Abstract
The inhibitor of DNA binding (Id) proteins are regulators of cell cycle and cell differentiation. Of all Id family proteins, Id1 is mostly linked to tumorigenesis, cellular senescence as well as cell proliferation and survival. Id1 is a stem cell-like gene more than a classical oncogene. Id1 is overexpressed in numerous types of cancers and exerts its promotion effect to these tumors through different pathways. Briefly, Id1 was found significantly correlated with EMT-related proteins, K-Ras signaling, EGFR signaling, BMP signaling, PI3K/Akt signaling, WNT and SHH signaling, c-Myc signaling, STAT3 signaling, RK1/2 MAPK/Egr1 pathway and TGF-β pathway, etc. Id1 has potent effect on facilitating tumorous angiogenesis and metastasis. Moreover, high expression of Id1 plays a facilitating role in the development of drug resistance, including chemoresistance, radiation resistance and resistance to drugs targeting angiogenesis. However, controversial results were also obtained. Overall, Id1 represent a promising target of anti-tumor therapeutics based on its potent promotion effect to cancer. Numerous drugs were found exerting their anti-tumor function through Id1-related signaling pathways, such as fucoidan, berberine, tetramethylpyrazine, crizotinib, cannabidiol and vinblastine.
Keywords: Id1, Cancer, Signaling pathway, Angiogenesis, Resistance, Target
Introduction
Inhibitor of differentiation or DNA binding (Id) was first isolated by Benezra, et al. in 1990 1. Id proteins belong to the helix-loop-helix (HLH) family. Proteins of HLH family contain an HLH dimerization domain which composed of two conserved amphipathic α helices separated by a loop and an adjacent region that contacts DNA 2. Basic HLH (bHLH) proteins bind to a DNA sequence known as an E-box or to the related N-box, which is found in the promoter-enhancer of expressed genes. The formation of heterodimers is an essential procedure for DNA binding and transcriptional activation in vivo 3. The bHLH transcription factors are inhibited by class V HLH protein, the Id proteins, which consist of four subtypes, namely Id1, Id2, Id3, and Id4. Id proteins lack a DNA binding domain, and they function as dominant negative regulators of basic HLH transcriptional factors through heterodimerizing with other bHLH factors such as MyoD 1 and E1A 4 and inhibiting their binding to DNA 5 . In humans the four Id genes are located on chromosomes 20q11 (Id1), 2p25 (Id2), 1p36.1 (Id3), and 6p21-p22 (Id4) 6-8 and Id proteins can be found both in the nucleus and in the cytoplasm 9.
Id proteins tightly regulate the expression of cell cycle regulators and orchestrate cell differentiation as well as cell linkage commitment 10, 11. Usually, Id gene expression is positively regulated in undifferentiated, highly proliferative, embryonic or cancer cells, especially for Id1, Id2 and Id3, as Id4 presents distinct functions from Id1, 2, 3 proteins 12. Of all the Id proteins, Id1 is the most extensively studied and mostly linked to tumorigenesis, cellular senescence as well as cell proliferation and survival 13, 14. This review will focus on the research progress of Id1 in the context of cancer and its treatment over the past decade.
Id1 in cancer-associated pathways
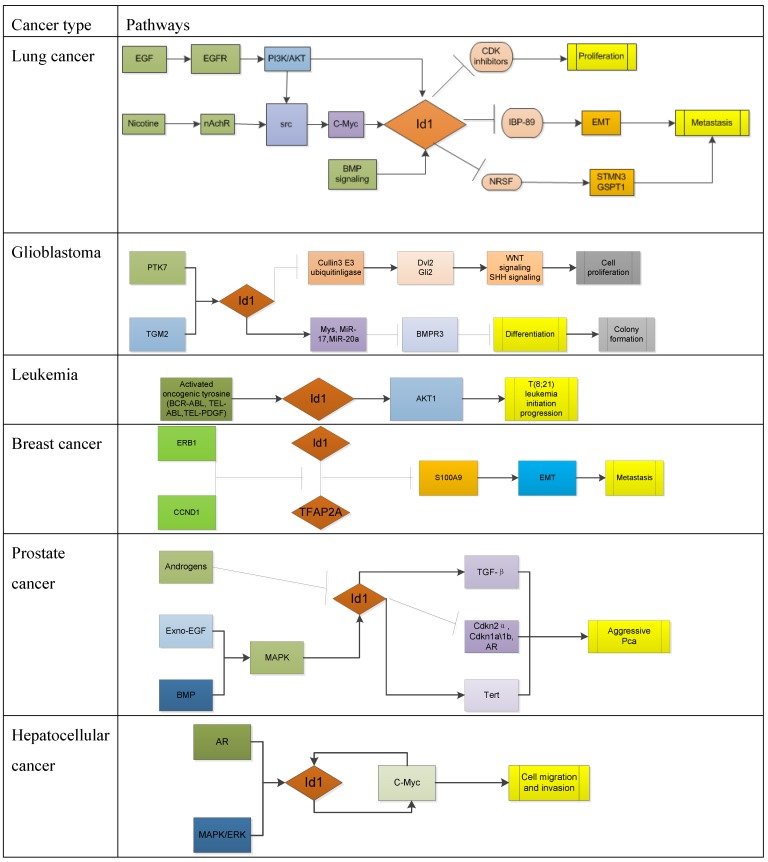
Oncogene is a gene that has the potential to cause cancer. In tumor cells, they are often mutated or overexpressed. Id1 does not strictly meet this classical definition of oncogene, because no tumor-associated mutations in Id1 gene have been observed 15. But Id proteins are overexpressed in over twenty types of cancer including breast cancer, prostate cancer, pancreatic cancer 16, ovarian cancer, endometrial cancer, bladder cancer, melanomas and lung cancer, etc. 17, and it is generally considered as tumor promoter 18. Id1 contributes to tumorigenesis mainly because its role in regulating proliferation and differentiation. Pathways involving Id1 in different types of tumor are described in the following subsections and briefly summarized in Table 1.
Table 1.
Id1 in different cancer-associated pathways
Lung cancer
Id1 promotes carcinogenesis and metastasis, and predicts prognosis of non-small cell lung cancer (NSCLC). Study shows that Id1 and Id3 co-expression is associated with a poor clinical outcome in patients with locally advanced NSCLC 19, 20. In analysis of 532 NSCLC patients' samples, Id1 was found significantly correlated with EMT-related proteins and it enables the tumor and the microenvironment to colonize the liver. Another study of high quality including 457 NSCLC patients also showed the independent prognostic value of Id1 levels for both stage I to stage IV patients that higher Id1 levels were associated with a shorter disease-free survival and overall survival in adenocarcinoma patients 21. Genetic loss of Id1 in the host tissue (Id1-/- mice) impaired liver colonization and increased survival of Id1-/-animals 22. Suppressing both Id1 and Id3 expression was accompanied by decreased angiogenesis and increased apoptosis and greatly reduced the average size of small cell lung cancer in nude mice 23.
In smokers, nicotine binds to pentameric nicotinic acetylcholine receptors (nAChRs) and promotes the growth and metastasis of lung cancers by modulating various signaling cascades, for instance, K-Ras signaling 24, 25. In non-smokers, epidermal growth factor receptor (EGFR) kinase domain mutations which have been established as valid predictors of increased sensitivity to EGFR kinase inhibitors are prevalent in lung cancers 26. Study shows that Id1 gene is a downstream mediator of both K-Ras and EGFR signaling. Both nicotine and epidermal growth factor (EGF) could induce the expression of Id1 in a Src-dependent manner 27. Additionally, BMP signaling in lung cancer cells increases expression of Id proteins and then Id1 regulates lung cancer cell cycle progression by activating CDK4/cyclin D1 and enriching cells at S and G2/M phases so that promotes cell proliferation and growth of lung cancer cells expressing stem cell markers, Oct4 or Nestin 28, 29. Id1 also induces the expression of Stathmin-like3 (STMN3), a microtubule destabilizing protein, and GSPT1, a protein involved in translation termination by down-regulating the expression of two transcriptional co-repressors, NRSF and ZBP89 27, 30 . Furthermore, Akt activation was observed to be involved in cell proliferation-promoting activity of Id1, which was blocked after treating Id1-overexpressing lung cancer cells with PI3K/Akt inhibitor wortmannin 28.
Glioblastoma
Glioblastoma (GBM) is grade IV glioma which is characterized by poor therapeutic response and poor overall survival. Differentiation therapy has been proposed as a promising strategy for GBM therapy, as upon differentiation, GBM cells lose tumorigenicity and become sensitive to chemotherapy and radiotherapy 31. Id1 serves as a main mediator that abrogates differentiation signals in glioblastoma stem cells (GSCs) and contributes to GBM initiation and chemoresistance in GBM. Knocking out of Id1 in GBM reduced tumor progression 32. Study shows that Id1 can simultaneously regulates stemness through WNT/SHH signaling and differentiation through bone morphogenetic protein receptor(BMPR)-mediated differentiation signaling in GSCs. Id1 activates WNT and SHH signaling by upregulating Dvl2 and Gli2 proteins through suppression of Cullin3 E3 ubiquitin ligase in a ligand-independent manner 33. Activation of WNT and SHH signaling increases the expression of Myc and its transcriptional targets miR-17 and miR-20a, then the two miRNAs inhibit expression of the differentiation-inducing receptor, BMPR2 34. In GBM cells, Id1 is also a potential downstream effector of protein tyrosine kinase 7(PTK7) and transglutaminase 2 (TGM2) which are highly expressed in CD44-high glioblastoma and predicts unfavorable prognosis. Depletion of PTK7 or TGM2 inhibitor treatment reduced Id1 expression and overexpression of Id1 mostly restored the cell proliferation and colony formation 35, 36. Id1 is regulated by ERK1/2 MAPK/Egr1 pathway and TGF-β pathway as well, activation of these pathways increases self-renewal capacity of GBM cells and resistance to radiation-induced DNA damage 37, 38.
Leukemia
Leukemia is a malignant hematological disorder characterized with different clinical manifestations, cellular and molecular mechanisms, and different response to therapy or risk of relapse. Overexpression of Id1 is seen in acute myeloid leukemia (AML) patients. Studies showed that high expression of Id1 is associated with poor prognosis in patients with AML, independently predicting shorter disease-free survival and overall survival 39, 40, especially for those with higher risk karyotype classification in young non-M3 patients 41. Noticeably, Id1 expression is not an independent prognostic factor in acute myeloid leukaemia with normal karyotype when CEBPA mutations were included in the analysis 42. Study suggests that Id1 is a key transcriptional regulator of hematopoietic stem cell lineage commitment and it can immortalize hematopoietic progenitors in vitro and promote a myeloproliferative disease in mice in vivo 14, 43. Oncogenic tyrosine kinases, such as BCR-ABL, TEL-ABL, TEL-PDGF beta R, and FLT3-ITD, play a major role in the development of hematopoietic malignancy. Id1 was identified as a common downstream target of constitutively activated oncogenic tyrosine kinases 44. Furthermore, loss of Id1 inhibited t(8;21) leukemia initiation and progression by abrogating AKT1 activation 45. It is different from other tumors that the differential role of Id1 in MLL-AF9-driven leukemia is basing on cell of origin. Study shows that mice receiving MLL-AF9-transduced fetal liver cells or bone marrow cells develop AML. Loss of Id1 significantly prolonged the median survival of mice receiving fetal liver cells but accelerated leukemogenesis in recipients of bone marrow cells 46. The effect of Id1 in leukemogenesis seems largely dependent on p21. The expression of p21 is extremely low in human fetal hematopoietic stem/progenitor cells, but it increases as the cells differentiate into myeloid cells 46, 47. Conclusion was also gained by researchers that low expression of Id1 was observed in most AML cell lines and human AML samples 48. This indicates that Id1 expression in AML is quite different from that reported in other human malignancies where the Id1 expression is up-regulated. But small sample size limited its reliability.
Breast cancer
High Id1 expression in breast cancer cell lines is associated with high aggressiveness and metastasis which is a major factor responsible for mortality in patients with breast cancer 15, 49. Id1 induces mammary tumorigenesis by increasing normal and malignant mammary stem cell activities in transgenic mice 50. Id1 also induces metastatic mammary carcinoma by cooperating with oncogenic Ras 51. Higher Id1 expression was associated with worse disease-free survival and overall survival 52.. Targeting Id1 expression in breast cancer cells reduces breast cancer metastasis in animal models. Breast tumors failed to grow and metastasize in Id1 (+/-) Id3 (-/-) mice. Mern, D.S., et al isolated a novel peptide aptamer, d1/3-PA7(LSAMAATLFAELGCHLSRWM), specifically interacting with Id1 and Id3 from randomized combinatorial expression library using yeast and mammalian two-hybrid systems. It can significantly provoke anti-proliferative and apoptotic effects in breast cancer cells 53. Gurrapu, S et al. suggests that Semaphorin 4C reverse signaling sustains the metastasis formation through induction of prometastatic genes including Id1 and Id3 54. Study indicated that Id1 promotes breast cancer metastasis by S100A9 regulation. Id1 interacts with TFAP2A to suppress S100A9 expression. Migratory, invasive phenotypes in vitro and metastasis in vivo induced by Id1 expression can be rescued by reestablishment of S100A9 expression 55. Id1 gene expression was also correlated with EMT-associated genes including, VIM, SNAI1, SNAI2, and TWIST1 56. Both ERβ1, a member of the nuclear receptor superfamily of ligand-regulated transcription factors and an important protein in regulating the progression of breast cancer 57 , and CCND1, a regulator of cyclin dependent kinase, regulate the migration and invasion of breast cancer cells in an Id1-dependent manner 56. Abl interactor 1 (Abi1) is a critical regulator of actin polymerization/depolymerization, involving in the abnormal development of cytoskeletal functions of breast cancer cells. Its regulating function of the invadopodia formation depends on the Id1, as well 58. Furthermore, addiction to the IGF2-Id1-IGF2 circuit is essential for maintenance of the breast cancer stem-like cells 59. But controversial conclusion was also gained, Zhou XL et al. showed increased Id1 mRNA levels were associated with higher relapse‑free survival rates in all patients with breast cancer through analysis of data from a set of publicly accessible databases 60. Even in a same study, paradoxical results were also acquired. Gurrapu, S et al. found that semaphorin 4C elicited Id1/3 dependent metastasis of breast cancer and prostate cancer, but in culture, they found that semaphorin 4C overexpression impaired cancer cell migration and invasivenesss 54. This means the effect of Id1 in the development of cancer is complicated, its role in the primary foci of cancer, the shedding and colonization of cancer cells, and the metastatic cancer needs further studied.
Prostate cancer
In mouse prostate model, overexpression of Id1 alone is not sufficient to drive neoplastic change 61, but Id1 is proved to regulate proliferation, apoptosis, and androgen-independence of prostate cancer (PCa) cell. Increased Id1 protein expression is strongly associated with increasing grade of PCa 62. And a study involving 52 prostate cancer patients showed higher Id1 RNA expression predicted a higher hazard ratio for progression and a shorter disease-free survival 63. Knocking out Id1 gene has an in-vivo preventive effect against the development of prostate cancer in mouse model 64. Id1 reduction is pre-requisite for inhibitory effects of TGFβ on cell proliferation and migration. Cross talk with MAPK, NFκB and TNFα promotes cell survival, proliferation, metastasis and androgen-independence 65, 66. Mechanism is related to attenuation of all three cyclin-dependent kinase inhibitors (Cdkn2b, -1a, and -1b) by increased Id1 expression 62. Id1 induced immortalization was also associated with decreased expression of Cdkn2a, Cdkn1a, androgen receptor (AR) and increased Tert expression. Network analysis indicates that Id1 promotes cancer morphology, cell cycle and epithelial to mesenchymal transition by influencing AP1, tnf, tgfbeta, PdgfBB and estradiol pathways 66. Intriguingly, study also showed that Id1 could down-regulate the ability of PC3 cells to form osteolytic lesions in vivo, although in the same study it was observed that knockdown of Id1 in PC3 cells inhibited the proliferation of cancer cells in vitro 67. While evidence also suggests that Id1 is a key factor in promoting cancer metastasis to lungs 68, the discrepancy of microenvironment in different sites may be the cause. Moreover, Id1 was showed mediating chemosensitivity enhancement. Patients with higher Id1 expression were found to be associated with longer relapse-free survival than patients without Id1 increase after neoadjuvant chemotherapy and radical prostatectomy. What's more, in the prostate cancer cell line LNCaP, docetaxel dose-dependently induced Id1 transcription and stable Id1 overexpression in LNCaP enhanced docetaxel-induced cytotoxicity 69. This means the role of Id1 in prostate cancer is complicated and should be analyzed in specific.
Cervical cancer
Human papillomavirus (HPV) infection is tightly associated with cervical cancer. High expression of Id1 protein was found to be correlated with E6 oncoprotein in high-risk HPV and HPV-immortalized cervical epithelial cells which suggested that Id1 plays an oncogenic role in HPV-related cervical carcinogenesis 70-72. Study shows that Id1 expression is an independent prognostic marker in early-stage cervical cancer, patients with strong or moderate expression of Id1 have a significant shorter overall survival time 73. Co-expression of Id1 and nuclear NF-κB p65 promotes progression and malignancy of cervical cancer 74. Moreover, inflammation exerts prominent function in tumorigenesis of cancer. A 37 kDa protein, annexin A1 (ANXA1), was found to be an anti-inflammatory mediator and expressed by tumor cells. Study showed that ANXA1 down-regulated Id1 gene expression and the Id1 pathway gene, BMPR1B, in cervical cancer 75. This indicates that Id1 also plays a role in tumor-associated inflammation.
Thyroid cancer
In human thyroid tissue, Id1 protein expression increases gradually from normal thyroid tissue, hyperplastic thyroid tissue to malignant thyroid tissue 76. But no significant association between Id1 protein expression level and tumor-node-metastasis stage, tumor size, primary tumor vs. lymph node metastasis, primary tumor vs. recurrent tumors, and extent of tumor differentiation was found which means Id1 is not a marker of aggressive phenotype in differentiated thyroid cancer 77. With regard to the association between Id1 level and overall survival or disease free survival for thyroid cancer patients needs to be further studied. Id1 gene expression was induced by many growth factors in various cell lines, including TGFβ1, PDGF, NGF, EGF, IGF-1, and estrogen 78. In thyroid cancer the Id1 mRNA expression was upregulated by thyroid-stimulating hormone (TSH) 79. And Id1 protein has been found to be an early target of TGFβ, it induces mesenchymal phenotype and promotes invasiveness of thyroid tumor cells 80.
Colorectal cancer
Higher Id1 expression in colorectal cancer specimens than in normal mucosal specimens was shown and high Id1 expression positively correlated with poor differentiation in colorectal cancer 81. Knock down of Id1 arrests the growth of colorectal cancer cells and suppressed hepatic metastasis in vivo 82, except for colitis-associated colorectal cancer 83. Downregulation of PCNA, survivin, CXCR4, MMP2 and MMP9 was found in Id1 knock down cells 82. Id1 maintains the stemness of colorectal cancer cells through the Id1-c-Myc-PLAC8 axis through activating the Wnt/β-catenin and Shh signaling pathways 81. Self-renewal and metastatic colonization of tumor-initiating cells in colorectal cancer were regulated by miR-371∼373/TGFβ receptor 2/Id1 signaling axis and p21/Id1 pathway 84, 85. Study also showed cell division cycle protein 27 (CDC27) mutation promoted metastasis and sphere-formation capacity of colorectal cancer cells in an Id1-dependent manner 86. In addition, p53/stat3/Id1 pathway mediates chemotherapeutic resistance of colorectal cancer 87.
Hepatocellular cancer (HCC)
Id1 is relevant to HCC dedifferentiation 88, Id1 levels are not only high in HCC cells, but also upregulated in HCV-infected hepatic cells or viral core gene-transfected cells, whereas they are very low in normal liver tissues 89. Moreover, overexpressed Id1 is associated with patients' prognosis and HBx expression in hepatitis B virus-related HCC. It contributes to the development of HCC with cirrhosis 90 and is a potential prognostic marker for HBV-related HCC 91. Patients with overexpression of Id1 had shorter disease-free and overall survival times 92. Indeed, Id1 promotes metabolic reprogramming in HCC cells 93. AR activity is associated with cancer development and progression. In HCC, AR contributes to the incidence of HCC. AR activation enhanced the expression of Id1, which led to increased HCC cell migration and invasion 94. Study found that Id1 is mediated by the MAPK/ERK pathway and associated with increased c-Myc levels in HCC. Id1 knockdown leads to c-Myc reduction as well as c-Myc knockdown leads to Id1 reduction. Moreover, Id1 may interact directly with c-Myc without inhibiting the transcriptional activity of c-Myc 93. However, a totally adverse conclusion was also acquired in other studies. Lei-lei Niu, et al. showed that Id1 protein was down-regulated in 15 out 20 HCC tumors compared to matched non-tumor tissues (even though the title of this paper is improper) 95. Damdinsuren B, et al. reported Id1 protein was highly expressed in non-tumor liver tissues with hepatitis and cirrhosis. The decreased expression of Id1 was observed in 372 liver HCC samples, compared to adjacent normal samples 88.
Id1 in tumor angiogenesis and metastasis
Sufficient nutrient supply guaranteed by new blood vessels is crucial to tumor progression and metastasis. Tumor angiogenesis is significantly triggered by the upregulation of vascular endothelial growth factor (VEGF). Study showed VEGF-A expression is regulated by TGF-β1 through Id1 pathway 96. Numerous studies indicated the important role of Id1 in angiogenesis, for example, in small cell lung cancer, suppressed expression of Id1 and Id3 was accompanied by decreased angiogenesis 23. Id1+/- Id3-/- mice fail to grow tumors due to poor vascularization and necrosis 97. MiR-885-3p downregulates Id1 by targeting BMPR1A, leading to impaired angiogenesis 98. High expressions of Id1 and matrix metalloproteinase 9 (MMP9) have tight correlations with the development and progression of colorectal adenocarcinoma and have positive correlations with microvascular density. Both of them may be involved in the microvascular generation, the invasion and hematogenous metastasis of colorectal carcinoma 99. Membrane degradation and cell migration were partly mediated by matrix metalloproteinases (MMPs). Id1 can increase MMP gene expression, leading to tumor cell invasion. High levels of Id1 and the membrane-type 1-MMP (MT1-MMP) or MMP1 were associated to breast cancer metastasis 100, 101. KLF17 is a zinc-finger protein acting as a metastasis suppressor. It can inhibit Id1 transcription through binding to its promoter region. KLF17 is significantly down-regulated in primary human breast cancer samples, thus leading to Id1 induction, which may promote primary tumor vascularization via VEGF production, breast cancer cell invasion and EMT 102.
Phenotypic plasticity, the epithelia-to-mesenchymal and mesenchymal-to-epithelial transition (EMT-MET) switch in particular, is required for cancer metastasis 103. Castañón E et al. analyzed samples of 532 NSCLC patients, they found Id1 significantly correlated with vimentin and other EMT-related proteins. The loss of Id1 decreased the levels of vimentin, integrinβ1, TGFβ1 and snail, both in vitro and in vivo. In their study, Id1 facilitated lung cancer liver colonization through activation of EMT program in tumor cells and establishment of the pre-metastatic niche 22. In breast cancer, Id1 induced by TGF-β opposes Twist1 and promotes metastatic colonization to lung via EMT 104. E47 protein (encoded by E2A gene) is a member of the class I bHLH transcription factors (also known as E protein). E47 has been described as a repressor of E-cadherin and inducer of EMT. Study found that E47 interacts with Id1 in E47 overexpressing MDCK cells that underwent EMT as well as in mesenchymal breast carcinoma and melanoma cell lines 105.
Id1 in therapeutic resistance
Numerous therapeutic methods were applied in anti-cancer treatment, such as chemotherapy, radiotherapy, targeted therapy and immunotherapy. But drug resistance which leads to a more aggressive cancer and poor prognosis is a severe limitation. Mechanism is associated with a sub-population of tumor cells with stem-like properties, cancer stem cells (CSCs) which are specifically endowed to resist or adapt to the standard therapies, leading to therapeutic resistance. Based on the fact that Id1 serves as a stem cell-like gene and knockdown of Id1 suppresses the expression of the key CSC-associated factors Nanog and octamer-binding protein 4 (Oct-4), a role of Id1 in the development of drug resistance has been suggested 106. Study demonstrated that knockdown of Id1 sensitized gastric cancer cells to cisplatin 106. miR381 interfered with NF-κB through repression of Id1 and thus re-sensitized A549/CDDP cells to cisplatin. Co-expression of Id1 reversed the enhancement of cisplatin cytotoxicity by miR-381 107. In NSCLC, Id1 and Id3 co-expression is associated with a poor clinical outcome in patients 19. High Id1 expression is a negative prognostic factor. However, it paradoxically predicts a favorable prognosis for adjuvant paclitaxel plus cisplatin therapy in surgically treated lung cancer patients 108. And Id1 overexpression increases gefitinib sensitivity in NSCLC, regardless of the mutational status of NSCLC. Mechanism is related to activation of RIP1/RIP3/MLKL pathway dependent necroptosis 109. In HCC, Id1 knockdown activates p16/IL6 axis and contributes to the resistance of HCC to sorafenib 95. Id1- induced pentose phosphate pathway activation confers chemoresistance to oxaliplatin and promotes HCC proliferation 110. In colorectal cancer, after long time 5-Fu selection, Id1 expression was top upregulated in colorectal cancer cells and more aggressive tumors was generated 111. Decreasing the expression of Id1 gene was able to restore the sensitivity to 5-Fluorouracil (5-FU) 112, 113. Mechanistically, study showed that Id1 conferred 5-FU chemoresistance through E2F1-dependent induction of thymidylate synthase expression 114. In GBM multiforme, Id1 induced by cyclooxygenase-2 (Cox-2)-derived prostaglandin E2 (PGE2) increases GBM self-renewal and radiation resistance. Mechanism was found to be via EP4-dependent activation of MAPK signaling and the Egr1 transcription factor 115. And the inhibition of Id1 enhances the effect of temozolomide, delays tumor recurrence, and prolongs survival 32. But an opposite conclusion was also acquired by Guo, Q et al. who found that GBM patients with high Id1 expression had better survival than patients with low Id1 expression since Id1 expression could increase the radiotherapy efficacy 116. Same conclusion was also obtained in a study of prostate cancer. They found stable Id1 overexpression in prostate cancer cell line LNCaP enhanced docetacel-induced cytotoxicity and patients with Id1 upregulation possess longer relapse-free survival than patients without Id1 increase 116.
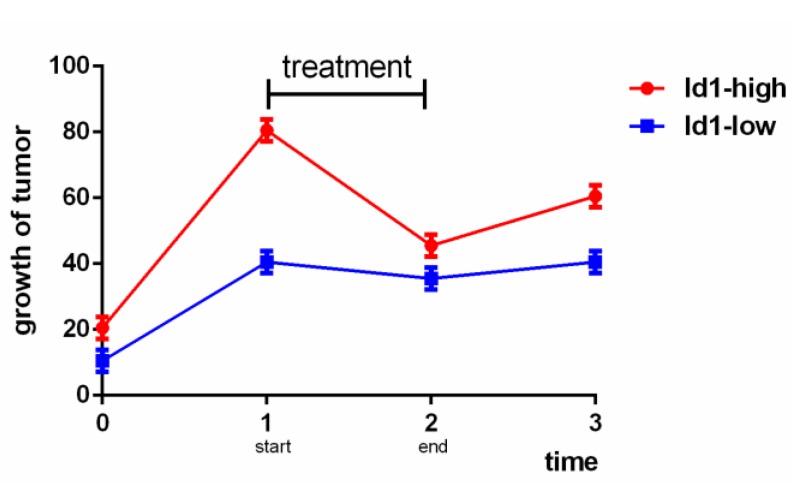
According to the above, we know that the role of Id1 in therapeutic resistance varies as cancer type varies. Mostly, Id1 is hazardous to the therapeutic resistance and prognosis of cancer, but in NSCLC, GBM and prostate cancer some shows that it is a sensitive marker to chemotherapy or radiotherapy. However, besides cancer types, the controversial conclusion may also related to the concrete expression amount of Id1, the duration of treatment, the observation time and specific medicine. Studies provided more details should be commenced and the balance between Id1 and treatment should be stressed because the relationships among Id1 expression, therapy and growth of tumor may present like Fig 1 below.
Figure 1.
Analog trend graph of relationships among Id1, therapy and growth of tumor.
Id1 as therapeutic targets
Regarding to low survival rate of patients with cancer, researches of novel, potent anti-tumor therapeutics are essential. The Id1 represent an interesting target for this purpose, as it is involved in cellular key events related to tumorigenesis and cancer progression 117. Anti-proliferative and chemosensitization effect of gamma-tocotrienol on breast cancer cells was mediated through downregulation of Id1 protein 118. HCC invasion was suppressed by fucoidan treatment both in vitro and in vivo which was related to NDRG-1/CAP43-dependent down-regulation of Id1 119. Berberine also suppressed the growth and development of lung metastases in HCC by inhibiting the expression of Id1. Berberine's anti-proliferative and anti-invasive activities could be partly rescued by Id1 overexpression 120. Tetramethylpyrazine inhibited the growth of lung cancer through disrupting angiogenesis via BMP/Smad/Id-1 signaling pathway 121. Crizotinib decreased Id1 levels in ALK- and MET-positive lung cancer cells and inhibited cell migration 122. Cannabidiol (CBD), a non-toxic, non-psychoactive cannabinoid and redox modulator, could inhibit GSCs survival, self-renewal and significantly increase the survival of GSC-bearing mice by activating p38 pathway and downregulating key stem cell regulators Sox2, Id1 and p-STAT3 123. Furthermore, vinblastine (VBL), a key microtubule inhibitor, was also confirmed that it downregulated Id1 in VBL-treated human cervical carcinoma cells 124. As to the therapy targeting Id1 in leukemia, pimozide, a known USP1 inhibitor was proved effective in inhibiting the growth of primary AML patient-derived leukemic cells 125. USP1 is a deubiquitinating enzyme, which removes polyubiquitin chains from the Id1 protein 126. Many other compounds exert their anti-tumor function via Id1-related signaling pathways or are found possessing effective regulation on Id1.
Conclusion
Id1 is a member of the HLH family which serves as a regulator of cell differentiation and cell linkage commitment. Generally, it is overexpressed in over twenty types of cancer and promotes growth and metastasis of cancer. Id1 potently induces angiogenesis and EMT. Its role in drug resistance is controversial, whereas most of studies suggested that Id1 is responsible for chemoresistance and radiation resistance. Id1 is a promising target of anti-tumor treatment as many compounds exert anti-tumor properties by mediating Id1-related pathways. Nevertheless, aiming to understand and solve controversial data, to answer open questions and to further validate Id1 as a therapeutic target of cancer and develop new drugs, more work should be done to explore the biological characteristics of Id1 and its relating signaling network.
Acknowledgments
This study was sponsored by Zhejiang Provincial Project for the Key Discipline of Traditional Chinese Medicine (Yong GUO, Grant No: 2017-XK-A09, http://www.zjwjw.gov.cn/); Project of Academic Experiences Inheritance from the Famous Traditional Chinese Medicine Doctor of Zhejiang Province, Yong GUO and Construction of Specialized Subject (Grant No: 2A11543); the National Natural Science Foundation of China (Grant No: 81973805); Zhejiang Provincial TCM Science and Technology Project (Grant No: 2015ZA088).
Abbreviations
- Id1
inhibitor of differentiation 1
- HLH
helix-loop-helix
- EMT
epithelial-mesenchymal transition
- bHLH
basic HLH
- NSCLC
non-small cell lung cancer
- nAChRs
nicotinic acetylcholine receptors
- EGFR
epidermal growth factor receptor
- EGF
epidermal growth factor
- STMN3
stathmin-like3
- GBM
glioblastoma
- GSCs
glioblastoma stem cells
- BMPR
bone morphogenetic protein receptor
- PTK7
protein tyrosine kinase 7
- TGM2
transglutaminase 2
- AML
acute myeloid leukemia
- PCa
prostate cancer
- HPV
human papillomavirus
- ANXA1
annexin A1
- TSH
thyroid-stimulating hormone
- CDC27
cycle protein 27
- HCC
hepatocellular cancer
- AR
androgen receptor
- VEGF
vascular endothelial growth factor
- MMP9
matrix metalloproteinase 9
- MMPs
matrix metalloproteinases
- MT1-MMP
membrane-type 1-MMP
- EMT-MET
epithelia-to-mesenchymal and mesenchymal-to-epithelial transition switch
- CSCs
cancer stem cells
- Oct-4
octamer-binding protein 4
- 5-FU
5-fluorouracil
- Cox-2
cyclooxygenase-2
- PGE2
prostaglandin E2
- CBD
Cannabidiol
- VBL
vinblastine
References
- 1.Benezra R, Davis RL, Lassar A. et al. Id - a negative regulator of helix-loop-helix dna-binding proteins - control of terminal myogenic differentiation. Ann Ny Acad Sci. 1990;599:1–11. doi: 10.1111/j.1749-6632.1990.tb42359.x. [DOI] [PubMed] [Google Scholar]
- 2.Lister J, Forrester WC, Baron MH. Inhibition of an erythroid differentiation switch by the helix-loop-helix protein Id1. J Biol Chem. 1995;270:17939–17946. doi: 10.1074/jbc.270.30.17939. [DOI] [PubMed] [Google Scholar]
- 3.Tournay O, Benezra R. Transcription of the dominant-negative helix-loop-helix protein Id1 is regulated by a protein complex containing the immediate-early response gene Egr-1. Mol Cell Biol. 1996;16:2418–2430. doi: 10.1128/mcb.16.5.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jen Y, Weintraub H, Benezra R. Overexpression of Id protein inhibits the muscle differentiation program: In vivo association of Id with E2A proteins. Genes Dev. 1992;6:1466–1479. doi: 10.1101/gad.6.8.1466. [DOI] [PubMed] [Google Scholar]
- 5.Sun XH, Copeland NG, Jenkins NA. et al. Id proteins Id1 and Id2 selectively inhibit DNA binding by one class of helix-loop-helix proteins. Mol Cell Biol. 1991;11:5603–5611. doi: 10.1128/mcb.11.11.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathew S, Chen W, Murty VV. et al. Chromosomal assignment of human ID1 and ID2 genes. Genomics. 1995;30:385–387. doi: 10.1006/geno.1995.0037. [DOI] [PubMed] [Google Scholar]
- 7.Deed RW, Hirose T, Mitchell EL. et al. Structural organisation and chromosomal mapping of the human Id-3 gene. Gene. 1994;151:309–314. doi: 10.1016/0378-1119(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 8.Pagliuca A, Bartoli PC, Saccone S. et al. Molecular cloning of ID4, a novel dominant negative helix-loop-helix human gene on chromosome 6p21.3-p22. Genomics. 1995;27:200–203. doi: 10.1006/geno.1995.1026. [DOI] [PubMed] [Google Scholar]
- 9.Ghil SH, Jeon YJ, Suh-Kim H. Inhibition of BETA2/NeuroD by id2. Exp Mol Med. 2002;34:367–373. doi: 10.1038/emm.2002.52. [DOI] [PubMed] [Google Scholar]
- 10.Perk J, Iavarone A, Benezra R. Id family of helix-loop-helix proteins in cancer. Nat Rev Cancer. 2005;5:603–614. doi: 10.1038/nrc1673. [DOI] [PubMed] [Google Scholar]
- 11.Norton JD. Id helix-loop-helix proteins in cell growth,differentiation and tumorigenesis. J Cell Sci. 2000;113:3897–3905. doi: 10.1242/jcs.113.22.3897. [DOI] [PubMed] [Google Scholar]
- 12.Patel D, Morton DJ, Carey J. et al. Inhibitor of differentiation 4 (ID4): From development to cancer. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2015;1855:92–103. doi: 10.1016/j.bbcan.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang J, Gordon GM, Nickoloff BJ. et al. The helix-loop-helix protein id-1 delays onset of replicative senescence in human endothelial cells. Lab Invest. 2002;82:1073–1079. doi: 10.1097/01.lab.0000022223.65962.3a. [DOI] [PubMed] [Google Scholar]
- 14.Suh HC, Leeanansaksiri W, Ji M. et al. Id1 immortalizes hematopoietic progenitors in vitro and promotes a myeloproliferative disease in vivo. Oncogene. 2008;27:5612–5623. doi: 10.1038/onc.2008.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roschger C, Cabrele C. The Id-protein family in developmental and cancer-associated pathways. Cell Commun Signal. 2017;15:7. doi: 10.1186/s12964-016-0161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y, Hu J, Chen F. et al. ID1 mediates escape from TGFβ tumor suppression in pancreatic cancer. Cancer Discov. 2020;10:142–157. doi: 10.1158/2159-8290.CD-19-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang G, Zhang Y, Xiong J. et al. Downregulation of Id1 by small interfering RNA in gastric cancer inhibits cell growth via the Akt pathway. Mol Med Rep. 2012;5:1075–1079. doi: 10.3892/mmr.2012.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma P, Chinaranagari S, Chaudhary J. Inhibitor of differentiation 4 (ID4) acts as an inhibitor of ID-1, -2 and -3 and promotes basic helix loop helix (bHLH) E47 DNA binding and transcriptional activity. Biochimie. 2015;112:139–150. doi: 10.1016/j.biochi.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castanon E, Bosch-Barrera J, Lopez I. et al. Id1 and Id3 co-expression correlates with clinical outcome in stage III-N2 non-small cell lung cancer patients treated with definitive chemoradiotherapy. J Transl Med. 2013;11:13. doi: 10.1186/1479-5876-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antonangelo L, Tuma T, Fabro A. et al. Id-1, Id-2, and Id-3 co-expression correlates with prognosis in stage I and II lung adenocarcinoma patients treated with surgery and adjuvant chemotherapy. Exp Biol Med (Maywood) 2016;241:1159–1168. doi: 10.1177/1535370216632623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponz-Sarvise M, Nguewa PA, Pajares MJ. et al. Inhibitor of differentiation-1 as a novel prognostic factor in NSCLC patients with adenocarcinoma histology and its potential contribution to therapy resistance. Clin Cancer Res. 2011;17:4155–4166. doi: 10.1158/1078-0432.CCR-10-3381. [DOI] [PubMed] [Google Scholar]
- 22.Castanon E, Soltermann A, Lopez I. et al. The inhibitor of differentiation-1 (Id1) enables lung cancer liver colonization through activation of an EMT program in tumor cells and establishment of the pre-metastatic niche. Cancer Lett. 2017;402:43–51. doi: 10.1016/j.canlet.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Chen D, Forootan SS, Gosney JR. et al. Increased expression of Id1 and Id3 promotes tumorigenicity by enhancing angiogenesis and suppressing apoptosis in small cell lung cancer. Genes Cancer. 2014;5:212–225. doi: 10.18632/genesandcancer.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albuquerque EX, Pereira EF, Alkondon M. et al. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue A, Nukiwa T. Gene mutations in lung cancer: Promising predictive factors for the success of molecular therapy. Plos Med. 2005;2:e13. doi: 10.1371/journal.pmed.0020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchetti A, Martella C, Felicioni L. et al. EGFR mutations in non-small-cell lung cancer: Analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol. 2005;23:857–865. doi: 10.1200/JCO.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 27.Pillai S, Rizwani W, Li X. et al. ID1 facilitates the growth and metastasis of Non-Small cell lung cancer in response to nicotinic acetylcholine receptor and epidermal growth factor receptor signaling. Mol Cell Biol. 2011;31:3052–3067. doi: 10.1128/MCB.01311-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng YJ, Tsai JW, Hsieh KC. et al. Id1 promotes lung cancer cell proliferation and tumor growth through Akt-related pathway. Cancer Lett. 2011;307:191–199. doi: 10.1016/j.canlet.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Langenfeld E, Deen M, Zachariah E. et al. Small molecule antagonist of the bone morphogenetic protein type I receptors suppresses growth and expression of Id1 and Id3 in lung cancer cells expressing Oct4 or nestin. Mol Cancer. 2013;12:129. doi: 10.1186/1476-4598-12-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nair S, Bora-Singhal N, Perumal D. et al. Nicotine-mediated invasion and migration of non-small cell lung carcinoma cells by modulating STMN3 and GSPT1 genes in an ID1-dependent manner. Mol Cancer. 2014;13:173. doi: 10.1186/1476-4598-13-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, McKay RM, Parada LF. Malignant glioma: Lessons from genomics, mouse models, and stem cells. Cell. 2012;149:36–47. doi: 10.1016/j.cell.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sachdeva R, Wu M, Smiljanic S. et al. ID1 is critical for tumorigenesis and regulates chemoresistance in glioblastoma. Cancer Res. 2019;79:4057–4071. doi: 10.1158/0008-5472.CAN-18-1357. [DOI] [PubMed] [Google Scholar]
- 33.Jin X, Jeon HM, Jin X. et al. The ID1-CULLIN3 axis regulates intracellular SHH and WNT signaling in glioblastoma stem cells. Cell Rep. 2016;16:1629–1641. doi: 10.1016/j.celrep.2016.06.092. [DOI] [PubMed] [Google Scholar]
- 34.Jin X, Jin X, Kim L. et al. Inhibition of ID1-BMPR2 intrinsic signaling sensitizes glioma stem cells to differentiation therapy. Clin Cancer Res. 2018;24:383–394. doi: 10.1158/1078-0432.CCR-17-1529. [DOI] [PubMed] [Google Scholar]
- 35.Liu Q, Zhang C, Yuan J. et al. PTK7 regulates Id1 expression in CD44-high glioma cells. Neuro Oncol. 2015;17:505–515. doi: 10.1093/neuonc/nou227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu J, Yang QY, Sai K. et al. TGM2 inhibition attenuates ID1 expression in CD44-high glioma-initiating cells. Neuro Oncol. 2013;15:1353–1365. doi: 10.1093/neuonc/not079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cook PJ, Thomas R, Kingsley PJ. et al. Cox-2-derived PGE2 induces Id1-dependent radiation resistance and self-renewal in experimental glioblastoma. Neuro Oncol. 2016;18:1379–1389. doi: 10.1093/neuonc/now049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anido J, Saez-Borderias A, Gonzalez-Junca A. et al. TGF-beta receptor inhibitors target the CD44(high)/Id1(high) Glioma-Initiating cell population in human glioblastoma. Cancer Cell. 2010;18:655–668. doi: 10.1016/j.ccr.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 39.Tang R, Hirsch P, Fava F. et al. High Id1 expression is associated with poor prognosis in 237 patients with acute myeloid leukemia. Blood. 2009;114:2993–3000. doi: 10.1182/blood-2009-05-223115. [DOI] [PubMed] [Google Scholar]
- 40.Tang R, Hirsch P, Fava F. et al. High Id1 expression is associated with poor prognosis in 237 patients with acute myeloid leukemia. Blood. 2009;114:2993–3000. doi: 10.1182/blood-2009-05-223115. [DOI] [PubMed] [Google Scholar]
- 41.Zhou JD, Yang L, Zhu XW. et al. Clinical significance of up-regulated ID1 expression in Chinese de novo acute myeloid leukemia. Int J Clin Exp Pathol. 2015;8:5336–5344. [PMC free article] [PubMed] [Google Scholar]
- 42.Damm F, Wagner K, Gorlich K. et al. ID1 expression associates with other molecular markers and is not an independent prognostic factor in cytogenetically normal acute myeloid leukaemia. Br J Haematol. 2012;158:208–215. doi: 10.1111/j.1365-2141.2012.09144.x. [DOI] [PubMed] [Google Scholar]
- 43.Lasorella A, Benezra R, Iavarone A. The ID proteins: Master regulators of cancer stem cells and tumour aggressiveness. Nat Rev Cancer. 2014;14:77–91. doi: 10.1038/nrc3638. [DOI] [PubMed] [Google Scholar]
- 44.Tam WF, Gu TL, Chen J. et al. Id1 is a common downstream target of oncogenic tyrosine kinases in leukemic cells. Blood. 2008;112:1981–1992. doi: 10.1182/blood-2007-07-103010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, Man N, Sun XJ. et al. Regulation of AKT signaling by Id1 controls t(8;21) leukemia initiation and progression. Blood. 2015;126:640–650. doi: 10.1182/blood-2015-03-635532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Man N, Sun X, Tan Y. et al. Differential role of Id1 in MLL-AF9-driven leukemia based on cell of origin. Blood. 2016;127:2322–2326. doi: 10.1182/blood-2015-11-677708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Gural A, Sun XJ. et al. The leukemogenicity of AML1-ETO is dependent on site-specific lysine acetylation. Science. 2011;333:765–769. doi: 10.1126/science.1201662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu WP, Scott SA, Dong WF. Induction of ID1 expression and apoptosis by the histone deacetylase inhibitor (trichostatin A) in human acute myeloid leukaemic cells. Cell Prolif. 2008;41:86–97. doi: 10.1111/j.1365-2184.2007.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teo WS, Nair R, Swarbrick A. New insights into the role of ID proteins in breast cancer metastasis: A MET affair. Breast Cancer Res. 2014;16:305. doi: 10.1186/bcr3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin DH, Park JH, Lee JY. et al. Overexpression of Id1 in transgenic mice promotes mammary basal stem cell activity and breast tumorigenesis. Oncotarget. 2015;6:17276–17290. doi: 10.18632/oncotarget.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swarbrick A, Roy E, Allen T. et al. Id1 cooperates with oncogenic Ras to induce metastatic mammary carcinoma by subversion of the cellular senescence response. Proc Natl Acad Sci U S A. 2008;105:5402–5407. doi: 10.1073/pnas.0801505105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wazir U, Jiang WG, Sharma AK. et al. The mRNA expression of inhibitors of DNA binding-1 and -2 is associated with advanced tumour stage and adverse clinical outcome in human breast cancer. Anticancer Res. 2013;33:2179–2183. [PubMed] [Google Scholar]
- 53.Mern DS, Hoppe-Seyler K, Hoppe-Seyler F. et al. Targeting Id1 and Id3 by a specific peptide aptamer induces E-box promoter activity, cell cycle arrest, and apoptosis in breast cancer cells. Breast Cancer Res Treat. 2010;124:623–633. doi: 10.1007/s10549-010-0810-6. [DOI] [PubMed] [Google Scholar]
- 54.Gurrapu S, Franzolin G, Fard D. et al. Reverse signaling by semaphorin 4C elicits SMAD1/5- and ID1/3-dependent invasive reprogramming in cancer cells. Sci Signal. 2019;12:v2041. doi: 10.1126/scisignal.aav2041. [DOI] [PubMed] [Google Scholar]
- 55.Gumireddy K, Li A, Kossenkov AV. et al. ID1 promotes breast cancer metastasis by S100A9 regulation. Mol Cancer Res. 2014;12:1334–1343. doi: 10.1158/1541-7786.MCR-14-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tobin NP, Sims AH, Lundgren KL. et al. Cyclin D1, Id1 and EMT in breast cancer. Bmc Cancer. 2011;11:417. doi: 10.1186/1471-2407-11-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Y, Ming J, Xu Y. et al. ERbeta1 inhibits the migration and invasion of breast cancer cells through upregulation of E-cadherin in a Id1-dependent manner. Biochem Biophys Res Commun. 2015;457:141–147. doi: 10.1016/j.bbrc.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 58.Sun X, Li C, Zhuang C. et al. Abl interactor 1 regulates Src-Id1-matrix metalloproteinase 9 axis and is required for invadopodia formation, extracellular matrix degradation and tumor growth of human breast cancer cells. Carcinogenesis. 2009;30:2109–2116. doi: 10.1093/carcin/bgp251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tominaga K, Shimamura T, Kimura N. et al. Addiction to the IGF2-ID1-IGF2 circuit for maintenance of the breast cancer stem-like cells. Oncogene. 2017;36:1276–1286. doi: 10.1038/onc.2016.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou XL, Zeng, Ye YH. et al. Prognostic values of the inhibitor of DNAbinding family members in breast cancer. Oncol Rep. 2018;40:1897–1906. doi: 10.3892/or.2018.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salomon R, Young L, Macleod D. et al. Probasin promoter-driven expression of ID1 is not sufficient for carcinogenesis in rodent prostate. J Histochem Cytochem. 2009;57:599–604. doi: 10.1369/jhc.2009.953182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma P, Patel D, Chaudhary J. Id1 and Id3 expression is associated with increasing grade of prostate cancer: Id3 preferentially regulates CDKN1B. Cancer Med. 2012;1:187–197. doi: 10.1002/cam4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ponz-Sarvise M, Castanon E, Panizo-Santos A. et al. Differential tumor expression of inhibitor of differentiation-1 in prostate cancer patients with extreme clinical phenotypes and prognostic implications. Clin Genitourin Cancer. 2014;12:87–93. doi: 10.1016/j.clgc.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 64.Ling YX, Tao J, Fang SF. et al. Downregulation of Id1 by small interfering RNA in prostate cancer PC3 cells in vivo and in vitro. Eur J Cancer Prev. 2011;20:9–17. doi: 10.1097/CEJ.0b013e32833ebaa0. [DOI] [PubMed] [Google Scholar]
- 65.Strong N, Millena AC, Walker L. et al. Inhibitor of differentiation 1 (Id1) and Id3 proteins play different roles in TGFbeta effects on cell proliferation and migration in prostate cancer cells. Prostate. 2013;73:624–633. doi: 10.1002/pros.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmidt M, Asirvatham AJ, Chaudhary J. Inhibitor of differentiation 1 (ID1) promotes cell survival and proliferation of prostate epithelial cells. Cell Mol Biol Lett. 2010;15:272–295. doi: 10.2478/s11658-010-0007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Z, Li K, Zhang X. et al. Effect of Id1 knockdown on formation of osteolytic bone lesions by prostate cancer PC3 cells in vivo. J Huazhong Univ Sci Technolog Med Sci. 2012;32:364–369. doi: 10.1007/s11596-012-0063-1. [DOI] [PubMed] [Google Scholar]
- 68.Gupta GP, Perk J, Acharyya S. et al. ID genes mediate tumor reinitiation during breast cancer lung metastasis. Proc Natl Acad Sci U S A. 2007;104:19506–19511. doi: 10.1073/pnas.0709185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geng H, Rademacher BL, Pittsenbarger J. et al. ID1 enhances docetaxel cytotoxicity in prostate cancer cells through inhibition of p21. Cancer Res. 2010;70:3239–3248. doi: 10.1158/0008-5472.CAN-09-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Darnel AD, Wang D, Ghabreau L. et al. Correlation between the presence of high-risk human papillomaviruses and Id gene expression in Syrian women with cervical cancer. Clin Microbiol Infect. 2010;16:262–266. doi: 10.1111/j.1469-0691.2009.02774.x. [DOI] [PubMed] [Google Scholar]
- 71.Li J, Xie L, Gan X. et al. Association of inhibitor of differentiation 1 expression with human papillomaviruses infections in cervical carcinoma. Int J Gynecol Cancer. 2011;21:1276–1281. doi: 10.1097/IGC.0b013e31821f7452. [DOI] [PubMed] [Google Scholar]
- 72.Xie L, Li J, Zhang Y. et al. Inhibitors of differentiation-1 promotes nitrosopyrrolidine-induced transformation of HPV 16-immortalized cervical epithelial cell. Cancer Sci. 2014;105:506–511. doi: 10.1111/cas.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schindl M, Oberhuber G, Obermair A. et al. Overexpression of Id-1 protein is a marker for unfavorable prognosis in early-stage cervical cancer. Cancer Res. 2001;61:5703–5706. [PubMed] [Google Scholar]
- 74.Wu Z, Li J, Zhang Y. et al. Synchronous coexpression of Id1 and nuclear NFkappaB p65 promotes cervical cancer progression and malignancy, and is associated with a poor prognosis and chemosensitivity. Oncol Rep. 2019;42:2075–2086. doi: 10.3892/or.2019.7301. [DOI] [PubMed] [Google Scholar]
- 75.Prates J, Franco-Salla GB, Dinarte DSA. et al. ANXA1Ac(2)(-)(2)(6) peptide reduces ID1 expression in cervical carcinoma cultures. Gene. 2015;570:248–254. doi: 10.1016/j.gene.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 76.Kebebew E. Id1 gene expression and regulation in human thyroid tissue. Thyroid. 2005;15:522–530. doi: 10.1089/thy.2005.15.522. [DOI] [PubMed] [Google Scholar]
- 77.Kebebew E, Peng M, Treseler PA. et al. Id1 gene expression is up-regulated in hyperplastic and neoplastic thyroid tissue and regulates growth and differentiation in thyroid cancer cells. J Clin Endocrinol Metab. 2004;89:6105–6111. doi: 10.1210/jc.2004-1234. [DOI] [PubMed] [Google Scholar]
- 78.Coppe JP, Smith AP, Desprez PY. Id proteins in epithelial cells. Exp Cell Res. 2003;285:131–145. doi: 10.1016/s0014-4827(03)00014-4. [DOI] [PubMed] [Google Scholar]
- 79.Kebebew E, Treseler PA, Duh QY. et al. The helix-loop-helix protein, Id-1, is overexpressed and regulates growth in papillary thyroid cancer. Surgery. 2003;134:235–241. doi: 10.1067/msy.2003.227. [DOI] [PubMed] [Google Scholar]
- 80.Ciarrocchi A, Piana S, Valcavi R. et al. Inhibitor of DNA binding-1 induces mesenchymal features and promotes invasiveness in thyroid tumour cells. Eur J Cancer. 2011;47:934–945. doi: 10.1016/j.ejca.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 81.Sun Y, Lai X, Yu Y. et al. Inhibitor of DNA binding 1 (Id1) mediates stemness of colorectal cancer cells through the Id1-c-Myc-PLAC8 axis via the Wnt/beta-catenin and Shh signaling pathways. Cancer Manag Res. 2019;11:6855–6869. doi: 10.2147/CMAR.S207167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lai X, Liao J, Lin W. et al. Inhibitor of DNA-binding protein 1 knockdown arrests the growth of colorectal cancer cells and suppresses hepatic metastasis in vivo. Oncol Rep. 2014;32:79–88. doi: 10.3892/or.2014.3172. [DOI] [PubMed] [Google Scholar]
- 83.Zhang N, Subbaramaiah K, Yantiss RK. et al. Id1 deficiency protects against tumor formation in Apc(Min/+) mice but not in a mouse model of Colitis-Associated colon cancer. Cancer Prev Res (Phila) 2015;8:303–311. doi: 10.1158/1940-6207.CAPR-14-0411. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Ullmann P, Rodriguez F, Schmitz M. et al. The miR-371 approximately 373 Cluster Represses Colon Cancer Initiation and Metastatic Colonization by Inhibiting the TGFBR2/ID1 Signaling Axis. Cancer Res. 2018;78:3793–3808. doi: 10.1158/0008-5472.CAN-17-3003. [DOI] [PubMed] [Google Scholar]
- 85.O'Brien CA, Kreso A, Ryan P. et al. ID1 and ID3 regulate the self-renewal capacity of human colon cancer-initiating cells through p21. Cancer Cell. 2012;21:777–792. doi: 10.1016/j.ccr.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 86.Qiu L, Tan X, Lin J. et al. CDC27 induces metastasis and invasion in colorectal cancer via the promotion of Epithelial-To-Mesenchymal transition. J Cancer. 2017;8:2626–2635. doi: 10.7150/jca.19381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu H, Yue X, Zhao Y. et al. LIF negatively regulates tumour-suppressor p53 through Stat3/ID1/MDM2 in colorectal cancers. Nat Commun. 2014;5:5218. doi: 10.1038/ncomms6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Damdinsuren B, Nagano H, Kondo M. et al. Expression of Id proteins in human hepatocellular carcinoma: Relevance to tumor dedifferentiation. Int J Oncol. 2005;26:319–327. [PubMed] [Google Scholar]
- 89.Kwon YC, Sasaki R, Meyer K. et al. Hepatitis c virus core protein modulates endoglin (CD105) signaling pathway for liver pathogenesis. J Virol. 2017;91:e1217–e1235. doi: 10.1128/JVI.01235-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matsuda Y, Yamagiwa S, Takamura M. et al. Overexpressed Id-1 is associated with a high risk of hepatocellular carcinoma development in patients with cirrhosis without transcriptional repression of p16. Cancer-Am Cancer Soc. 2005;104:1037–1044. doi: 10.1002/cncr.21259. [DOI] [PubMed] [Google Scholar]
- 91.Hampl R, Picha J, Chundela B. et al. Radioimmunoassay of nortestosterone and related steroids. J Clin Chem Clin Biochem. 1979;17:529–532. doi: 10.1515/cclm.1979.17.8.529. [DOI] [PubMed] [Google Scholar]
- 92.Ding R, Han S, Lu Y. et al. Overexpressed Id-1 is associated with patient prognosis and HBx expression in hepatitis B virus-related hepatocellular carcinoma. Cancer Biol Ther. 2010;10:299–307. doi: 10.4161/cbt.10.3.12454. [DOI] [PubMed] [Google Scholar]
- 93.Sharma BK, Kolhe R, Black SM. et al. Inhibitor of differentiation 1 transcription factor promotes metabolic reprogramming in hepatocellular carcinoma cells. Faseb J. 2016;30:262–275. doi: 10.1096/fj.15-277749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ao J, Meng J, Zhu L. et al. Activation of androgen receptor induces ID1 and promotes hepatocellular carcinoma cell migration and invasion. Mol Oncol. 2012;6:507–515. doi: 10.1016/j.molonc.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Niu LL, Cheng CL, Li MY. et al. ID1-induced p16/IL6 axis activation contributes to the resistant of hepatocellular carcinoma cells to sorafenib. Cell Death Dis. 2018;9:852. doi: 10.1038/s41419-018-0926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Young VJ, Ahmad SF, Brown JK. et al. Peritoneal VEGF-A expression is regulated by TGF-beta1 through an ID1 pathway in women with endometriosis. Sci Rep. 2015;5:16859. doi: 10.1038/srep16859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lyden D, Young AZ, Zagzag D. et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401:670–677. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- 98.Xiao F, Qiu H, Cui H. et al. MicroRNA-885-3p inhibits the growth of HT-29 colon cancer cell xenografts by disrupting angiogenesis via targeting BMPR1A and blocking BMP/Smad/Id1 signaling. Oncogene. 2015;34:1968–1978. doi: 10.1038/onc.2014.134. [DOI] [PubMed] [Google Scholar]
- 99.Wu XL, Xue J, Wang LK. et al. Expressions of inhibitors of DNA binding-1 and matrix metalloproteinase-9 in colorectal adenocarcinoma tissues and their correlations with microvessel density. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2016;38:696–701. doi: 10.3881/j.issn.1000-503X.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 100.Fong S, Itahana Y, Sumida T. et al. Id-1 as a molecular target in therapy for breast cancer cell invasion and metastasis. Proc Natl Acad Sci U S A. 2003;100:13543–13548. doi: 10.1073/pnas.2230238100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Minn AJ, Gupta GP, Siegel PM. et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gumireddy K, Li A, Gimotty PA. et al. KLF17 is a negative regulator of epithelial-mesenchymal transition and metastasis in breast cancer. Nat Cell Biol. 2009;11:1297–1304. doi: 10.1038/ncb1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brabletz T. To differentiate or not-routes towards metastasis. Nat Rev Cancer. 2012;12:425–436. doi: 10.1038/nrc3265. [DOI] [PubMed] [Google Scholar]
- 104.Stankic M, Pavlovic S, Chin Y. et al. TGF-beta-Id1 signaling opposes Twist1 and promotes metastatic colonization via a mesenchymal-to-epithelial transition. Cell Rep. 2013;5:1228–1242. doi: 10.1016/j.celrep.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cubillo E, Diaz-Lopez A, Cuevas EP. et al. E47 and Id1 interplay in epithelial-mesenchymal transition. Plos One. 2013;8:e59948. doi: 10.1371/journal.pone.0059948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li L, Wei X, Wu B. et al. SiRNA-mediated knockdown of ID1 disrupts Nanog- and Oct-4-mediated cancer stem cell-likeness and resistance to chemotherapy in gastric cancer cells. Oncol Lett. 2017;13:3014–3024. doi: 10.3892/ol.2017.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang RS, Zheng YL, Zhao J. et al. MicroRNA-381 suppresses the growth and increases cisplatin sensitivity in non-small cell lung cancer cells through inhibition of nuclear factor-kappaB signaling. Biomed Pharmacother. 2018;98:538–544. doi: 10.1016/j.biopha.2017.12.092. [DOI] [PubMed] [Google Scholar]
- 108.Cheng Y, Lee Y, Chiu W. et al. High Id1 expression, a generally negative prognostic factor, paradoxically predicts a favorable prognosis for adjuvant paclitaxel plus cisplatin therapy in surgically treated lung cancer patients. Oncotarget. 2014;5:11564. doi: 10.18632/oncotarget.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tan HY, Wang N, Chan YT. et al. ID1 overexpression increases gefitinib sensitivity in non-small cell lung cancer by activating RIP3/MLKL-dependent necroptosis. Cancer Lett. 2020;475:109–118. doi: 10.1016/j.canlet.2020.01.025. [DOI] [PubMed] [Google Scholar]
- 110.Yin X, Tang B, Li JH. et al. ID1 promotes hepatocellular carcinoma proliferation and confers chemoresistance to oxaliplatin by activating pentose phosphate pathway. J Exp Clin Cancer Res. 2017;36:166. doi: 10.1186/s13046-017-0637-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Francipane MG, Bulanin D, Lagasse E. Establishment and characterization of 5-Fluorouracil-Resistant human colorectal cancer Stem-Like cells: Tumor dynamics under selection pressure. Int J Mol Sci. 2019. 20. [DOI] [PMC free article] [PubMed]
- 112.Przybyla T, Sakowicz-Burkiewicz M, Maciejewska I. et al. Suppression of ID1 expression in colon cancer cells increases sensitivity to 5-fluorouracil. Acta Biochim Pol. 2017;64:315–322. doi: 10.18388/abp.2016_1421. [DOI] [PubMed] [Google Scholar]
- 113.Romano G, Santi L, Bianco MR. et al. The TGF-beta pathway is activated by 5-fluorouracil treatment in drug resistant colorectal carcinoma cells. Oncotarget. 2016;7:22077–22091. doi: 10.18632/oncotarget.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li B, Xu WW, Guan XY. et al. Competitive binding between id1 and E2F1 to cdc20 regulates E2F1 degradation and thymidylate synthase expression to promote esophageal cancer chemoresistance. Clin Cancer Res. 2016;22:1243–1255. doi: 10.1158/1078-0432.CCR-15-1196. [DOI] [PubMed] [Google Scholar]
- 115.Cook PJ, Thomas R, Kingsley PJ. et al. Cox-2-derived PGE2 induces Id1-dependent radiation resistance and self-renewal in experimental glioblastoma. Neuro Oncol. 2016;18:1379–1389. doi: 10.1093/neuonc/now049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guo Q, Guo P, Mao Q. et al. ID1 affects the efficacy of radiotherapy in glioblastoma through inhibition of DNA repair pathways. Med Oncol. 2013;30:325. doi: 10.1007/s12032-012-0325-6. [DOI] [PubMed] [Google Scholar]
- 117.Lyden D, Young AZ, Zagzag D. et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401:670–677. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- 118.Yap WN, Zaiden N, Tan YL. et al. Id1, inhibitor of differentiation, is a key protein mediating anti-tumor responses of gamma-tocotrienol in breast cancer cells. Cancer Lett. 2010;291:187–199. doi: 10.1016/j.canlet.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 119.Cho Y, Cho EJ, Lee JH. et al. Fucoidan-induced ID-1 suppression inhibits the in vitro and in vivo invasion of hepatocellular carcinoma cells. Biomed Pharmacother. 2016;83:607–616. doi: 10.1016/j.biopha.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 120.Tsang CM, Cheung KC, Cheung YC. et al. Berberine suppresses Id-1 expression and inhibits the growth and development of lung metastases in hepatocellular carcinoma. Biochim Biophys Acta. 2015;1852:541–551. doi: 10.1016/j.bbadis.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 121.Jia Y, Wang Z, Zang A. et al. Tetramethylpyrazine inhibits tumor growth of lung cancer through disrupting angiogenesis via BMP/Smad/Id-1 signaling. Int J Oncol. 2016;48:2079–2086. doi: 10.3892/ijo.2016.3443. [DOI] [PubMed] [Google Scholar]
- 122.Stutz E, Gautschi O, Fey MF. et al. Crizotinib inhibits migration and expression of ID1 in MET-positive lung cancer cells: Implications for MET targeting in oncology. Future Oncol. 2014;10:211–217. doi: 10.2217/fon.13.179. [DOI] [PubMed] [Google Scholar]
- 123.Singer E, Judkins J, Salomonis N. et al. Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 2015;6:e1601. doi: 10.1038/cddis.2014.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang J, Yan B, Liu SM. et al. Transcriptomic and functional pathway analysis of human cervical carcinoma cancer cells response to microtubule inhibitor. J Cancer. 2015;6:930–937. doi: 10.7150/jca.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mistry H, Hsieh G, Buhrlage SJ. et al. Small-molecule inhibitors of USP1 target ID1 degradation in leukemic cells. Mol Cancer Ther. 2013;12:2651–2662. doi: 10.1158/1535-7163.MCT-13-0103-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Williams SA, Maecker HL, French DM. et al. USP1 deubiquitinates ID proteins to preserve a mesenchymal stem cell program in osteosarcoma. Cell. 2011;146:918–930. doi: 10.1016/j.cell.2011.07.040. [DOI] [PubMed] [Google Scholar]