Abstract
Purpose
Red blood cell (RBC) folate indicates long-term folate intake, and methylenetetrahydrofolate reductase (MTHFR) gene is the main gene affecting folate status. Increasing evidence suggests an association between gestational diabetes mellitus (GDM) and increased folate levels. Whether RBC folate concentrations in the first trimester of pregnancy or polymorphisms of MTHFR C677T (rs1801133) affect GDM risk in Chinese pregnant women remains unknown. Therefore, we analyzed the associations of RBC folate concentrations and rs1801133 polymorphisms with GDM risk among pregnant women in China.
Methods
A total of 366 women with a singleton pregnancy were followed prospectively from their first prenatal visit to delivery. RBC folate concentrations and rs1801133 polymorphisms were assessed during the first trimester of pregnancy. Binary logistic regression analyses were performed to determine the odds ratios (ORs) of GDM and 95% confidence intervals (CIs) by using the RBC folate concentration quartiles and rs1801133 polymorphisms.
Results
Participants with the TT genotype had the highest RBC folate concentrations. Those with heterozygous or homozygous variants did not have a significantly higher risk of GDM than did women with C alleles. After adjustments for covariates, women in the highest quartile for RBC folate concentration had a higher risk of GDM (adjusted OR = 2.473, 95% CI = 1.013–6.037, P = 0.047) than did those in the lowest quartile, but this association was nonsignificant after adjustment for rs1801133 polymorphisms.
Conclusion
Higher RBC folate, partly caused by MTHFR 677C→T, may be associated with increased GDM risk, even in early pregnancy. Assessing RBC folate status and appropriately supplementing folate during early pregnancy, particularly for patients with MTHFR 677C→T, may prevent GDM. Further studies with larger populations are warranted.
Keywords: gestational diabetes mellitus, folic acid, folate, red blood cell folate, methylenetetrahydrofolate reductase, MTHFR
Introduction
Gestational diabetes mellitus (GDM) is common during pregnancy. Although GDM affects approximately 15% of pregnant women globally,1 its prevalence in Asian countries can reach 17–20%.2,3 GDM is defined as impaired glucose intolerance and insulin resistance with onset or recognition during pregnancy,4 and it has various negative implications for mothers and their children. For mothers, GDM is associated with higher rates of preeclampsia, cesarean deliveries, shoulder dystocia, and type 2 diabetes mellitus in the postpartum period.5,6 In addition, children born to mothers with GDM are more likely to develop obesity, impaired glucose tolerance (IGT), and type 2 diabetes in childhood or early adulthood.5,7,8 Overweight, obesity, and IGT are significant risk factors for GDM, causing a vicious intergenerational cycle of obesity and diabetes. Therefore, effective interventions to treat and prevent GDM are required to halt this cycle.9 Currently, main preventive measures focus on reasonably controlling weight gain during pregnancy, a main modifiable risk factor for GDM. However, increasing evidence of the relationship between high folate levels and GDM has emerged.
Folic acid (FA) can prevent neural tube defects (NTDs), and FA supplements before and during pregnancy are recommended globally.10 Since increased FA consumption has become common among pregnant women, the potential adverse effects of FA supplementation or elevated folate levels in mothers on insulin resistance in their children are concerning.11,12 Studies from Asian countries have reported that FA supplementation in early pregnancy and higher plasma folate concentrations are associated with higher risks of GDM.13,14 Furthermore, higher dosages (≥800 μg/d) of FA supplements and longer supplementation durations are associated with higher GDM risks.15 Therefore, evaluating the folate levels of pregnant women is necessary. The determinants of folate status may be multifactorial, including genetic, biological, and socioeconomic components.16 Inheritance of the specific genetic variant methylenetetrahydrofolate reductase (MTHFR) C677T (rs1801133) in the gene encoding the MTHFR enzyme is considered the strongest determinant of folate status in women of reproductive age.16,17 In clinical practice, mutations in folate-associated genes, among which MTHFR C677T is the most crucial, are commonly detected in women with adverse pregnancy histories.
Folate status can be modulated through the appropriate dosage and duration of FA supplementation, and several methods are used to evaluate folate levels, such as measuring folate in urine, serum, and red blood cells (RBCs).18 Serum folate rapidly responds to folate intake or FA supplementation, whereas RBC folate indicates long-term folate status and responds mainly to supplementation and fortification.16 The World Health Organization (WHO) provided a reference for folate status and defined RBC folate concentrations of ≥906 nmol/L as optimal for preventing NTDs.16 However, a Chinese study reported that RBC folate concentrations of ≥906 nmol/L during the second trimester of pregnancy significantly increase GDM risk.19 Because FA supplementation in early pregnancy is associated with GDM risk,13 and no relevant study has accounted for folate-associated genes, such as MTHFR C677T, we hypothesized that higher concentrations of RBC folate in early pregnancy and rs1801133 polymorphisms affect subsequent GDM development. Therefore, we observed the associations of RBC folate concentrations in the first trimester of pregnancy and rs1801133 polymorphisms with subsequent GDM risk among pregnant women in China. Data from a mother–child cohort study in which the correlation between single-nucleotide polymorphisms (SNPs) in nutrient-associated genes and maternal nutritional status was investigated.
Materials and Methods
Ethical Statement
The study protocol was approved by the Ethics Committee of Peking Union Medical College Hospital of the Chinese Academy of Medical Science (Unique Protocol ID: hs-1646) and registered on www.ClinicalTrials.gov (registration ID: NCT03651934). This study was conducted in accordance with both the Declaration of Helsinki, as revised in 1983, and the guidelines of the center’s institutional review board. All participants received details of the study and provided written informed consent.
Study Population
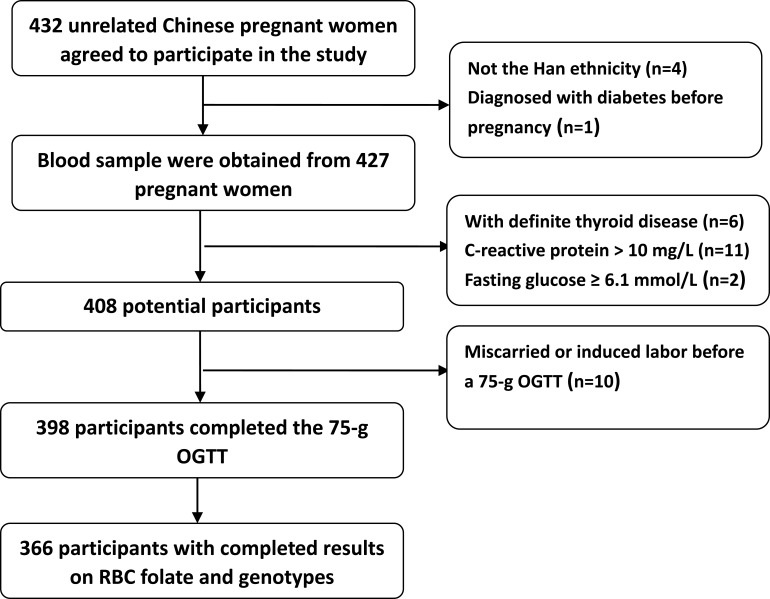
Women in early pregnancy were recruited in October and December 2018 at the Shunyi District Maternal and Child Health Hospital (Beijing, China). Participants were Chinese residents, had established prenatal records before recruitment, and intended to deliver in the same hospital. Women were excluded if they (1) did not have a singleton pregnancy, (2) were not of the Han ethnicity (to prevent confounding by ethnicity), (3) had lab-tested fasting glucose ≥ 6.1 mmol/L or HbA1c > 6.5% or received a diagnosis of diabetes before pregnancy, (4) had a history of autoimmune diseases (such as systemic lupus erythematosus) or currently used corticosteroids, (5) had definite hyperthyroidism or hypothyroidism, (6) had miscarried or induced labor before the 75-g oral glucose tolerance test (OGTT) at 24 to 28 weeks’ gestation, (7) had a history of liver or renal insufficiency or presumed acute inflammation (C-reactive protein [CRP] > 10 mg/L), or (8) had incomplete RBC folate or MTHFR C677T gene measurement records. A total of 432 pregnant women agreed to participate at baseline. Trained researchers used a standard questionnaire to collect participants’ age, ethnicity (self-reported), smoking habits (yes/no), drinking habits (yes/no), physical activity (0–150 mins or ≥150 mins of weekly moderate exercise [such as fast walking, jogging, or aerobics]), parity (nullipara, secundipara, or multipara [>two deliveries]), family history of diabetes (yes/no), and use of FA supplements at enrollment (<400 or ≥400 μg/d). Height was measured to the nearest 0.1 cm with a portable stadiometer. Weight was measured in an upright position to the nearest 0.1 kg with a calibrated scale. Body mass index (BMI) was calculated as weight (kg)/height (m2). During the entire pregnancy, routine prenatal examinations for each participant were performed in the same hospital. At 24 to 28 weeks’ gestation, a 75-g OGTT was conducted for all participants. GDM was diagnosed using the following glucose-level thresholds of the Implementation of the International Association of Diabetes and Pregnancy Study Groups and WHO: fasting plasma glucose (FPG) ≥ 5.1 mmol/L, 1-h plasma glucose (PG) ≥ 10.0 mmol/L, and 2-h PG ≥ 8.5 mmol/L. All related data were obtained from medical records. A total of 366 women with complete data were eligible to participate in this study. A participant inclusion flowchart is presented in Figure 1.
Figure 1.
Participant flowchart.
Blood-Sample Measurements
Blood samples were retrieved from participants during their first visit before 12 weeks’ gestation after an overnight (≥8 h) fast. Concentrations of plasma folate, RBC folate, and vitamin B12 were quantified through chemiluminescence assay using a Beckman Coulter DxI 800 chemistry analyzer (Beckman Coulter Inc., Brea, CA, USA). CRP was measured using a Beckman Coulter AU5800 chemistry analyzer (Beckman Coulter Inc., Brea, CA, USA) and its supporting reagent. Homocysteine concentrations were measured using an enzymatic assay on a Beckman Coulter DX1 800 automatic chemistry analyzer (Beckman Coulter Inc., Brea, CA, USA). Plasma glucose measurements in the OGTT were conducted on a Beckman Coulter AU2700 chemistry analyzer (Beckman Coulter Inc., Brea, CA, USA). Homeostasis model assessment-insulin resistance (HOMA-IR) and homeostasis model assessment-β (HOMA-β) were calculated as follows:20 HOMA-IR = (fasting plasma glucose [mmol/L] × fasting serum insulin [mIU/mL])/22.5, and HOMA-β = (20 × fasting serum insulin [mIU/mL])/(fasting plasma glucose [mmol/L] − 3.5).
DNA Extraction
DNA was extracted from saliva or an oral swab sample with an Auto-Pure 96 automatic extractor and then quantified using a Tecan Infinite multifunction enzyme-labeling instrument for concentration, and A260/A280 and A260/A230 ratios were calculated for array testing. Extracted DNA was amplified before fragmentation. Resuspended DNA samples were loaded into a Tecan Freedom Evo liquid-processing workstation for overnight hybridization with an Illumina array in an Illumina Hybridization Oven at room temperature. After hybridization, the chip was washed to remove excess nonhybridized nucleic acid fragments. Subsequent extension and dyeing procedures were performed on the same workstation. The final coated array was scanned using an Illumina iSCAN scanner and analyzed to obtain the genotypes of the MTHFR C677T loci.
Statistical Analysis
Statistical analysis was performed using SPSS (version 16.0, Chicago, IL, USA). Normally distributed variables are presented as means (standard deviations), whereas skewed variables are presented as medians (interquartile range, 25–75%). Categorical variables are expressed as frequencies or percentages and were examined using chi-square tests. Independent-sample t tests or the Mann–Whitney U-tests, respectively, were used to compare variables with normal or skewed distributions between the GDM and non-GDM groups. Differences in concentrations of RBC folate as well as homocysteine between the GDM and non-GDM groups or within each group according to genotype were analyzed using a univariate analysis of variance (UNIANOVA) adjusted for age, BMI, and FA supplement use (<400 or ≥400 μg/d). Spearman correlation analyses were performed to detect associations of RBC folate concentrations with HOMA-IR, CRP, age, and BMI. RBC folate concentrations were divided into quartiles (Q1, <509.0 nmol/L; Q2, 509.0–647.9 nmol/L; Q3, 648.0–862.4 nmol/L; and Q4, ≥862.5 nmol/L). Binary logistic regression analyses were used to determine odds ratios (ORs) and 95% confidence intervals (CIs) of the associations of GDM with RBC folate levels (as quartiles or a continuous variable) as well as MTHFR C677T SNP, with or without adjustments for covariates; the dominant [(CT+TT): CC] and additive [(CC+TT): CT] models for the MTHFR C677T gene loci were also analyzed. All reported P values were two-tailed, and P < 0.05 was considered statistically significant.
Results
Baseline Characteristics and GDM Incidence
Of the 366 eligible women, 67 (18.3%) received diagnoses of GDM. Because none of the participants smoked or consumed alcohol for at least 3 months before enrollment, we do not present the data on smoking and alcohol consumption. Compared with participants in the non-GDM group, those in GDM group were significantly older, were more likely to have family history of diabetes, and had higher BMI, HOMA-IR, and CRP values (P < 0.01 for all). Participants in the GDM group tended to have higher RBC folate concentrations (P = 0.069) but less exercise time per week (P = 0.01). No significant differences in parity, FA supplement use (at enrollment), hemoglobin concentration, serum folate level, serum vitamin B12 level, serum homocysteine level, HOMA-β, or MTHFR C677T genotype were observed between the groups (Table 1).
Table 1.
Maternal Characteristics, RBC Folate Concentrations, and MTHFR C677T Polymorphisms in the GDM and Non-GDM Groups
| Maternal Characteristics | GDM (n=67) | Non-GDM (n=299) | P |
|---|---|---|---|
| Age (years) | 30.5 (4.0) | 28.9 (3.5) | 0.001 |
| <30 | 30 (44.8) | 122 (40.8) | 0.551 |
| ≥30 | 37 (55.2) | 177 (59.2) | |
| BMI at enrollment (kg/m2) | 24.3 (3.6) | 22.4 (3.6) | <0.001 |
| <24 | 37 (55.2) | 216 (72.2) | 0.006 |
| ≥24 | 30 (44.8) | 83 (27.8) | |
| Parity | |||
| Nullipara | 37 (55.2) | 180 (60.2) | 0.454 |
| Secundipara | 30 (44.8) | 119 (39.8) | |
| Multipara | 0 (0.0) | 0 (0.0) | - |
| Family history of diabetes | 12 (17.9) | 21 (7.0) | 0.005 |
| Physical Activity | |||
| 0–150 minutes per week | 45 (67.2) | 149 (49.8) | 0.01 |
| ≥150 minutes per week | 22 (32.8) | 150 (50.2) | |
| Folic Acid Supplements at Enrollment | |||
| <400μg | 24 (35.8) | 97 (32.4) | 0.595 |
| ≥400μg | 43 (64.2) | 202 (67.6) | |
| Hemoglobin (g/L) | 132.4 (8.9) | 131.3 (10.8) | 0.448 |
| Serum folate (nmol/L) | 23.9 (14.1–24.0) | 21.4 (16.0–24.0) | 0.454 |
| C-reactive protein (mg/L) | 3.3 (1.6–5.9) | 1.9 (0.9–3.5) | <0.001 |
| HOMA-IR | 2.1 (1.4–3.0) | 1.4 (1.0–2.1) | <0.001 |
| HOMA-β | 169.1 (121.4–282.4) | 168.2 (111.2–279.6) | 0.698 |
| Serum homocysteine (μmol/L) | 9.1 (1.9) | 9.1 (2.8) | 0.978 |
| Serum vitamin B12 (pmol/L) | 237.3 (101.8) | 241.0 (108.9) | 0.801 |
| RBC folate (nmol/L) | 755.1 (276.0) | 690.6 (258.3) | 0.069 |
| MTHFR Polymorphisms | 0.384 | ||
| CC | 8 (12.0) | 56 (18.7) | |
| CT | 35 (52.2) | 151 (50.5) | |
| TT | 24 (35.8) | 92 (30.8) |
Abbreviations: HOMA-IR, homeostasis model assessment-insulin resistance; RBC, red blood cell; HOMA-β, homeostasis model assessment-β.
We compared concentrations of RBC folate and homocysteine as well as HOMA-IR among the participants according to rs1801133 genotype (Table 2), revealing a significant difference in the overall concentration of RBC folate and homocysteine among the three genotypes (P < 0.01 for all).
Table 2.
HOMA-IR, RBC Folate, and Homocysteine Concentrations by Genotype
| Variables | CC | CT | TT | P |
|---|---|---|---|---|
| RBC folate (nmol/L)a | ||||
| GDM | 717.1 (233.0) | 667.2 (238.4) | 895.9 (291.7) | 0.016 |
| Non-GDM | 596.2 (195.2) | 645.7 (220.3) | 816.3 (297.5) | <0.001 |
| All | 611.5 (202.4) | 649.8 (223.3) | 832.9 (296.8) | <0.001 |
| Homocysteine (μmol/L)b | ||||
| GDM | 8.6 (1.3) | 9.0 (1.6) | 9.2 (2.4) | 0.688 |
| Non-GDM | 8.7 (1.4) | 8.6 (1.2) | 10.0 (4.5) | 0.002 |
| All | 8.7 (1.3) | 8.7 (1.3) | 9.8 (4.2) | 0.001 |
| HOMA-IRc | ||||
| GDM | 1.91 (0.96–4.66) | 2.23 (1.45–2.84) | 1.89 (1.38–3.31) | 0.866 |
| Non-GDM | 1.66 (1.15–2.24) | 1.29 (0.93–1.92) | 1.47 (1.05–2.07) | 0.077 |
| All | 1.66 (1.13–2.45) | 1.42 (0.98–2.31) | 1.53 (1.10–2.26) | 0.347 |
Notes: a,bAdjusted for age, BMI, and use of FA supplements. cComparisons made using the Kruskal–Wallis H-test.
Associations of RBC Folate Concentration with GDM Risk, HOMA-IR, and Inflammatory Markers
The Spearman correlation analysis indicated that RBC folate level was not significantly correlated with age (coefficient = 0.061, P = 0.241), BMI (coefficient = −0.032, P = 0.541), HOMA-IR (coefficient = 0.039, P = 0.455), or CRP (coefficient = 0.019, P = 0.722).
RBC folate concentrations were divided into quartiles according to the cutoff points of the distribution for this entire study population, and the lowest quartile was used as a reference. Binary logistic analyses indicated that women with RBC folate concentrations in the highest quartile had a higher risk of GDM (adjusted OR = 2.473, 95% CI = 1.013–6.037, P = 0.047) than did those with RBC folate concentrations in the lowest quartile, after adjustments for age, BMI, physical activity, family history of diabetes, parity, FA supplement use at enrollment, and HOMA-IR, CRP, hemoglobin, serum vitamin B12, and serum homocysteine level (Table 3). After further adjustment for rs1801133 SNPs, this association became nonsignificant (adjusted OR = 2.251, 95% CI = 0.890–5.696, P = 0.087). When RBC folate was regarded as a continuous variable, it was not linearly associated with GDM risk (Table 3).
Table 3.
Association of GDM Risk with RBC Folate Levels
| RBC Folate levels | GDM, n (%) | Model Onea | Model Twob | ||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Q1(<509.0nmol/L) | 12 (13.2) | 1.00 (Reference) | – | 1.00 (Reference) | – |
| Q2 (509.0–647.9nmol/L) | 15(16.3) | 1.417 (0.612–3.277) | 0.416 | 1.354 (0.530–3.456) | 0.526 |
| Q3 (648.0–862.4nmol/L) | 17(18.7) | 1.671 (0.735–3.800) | 0.173 | 1.374 (0.546–3.455) | 0.500 |
| Q4 (≥862.5nmol/L) | 24(26.1) | 2.567 (1.173–5.619) | 0.018 | 2.473 (1.013–6.037) | 0.047 |
| As a continuous variable (SD, 262.4 nmol/L) | – | 1.001 (1.000–1.002) | 0.071 | 1.001 (1.000–1.002) | 0.121 |
Notes: aWithout adjustment for covariates. bAdjusted for age, physical activity, BMI, parity, family history of diabetes, use of folic acid supplements, HOMA-IR, C-reactive protein, hemoglobin, vitamin B12, and serum homocysteine.
Associations Between MTHFR SNP and GDM Risk
In the study population, the CC, CT, and TT C677T genotypes had frequencies of 17.5%, 50.8%, and 31.7%, respectively. No significant differences were discovered between the observed and genotype distributions expected according with the Hardy–Weinberg equilibrium (P > 0.05).
We analyzed the associations of the CT and TT rs1801133 genotypes with GDM risk, with the CC genotype as a reference. After adjustment for covariates in the logistic regression analysis, women with the CT or TT genotypes of rs1801133 did not have a significantly higher risk of GDM than did women with C alleles. Furthermore, in the analysis of both mutant genotypes (CT or TT), women without the C alleles did not have a significantly higher risk of GDM than did women with C alleles (all P > 0.05), after adjustment for covariates. Finally, with the CT genotype was used as a reference, women with the CC or TT genotype did not have a significantly higher risk of GDM than did women with the CT genotype (Table 4).
Table 4.
Association of MTHFR SNPs with GDM Risk
| SNPs | Loci | GDM, n (%) | Non-GDM, n (%) | Adjusted Modela | |
|---|---|---|---|---|---|
| OR (95% CI) | P | ||||
| Genotype | CC | 8 (12.0) | 56(18.7) | 1.000 | |
| CT | 35(52.2) | 151(50.5) | 2.162(0.842–5.551) | 0.109 | |
| TT | 24(35.8) | 92(30.8) | 2.391(0.875–6.529) | 0.089 | |
| Dominant model | CC | 8 (12.0) | 56(18.7) | ||
| CT+TT | 59(88.0) | 243(81.3) | 2.241(0.901–5.578) | 0.083 | |
| Additive model | CT | 35(52.2) | 151(50.5) | ||
| CC+TT | 32(47.8) | 148(49.5) | 0.822(0.452–1.497) | 0.522 | |
Note: aAdjustments for age, physical activity, BMI, parity, family history of diabetes, use of FA supplements, HOMA-IR, C-reactive protein, hemoglobin, vitamin B12, and serum homocysteine.
Discussion
China currently has no policy regarding food fortification with FA, and insufficient dietary FA intake and FA deficiency remain common. FA supplementation before and during early pregnancy may lead to significantly higher folate levels among pregnant women than among the general population. Because the current dosage (200–5000 μg/d) and courses of FA supplements before and during early pregnancy are relatively broad, excessive FA supplementation in early pregnancy requires attention.
To our knowledge, this is the first study to analyze the association between maternal RBC folate concentrations in the first trimester of pregnancy and GDM risk. Our findings suggest that high RBC folate levels, even in early pregnancy, may be associated with an increased risk of GDM. This evidence strengthens a previous finding that daily FA supplement use in the first trimester is associated with a higher risk of GDM,13 despite differences in folate evaluation between our studies.
Although the underlying mechanism by which high folate levels affect GDM susceptibility remains unclear, two possible explanations have been proposed.13 The first is an imbalance between vitamin B12 and folate. High folate levels may exacerbate the metabolic effects of vitamin B12 deficiency21 and affect the pathogenesis of GDM by impairing insulin resistance. Several studies have confirmed the possibility of this mechanism.11,22,23 Furthermore, the combination of vitamin B12 deficiency and high plasma folate concentrations was associated with a higher risk of GDM compared with normal vitamin B12 status and high folate concentrations.14 This adds evidence to the two studies. A UK population study24 reported that women with vitamin B12 deficiency rather than those with high folate levels were more likely to have obesity and GDM. However, we demonstrated that women whose RBC folate status was in the highest quartile had higher odds of GDM than did women whose RBC folate status was in the lowest quartile, independent of serum vitamin B12 and other covariates. Several factors may contribute to the inconsistency of the aforementioned findings. First, the time windows for determining folate status and serum vitamin B12 were different. Second, differences in participant ethnicities or dietary patterns may partly account for the inconsistent findings. Third, none of these studies, including the current study, included details on the duration of folate intake, which is crucial to the association of FA intake with adverse pregnancy outcomes,25 suggesting that differences in FA intake duration may affect the results.
The folate status of the participants in our study was assessed using RBC folate, which is indicative of long-term folate status and responds mainly to supplementation and fortification.16 In this case, RBC folate is an ideal index of effectiveness of folate supplementation because food fortification with FA has not been implemented in China, and FA supplements were the only source of synthetic FA used by the study population. Furthermore, a Chinese study confirmed that high-dose (≥800 μg/d) FA supplementation from prepregnancy to midpregnancy is significantly associated with a higher GDM risk,15 supporting our findings.
The second possible explanation for the mechanism underlying the association between high folate levels and an increased risk of GDM is the harmful effect of unmetabolized plasma FA, which is associated with reduced cytotoxicity of natural killer cells.26 This reduced cytotoxicity may be involved in the pathogenesis of GDM.27 However, studies on the effect of long-term high-FA intake on the immune function and health of pregnant women are warranted.
The MTHFR gene has been mapped to chromosomal region lp36.3 and comprises 11 exons encoding 5′,10′-MTHFR,28 a crucial regulatory enzyme in folate metabolism that converts 5′,10′-MTHFR into 5′-MTHFR, which is the methyl donor for the remethylation of homocysteine to methionine.29 MTHFR 677C→T is a common missense mutation resulting in the substitution of alanine with valine at amino acid position 222. Inheritance of the recessive T allele reduces enzyme activity and increases homocysteine concentrations,30 which is associated with insulin resistance.31,32
We observed no significant differences in HOMA-IR among the three genotypes. However, overall homocysteine concentrations in women with the TT genotype were significantly higher than those with the CT or CC genotypes (P = 0.001), after adjustment for age, BMI, and FA supplement use. These results are consistent with previous findings.30 In addition, carriers of the TT genotype typically have lower folate levels than do C allele carriers.17 However, we demonstrated that RBC folate concentrations in the TT group were generally higher than those in the other two groups when adjusted for age, BMI, and FA supplement use. This suggests that the TT genotype is related to increased RBC folate; however, dietary factors and FA supplementation duration were not quantitatively evaluated. Our findings are supported by a Chinese study in which RBC folate levels were significantly higher in individuals with the TT genotype than in those with the CC genotype.33
The effects of MTHFR C677T SNPs on GDM risk were analyzed in our study. Currently, only one study from India has researched the relationship between MTHFR C677T polymorphisms and GDM risk.34 That study reported no significant difference in the allele or genotype frequencies of MTHFR C677T polymorphisms between patients with and without GDM. This result is consistent with our findings. Furthermore, we performed binary logistic regressions to determine the ORs and 95% CIs of GDM risk according to MTHFR C677T polymorphisms. When the CC genotype was used as a reference, we observed that women with the CT or TT genotypes did not have a significantly higher risk of GDM, after adjustment for covariates. Furthermore, when analyzing the dominant and additive models for the MTHFR C677T gene loci, we observed a negative association between GDM risk and MTHFR C677T polymorphisms in this population.
We demonstrated that women whose RBC folate concentrations were in the highest quartile had a higher risk of GDM (P = 0.047) than did those whose RBC folate concentrations were in the lowest quartile, after adjustments for covariates. Moreover, rs1801133 SNPs were unlikely to be associated with GDM risk in this Chinese population. However, when rs1801133 polymorphisms were regarded as a covariate in the model of the relationship between RBC folate status and GDM risk, the GDM risk of women in the highest quartile of RBC folate was not significantly higher than that of women in the lowest quartile (adjusted OR = 2.251, 95% CI = 0.890–5.696, P = 0.087). Because women with the T allele genotype had the highest concentration of RBC folate in this population, rs1801133 SNPs may affect the association between RBC folate and GDM risk by influencing folate status.
Conclusion
The strengths of this study were its prospective design, novel analysis of the associations of RBC folate status in the first trimester of pregnancy and rs1801133 polymorphisms with GDM risk, and careful recording of obstetric outcomes by researchers blinded to folate status and rs1801133 genotype. However, our study has several limitations. First, the sample size was relatively small. Although high RBC folate status in early pregnancy was associated with an increased risk of GDM in this population, the significance was weak. Studies with larger sample sizes are warranted. However, our findings correspond to those of a Chinese study in which high-dosage (≥800 μg/d) FA supplementation from prepregnancy to midpregnancy was significantly associated with higher GDM risk.15 Second, we measured RBC folate concentration only once during early pregnancy. However, increased folate levels in the second or the third trimester of pregnancy are also associated with high GDM risk. Third, dietary folate intake and duration of FA supplementation were not quantitatively evaluated. Despite these shortcomings, our study suggests for the first time that higher maternal RBC folate levels during early pregnancy are associated with greater GDM risks, and this association may be affected by rs1801133 polymorphisms. Therefore, folate status assessment using RBC concentrations and subsequent administration of appropriate folate supplements during early pregnancy may help prevent GDM.
Acknowledgments
We thank Shanghai GeneX Biotech Co., Ltd and Beijing Malt Health Management Co. Ltd for their free technical support. We also acknowledge Li ShanShan, Wang BingXin, Li BaoLei, Li Rui, Luo Haoze, Zhou Zikun, and Bao Yuanyuan, who helped with collecting anonymous data from routinely collected maternity records.
Abbreviations
GDM, gestational diabetes mellitus; MTHFR, methylenetetrahydrofolate reductase; RBC, red blood cell; FA, folic acid; FPG, fasting plasma glucose; IGT, impaired glucose tolerance; CRP, C-reactive protein; OGTT, oral glucose tolerance test; NTD, neural tube defect; HOMA-IR, homeostasis model assessment-insulin resistance; HOMA-β, homeostasis model assessment-β; SNP, single-nucleotide polymorphism.
Author Contributions
All authors contributed to data analysis, article drafting, and revision. All authors approved the final version for publishing and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16(1):7. doi: 10.1007/s11892-015-0699-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu WW, Yang HX, Wei YM, et al. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in china. Diabetes Care. 2013;36:586–590. doi: 10.2337/dc12-1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014;103(2):176e85. doi: 10.1016/j.diabres.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 4.Mishra S, Rao CR, Shetty A. Trends in the diagnosis of gestational diabetes mellitus. Scientifica (Cairo). 2016;2016:5489015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(Suppl 3):S173–S211. [DOI] [PubMed] [Google Scholar]
- 6.Farrar D, Simmonds M, Bryant M, et al. Hyperglycaemia and risk of adverse perinatal outcomes: systematic review and meta-analysis. BMJ. 2016;354:i4694. doi: 10.1136/bmj.i4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowe WL Jr, Scholtens DM, Kuang A, et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019;42(3):372–380. doi: 10.2337/dc18-1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Cianni G, Lacaria E, Lencioni C, Resi V. Preventing type 2 diabetes and cardiovascular disease in women with gestational diabetes – the evidence and potential strategies. Diabetes Res Clin Pract. 2018;145:184–192. doi: 10.1016/j.diabres.2018.04.021 [DOI] [PubMed] [Google Scholar]
- 9.Page KA, Buchanan TA. The vicious cycle of maternal diabetes and obesity: moving from “what” to “how” and “why.”. J Pediatr. 2011;158:872–873. doi: 10.1016/j.jpeds.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 10.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Folic acid supplementation for the prevention of neural tube defects: US preventive services task force recommendation statement. JAMA. 2017;317:183–189. doi: 10.1001/jama.2016.19438 [DOI] [PubMed] [Google Scholar]
- 11.Yajnik CS, Deshpande SS, Jackson AA, et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia. 2008;51:29–38. doi: 10.1007/s00125-007-0793-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, He Y, Sun X, He Y, Li Y, Sun C. Maternal high folic acid supplement promotes glucose intolerance and insulin resistance in male mouse offspring fed a high-fat diet. Int J Mol Sci. 2014;15:6298–6313. doi: 10.3390/ijms15046298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu B, Ge X, Huang K, et al. Folic acid supplement intake in early pregnancy increases risk of gestational diabetes mellitus: evidence from a prospective cohort study. Diabetes Care. 2016;39:e36–7. doi: 10.2337/dc15-2389 [DOI] [PubMed] [Google Scholar]
- 14.Lai JS, Pang WW, Cai S, et al. High folate and low vitamin B12 status during pregnancy is associated with gestational diabetes mellitus. Clin Nutr. 2018;37:940–947. doi: 10.1016/j.clnu.2017.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, Zhang Y, Huang L, et al. High-dose folic acid supplement use from prepregnancy through midpregnancy is associated with increased risk of gestational diabetes mellitus: a prospective cohort study. Diabetes Care. 2019;42:e113–e115. doi: 10.2337/dc18-2572 [DOI] [PubMed] [Google Scholar]
- 16.Cordero AM, Crider KS, Rogers LM, Cannon MJ, Berry RJ. Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects: World Health Organization guidelines. MMWR Morb Mortal Wkly Rep. 2015;64:421–423. [PMC free article] [PubMed] [Google Scholar]
- 17.Botto LD, Yang Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol. 2000;151(9):862–877. doi: 10.1093/oxfordjournals.aje.a010290 [DOI] [PubMed] [Google Scholar]
- 18.Pfeiffer CM, Fazili Z. Folate analytical methodology In: Baily LB, editor. Folate in Health and Diseases. 2nd ed. Boca Raton: CRC Press; 2010:517–574. [Google Scholar]
- 19.Xie K, Xu P, Fu Z, et al. Association of maternal folate status in the second trimester of pregnancy with the risk of gestational diabetes mellitus. Food Sci Nutr. 2019;7:3759–3765. doi: 10.1002/fsn3.1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 21.Selhub J, Morris MS, Jacques PF. In vitamin B12 deficiency, higher serum folate is associated with increased total homocysteine and methylmalonic acid concentrations. Proc Natl Acad Sci U S A. 2007;104:19995–20000. doi: 10.1073/pnas.0709487104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Idzior-Walus B, Cyganek K, Sztefko K, et al. Total plasma homocysteine correlates in women with gestational diabetes. Arch Gynecol Obstet. 2008;278:309e13. doi: 10.1007/s00404-008-0571-1 [DOI] [PubMed] [Google Scholar]
- 23.Krishnaveni GV, Hill JC, Veena SR, et al. Low plasma vitamin B12 in pregnancy is associated with gestational ‘diabesity’ and later diabetes. Diabetologia. 2009;52:2350–2358. doi: 10.1007/s00125-009-1499-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sukumar N, Venkataraman H, Wilson S, et al. Vitamin B12 status among pregnant women in the UK and Its Association with Obesity and Gestational Diabetes. Nutrients. 2016;8:pii: E768. doi: 10.3390/nu8120768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Ge X, Zhu B, et al. Maternal continuing folic acid supplementation after the first trimester of pregnancy increased the risk of large-for-gestational-age birth: a population-based birth cohort study. Nutrients. 2016;8(8):pii: E493. doi: 10.3390/nu8080493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Troen AM, Mitchell B, Sorensen B, et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr. 2006;136(1):189–194. doi: 10.1093/jn/136.1.189 [DOI] [PubMed] [Google Scholar]
- 27.Zhao YH, Wang DP, Zhang LL, Zhang F, Wang DM, Zhang WY. Genomic expression profiles of blood and placenta reveal significant immune-related pathways and categories in Chinese women with gestational diabetes mellitus. Diabet Med. 2011;28:237–246. doi: 10.1111/j.1464-5491.2010.03140.x [DOI] [PubMed] [Google Scholar]
- 28.Rai V. Folate pathway gene MTHFR C677T polymorphism and risk of lung cancer in Asian populations. Asian Pac J Cancer Prev. 2014;15(21):9259–9264. doi: 10.7314/APJCP.2014.15.21.9259 [DOI] [PubMed] [Google Scholar]
- 29.Liu NB, Li J, Qi JF, Zhang ZZ, Wu X, Zhang JH. Methylenetetrahydrofolate reductase 677TT genotype may be associated with an increased lung cancer risk in North China: an updated meta-analysis. Med Sci Monit. 2014;20:2817–2823. doi: 10.12659/MSM.892050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crider KS, Zhu JH, Hao L, et al. MTHFR 677C→T genotype is associated with folate and homocysteine concentrations in a large, population-based, double-blind trial of folic acid supplementation. Am J Clin Nutr. 2011;93:1365–1372. doi: 10.3945/ajcn.110.004671 [DOI] [PubMed] [Google Scholar]
- 31.Weiss N, Heydrick SJ, Postea O, Keller C, Jr JF K, Loscalzo J. Influence of hyperhomocysteinemia on the cellular redox state-impact on homocysteine-induced endothelial dysfunction. Clin Chem Lab Med. 2003;41:1455e61. doi: 10.1515/CCLM.2003.223 [DOI] [PubMed] [Google Scholar]
- 32.Meigs JB, Jacques PF, Selhub J, et al. Fasting plasma homocysteine levels in the insulin resistance syndrome: the Framingham offspring study. Diabetes Care. 2001;24(8):1403e10. doi: 10.2337/diacare.24.8.1403 [DOI] [PubMed] [Google Scholar]
- 33.Ni J, Zhang L, Zhou T, et al. Association between the MTHFR C677T polymorphism, blood folate and vitamin B12 deficiency, and elevated serum total homocysteine in healthy individuals in Yunnan Province, China. J Chin Med Assoc. 2017;80:147–153. doi: 10.1016/j.jcma.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 34.Khan IA, Shaik NA, Kamineni V, Jahan P, Hasan Q, Rao P. Evaluation of gestational diabetes mellitus risk in South Indian women based on MTHFR (C677T) and FVL (G1691A) mutations. Front Pediatr. 2015;3:34. doi: 10.3389/fped.2015.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]