) and prevalence (existing users) of TT according to demographic characteristics, comorbidities, and potential indications.
Results:
There were 392,698 incident TT users during 88 million person-years. TT users were predominantly younger, white non-Hispanic, and located in South and West US Census regions. On average, TT dramatically increased during 2007–2014 (average annual percent change=15.5%), despite a decrease in 2014. In 2014, the most common recorded potential indications for any TT were hypogonadism (48%), fatigue (18%), erectile dysfunction (15%), depression (4%), and psychosexual dysfunction (1%). Laboratory tests to measure circulating testosterone concentrations for TT were infrequent with 35% having had at least one testosterone test in the 120 days preceding TT, 4% the recommended two pre-TT tests, and 16% at least one pre-TT test and at least one post-TT test.
Conclusions:
TT remains common in the older US male population, despite a recent decrease. Although TT prescriptions are predominantly for hypogonadism, a substantial proportion appear to be for less specific conditions. Testosterone tests amongst men prescribed TT appear to be infrequent.
Keywords: Testosterone, Hypogonadism, Medicare, Trends, Epidemiology
Introduction
Endogenous testosterone is an anabolic steroid hormone essential to several human functions including secondary male characteristics, spermatogenesis, hematopoiesis, sexual function, physical performance, muscle strength, lean body mass and cognition. Hypogonadism is clinically defined as confirmed morning serum testosterone below 300 ng/d due to disorders of the testicles, pituitary gland, or brain. Hypogonadism has been linked with sexual dysfunction, negative mood states, decreased bone density, and body lean mass; however, its association with cardiovascular disease and cancer remains controversial.1–4 Serum testosterone concentrations decline 0.4–2.6% per year of age in men.5, 6 Among men 65 years and older, almost 10–14% have low serum testosterone concentrations indicative of hypogonadism7, and approximately 5% exhibit symptoms associated with hypogonadism.8
In recent years, prescriptions for testosterone therapy (TT) have increased rapidly.9 Diversification of TT to non-injectable forms and extensive direct-to-consumer marketing with increasing off-label use10, 11 have likely contributed to these changes. A study of Truven Health MarketScan commercial claims database12showed that TT use during 2003–2013 increased four-fold (29 to 118/10,000 person-years) among younger men and three-fold (125 to 374/10,000 person-years) among older men.12 The study also found that use of topical gels and transdermal patches reduced during 2003–2013, whereas use of testosterone injections and implantable pellets increased among younger men.12 However, they did not study testosterone use among men aged at 65 years or older, who could have important and different utilization patterns due to age related comorbidities (e.g., hypogonadism, fatigue, erectile dysfunction, and psychosexual dysfunction) and change in insurance reimbursement.13 Moreover, there has been limited study of trends of TT by indication for prescription as well as the recommended tests required to accurately diagnose hypogonadism as the underlying condition.
To address these gaps, we assessed trends in use of TT by demographic characteristics, comorbidities, and potential indications in the older US male population using Medicare data for the period 1999–2014.
Materials and Methods
Data sources
Medicare is a US federal medical insurance program for individuals aged 65 years or older, select disabled adults and patients with end-stage renal disease. This program includes 97% of the US population aged 65 years or older and has three main parts: Part A covers inpatient stays, Part B covers outpatient health care, and Part D—added in 2006—covers prescription drugs. We constructed an open cohort consisting of all male Medicare beneficiaries who were 65 years or older, had procedure/diagnostic claims in Parts A/B during 1999–2014, or had prescription claims in Part D during 2006–2014, and were not enrolled in Medicare capitated care (HMO). We linked the county code of enrollee’s residence in Medicare to the 2005–2009 American Community Survey (ACS) for a composite index of socioeconomic status (SES) 14, 15.
Testosterone Therapy
The four major routes of TT administration are intramuscular injection, implantable pellet, oral (including buccal), and transdermal. Injection/implant medical procedures were searched by Health Care Procedure Coding System (HCPCS) codes in Medicare Parts A and B (Supplemental Table 1). Oral and transdermal medication prescriptions were searched by National Drug Code in Medicare Part D (Supplemental Table 2).
Demographics, Comorbidities, Potential Indications, and Surveillance
From the Medicare files and the linked ACS, we extracted demographic characteristics (calendar year and age of Medicare enrollment, race, census region, and county-level SES), comorbidity diagnoses and lifestyle variables ever preceding TT initiation (hypertension, diabetes, hyperlipidemia, major cardiovascular diseases, prostatic hyperplasia/lower urinary syndrome, inflammatory prostate, family history of prostate cancer, Charlson- Klabunde comorbidity index [0, 1, ≥2] not including cancer diagnosis16, obesity/overweight, and tobacco use), relevant medical complaints within 120 days preceding TT initiation (i.e., hypogonadism, fatigue, depression, erectile dysfunction, and psychosexual dysfunction), and laboratory tests for free or total testosterone within 120 days before and/or after TT initiation.
Data Analysis
To allow for a reasonable retrospective period to capture information on comorbidities and lifestyle factors, we required men to be covered by Medicare for at least 12 months prior to Jan 1st of the calendar year of analysis.
To estimate annual incidence (new user) rates of TT, new users were defined as men initiating TT following a minimum 120-day washout period free of TT. Incidence was calculated as the number of men with an incident prescription of TT in the calendar year over person-time at risk among eligible individuals in Medicare (Equation 1). Men with an incident TT prescription stopped contributing person-time to the denominator from the date of incident prescription until the end of a 120-day washout period.
Equation 1:
To estimate annual prevalence (existing users) of TT use, we required men to be covered by Medicare for at least 30 days in the calendar year of analysis. Prevalence was calculated as the number of men who ever received TT in the calendar year divided by the total number of men on June 30th of the given calendar year (Equation 2).
Equation 2:
Annual incidence and prevalence were age-standardized to the 2000 US standard population and expressed per 100,000 person-years. Counts fewer than ten were suppressed as per confidentiality requirements. Calculation of prevalence from 2006 onwards and incidence from 2007 onwards (to allow for the 12-month requirement in equation 1) were based on male beneficiaries who had Part A/B and Part D coverages to enable direct comparisons among TT routes of administration. Because of sparse data for oral TT, we only reported overall age-standardized incidence and prevalence for this medication type.
We quantified the trends in age-standardized incidence and prevalence using joinpoint regression analysis17. Each joinpoint denotes a statistically significant change in the trend. Trends were quantified using annual percent change (APC) for individual time intervals determined by the best-fit joinpoint model. We also calculated average annual percent change (AAPC) to summarize trends in the most recent eight-year period (2007–2014; starting one year after Part D). AAPC is the weighted average of the APCs. R18 and Joinpoint17 were used for data analysis.
Results
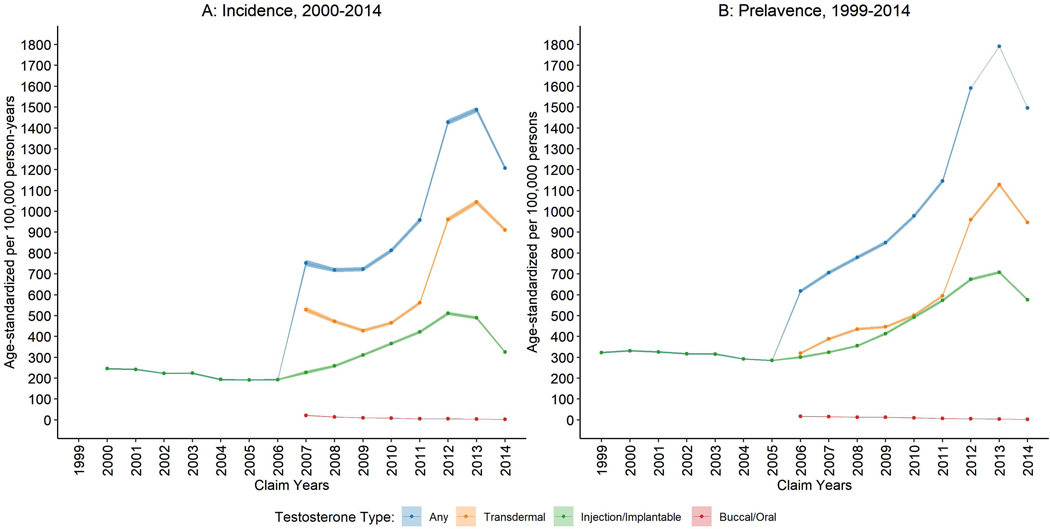
In this Medicare cohort, we observed 392,698 incident TT users during 88 million person-years providing a crude new user (incidence) rate of 444 per 100,000 person-years. Annual numbers and age-standardized rates of new and existing users of TT are shown in Figure 1 and Table 1. New user rates of TT injection/implant dropped continually from 246 to 194 per 100,000 person-years during 2000–2006 (APC = −4.6%, 95%CI: −6.2, −3.0) followed by a surge to a peak of 511 in 2012 (APC = 18.7%, 95%CI: 16.2, 21.3), before decreasing rapidly to 325 in 2014 (APC = −18.4%, 95%CI: −25.9, −10.1). The new user rate of transdermal TT started high at 529 per 100,000 person-years in 2007 and dramatically increased during the period assessed (APC = 13.2%, 95%CI: 4.1, 23.0) with a peak of 1,045 in 2013. The use of oral TT was not common in this population, with a new user rate that declined from 22 in 2007 to 3 per 100,000 person-years in 2014 (APC = −25.0%, 95%CI: −27.7, −22.2). In the most recent eight years (2007–2014), new user rates of any TT increased by 15.5% per year (AAPC; 95%CI: 5.8%, 26.1%); specifically, injection/implant increased by 6.7% (95%CI: 3.9%, 9.5%) and transdermals increased by 13.2% (95%CI: 4.1%, 23.0%). Existing user (prevalence) trends mirrored these new user trends.
Figure 1.
Age-standardized rates of (A) new (2000–2014) and (B) existing (1999–2014) users of testosterone therapy among United States male Medicare beneficiaries
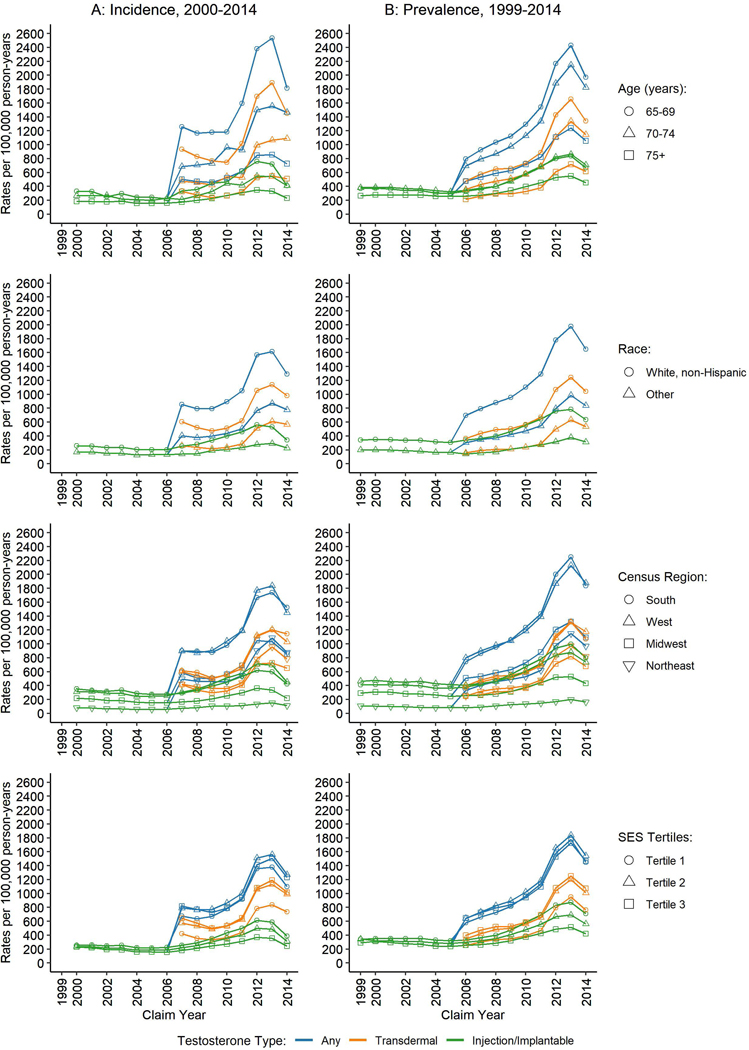
There were notable differences in TT use by population demographics (Figure 2). TT users were predominantly younger (ages 65–69 years), white non-Hispanic, and located in South and West US Census regions. These differences remained when assessing specific TT types. There was a notable drop in new TT users for the youngest age group during 2013–2014, compared with relatively stable trends at older ages. White non-Hispanic men were twice as likely as other racial/ethnic groups to receive TT. Men in higher tertiles of SES were more likely to use transdermal TT; men in the lowest tertile were more likely to use injection/implant TT.
Figure 2.
Age-standardized rates of (A) new (2000–2014) and (B) existing (1999–2014) users of testosterone therapy among United States male Medicare beneficiaries by population demographics
Men with a Charlson index ≥2 versus 0 were approximately twice as likely to initiate TT (data not shown). Most new TT users had a history of hyperlipidemia (~90%) or hypertension (~89%) (Supplementary Figure 1). Approximately half of new users ever had a diagnosis of cardiovascular disease or diabetes. More than 50% of new users ever had an enlarged prostate, and less than 20% ever had a symptomatic inflamed prostate.
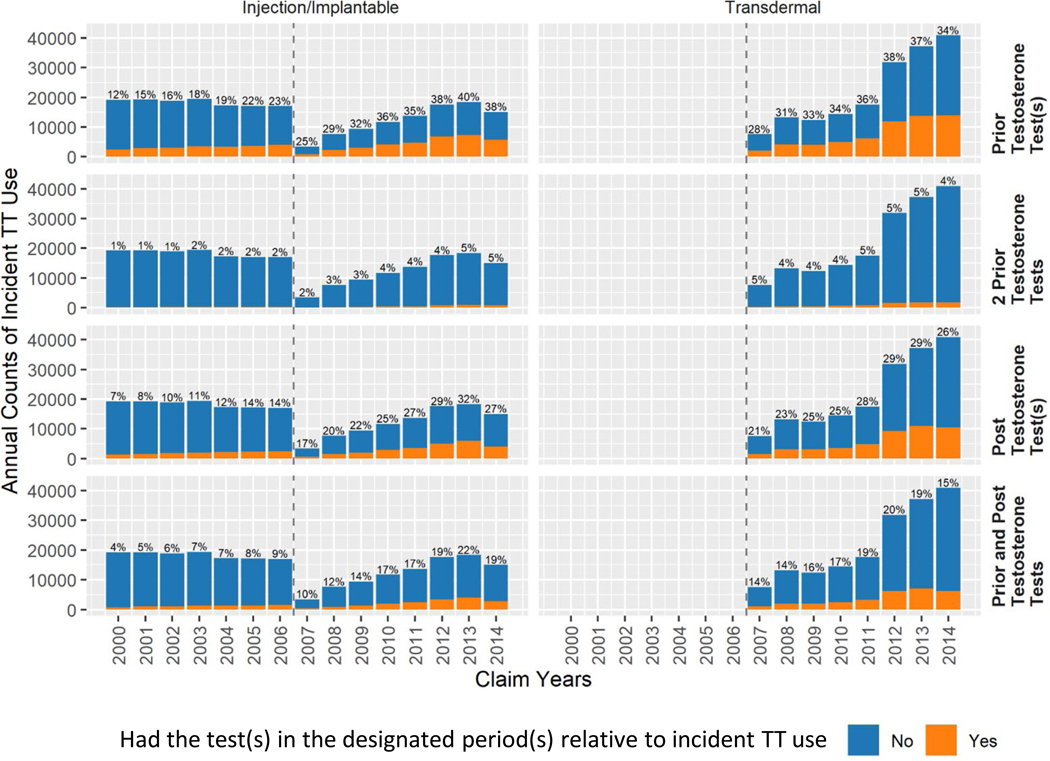
Hypogonadism was the most common of the recorded potential indications in the 120 days prior to TT initiation (Supplementary Figure 2; ~50% in 2014), followed by fatigue (~20%), erectile dysfunction (~15%), depression (~4%), and psychosexual dysfunction (~1%). The proportion of hypogonadism diagnoses among new users increased from 28% to 50% during 2007–2014 for injection/implant TT users and 32% to 49% for transdermal TT users. In line with hypogonadism changes over time, having received at least one testosterone test in the 120 days prior to TT increased from 25% to 38% for injection/implant and 28% to 34% for transdermal during 2007–2014 (Figure 3). Testosterone monitoring within 120 days after TT initiation also increased during this period. The recommended two testosterone19 tests within 120 days prior to TT initiation was rare (~4%), and paired pre- and post-tests for testosterone concentration were found in only ~16% of new TT users.
Figure 3.
Annual counts and percentages of new users of testosterone therapy among United States male Medicare beneficiaries by receipt of testosterone test(s) in the 120 days before or after testosterone therapy initiation, 2000–2014
Discussion
This study of Medicare data shows that TT use in the older US male population underwent dramatic increases followed by a recent plateau and small decrease during the period 2006–2013. Injectable and transdermal TT were the predominant types underlying these trends. Our study also shows that non-white Hispanics used TT nearly twice as much as other racial-ethnic groups. Although hypogonadism appeared to be the predominant indication for TT prescription, less specific conditions like fatigue and erectile dysfunction are inferred as possible indications for a substantial proportion of TT prescriptions. Testosterone tests amongst men prescribed TT were infrequent in these data.
We observed 392,698 incident TT users during 88 million person-years providing for a crude new user (incidence) rate of 444 per 100,000 person-years. Our results also found that use of any TT rose rapidly during 2006–2013 followed by a decline in 2014 among the older male Medicare population. This is in line with other studies to have assessed TT use among older adults or across all age groups.12, 13, 20 A previous study by Rao et al12 reported that, from 2003 to 2013, the rate of TT use among men increased from 29.2 to 118.1/10,000 person-years, almost a 4-fold increase during 10 years. Similarly Layton et al compared TT use among older adults in the United States and United Kingdom and found that TT initiation in the United States increased from 20.2 to 75.7/10,000 person-years during 2000–2011, with the most rapid increase observed in 2008.20 Finally, Baillargeon, et al also found increasing TT use among men 30 years and older from 2002 to 2013 and then a decline in use thereafter among 10 million men in a commercial health database.21
We evaluated use of TT in three major administration routes: transdermal, injection/implantable, and oral/buccal. Use of transdermal and injection/implantable modes of TT showed steady increases during 2006–2013 before declining in 2014, whereas use of oral TT remained low and exhibited a steady decline during 2006–2014. This is in line with previous findings from Baillargeon et al who reported similar increases injectable and gel formulations of TT and stable trends of oral and patch use of TT among US adults. 13 Similarly, Piszczek et al reported trends in TT use among Canadian men aged 66 years and older, finding that topical and injectable forms of TT increased during 1997–2012, while trends of oral forms were stable. 22
Hypogonadism, when defined simply as a serum total testosterone concentration of <300 ng/dL, may have a prevalence of up to 20% in men 60 years and older which may increase to ~50% among men 80 years and older.23 In our study, the most common potential indication for TT use appeared to be hypogonadism (50%)—as would be expected—followed by fatigue (20%), erectile dysfunction (15%), depression (4%) and psychosocial dysfunction (1%). This is in line with prior work by Baillargeon et al who reported similar but higher prevalence’s of the same indications associated with TT use in their patient population.13
Our study indicates that few men receive the Endocrine Society recommended24 two endogenous testosterone tests prior to receiving a TT prescription, and 2/3 of men were not tested at all, which is higher than the 30–40% estimates from two recent overlapping studies using Truven Health Analytics which includes commercial (Marketscan) and employer-based Medicare Supplemental Insurance databases20, 25. In a third recent study of Truven, the authors report that the proportion of men receiving TT who had at least one prior testosterone test increased from 33% to 80% during 2003–201312. Although we also observed an increase in Medicare data, it only peaked at 1/3 of men in 2014. The differences in estimates from our Medicare study and the prior studies of Truven are likely attributable to differences in accessibility (employer-based/federal), age distribution, and additional insurance coverage particularly in Medicare beneficiaries.
The primary lmitations of our study is that we lack information from any additional insurance and we rely solely upon Medicare billing data to infer indications and evaluate testosterone tests. Other limitations include the inability to assess TT use and trends in men aged less than 65 years, and current research data availability only through 2014. A major strength of our study is that Medicare is a large, longitudinal data source that can be used to study a variety of factors and associations in a nationally representative sample of older adults in the US.
Conclusions
In conclusion, our study showed that use of TT increased from 2000–2013 and then showed a decline in 2014. Although hypogonadism appeared to be the primary indication, TT also appeared to be prescribed for less specific conditions, often without the pre-requisite testosterone tests. A definitive diagnosis of hypogonadism should be made, and the potential benefits and harms should be discussed and weighed, prior to providing a TT prescription.
Supplementary Material
Supplementary Figure 1. Annual counts and percentages of new users of testosterone therapy among United States male Medicare beneficiaries by medical conditions in the 120 days before testosterone therapy initiation, 2000–2014
Supplementary Figure 2. Annual counts and percentages of new users of testosterone therapy among United States male Medicare beneficiaries by potential indications in the 120 days before testosterone therapy initiation, 2000–2014
Acknowledgement:
The authors thank Mrs. Ruth Parsons and Winnie Ricker at Information Management Services, Inc. for their expertise in Medicare data, and Dr. Barry I. Graubard of the Biostatistics Branch, Division of Cancer Epidemiology and Genetics, NCI for his valued statistical expertise and input.
Funding: This research was supported by the Sallie Rosen Kaplan Fellowship for Women Scientists (CKZ) and the Intramural Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
References
- 1.Lopez DS, Advani S, Tsilidis KK et al. : Endogenous and exogenous testosterone and prostate cancer: decreased-, increased- or null-risk? Transl Androl Urol, 6: 566, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maggio M, Lauretani F, Ceda GP et al. : Relationship between low levels of anabolic hormones and 6-year mortality in older men: the aging in the Chianti Area (InCHIANTI) study. Archives of internal medicine, 167: 2249, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araujo AB, Dixon JM, Suarez EA et al. : Endogenous Testosterone and Mortality in Men: A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab, 96: 3007, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Traish AM, Miner MM, Morgentaler A. et al. : Testosterone deficiency. The American journal of medicine, 124: 578, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Harman SM, Metter EJ, Tobin JD.et al. : Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab, 86: 724, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Wu FC, Tajar A, Pye SR et al. : Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab, 93: 2737, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Ross A, Bhasin S: Hypogonadism: Its Prevalence and Diagnosis. Urol Clin North Am, 43: 163, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Araujo AB, Wittert GA: Endocrinology of the aging male. Best Pract Res Clin Endocrinol Metab, 25: 303, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Handelsman DJ: Global trends in testosterone prescribing, 2000–2011: expanding the spectrum of prescription drug misuse. Med J Aust, 199: 548, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Layton JB, Kim Y, Alexander GC et al. : Association Between Direct-to-Consumer Advertising and Testosterone Testing and Initiation in the United States, 2009–2013. JAMA, 317: 1159, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bandari J, Ayyash OM, Emery SL et al. : Marketing and Testosterone Treatment in the USA: A Systematic Review. Eur Urol Focus, 3: 395, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Rao PK, Boulet SL, Mehta A. et al. : Trends in Testosterone Replacement Therapy Use from 2003 to 2013 among Reproductive-Age Men in the United States. J Urol, 197: 1121, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baillargeon J, Urban RJ, Ottenbacher KJ et al. : Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med, 173: 1465, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu M, Tatalovich Z, Gibson JT et al. : Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes Control, 25: 81, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Yost K, Perkins C, Cohen R.et al. : Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control, 12: 703, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Klabunde CN, Potosky AL, Legler JM et al. : Development of a comorbidity index using physician claims data. J Clin Epidemiol, 53: 1258, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, Fay MP, Feuer EJ et al. : Permutation tests for joinpoint regression with applications to cancer rates. Stat Med, 19: 335, 2000 [DOI] [PubMed] [Google Scholar]
- 18.R Core Team: R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2017. https://www.R-project.org/ [Google Scholar]
- 19.Bhasin S, Cunningham GR, Hayes FJ et al. : Testosterone Therapy in Adult Men with Androgen Deficiency Syndromes: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism, 91: 1995, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Layton JB, Li D, Meier CR et al. : Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab, 99: 835, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baillargeon J, Kuo Y, Westra JR et al. : Testosterone prescribing in the united states, 2002–2016. JAMA, 320: 200, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piszczek J, Mamdani M, Antoniou T. et al. : The impact of drug reimbursement policy on rates of testosterone replacement therapy among older men. PLoS One, 9: e98003, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desroches B, Kohn TP, Welliver C. et al. : Testosterone therapy in the new era of Food and Drug Administration oversight. Translational andrology and urology, 5: 207, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhasin S, Brito JP, Cunningham GR et al. : Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab, 2018 [DOI] [PubMed] [Google Scholar]
- 25.Muram D, Zhang X, Cui Z. et al. : Use of Hormone Testing for the Diagnosis and Evaluation of Male Hypogonadism and Monitoring of Testosterone Therapy: Application of Hormone Testing Guideline Recommendations in Clinical Practice. J Sex Med, 12: 1886, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Annual counts and percentages of new users of testosterone therapy among United States male Medicare beneficiaries by medical conditions in the 120 days before testosterone therapy initiation, 2000–2014
Supplementary Figure 2. Annual counts and percentages of new users of testosterone therapy among United States male Medicare beneficiaries by potential indications in the 120 days before testosterone therapy initiation, 2000–2014