Abstract
Recently, it has been proposed the association of a common deletion affecting toll-like receptor 2 promoter (-196 to -177) to type 2 diabetes mellitus risk. However, genotyping results show a significant deviation from the HardyWeinberg Equilibrium (HWE). The law of Hardy-Weinberg shows that for an autosomal biallelic marker with allele frequencies fA=p and fa=q, the proportion of subjects with genotypes AA, Aa, and aa should follow the following: fAA=p2 , fAa=2pq, and faa=q2 . Departure from HWE or Hardy-Weinberg Disequilibrium (HWD) in a human control population can be caused by natural factors such as selective pressure against a certain genotype. However their prevalence is scarce and magnitude of effect over the HWE are small. Other factors such as inbreeding caused by consanguinity, population stratification, or technical problems in genotyping are more usual. Nevertheless, if the control population follows a perfect HWE, the presence HWD among patients might be explained by the genetic association and evidencing a real link between the locus and the trait under study. However, HWD affecting both cases and controls, such as the one reported might be explained by one of the aforementioned issues.
Genetic case-control studies have been proven as a powerful strategy to decipher the biochemical pathways underlying complex diseases. The basis of this approach is to find whether patients share an ancestral haplotype harboring either a common risk factor or directly a causative mutation. This implies sharing a determined genotype due to the fact of being patients (identical by state) rather than being relatives (identical by descent). Geneticists determine the genotypes and then compare allele frequencies in unrelated patients and control series. Whenever the mutant allele is in linkage disequilibrium with the causative mutation, we observe a statistically significant increase on its allele frequency among cases. However, independent of the allele frequency and the locus under analysis, any genetic marker mapping the autosomal chromosome shall follow the Hardy-Weinberg Equilibrium (HWE) (1, 2). This apparently simple rule states that for a biallelic locus with frequencies p and q respectively, genotypic frequencies must follow the p2, 2pq and q2. Disturbances of the Hardy-Weinberg Equilibrium (HWD) occur when natural selection operates over a particular genotype giving a differential fitness to any of them, such in the case of the hemoglobin (Hb) locus, associated to sickle cell anemia and resistance to Plasmodium falciparum infection (3). However, these cases arescarce in the literature, and large series are required to find statistically significant results. More often, HWD evidences population stratification. If a study series comprises subjects with different genetic background differing in their allele frequencies, their mix would exhibit a HWD. This is the called Wahlund effect and happens when each population, independently, fit HWE (4). Alternatively, HWD might be due to the existence of inbreeding in the series. This is evidenced by a reduction of the heterozygosity within a population (5). Finally, the HWD might be consequence of genotyping problems. A cross contamination typically pops up due to an excess of heterozygotes, while a reduced sensitivity of the mutant allele results in a lower mutant homozygotes frequency. Alternatively, the inclusion of duplicates in the genotyping series might also be associated to HWD.
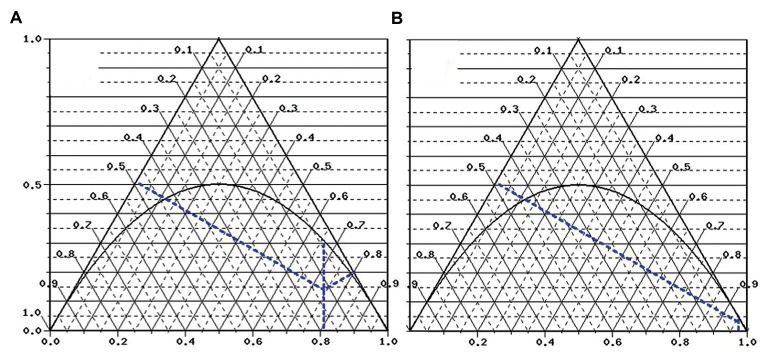
Recently, Ermi؛ Karaali et al. (6) studied the role of a common Toll-like receptor 2 promoter deletion (196 to-177) in type 2 diabetes risk (T2DM). The rational underlying work roots on the contrasted role of TLR2 on human innate immunity. They performed a casecontrol study determining the presence of this promoter deletion among 100 cases and 98 age-matched controls, and concluded that the deletion allele was associated to T2DM risk. However, this conclusion should betaken with caution. Both cases and controls show a remarkable HWD (X2 P=4.99×10-8 and 1.4×10-4 for cases and controls, respectively). The nature of this HWD relies on an underrepresentation of heterozygotes in both cases and controls that can be illustrated in the De Finetti diagram (Fig.1). The observed heterozygotes among controls only represent 61% from what expected according to HWE (3 vs. 4.87) and this effect was more accused within the T2DM series with only 45.5% from what expected (14 vs. 30.78). Therefore inbreeding coefficient estimates range between F=0.545 and F=0.384 (cases and controls, respectively). TLR2 -196 to -177 has been extensively determined in different studies with no evident HWD and, whenever reported, this was constrained to cases (Table 1). It has been recently reported that besides using the information from 2,405 subjects available from the 1,000-genome project release 3, no HWD is found for control populations of Europe, Africa, Asia or America at this locus (7). This suggests that Ermi؛ Karaali et al. (6) might have fallen in at least one of the aforementioned problems associated to HWD. It should be highlighted that we cannot rule out the possibility that functional variants affecting TLR2 physiology might be associated to T2DM. However, this potential role of TLR2 -196 to -177 deletion shall be repeated following the adequate controls in order to determine its potential implication in T2DM risk.
Fig.1.
De Finetti representation of Ermi؛ Karaali et al. (6) results of TLR2 -196 to -177 variant on T2DM risk. De Finetti representation (8) of A. Both cases and B. Controls from Ermi؛ Karaali et al. (6). The X-axis represents the frequency of the Ins allele. The intersection of the parabola and vertical line represents the frequency of genotype Ins/Del under f Hardy-Weinberg Equilibrium.
Table 1.
HWE was assayed using the freely available resource at https://ihg.gsf.de/cgi-bin/hw/hwa2.pl from the University of Munich. P values were calculated using Pearson’s goodness-of-fit chi-square with one degree of freedom
| Disease | Subjects | Ins/Ins | Ins/Del | Del/Del | MAF | HWE | Country | Reference |
|---|---|---|---|---|---|---|---|---|
| P value | ||||||||
| T2DM | Controls | 94 | 3 | 1 | 0.03 | 1.42×10-4 | Turkey | (6) |
| Cases | 74 | 14 | 12 | 0.19 | 4.99×10-8 | |||
| Parkinson’s disease | Controls | 95 | 21 | 2 | 0.11 | 0.511 | Greece | (9) |
| Cases | 156 | 52 | 7 | 0.15 | 0.309 | |||
| VIH susceptibility | Controls | 189 | 65 | 3 | 0.14 | 0.318 | Spain | (7) |
| Cases | 160 | 18 | 0 | 0.05 | 0.477 | |||
| General population | Controls | 208 | 85 | 11 | 0.18 | 0.531 | Poland | (10) |
| Gastric cancer | Controls | 75 | 65 | 8 | 0.27 | 0.202 | Japan | (11) |
| Cases | 126 | 112 | 51 | 0.37 | 4.1×10-3 | |||
| Alzheimer’s disease | Controls | 172 | 168 | 60 | 0.36 | 0.077 | China | (12) |
| Cases | 150 | 161 | 89 | 0.42 | 4.37×10-4 | |||
Acknowledgements
The author has no financial support to disclose with respect to this manuscript and declares no conflict of interest. The author would like to thank Dr. Bravo for critically reading this letter.
References
- 1.Hardy GH. Mendelian proportions in a mixed population. Science. 1908;28(706):49–50. doi: 10.1126/science.28.706.49. [DOI] [PubMed] [Google Scholar]
- 2.Weinberg W. About proof of heredity in humans. Germany: The association for national natural history in württemberg. 1908;64:368–382. [Google Scholar]
- 3.Piel FB, Adamkiewicz TV, Amendah D, Williams TN, Gupta S, Grosse SD. Observed and expected frequencies of structural hemoglobin variants in newborn screening surveys in Africa and the Middle East: deviations from Hardy-Weinberg equilibrium. Genet Med. 2016;18(3):265–274. doi: 10.1038/gim.2015.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wahlund S. Composition of population and correlation phenomenon from the viewpoint of heredity. Hereditas. 1928;11:65–106. [Google Scholar]
- 5.Falconer DS, Mackay TFC. Introduction to quantitative genetics.Oxford: Blackwell’s Oxford. Oxford: Blackwell’s Oxford; 1996. [Google Scholar]
- 6.Ermiş Karaali Z, Candan G, Aktuğlu MB, Velet M, Ergen A. Toll-like receptor 2 (TLR-2) gene polymorphisms in type 2 diabetes mellitus. Cell J. 2019;20(4):559–563. doi: 10.22074/cellj.2019.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Royo JL, Alarcón-Martín E, Díaz-Fuentes J, Colmenero JD, Bravo MJ. Discordance in TLR2 (-196 to -174) polymorphism effect on HIV infection risk. J Gene Med. 2018;20(10-11):e3051–e3051. doi: 10.1002/jgm.3051. [DOI] [PubMed] [Google Scholar]
- 8.Cannings C, Edwards AW. Natural selection and the De Finetti diagram. Ann Hum Genet. 1968;31(4):421–428. doi: 10.1111/j.1469-1809.1968.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 9.Kalinderi K, Bostantjopoulou S, Katsarou Z, Fidani L. TLR9 -1237 T/C and TLR2 -194 to -174 del polymorphisms and the risk of Parkinson’s disease in the Greek population: a pilot study. Neurol Sci. 2013;34(5):679–682. doi: 10.1007/s10072-012-1106-x. [DOI] [PubMed] [Google Scholar]
- 10.Lewandowska M, Garczyńska P, Jędrychowska-Dańska K, Kopczyńska P, Masłowska A, Witas H. Frequency of P2RX7 A1513C and TLR2 -196 to -174 ins/del in healthy Polish individuals. Int J Immunogenet. 2015;42(3):195–199. doi: 10.1111/iji.12185. [DOI] [PubMed] [Google Scholar]
- 11.Tahara T, Arisawa T, Wang F, Shibata T, Nakamura M, Sakata M, et al. Toll-like receptor 2 -196 to 174del polymorphism influences the susceptibility of Japanese people to gastric cancer. Cancer Sci. 2007;98(11):1790–1794. doi: 10.1111/j.1349-7006.2007.00590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu JT, Mou SM, Wang LZ, Mao CX, Tan L. Toll-like receptor 2 -196 to -174 del polymorphism influences the susceptibility of Han Chinese people to Alzheimer’s disease. J Neuroinflammation. 2011;8:136–136. doi: 10.1186/1742-2094-8-136. [DOI] [PMC free article] [PubMed] [Google Scholar]