Abstract
Objective(s):
Seizure detection during online recording of electrophysiological parameters is very important in epileptic patients. In the present study, online analysis of field potential recordings was used for detecting spontaneous seizures in epileptic animals.
Materials and Methods:
Epilepsy was induced in rats by pilocarpine injection. During the chronic period of the pilocarpine model, local field potential (LFP) recording was run for at least 24 hr. At the same time, video monitoring of the animals was done to determine the real time of seizure occurrence. Both power and sample entropy of LFP were used for online analysis.
Results:
Obtained results showed that changes in LFP power are a better index for seizure detection. In addition, when we used one hundred consecutive epochs (each epoch equals 10 ms) of LFP for data analysis, the best detection was achieved.
Conclusion:
It may be suggested that power is a suitable parameter for online analysis of LFP in order to detect the spontaneous seizures correctly.
Key Words: Entropy, Local field potentials, Pilocarpine, Power, Seizure detection
Introduction
With 68.8/100000 victims, epilepsy is one of the most common neural diseases (1). In most cases, the cause of epilepsy is unknown, but events such as brain damage, stroke, and trauma may be the reason in some cases (2). Many developments have occurred in epilepsy treatment during recent years (3). During the past decade, many novel anticonvulsant drugs have been introduced to the market, yet a third of epileptic patients still suffer from seizures (4). However, 30% of patients are drug-resistant, and many others cannot undergo surgery because the focal-point of their seizure-attack is unidentified (5). Therefore, it is necessary to find new treatments for these patients. Deep brain stimulation (DBS) has been recently studied in many experimental models of epilepsy as an alternative therapy for drug-resistant epilepsies.
Among different laboratory models of epilepsy, the pilocarpine model is very similar to human temporal lobe seizures according to its behavioral, electrophysiological, and morphological properties (6). Various parts of the limbic system, including the amygdala and hippocampus, have an important role in the spreading of seizures (7). Synchronized and overactive neurons of these regions can be targeted for studying brain dynamics by using spatiotemporal signals, such as local field potential (LFP) recordings (8). After the discovery of electro-encephalography, LFP recording has been extensively used for studying brain activity (9). LFP is used to record all frequency ranges of brainwaves and can represent brain activity.
In the past two decades, low-frequency stimulation (LFS), as an effective anti-convulsant pattern of DBS, has been investigated by many researchers for curing drug-resistant epileptic patients (10). Seizure-detecting systems constantly monitor seizure activity and have a significant role in managing and controlling seizure. Following detection, brain stimulation can be applied as a curing option as soon as the clinical symptoms are revealed. This method results in limited side-effects compared to epilepsy surgery and prevents seizure attacks (11).
Two methods of stimulation have been used to stimulate the brain for curing epilepsy during the past decades: the open-loop and the closed-loop. The open-loop method applies a pre-determined stimulating protocol regardless of clinical symptoms (12); however, the closed-loop method applies its stimulation during a seizure attack (13). In the open-loop method, the brain is stimulated in a semi-constant (12, 14) or alternating schedule in a given time (15). This daily pattern changes depending on each patient and time of therapy (16). The open-loop does not have a smart-mechanism to determine the condition of the brain for stimulation.
The closed-loop method has three subsystems: the brain-recording system, seizure-detecting system, and stimulation program. Analysis is performed simultaneously with LFPs recording , and stimulation is applied once a seizure is detected (13, 17). Nevertheless, the closed-loop method has advantages including: better capability to prevent the spreading of seizure, fewer stimulations (only on previously scheduled moments), fewer undesired effects (the open-circuit method may impair normal brain activity), fewer number of stimulation which decreases the battery usage and therefore fewer surgery will be needed for battery exchanging, the ability to study seizure frequency, and other parameters for detecting seizures (18, 19). Several algorithms have been produced in the past decades for detecting and predicting seizures (20–22). Most of these studies are based on previously available data (23, 24).
Detecting seizures enables us to overcome some of the challenges faced in managing the epilepsy curing procedure. This information can be utilized for exact detection of seizures and to provide proper curing in a suitable time for epilepsy. In the present research, we tried to find an appropriate algorithm for seizure detection in the pilocarpine epilepsy model in rats. Finding this algorithm is very important for use in future studies on closed-loop electrical stimulation of epileptic subjects.
Materials and Methods
Animals
21 male Wistar rats (weighing 200–250 g), obtained from the Razi Institute (Karaj, Iran), were used. Animals were maintained under the standard conditions, 22–25 °C temperature, 12 hr light/dark, and access to food and water ad libitum. All manipulations were in line with the ethical guidelines approved in advance by the Ethical Committee of the Faculty of Medical Sciences, Tarbiat Modares University according to the NIH Guide for the Care and Use of Laboratory Animals.
Chronic Seizure Induction
For epilepsy induction, animals received lithium chloride (212 mg/kg, IP) and pilocarpine hydrochloride (150 mg/kg, IP) 24 hr later. Methylscopolamine bromide (1 mg/kg, IP) was also administered 30 min before pilocarpine hydrochloride in order to limit the peripheral effects of pilocarpine. Diazepam (4 mg/kg) was administered one hour after the first seizure experience to control the severity of status epilepticus and to prevent animal death. Rats developed diarrhea and showed other signs of cholinergic stimulation 5 min after pilocarpine injection. They exhibited head nodding, scratching, chewing, and exploratory behavior during the next 15–20 min. Pilocarpine treatment induced the following behavioral changes: akinesia, facial automatisms, and limbic seizures consisting of forelimb clonus with rearing, salivation, masticatory jaw movements, and falling. In fact, pilocarpine side effects were restricted to the first week after its administration, while the recurrent seizures started around 30–60 min after the pilocarpine administration, and the animals were unresponsive to their environment. The initial acute insult was followed by a seizure-free phase (silent) and, finally, a chronic period characterized by the occurrence of spontaneous seizures; the chronic phase started 45–60 days after the first attack. Only 7 out of 21 rats showed epileptic behaviors.
Surgery
Under 100 mg/Kg ketamine (10%, Alfasan, The Netherlands) and 10 mg/Kg xylazine (20%, Alfasan, The Netherlands), animals underwent stereotaxic implantation with a monopolar recording electrode in the CA1 region of the right ventral hippocampus (coordinates: A, -6.0 mm; L, 5.5mm; and V, 6.8 below dura) according to the atlas of Paxinos and Watson (1985). Recording electrode (stainless steel, Teflon coated, 127 µm in diameter, A.M. Systems, USA) and reference electrode (connected to the skull by a miniature screw) were insulated except at their tips. Three screws were planted into the skull as anchors. All electrodes were connected to pins of a lightweight multichannel miniature socket as a head-stage and fixed on the skull with dental acrylic. The animals were allowed 10 days as post-surgery recovery period before starting the experiments.
Local field potential recording
LLFPs were recorded from the CA1 region of the hippocampus. After recovery, hippocampal LFPs were recorded when the animals were put in a Plexiglas recording box inside a Faraday’s cage. The rat’s head-stage was connected to a flexible, shielded cable, and the animal was allowed to move freely during recording in the recording box. Signals were filtered at 3 kHz, amplified, and digitized (at 10 kHz) using a PC-based data acquisition system (BIODAC ES1721, TRITA WaveGram CO., Tehran, Iran) and were continuously monitored and stored on disk.
Hippocampal LFPs were recorded 24 hr a day for one week. Simultaneously, 24 hr video recording was run to evaluate the occurrence of seizure attacks based on animal behaviors. Seizure behaviors were detected according to the Racine’s scales (25).
Seizure detector
For seizure detecting, a detection algorithm based on comparing either the short-term total power or the short-term sample entropy of signal to a threshold value was used. Both the total power and the entropy can be potentially suitable indices for seizure detection as their values change during seizure occurrence. The power, which is related to the amplitude of the LFP waves, increases by seizure. The sample entropy, which is calculated as the negative natural logarithm of an estimated conditional probability that subseries of length match point-wise within a tolerance , also match at the next point, decreases during seizure.
The calculation of short-term power and short-term sample entropy values was done by windowing analysis of signal. We analyzed the LFP signals and epileptic waves by using a MATLAB toolbox named signal processing toolbox. We used the pwelch function of the MATLAB software package to calculate the short-time power. In addition, the sampen function, which is available on the physio.net site for the MATLAB software package, was used for calculating the sample entropy. The length of the time-windows for both methods was 2 sec and the overlap between windows was 95%. In the pwelch method, which includes an additional segmentation, the length of each segment was 1 sec and the overlap between segments was 75%.
To detect the onset of seizures, the average of the desired parameter (either power or sample entropy) was calculated for a long time (10 sec in our study) of signal as base value of that parameter. In each data update (0.1 sec in our study), the instantaneous value of power or sample entropy was calculated and was compared to a threshold value. The threshold level was considered as 3 times increase in power (or 0.25 time decrease in sample entropy). The greater the magnitude of the threshold coefficient, the harder conditions were considered for seizure detection. Therefore, in this condition, seizure may be detected with more latency, but more confidently.
In this study a closed-loop system was used for seizure detection in freely moving animals. Therefore, the confidence coefficient of detection should be high enough to avoid misdiagnosis of the movement artifacts of the animal. For this purpose, we considered a time interval during which the short-term values should stay greater than (for power) or less than (for sample entropy) the threshold value. It should be noted that if in any moment of this interval, the momentum value of power is less than the threshold value, the interval will begin from zero again. The longer the length of this interval, the higher the reliability of the detection and lower the misdiagnosis of environmental artifacts and noises; but more latency in the time of seizure detection. The flowchart of the used algorithm is shown in Figure 1.
Figure 1.
A) Schematic figure showing the setup used for online analysis of LFP recorded from an epileptic animal accompanied by the video monitoring of rat. B) Block diagram showing the algorithm used for online analysis of data
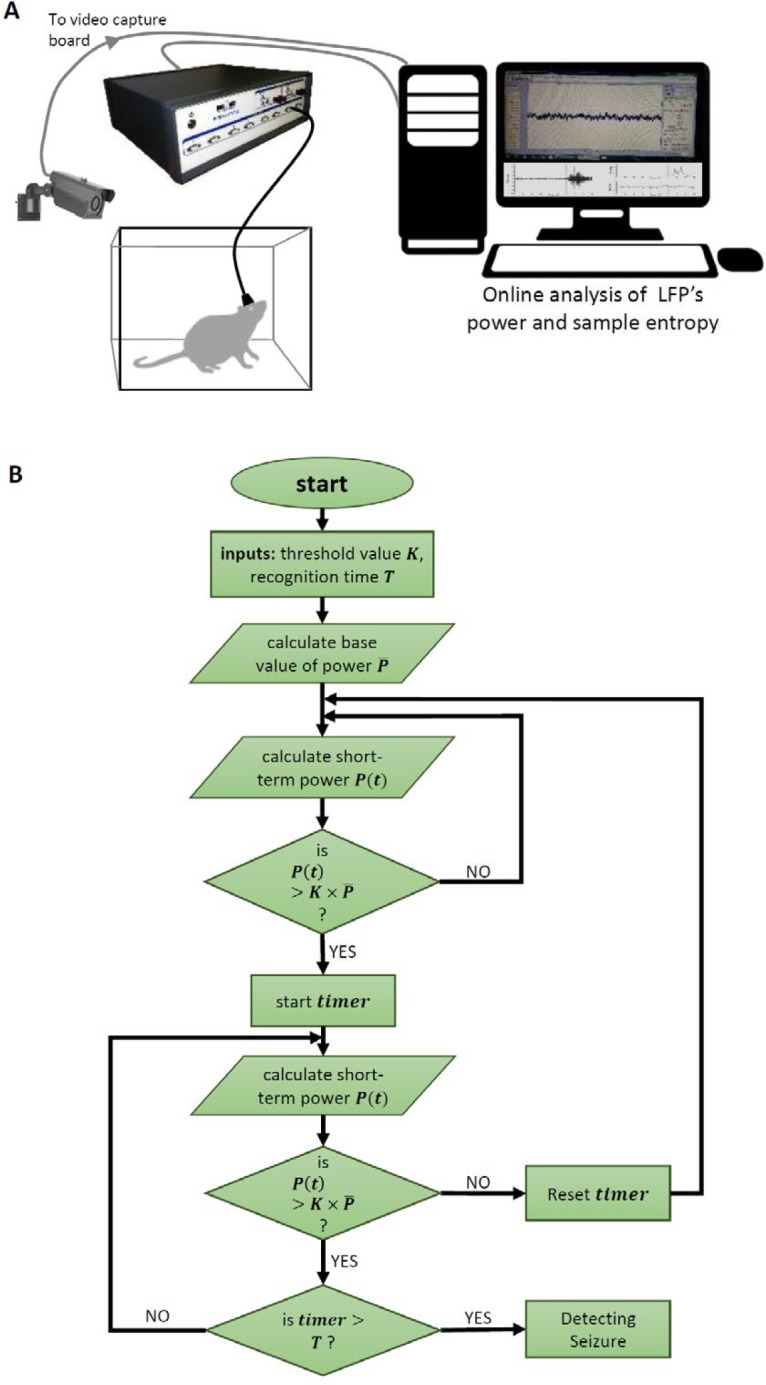
LFP: Local field potential
Statistical analysis
All obtained data were analyzed via the MATLAB (MathWorks) software package, and are presented as means±SEM. Statistical comparison between delay time of seizure detection by power or sample entropy analysis was performed using Graphpad Prism software, version 6.01 for Windows (Graphpad, CA, USA). The level of significance was P<0.05.
Results
As explained previously, the online analysis of power and entropy of hippocampal LFPs were run using the MATLAB software package. During seizure induction, the occurrence of ictal discharges resulted in huge fluctuations in LFP recording and was accompanied with changes in power and entropy. To determine that between sample entropy or power of LFP which one is a better parameter for seizure detection, we compared the online detection of 16 seizures in 6 epileptic rats. Obtained results revealed that LFP power is a better indicator for online seizure detection compared to LFP entropy. As Figure 2 shows, the changes in power were correctly matched with seizure occurrence in all of the cases while there was no relationship between the changes in entropy and seizure occurrence in some cases. Table 1 shows the number of correct and incorrect detections of seizures in these two methods illustrating power as a better indicator for seizure detection.
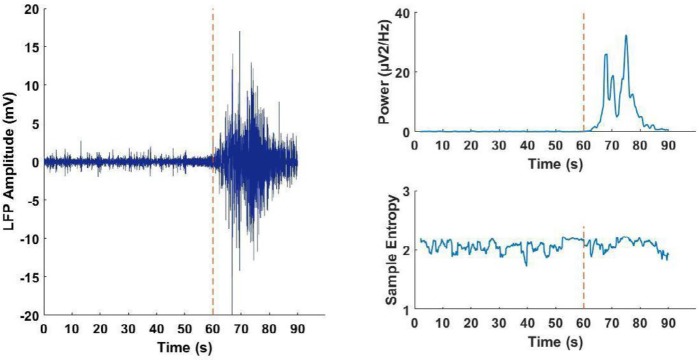
Figure 2.
Using the change in wave power and sample entropy for seizure detection in freely moving animals. A) Left: Sample record of LFS from the hippocampal CA1 region during occurrence of spontaneous seizure. Red dashed line shows the time of seizure initiation. Right: Sample online analysis of changes in LFP power and sample entropy for detecting the seizure initiation. Dashed line shows the real initiation time of seizure and arrows show the detection time according to online analysis of power (above) or sample entropy (below). B) Delay in seizure detection time according to online analysis of power or sample entropy of LFP. Sixty sec was considered as cut-off time for seizure detection. As the graph shows, online analysis of power is more suitable for seizure detection
LFP: local field potentials
Table 1.
The percentage of correct and wrong seizure detection by using either power or entropy of local field potentials
| Wrong detections | Correct detections | No. of seizures | Detection method | |
|---|---|---|---|---|
| 0 | 100 | 16 | Power | |
| 31.25 | 68.75 | 16 | Entropy | |
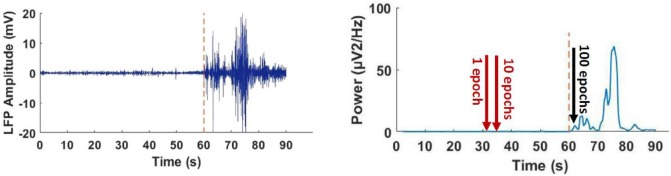
There were also some non-seizure noises in LFPs, for example from animal movement or electronics instruments. To find a way for correct detection of seizure behavior, the power or entropy of the changes were assessed online in 10 ms epochs. A five times increase in power or entropy compared to baseline, was considered as criteria of seizure induction. Figure 3 shows that when the software calculated this increase in one or 10 consecutive epochs, both seizure and non-seizure induced fluctuations were detected as seizure. However, after increasing the number of epochs to 100, correct detection occurred (Figure 3).
Figure 3.
Effect of change in the number of epochs (10 ms) when LFP power was used for seizure detection in sample LFS recorded during spontaneous seizure recording (left). Red dashed line shows the time of seizure initiation. Using one or ten epochs (red arrows in the right graph) resulted in incorrect detection of seizure. Using one hundred epochs (black arrows in the right graph) resulted in correct detection of seizure
LFP: local field potentials; LFS: low frequency stimulation
Discussion
The main aim of this study was to find a method for online detection of seizure attacks according to LFP recording. We used the online analysis of power and entropy of LFP waves. Our study showed that LFP power was a more reliable criteria compared to its entropy.
Many studies have been done to find the best methods for seizure detection and/or anticipation. However, in most of them the rout of analysis was according to the offline EEG analysis. During the online detection of seizure, one important problem is the ability to distinguish between seizure- and nonseizure-induced fluctuations in LFP waves. This is a big problem especially in preclinical experiments with animals. During freely moving behaviors, there are a lot of sources which can produce noises in the LFP, such as sudden and rapid movements of animal, while in human EEG there is no movement-induced noises during interictal periods.
In this study we recorded LFPs from the ventral hippocampus. In pilocarpine model of epilepsy, the chemical is distributed to all brain regions, but those regions which are susceptible to seizure initiation, will exert the main role. The hippocampus is one of the most important focal points of the seizure. It has been reported that in the pilocarpine model, the initial origins of the seizure are the ventral hippocampus and ventral subiculum (equivalent to the anterior hippocampus in humans) (26).
Artificial neural networks use a variety of criteria to detect the seizures. We used wave entropy and power to detect seizure. Algorithms designed based on artificial neural networks usually use complex signal analysis methods that are suitable for offline seizure detection. However, these complex analyses take time and may not be suitable for online seizure detection. Considering this fact, analysis of the wave power is a suitable manner for online detection of seizure activity, as it has low complexity (27). The characteristics of various band frequencies, such as the delta and theta waves change continuously (28). Accordingly, in this study, the total power frequency band was considered for analysis, which was a more stable parameter. The results of the present study revealed that wave power increases when a seizure begins to initiate. Novak et al. have shown that wave power increases 30 sec before onset of seizure activity and quickly drops once seizure ends (29).
Acharya et al. (2015) have shown that among the various entropy measures used for detecting seizure, RE, SEN, and PE are the best types (30). Therefore, we also used sample entropy to detect seizures. Our data revealed that sample entropy is not a suitable criteria for detecting seizure activity online. Nevertheless, some studies have shown that sample entropy is a suitable and reliable criteria for online detection of seizures (31). Jouny et al. (2012) have found that sample entropy changes in partial seizures and mesial onset seizures. Perhaps one of the reasons is that seizure with sample entropy does not fully perform in epilepsy of the pilocarpine model, which is a complex epilepsy (28).
Seizure detection by EEG of LFP recording is very important in closed-loop circuit for using brain stimulation as a potential anticonvulsive agent. The time of brain stimulation is very important for maximum effectiveness. We previously showed that application of low-frequency stimulation (as an anti-seizure agent) at the beginning of epileptiform activity is more effective compared to its application before or several minutes after the onset of seizure (32) . Therefore, correct detection of seizure can be used in a closed-loop circuit for applying the brain stimulations at the beginning of epileptic seizures.
Conclusion
Simple algorithms must be used for online detection of seizure. We demonstrated that LFP wave power is a more reliable criteria than entropy. Noises caused by animal behavior are best omitted using software modifications. Modifying algorithms related to power is more reliable since changing entropy algorithm resulted in false seizure detection.
Acknowledgment
This study was supported by a grant (#IG-37909) from Tarbiat Modares University, Tehran, Iran and a grant (#957513) from National Institute for Medical Research Development (NIMAD), Iran.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Christensen J, Vestergaard M, Pedersen MG, Pedersen CB, Olsen J, Sidenius P. Incidence and prevalence of epilepsy in Denmark. Epilepsy Res. 2007;76:60–65. doi: 10.1016/j.eplepsyres.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Herman ST. Epilepsy after brain insult: targeting epileptogenesis. Neurology. 2002;59:21–26. doi: 10.1212/wnl.59.9_suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- 3.Chang BS, Lowenstein DH. Epilepsy. N Engl J Med. 2003;349:1257–1266. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- 4.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 5.Sillanpaa M, Jalava M, Kaleva O, Shinnar S. Long-term prognosis of seizures with onset in childhood. N Engl J Med. 1998;338:1715–1722. doi: 10.1056/NEJM199806113382402. [DOI] [PubMed] [Google Scholar]
- 6.Scorza FA, Arida RM, Naffah-Mazzacoratti MdG, Scerni DA, Calderazzo L, Cavalheiro EA. The pilocarpine model of epilepsy: what have we learned? An Acad Bras Cienc. 2009;81:345–365. doi: 10.1590/s0001-37652009000300003. [DOI] [PubMed] [Google Scholar]
- 7.Cendes F, Andermann F, Gloor P, Evans A, Jones-Gotman M, Watson C et al. MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology. 1993;43:719–725. doi: 10.1212/wnl.43.4.719. [DOI] [PubMed] [Google Scholar]
- 8.Boulton AA, editor. Neurophysiological Techniques: Applications to Neural Systems. Totowa, NJ: Humana Press; 1991. [Google Scholar]
- 9.Kajikawa Y, Schroeder CE. How local is the local field potential? Neuron. 2011;72:847–858. doi: 10.1016/j.neuron.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kile KB, Tian N, Durand DM. Low frequency stimulation decreases seizure activity in a mutation model of epilepsy. Epilepsia. 2010;51:1745–1753. doi: 10.1111/j.1528-1167.2010.02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramgopal S, Thome-Souza S, Jackson M, Kadish NE, Sánchez Fernández I, Klehm J et al. Seizure detection, seizure prediction, and closed-loop warning systems in epilepsy. Epilepsy Behav. 2014;37:291–307. doi: 10.1016/j.yebeh.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 12.Elliott RE, Morsi A, Tanweer O, Grobelny B, Geller E, Carlson C et al. Efficacy of vagus nerve stimulation over time: review of 65 consecutive patients with treatment-resistant epilepsy treated with VNS 10 years. Epilepsy Behav. 2011;20:478–483. doi: 10.1016/j.yebeh.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 13.Morrell M. Brain stimulation for epilepsy: Can scheduled or responsive neurostimulation stop seizures? Curr Opin Neurol. 2006;19:164–168. doi: 10.1097/01.wco.0000218233.60217.84. [DOI] [PubMed] [Google Scholar]
- 14.Rashid S, Pho G, Czigler M, Werz MA, Durand DM. Low frequency stimulation of ventral hippocampal commissures reduces seizures in a rat model of chronic temporal lobe epilepsy. Epilepsia. 2012;53:147–156. doi: 10.1111/j.1528-1167.2011.03348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodaie M, Wennberg RA, Dostrovsky JO, Lozano AM. Chronic anterior thalamus stimulation for intractable epilepsy. Epilepsia. 2002;43:603–608. doi: 10.1046/j.1528-1157.2002.26001.x. [DOI] [PubMed] [Google Scholar]
- 16.Pavlova MK, Shea SA, Bromfield EB. Day/night patterns of focal seizures. Epilepsy Behav. 2004;5:44–49. doi: 10.1016/j.yebeh.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Good LB, Sabesan S, Marsh ST, Tsakalis K, Treiman D, Iasemidis L. Control of synchronization of brain dynamics leads to control of epileptic seizures in rodents. Int J Neural Syst. 2009;19:173–196. doi: 10.1142/S0129065709001951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun FT, Morrell MJ. The RNS System: Responsive cortical stimulation for the treatment of refractory partial epilepsy. Expert Rev Med Devices. 2014;11:563–572. doi: 10.1586/17434440.2014.947274. [DOI] [PubMed] [Google Scholar]
- 19.Fisher RS, Velasco AL. Electrical brain stimulation for epilepsy. Nat Rev Neurol. 2014;10:261–270. doi: 10.1038/nrneurol.2014.59. [DOI] [PubMed] [Google Scholar]
- 20.Schad A, Schindler K, Schelter B, Maiwald T, Brandt A, Timmer J et al. Application of a multivariate seizure detection and prediction method to non-invasive and intracranial long-term EEG recordings. Clin Neurophysiol. 2008;119:197–211. doi: 10.1016/j.clinph.2007.09.130. [DOI] [PubMed] [Google Scholar]
- 21.Aarabi A, Fazel-Rezai R, Aghakhani Y. A fuzzy rule-based system for epileptic seizure detection in intracranial EEG. Clin Neurophysiol. 2009;120:1648–1657. doi: 10.1016/j.clinph.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Salam M, Sawan M, Nguyen D. Low-power implantable device for onset detection and subsequent treatment of epileptic seizures: A review. J Helthc Eng. 2010;1:169–184. [Google Scholar]
- 23.Salam MT, Sawan M, Dang KN. A novel low-power-implantable epileptic seizure-onset detector. IEEE Trans Biomed Circuits Syst. 2011;5:568–578. doi: 10.1109/TBCAS.2011.2157153. [DOI] [PubMed] [Google Scholar]
- 24.Safi-Harb M, Salam MT, Nguyen DK, Sawan M. An implantable seizure-onset detector based on a dual-path single-window count-based technique for closed-loop applications. IEEE J Emerg. Sel. Topics Circuits Syst. 2011;1:603–612. [Google Scholar]
- 25.Racine RJ. Modification of seizure activity by electrical stimulation: II Motor seizure. Electroencephalography and Clinical Neurophysiology. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 26.Toyoda I, Bower MR, Leyva F, Buckmaster PS. Early activation of ventral hippocampus and subiculum during spontaneous seizures in a rat model of temporal lobe epilepsy. J Neurosci. 2013;33:11100–11115. doi: 10.1523/JNEUROSCI.0472-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imtiaz SA, Logesparan L, Rodriguez-Villegas E. Performance-power consumption tradeoff in wearable epilepsy monitoring systems. IEEE J Biomed Health Inform. 2015;19:1019–1028. doi: 10.1109/JBHI.2014.2342501. [DOI] [PubMed] [Google Scholar]
- 28.Jouny CC, Bergey GK. Characterization of early partial seizure onset: Frequency, complexity and entropy. Clin Neurophysiol. 2012;123:658–669. doi: 10.1016/j.clinph.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novak V, Reeves AL, Novak P, Low PA, Sharbrough FW. Time-frequency mapping of R-R interval during complex partial seizures of temporal lobe origin. J Auton Nerv Syst. 1999;77:195–202. [PubMed] [Google Scholar]
- 30.Acharya UR, Fujita H, Sudarshan VK, Bhat S, Koh JE. Application of entropies for automated diagnosis of epilepsy using EEG signals: A review. Knowledge-based systems. 2015;88:85–96. [Google Scholar]
- 31.Song Y, Crowcroft J, Zhang J. Automatic epileptic seizure detection in EEGs based on optimized sample entropy and extreme learning machine. J Neurosci Methods. 2012;210:132–146. doi: 10.1016/j.jneumeth.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Ghasemi Z, Naderi N, Shojaei A, Ahmadirad N, Raoufy MR, Mirnajafi-Zadeh J. Low frequency electrical stimulation attenuated the epileptiform activity-induced changes in action potential features in hippocampal CA1 pyramidal neurons. Cell J. 2018;20:355–360. doi: 10.22074/cellj.2018.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]