Abstract
Purpose
The aim was to compare outcomes in adult patients with inflammatory bowel disease (IBD) who switched to infliximab-dyyb with those who remained on reference product (RP) infliximab in the United States (US) in a retrospective, propensity score-matched, non-inferiority cohort trial.
Methods
This study was a retrospective, non-inferiority study conducted within a US integrated healthcare system and included adult patients with a confirmed diagnosis of Crohn’s disease or ulcerative colitis. A 1:1 propensity score matching was utilized to match patients who switched to infliximab-dyyb during the period April 2016–March 2018 to patients who remained on RP infliximab. The non-inferiority margin was set at + 10% of the upper limit. The primary outcome was a composite measure of disease worsening requiring acute care after the index date of switching to infliximab-dyyb or continuing RP infliximab. Disease worsening requiring acute care was defined as any IBD-related emergency room visit, hospitalization, or surgery. The secondary outcome was the composite measure of disease worsening requiring acute care or treatment failure. A switch to another biologic or tofacitinib was a proxy for treatment failure. All patients were followed for up to 9 months.
Results
After propensity score matching, the matched cohort included 1409 patients in the infliximab-dyyb group and 1409 patients in the RP infliximab group. The overall mean age (± standard deviation) was 47.7 ± 17.0 years, 50.9% of patients were of male gender, and 51.8% of patients had Crohn’s disease, while the remainder of the cohort had ulcerative colitis. There were 144 patients (10.2%) in the infliximab-dyyb group and 245 patients (17.4%) in the RP infliximab group who experienced disease worsening requiring acute care (P < 0.01 for non-inferiority). There were 347 patients (24.6%) in the infliximab-dyyb group who experienced disease worsening requiring acute care or treatment failure compared to 375 patients (26.6%) who remained on RP infliximab (P < 0.01 for non-inferiority).
Conclusion
There was no increased risk of (1) disease worsening requiring acute care or (2) disease worsening requiring acute care or treatment failure in patients with IBD who switched from RP infliximab to infliximab-dyyb when compared to patients who remained on RP infliximab in this US population. Infliximab-dyyb is an option for patients with IBD who need to use RP infliximab.
Key Points
| The indication for use of infliximab-dyyb in inflammatory bowel disease (IBD) was approved based on extrapolation of data from reference product (RP) infliximab by the US Food and Drug Administration. |
| This study evaluated the outcomes of patients with IBD who switched from RP infliximab to the biosimilar, infliximab-dyyb. |
| Comparing patients in the infliximab-dyyb group to a matched group of patients with IBD who remained on RP infliximab, the study demonstrated that infliximab-dyyb was non-inferior to RP infliximab in a real-world setting in the United States. |
| The biosimilar infliximab-dyyb is another treatment option for patients with IBD as it has similar effectiveness to RP infliximab. |
Introduction
It was estimated that 1.3%, or 3 million, of Americans were suffering from inflammatory bowel disease (IBD) in 2015 [1]. The incidence of Crohn’s disease (CD) and ulcerative colitis (UC) in North America was 3.1–20.2 cases per 200,000 person-years and 2.2–19.2 cases per 100,000 person-years, respectively, and was believed to be higher than in other countries [2]. Most patients have disease onset around the age of 30–40 years and require medication for long-term management in order to achieve and maintain remission [1, 3]. Patients with IBD may experience relapses and accrue high healthcare costs due to frequent hospitalizations and surgeries. A recent study reported an additional mean annual medical expenditure of US$23,000 per patient diagnosed with IBD between 2007 and 2016 [4], and there was a trend of increasing annual costs of care between 2014 and 2019 [5]. Mild to moderate disease is typically managed by aminosalicylates [3, 6]. It was estimated that 1–2% of UC patients have severe disease, while 11% of CD patients have chronically active disease [7]. These severe patients are typically managed by costly agents, such as anti-tumor necrosis factor agents like infliximab [3, 6]. Infliximab reference product (RP) (Remicade™, Janssen Biotech, Horsham, PA, USA) [8] was approved in 1999 for use in CD and was later approved for UC based on efficacy demonstrated in pivotal trials [9, 10]. Infliximab has been recommended by the American College of Gastroenterology for use in severe IBD to induce remission [11, 12]. A reduction in hospitalizations and surgical interventions has been demonstrated in patients with IBD who were treated with infliximab [13]. Since infliximab is a long-term maintenance therapy for these patients, it is often a cost burden for these patients. The recently approved biosimilar infliximab-dyyb (Inflectra®, Pfizer, NY, NY) [14] in the United States (US) is a less costly alternative to RP infliximab and may help alleviate some of this burden for patients with IBD.
Biosimilars have been used for over a decade in Europe; the first biosimilar approved by the European Medicines Agency (EMA) was somatropin (Omnitrope®, Sandoz GmbH, Austria) in 2006 [15]. However, the first biosimilar, filgrastim-sndz (Zarxio®, Sandoz, Princeton, NJ, USA), was not approved by the US Food and Drug Administration (FDA) until 2015 [16]. Biological products (or biologics), such as RP infliximab, are a growing class of therapeutic agents for various disease states. According to the FDA, biosimilars are highly similar to and have no clinically meaningful differences from the existing FDA-approved RPs [17]. Consequently, biosimilars have the potential to increase patient access to these therapeutic options at reduced costs as a result of competition.
Infliximab-dyyb, a monoclonal antibody biosimilar, received approval from the EMA in 2013 [15] and from the FDA in 2016 [16]. Its label carried all adult indications of the RP infliximab. The indication of use in IBD was approved via extrapolation, which implied infliximab-dyyb was approved for an indication of the RP infliximab even if it was not directly studied for that indication. The FDA states that extrapolation is based on all available data from analytical, animal, and clinical pharmacology studies, previous findings of safety and efficacy for other approved indications, and scientific justification [18]. The absence of regulatory trial data for infliximab-dyyb use in IBD has created concerns regarding safety and efficacy amongst gastroenterologists and patients in the US [19]. More specifically, there has been more hesitance surrounding switching patients who were stable on RP infliximab to infliximab-dyyb, as opposed to initiating patients on infliximab-dyyb for IBD. Two prospective randomized controlled non-inferiority studies on switching RP infliximab to infliximab-dyyb have been published. In the NOR-SWITCH study, patients who switched from RP infliximab to infliximab-dyyb were compared to those who remained on RP infliximab in study centers in Norway. The study enrolled 482 patients with CD, UC, rheumatoid arthritis, psoriatic arthritis, and chronic plaque psoriasis. The authors concluded switching to infliximab-dyyb was non-inferior to patients who remained on RP infliximab [20]. The second trial, by Ye et al., reported interim results (at 30 weeks) of a study which enrolled 220 patients with CD from 16 countries who were randomly assigned to initiate either RP infliximab or infliximab-dyyb [21]. The study demonstrated that the two therapies were non-inferior to each other at week 6, 14, and 30 on clinical efficacy and safety. Half of the patients in each arm would have the therapy switched at week 30, but the results have not been presented yet. Recently, more studies have been published regarding the experience of patients with IBD who switched to infliximab-dyyb from RP infliximab [22–34]. These included one meta-analysis [22], many single group observational studies [23–32], and two studies with comparison groups [33, 34]. The length of follow-up ranged from 4 months [23] to 5 years [24]. Patients with IBD were recruited from Europe [25–34] and Asia [23, 24]. All except one study [34] have demonstrated no differences or non-inferiority in effectiveness and safety between RP infliximab and infliximab-dyyb.
Due to the lack of US-based data and a lack of independent comparison groups in most of the published studies [23–32], the aim of the current study was to compare the real-world effectiveness of switching from RP infliximab to infliximab-dyyb versus remaining on RP infliximab in patients with IBD within a US integrated healthcare system using a non-inferiority propensity score-matched cohort design.
Methods
Study Design
This was a retrospective, non-inferiority cohort study conducted within Kaiser Permanente Northern California (KPNC) and Southern California (KPSC) regions. Kaiser Permanente is a national integrated healthcare system that serves over 12 million members in the US; among them, 9 million members are in the KPNC and KPSC regions in California. Members receive hospital, ambulatory, pharmacy, and ancillary care services within the same network, and data are captured for all services in an electronic medical record system. This study was approved by the KPNC and KPSC institutional review boards, and a waiver of informed consent was received.
Study Population
Electronic databases were used to identify eligible patients who received RP infliximab or infliximab-dyyb during the period January 1, 2013 through March 31, 2018, the cohort identification period. The index date was defined as the first date of infliximab-dyyb infusion for patients who switched from RP infliximab to infliximab-dyyb during the time period, while the index date for the RP infliximab group was a randomly selected infusion date during the cohort identification period. Patients were included if they were age 18 years or older, had a diagnosis of either CD or UC within 6 months prior to the index date, and had received RP infliximab for at least 3 months. IBD diagnosis was identified based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM 555.x and 556.x) or the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM K50.x and K51.x).
Each patient who switched from RP infliximab to infliximab-dyyb was matched to a unique patient who remained on RP infliximab using propensity score at a 1:1 ratio. Propensity score matching [35] was used to minimize the difference of each confounding variable between patients who switched to infliximab-dyyb and patients who remained on RP infliximab, by using a nearest-neighbor matching method without replacement. The score was calculated using the following variables: age within 2 years of index date, gender, white race, type of IBD (UC or CD), IBD-related surgery within 2 years of index date, Charlson Comorbidity Index (CCI) calculated using data from 12 months prior to index date, dose of RP infliximab or infliximab-dyyb on the index date, and use of IBD medications including immunomodulators, non-infliximab biologics, or tofacitinib within 6 months prior to the index date. IBD-related surgeries that were evaluated included colectomy, colostomy, hemicolectomy, ileostomy, ileocolectomy, and proctocolectomy. Immunomodulators included azathioprine, basiliximab, cyclosporine, hydroxychloroquine, mercaptopurine, methotrexate, mycophenolate, sirolimus, and tacrolimus. Non-infliximab biologics included abatacept, adalimumab, belatacept, belimumab, certolizumab, etanercept, glatiramer, golimumab, natalizumab, tocilizumab, ocrelizumab, ustekinumab, and vedolizumab. Patients without continuous enrollment and drug benefit for at least 6 months immediately before the index date were excluded. Patients were followed until a switch in RP infliximab or infliximab-dyyb therapy, end of membership, death, or up to 9 months, whichever occurred first. A follow-up period of 9 months was selected because the data collection concluded on December 31, 2018. Other covariables were collected on the cohort, including length of IBD history, length and frequency of RP infliximab usage prior to the index date, history of tobacco use, and recent or concomitant usage of aminosalicylates and systemic steroid, defined as in excess of 1800 mg prednisone dose equivalents within 6 months prior to the index date (more than 10 mg prednisone dose equivalents per day for 6 months).
Outcome Measures and Definitions
The primary outcome was a composite endpoint of disease worsening requiring acute care during study follow-up and was defined as IBD-related emergency room visits, hospitalizations, or surgeries. The secondary outcome was a composite endpoint of disease worsening requiring acute care or treatment failure. Treatment failure was defined as a patient switching to tofacitinib or other biologics, which included abatacept, adalimumab, belatacept, belimumab, certolizumab, etanercept, glatiramer, golimumab, natalizumab, tocilizumab, ocrelizumab, ustekinumab, vedolizumab, and RP infliximab (study group only).
Statistical Analysis
Based on results from the NOR-SWITCH study [20], we assumed 36% of patients with CD and 12% of patients with UC would experience disease worsening; 485 patients with CD and 222 patients with UC in each group would be required to detect a 10% non-inferiority upper limit margin. While the NOR-SWITCH study used a 15% margin, we chose a smaller non-inferiority upper limit margin at 10% because of the retrospective nature of the study. To compare baseline characteristics, Chi-square tests were used for categorical variables and t tests were used for continuous variables. If non-inferiority in either the primary or secondary outcome between the two groups was met, two-tailed testing using Chi-square tests would be used. Conditional logistic regression was performed to estimate the adjusted odds ratio (OR) for both primary and secondary outcomes comparing patients who switched to infliximab-dyyb and patients who remained on RP infliximab. The regression adjusted for age, gender, white race, CCI, tobacco use, Kaiser Permanente region (KPNC vs. KPSC), type of IBD, length of IBD history, RP infliximab or infliximab-dyyb dose on index date, number of RP infliximab infusions within the prior 3 months, disease worsening requiring acute care within the prior 6 months, and use of aminosalicylates, immunomodulators, biological products, and systemic steroids (excluding oral budesonide) in excess of 1800 mg prednisone dose equivalents within the prior 6 months, which was a proxy for a patient requiring prednisone as maintenance therapy.
In addition, subgroup analysis was repeated for all the outcomes against the matched patients who were not exposed to systemic steroids, defined as in excess of 1800 mg prednisone dose equivalents within 6 months prior to index date. The analysis was performed because patients exposed to steroids were more likely to be in the RP infliximab group, and we were unable to balance the difference by propensity score matching. All statistical analyses were performed using SAS statistical software version 9.4 (SAS Institute, Cary, NC). A significance level of 0.05 was used for all analyses.
Results
Patients
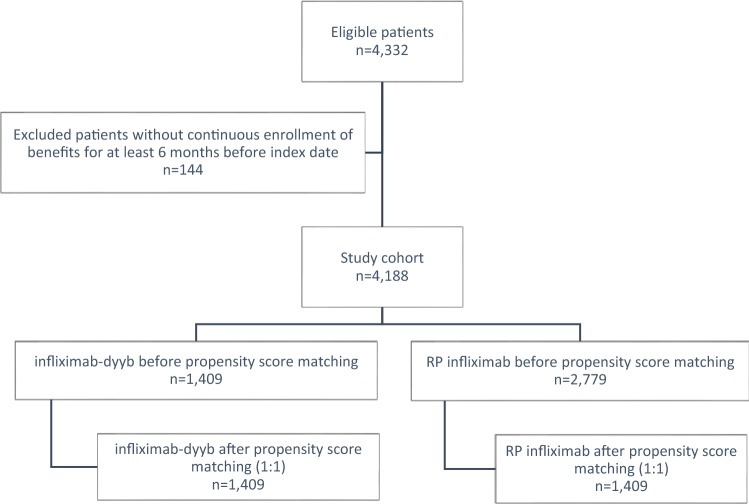
After applying eligibility criteria, 4188 patients were identified for the study cohort (Fig. 1). Among them, there were 1409 patients who switched to infliximab-dyyb and 2779 patients in the RP infliximab group. The patients in the infliximab-dyyb group were older (mean age ± standard deviation, 47.8 ± 16.9 vs. 42.6 ± 16.9 years old, P < 0.01), had a higher proportion of male patients (52% vs. 48%, P = 0.04), a lower rate of recent or concomitant use of immunomodulators (29% vs. 32%, P = 0.01), and more patients on a higher dose of infliximab-dyyb on index date (P < 0.01) when compared to the RP infliximab group. The baseline characteristics of the pre-matched cohort are summarized in Table 1.
Fig. 1.
Cohort flow diagram. RP reference product
Table 1.
Comparison of baseline characteristics for patients with inflammatory bowel disease (IBD) in the infliximab-dyyb group versus reference product (RP) infliximab group before propensity score matching (PSM) and after PSM
| Baseline characteristic | Infliximab-dyyb (n = 1409) | Before PSM | After PSM | |||
|---|---|---|---|---|---|---|
| RP infliximab (n = 2779) | P value | RP infliximab (n = 1409) | P value | |||
| Matched variables | Age, mean ± SD | 47.8 ± 16.9 | 42.6 ± 16.9 | < 0.01 | 47.5 ± 17.1 | 0.61 |
| Male | 727 (52) | 1340 (48) | 0.04 | 708 (50) | 0.47 | |
| White race | 940 (67) | 1886 (68) | 0.15 | 986 (70) | 0.06 | |
| CCI, median (IQR) | 0 (0.1) | 0 (0.1) | < 0.01 | 0 (0.1) | 0.55 | |
| Crohn’s disease | 728 (52) | 1528 (55) | 0.04 | 731 (52) | 0.91 | |
| Ulcerative colitis | 681 (48) | 1251 (45) | 678 (48) | |||
| Recent IBD-related surgery | 35 (2.5) | 56 (2.0) | 0.33 | 28 (2.0) | 0.37 | |
| Recent/concomitant IBD medication | ||||||
| Immunomodulator | 403 (29) | 899 (32) | 0.01 | 400 (28) | 0.90 | |
| Non-infliximab biologic or tofacitinib | 18 (1) | 133 (5) | < 0.01 | 27 (2) | 0.18 | |
| Dose at index date | ||||||
| < 300 mg | 29 (2) | 113 (4) | < 0.01 | 35 (2) | 0.70 | |
| 300–500 mg | 998 (71) | 2059 (74) | 1002 (71) | |||
| > 500 mg | 382 (27) | 607 (22) | 372 (26) | |||
| Unmatched variables | Length of IBD history (years), mean ± SD | 8.1 ± 5.8 | 5.7 ± 5.4 | < 0.01 | 7.0 ± 5.6 | < 0.01 |
| Infliximab use (years), mean ± SD | 3.3 ± 2.4 | 1.6 ± 1.9 | < 0.01 | 2.2 ± 2.0 | < 0.01 | |
| Currently or past history of smoking | 481 (34) | 988 (36) | 0.36 | 544 (39) | 0.01 | |
| Use of steroida | 60 (4) | 916 (33) | < 0.01 | 197 (14) | < 0.01 | |
| Recent/concomitant use of aminosalicylates | 299 (21) | 1069 (38) | < 0.01 | 359 (25) | < 0.02 | |
Values are n (%) unless otherwise stated
Immunomodulators included azathioprine, basiliximab, cyclosporine, hydroxychloroquine, mercaptopurine, methotrexate, mycophenolate, sirolimus, and tacrolimus
Non-infliximab biologics included abatacept, adalimumab, belatacept, belimumab, certolizumab, etanercept, glatiramer, golimumab, natalizumab, tocilizumab, ocrelizumab, ustekinumab, and vedolizumab
CCI Charlson Comorbidity Index, IQR interquartile range, SD standard deviation
aUse of steroids = a cumulative of > 1800 mg prednisone dose equivalents of systemic steroid within 6 months prior to index date
After propensity score matching, the matched cohort included 1409 patients in the study group and 1409 patients in the control group. There were no differences between the two groups in the matched variables, which included age, gender, race, CCI, type of IBD, recent IBD-related surgeries, recent or concomitant IBD maintenance medications, and dose of infliximab on index date (Table 1). The overall mean age for the final study cohort (n = 2818) was 47.7 ± 17.0 years, and 50.9% of patients were male. In terms of the type of IBD, 51.8% of patients had CD, while the remainder of the cohort had UC. The patients were generally healthy, with an overall median (interquartile range) CCI of 0 (0–1), with 2.2% who had prior IBD-related surgery. However, there were differences in baseline characteristics for variables that could not be matched (Table 1). Patients in the infliximab-dyyb group had been using RP infliximab longer (3.3 ± 2.4 vs. 2.2 ± 2.0 years, P < 0.01) and had a longer history of IBD disease (8.1 ± 5.8 vs. 7.0 ± 5.6 years, P < 0.01). A greater proportion of patients in the RP infliximab group had a history of tobacco use (39% vs. 34%, RP infliximab vs. infliximab-dyyb, P = 0.01), previous use of steroids as maintenance therapy (14% vs. 4%, P < 0.01), and previous use of aminosalicylates (25% vs. 21%, P < 0.02). Approximately 93% of the patients were followed for the entire 9-month follow-up period.
Primary Outcome
There were 144 patients (10.2%) in the infliximab-dyyb group who experienced disease worsening requiring acute care compared to 245 patients (17.4%) in the RP infliximab group (P < 0.01 for non-inferiority). Non-inferiority was met, as the difference in the proportion of patients with the composite endpoint for disease worsening requiring acute care (defined as patients who experienced IBD-related hospitalizations, emergency room visits, or surgery) was − 7.2%, which fell within the prespecified non-inferiority margin of + 10%. In the two-tailed analysis, there was a significantly lower proportion of patients who switched to infliximab-dyyb that experienced disease worsening requiring acute care compared to patients who remained on RP infliximab (P < 0.01) (Table 2). We further broke down the composite endpoints into each individual event. A significantly lower proportion of patients who switched to infliximab-dyyb experienced IBD-related hospitalizations (1.4% vs. 3.4%, P < 0.01), emergency room visits (10.0% vs. 15.7%, P < 0.01), and surgeries (0.9% vs. 4.0%, P < 0.01) when compared to the RP infliximab group.
Table 2.
Unadjusted outcomes: composite endpoint of disease worsening requiring acute care and composite endpoint of disease worsening or treatment failure
| Infliximab-dyyb (n = 1409) | RP infliximab (n = 1409) | Non-inferiority test P value | Superiority test P value | |
|---|---|---|---|---|
| Composite endpoint of IBD worsening requiring acute carea, n (%) | 144 (10.2) | 245 (17.4) | < 0.01 | < 0.01 |
| Hospitalization | 20 (1.4) | 48 (3.4) | < 0.01 | < 0.01 |
| Emergency room visit | 141 (10.0) | 221 (15.7) | < 0.01 | < 0.01 |
| Surgery | 12 (0.9) | 57 (4.0) | < 0.01 | < 0.01 |
| Composite endpoint of IBD worsening requiring acute care or treatment failureb, n (%) | 347 (24.6) | 375 (26.6) | < 0.01 | 0.23 |
| Switching therapy | 221 (15.7) | 163 (11.6) | < 0.01 | < 0.01 |
IBD inflammatory bowel disease, RP reference product
aComposite endpoint of IBD worsening requiring acute care was defined as patients who experienced IBD-related hospitalization, emergency room visit, or surgery
bComposite endpoint of IBD worsening requiring acute care or treatment failure was defined as patients who experienced IBD-related hospitalization, emergency room visit, surgery, or switching IBD therapy
In the conditional logistic regression comparing patients who switched to infliximab-dyyb with patients who remained on RP infliximab, patients in the infliximab-dyyb group were 50% less likely to experience disease worsening requiring acute care (OR = 0.50; 95% CI 0.36–0.68, P < 0.01) (Table 3). Significant positive predictors of disease worsening requiring acute care in the study cohort included higher CCI (OR = 1.46; 95% CI 1.14–1.85, P < 0.01), use of acute care within 6 months before index date (OR = 2.28; 95% CI 1.40–3.72, P < 0.01), and steroid use in excess of 1800 mg of prednisone dose equivalents within 6 months prior to index date (OR = 3.29; 95% CI 1.43–7.57, P < 0.01). There was no difference in disease worsening rate between UC and CD (OR = 0.93; 95% CI 0.63–1.36, P = 0.70).
Table 3.
Adjusted odds ratio of composite endpoint of disease worsening requiring acute care (primary outcome) and composite endpoint of disease worsening or treatment failure (secondary outcome) using conditional logistic regression
| Composite endpoint of disease worsening requiring acute care (primary outcome) | Composite endpoint of disease worsening requiring acute care or treatment failure (secondary outcome) | |||
|---|---|---|---|---|
| Odds ratio, 95% CI | P value | Odds ratio, 95% CI | P value | |
| Infliximab-dyyb vs. RP infliximab | 0.50 (0.36–0.68) | < 0.01 | 0.95 (0.77–1.17) | 0.63 |
| CCI (reference = 0) | 1.46 (1.14–1.85) | < 0.01 | 1.11 (0.95–1.30) | 0.19 |
| Use of IBD-related acute care within prior 6 months | 2.28 (1.40–3.72) | < 0.01 | 1.93 (1.33–2.78) | < 0.01 |
| Use of steroid within prior 6 monthsa | 3.29 (1.43–7.57) | < 0.01 | 2.22 (1.28–3.84) | < 0.01 |
| Ulcerative colitis (vs. Crohn’s disease) | 0.93 (0.63–1.36) | 0.70 | 0.94 (0.71–1.24) | 0.64 |
Additional covariables in the conditional logistic regression included age, sex, race, smoking status, Kaiser Permanente region (KPNC vs. KPSC), length of IBD history, infliximab dose on index date, number of RP infliximab infusions within the prior 3 months, and use of aminosalicylates, immunomodulators, or biological products within prior 6 months
CCI Charlson Comorbidity Index, CI confidence interval, IBD inflammatory bowel disease, KPNC Kaiser Permanente Northern California, KPSC Kaiser Permanente Southern California, RP reference product
aUse of steroids = a cumulative of > 1800 mg prednisone dose equivalents of systemic steroid within 6 months prior to index date
Secondary Outcome
There were 347 patients (24.6%) in the infliximab-dyyb group who experienced disease worsening requiring acute care or treatment failure compared to 375 patients (26.6%) who remained on RP infliximab (Table 2). In the non-inferiority analysis, the difference in the proportion of patients who achieved the primary outcome was − 2.0%, which was within the prespecified + 10% non-inferiority margin (P < 0.01). In addition, a two-tailed analysis was performed which showed no statistical differences between the two groups (P = 0.23). Although more patients in the infliximab-dyyb group switched therapy to another biologic compared to the RP infliximab group (15.7% vs. 11.6%, P < 0.01), 77% of patients who switched to another biologic in the infliximab-dyyb group switched back to RP infliximab, while 100% of patients who switched to another biologic in the RP infliximab group switched to another non-infliximab biologic.
In the conditional logistic regression comparing patients who switched to infliximab-dyyb with patients who remained on RP infliximab, there were no differences in the odds of patients reaching the composite endpoint of disease worsening requiring acute care or treatment failure (OR = 0.95; 95% CI 0.77–1.17 P = 0.63) (Table 3). Significant positive predictors of the secondary outcome in the study cohort included use of acute care within 6 months before index date (OR = 1.93; 95% CI 1.33–2.78, P < 0.01) and steroid use in excess of 1800 mg of prednisone dose equivalents within 6 months prior to index date (OR = 2.22; 95% CI 1.28–3.84, P < 0.01).
Subgroup Analysis in Patients without Prior Chronic Steroid Use
There were 1192 matched pairs of patients (n = 2384) who had no chronic steroid use in the 6 months prior to the index date. For the primary outcome, 174 patients (14.6%) who received RP infliximab compared to 114 patients (9.6%) who switched to infliximab-dyyb experienced disease worsening requiring acute care (P < 0.01 for non-inferiority). A significantly lower proportion of patients who switched to infliximab-dyyb experienced emergency room visits (9.3% vs. 13.2%, P < 0.01) and IBD-related surgery (0.8% vs. 2.2%, P < 0.01) when compared to the RP infliximab group. There were no differences in hospitalizations (1.2% vs. 2.0%, P = 0.10) between the two groups. For the secondary outcome, 279 patients (23.4%) in the RP infliximab group compared to 283 patients (23.7%) who switched to infliximab-dyyb experienced disease worsening requiring acute care or treatment failure (P < 0.01 for non-inferiority). There was a greater proportion of patients who switched to another biologic (15.4% vs. 10.7%, P < 0.01) compared to patients who remained on RP infliximab.
Discussion
In this retrospective, non-inferiority cohort study, we evaluated the effectiveness of switching from RP infliximab to infliximab-dyyb in patients with IBD. Non-inferiority for both the primary and secondary outcomes was demonstrated; patients who switched to infliximab-dyyb had no increased risk of either (1) disease worsening requiring acute care or (2) disease worsening requiring acute care or treatment failure, when compared to patients who remained on RP infliximab. To our knowledge, this is the first study that reflects real-world practice in an integrated healthcare system within the US. Furthermore, our study is the largest study to date in the US regarding switching from RP infliximab to infliximab-dyyb in patients with IBD. The study was also conducted in an integrated health plan, which allowed for the opportunity to provide all healthcare services under one umbrella and a continuity of available data. The ability to use electronic data and propensity score matching to balance confounders and identify a comparison group in demonstrating non-inferiority was a strength of our study. Our study provided support with regards to the FDA’s utilization of extrapolation to approve infliximab-dyyb for use in IBD. Our results were consistent with findings from previous studies in other countries, [20–33] which found no differences or non-inferiority between RP infliximab and infliximab-dyyb in various endpoints including the use of subjective measurement scales or electronic data. There was only one published study [34] which found unfavorable results in infliximab-dyyb. However, in this cohort study that compared patients who switched from RP infliximab to infliximab-dyyb versus those who remained on RP infliximab, the patients were not matched and had baseline differences.
There were some key limitations of the study. First, due to the retrospective study design, there was a potential for selection bias. It was generally believed that patients who were selected to switch to infliximab-dyyb were more stable and had less risk of relapse. We utilized propensity score matching to control for some of these confounding variables, such as age, gender, race, disease burden, type of IBD, concomitant or recent IBD medication, and dose of infliximab on index date. For variables that we could not match, such as use of systemic steroid within the prior 6 months, we performed a subgroup analysis, which showed the same result as the main cohort. In addition, the patients who switched to infliximab-dyyb had a significantly longer history of the disease and prior use of RP infliximab. This might be an indicator that they were more stable patients. However, unlike diabetes, the length of disease is not indicative of severity of the disease. Moreover, since our study utilized electronic data and did not have patient-reported outcomes, it might underestimate the rate of disease worsening in situations where symptoms were not severe enough to require acute care. Although various events and outcomes were used to define disease worsening using electronic data, we did not perform chart reviews in this study to validate disease worsening. However, given that our findings were similar to the majority of reported studies that utilized patient-reported outcomes [20–33], the ability to use electronic data allowed us to capture data on a much larger cohort of patients in a timely manner. In our healthcare system, the decision to switch from RP infliximab to infliximab-dyyb required physician approval and patient agreement, but other patient-specific factors were also considered. In some cases, pharmacists followed up with patients and assisted in monitoring to help guide the switching process. With closer follow-up in the infliximab-dyyb group, this might potentially result in higher rates of remission in the infliximab-dyyb group.
We defined treatment failure as switching to another biologic because chart review was not done to confirm if patients were not responding to or failing the therapy. We observed that 77% of patients who switched therapy in the infliximab-dyyb group were switched to RP infliximab. Since the reason for switchback may not be an indicator for disease worsening but rather a patient preference, the switch from infliximab-dyyb to RP infliximab might be a nocebo effect [36, 37], which could have inflated the treatment failure rate in the infliximab-dyyb group. Lastly, the follow-up period of the study was only 9 months, and it might not be long enough to evaluate disease worsening or the full effect from the therapies. Longer follow-up would be needed for future studies.
Conclusion
This study examined the effectiveness of switching from RP infliximab to infliximab-dyyb in patients with IBD. When defining disease worsening as any IBD-related emergency room visit, hospitalization, or surgery, we found that there was no increased risk for patients who switched to infliximab-dyyb compared to patients who remained on RP infliximab. This was the largest study reported to date in the US population regarding switching patients with IBD from RP infliximab to infliximab-dyyb, and it demonstrated that infliximab-dyyb could be another option in patients with IBD.
Acknowledgements
We would like to thank Michele Spence, PhD, Thomas Delate, PhD, and Patricia Powers, PharmD, who were consulted on the study design.
Compliance with Ethical Standards
Funding
No funding was provided to support this research study.
Conflict of interest
The authors, Stephanie L. Ho, Fang Niu, Suresh Pola, Fernando S. Velayos, Xian Ning, and Rita L. Hui, declare that they have no potential conflicts of interest that might be relevant to the contents of this article.
Ethics approval
The study protocol was reviewed and approved by the Institutional Review Boards of Kaiser Permanente Northern California and Kaiser Permanente Southern California. Informed consent was waived for the study.
Author contributions
All authors participated in drafting or revising the manuscript, approved the final version of the paper, and agree to be accountable for all aspects of the work. RH is the guarantor for the overall content.
Footnotes
The original version of this article was revised due to a retrospective Open Access order.
Change history
4/6/2020
The article Effectiveness of Switching from Reference Product Infliximab to Infliximab-Dyyb in Patients with Inflammatory Bowel Disease in an Integrated Healthcare System in the United States: A Retrospective, Propensity Score-Matched, Non-Inferiority Cohort Study, written by Stephanie L. Ho, Fang Niu, Suresh Pola, Fernando S. Velayos, Xian Ning and Rita L. Hui, was originally published electronically on 26 February 2020 without open access.
Contributor Information
Stephanie L. Ho, Email: Stephanie.L.Ho@kp.org
Fang Niu, Email: Fang.Niu@kp.org.
Suresh Pola, Email: Suresh.Pola@kp.org.
Fernando S. Velayos, Email: Fernando.Velayos@kp.org
Xian Ning, Email: Jennie.X.Ning@kp.org.
Rita L. Hui, Email: Rita.L.Hui@kp.org
References
- 1.Centers for Disease Control and Prevention. Data and statistics: inflammatory bowel disease (IBD) prevalence in the United States. 2019. https://www.cdc.gov/ibd/data-statistics.htm. Accessed 26 Dec 2019.
- 2.McDowell C, Haseeb M. Inflammatory bowel disease (IBD). StatPearls (Internet). Treasure Island (FL): StatPearls Publishing; 2019. [PubMed]
- 3.Tripathi K, Feuerstein JD. New developments in ulcerative colitis: latest evidence on management, treatment, and maintenance. Drugs Context. 2019;8:212572. doi: 10.7573/dic.212572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park KT, Ehrlich OG, Allen JI, Meadows P, Szigethy EM, Henrichsen K, et al. The cost of inflammatory bowel disease: an initiative from the Crohn’s & Colitis Foundation. Inflamm Bowel Dis. 2020;26(1):1–10. doi: 10.1093/ibd/izz104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu F, Liu Y, Wheaton AG, Rabarison KM, Croft JB. Trends and factors associated with hospitalization costs for inflammatory bowel disease in the United States. Appl Health Econ Health Policy. 2019;17(1):77–91. doi: 10.1007/s40258-018-0432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gajendran M, Loganathan P, Catinella AP, Hashash JG. A comprehensive review and update on Crohn’s disease. Dis Mon. 2018;64(2):20–57. doi: 10.1016/j.disamonth.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Crohn’s & Colitis Foundation of America. The facts about inflammatory bowel diseases. 2014. https://www.crohnscolitisfoundation.org/sites/default/files/2019-02/Updated%20IBD%20Factbook.pdf. Accessed 26 Dec 2019.
- 8.Prescribing Information for Remicade™, Janssen Biotech, Horsham. 2013. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103772s5359lbl.pdf. Accessed 26 Dec 2019.
- 9.Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. N Engl J Med. 1997;337(15):1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 10.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 11.Kornbluth A, Sachar DB, Practice Parameters Committee of the American College of Gastroenterology Ulcerative colitis practice guidelines in adults: American College of Gastroenterology. Practice Parameters Committee. Am J Gastroenterol. 2010;105(3):501–523. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113(4):481–517. doi: 10.1038/ajg.2018.27. [DOI] [PubMed] [Google Scholar]
- 13.Costa J, Magro F, Caldeira D, Alarcão J, Sousa R, Vaz-Carneiro A. Infliximab reduces hospitalizations and surgery interventions in patients with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2013;19(10):2098–2110. doi: 10.1097/MIB.0b013e31829936c2. [DOI] [PubMed] [Google Scholar]
- 14.Prescribing Information for Inflectra®, Pfizer, New York. 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125544s000lbl.pdf. Accessed 26 Dec 2019.
- 15.European Medicines Agency. Biosimilar medicines: overview. 2017. https://www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines-overview. Accessed 26 Dec 2019.
- 16.U.S. Food and Drug Administration. Biosimilar product information. 2019. https://www.fda.gov/drugs/biosimilars/biosimilar-product-information. Accessed 26 Dec 2019.
- 17.U.S. Food and Drug Administration. Industry information and guidance. 2018. https://www.fda.gov/drugs/biosimilars/industry-information-and-guidance. Accessed 26 Dec 2019.
- 18.U.S. Food and Drug Administration. Biosimilar development, review, and approval. 2017. https://www.fda.gov/drugs/biosimilars/biosimilar-development-review-and-approval. Accessed 26 Dec 2019.
- 19.Tesser JR, Furst DE, Jacobs I. Biosimilars and the extrapolation of indications for inflammatory conditions. Biologics. 2017;11:5–11. doi: 10.2147/BTT.S124476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jørgensen KK, Olsen IC, Goll GL, Lorentzen M, Bolstad N, Haavardsholm EA, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017;389(10086):2304–2316. doi: 10.1016/S0140-6736(17)30068-5. [DOI] [PubMed] [Google Scholar]
- 21.Ye BD, Pesegova M, Alexeeva O, Osipenko M, Lahat A, Dorofeyev A, et al. Efficacy and safety of biosimilar CT-P13 compared with originator infliximab in patients with active Crohn’s disease: an international, randomised, double-blind, phase 3 non-inferiority study. Lancet. 2019;393(10182):1699–1707. doi: 10.1016/S0140-6736(18)32196-2. [DOI] [PubMed] [Google Scholar]
- 22.Ebada MA, Elmatboly AM, Ali AS, Ibrahim AM, Fayed N, Faisal AF, et al. An updated systematic review and meta-analysis about the safety and efficacy of infliximab biosimilar, CT-P13, for patients with inflammatory bowel disease. Int J Colorectal Dis. 2019;34(10):1633–1652. doi: 10.1007/s00384-019-03354-7. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa T, Kobayashi T, Nishikawa K, Yamada F, Asai S, Sameshima Y, et al. Infliximab biosimilar CT-P13 is interchangeable with its originator for patients with inflammatory bowel disease in real world practice. Intest Res. 2019;17(4):504–515. doi: 10.5217/ir.2019.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim NH, Lee JH, Hong SN, Yoon H, Kang HW, Lee SH, et al. Long-term efficacy and safety of CT-P13, a biosimilar of infliximab, in patients with inflammatory bowel disease: a retrospective multicenter study. J Gastroenterol Hepatol. 2019;34(9):1523–1532. doi: 10.1111/jgh.14645. [DOI] [PubMed] [Google Scholar]
- 25.Bronswijk M, Moens A, Lenfant M, Tops S, Compernolle G, Van Assche G, et al. Evaluating efficacy, safety, and pharmacokinetics after switching from infliximab originator to biosimilar CT-P13: experience from a large tertiary referral center. Inflamm Bowel Dis. 2020;26(1):161. doi: 10.1093/ibd/izz271. [DOI] [PubMed] [Google Scholar]
- 26.Gheorghe C, Svoboda P, Mateescu B. Effectiveness and safety of biosimilar infliximab (CT-P13) in a real-life setting in patients with Crohn’s disease or ulcerative colitis. J Drug Assess. 2019;8(1):129–134. doi: 10.1080/21556660.2019.1626735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Hoeve K, Dreesen E, Hoffman I, Van Assche G, Ferrante M, Gils A, et al. Efficacy, pharmacokinetics, and immunogenicity is not affected by switching from infliximab originator to a biosimilar in pediatric patients with inflammatory bowel disease. Ther Drug Monit. 2019;41(3):317–324. doi: 10.1097/FTD.0000000000000601. [DOI] [PubMed] [Google Scholar]
- 28.Ilias A, Szanto K, Gonczi L, Kurti Z, Golovics PA, Farkas K, et al. Outcomes of patients with inflammatory bowel diseases switched from maintenance therapy with a biosimilar to Remicade. Clin Gastroenterol Hepatol. 2019;17(12):2506–2513. doi: 10.1016/j.cgh.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 29.Plevris N, Jones GR, Jenkinson PW, Lyons M, Chuah CS, Merchant LM, et al. Implementation of CT-P13 via a managed switch programme in Crohn’s disease: 12-month real-world outcomes. Dig Dis Sci. 2019;64(6):1660–1667. doi: 10.1007/s10620-018-5406-8. [DOI] [PubMed] [Google Scholar]
- 30.Høivik ML, Buer LCT, Cvancarova M, Warren DJ, Bolstad N, Moum BA, et al. Switching from originator to biosimilar infliximab—real world data of a prospective 18 months follow-up of a single-center IBD population. Scand J Gastroenterol. 2018;53(6):692–699. doi: 10.1080/00365521.2018.1463391. [DOI] [PubMed] [Google Scholar]
- 31.Strik AS, van de Vrie W, Bloemsaat-Minekus JPJ, Nurmohamed M, Bossuyt PJJ, Bodelier A, et al. Serum concentrations after switching from originator infliximab to the biosimilar CT-P13 in patients with quiescent inflammatory bowel disease (SECURE): an open-label, multicentre, phase 4 non-inferiority trial. Lancet Gastroenterol Hepatol. 2018;3(6):404–412. doi: 10.1016/S2468-1253(18)30082-7. [DOI] [PubMed] [Google Scholar]
- 32.Guerra Veloz MF, Argüelles-Arias F, Castro Laria L, Maldonado Pérez B, Benítez Roldan A, Perea Amarillo R, et al. Loss of efficacy and safety of the switch from infliximab original to infliximab biosimilar (CT-P13) in patients with inflammatory bowel disease. World J Gastroenterol. 2018;24(46):5288–5296. doi: 10.3748/wjg.v24.i46.5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petitdidier N, Tannoury J, de’Angelis N, Gagniere C, Hulin A, Rotkopf H, et al. Patients’ perspectives after switching from infliximab to biosimilar CT-P13 in patients with inflammatory bowel disease: a 12-month prospective cohort study. Dig Liver Dis. 2019;51(12):1652–1660. doi: 10.1016/j.dld.2019.08.020. [DOI] [PubMed] [Google Scholar]
- 34.Chaparro M, Garre A, Guerra Veloz MF, Vázquez Morón JM, De Castro ML, Leo E, et al. Effectiveness and safety of the switch from Remicade® to CT-P13 in patients with inflammatory bowel disease. J Crohns Colitis. 2019;13(11):1380–1386. doi: 10.1093/ecco-jcc/jjz070. [DOI] [PubMed] [Google Scholar]
- 35.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleischmann R, Jairath V, Mysler E, Nicholls D, Declerck P. Nonmedical switching from originators to biosimilars: does the nocebo effect explain treatment failures and adverse events in rheumatology and gastroenterology? Rheumatol Ther. 2020;7(1):35–64. doi: 10.1007/s40744-019-00190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colloca L, Panaccione R, Murphy TK. The clinical implications of nocebo effects for biosimilar therapy. Front Pharmacol. 2019;29(10):1372. doi: 10.3389/fphar.2019.01372. [DOI] [PMC free article] [PubMed] [Google Scholar]