Abstract
The glucagon-like peptide-1 receptor (GLP-1R) agonist liraglutide is approved for the treatment of obesity; however, there is still much to be learned regarding the neuronal sites of action that underlie its suppressive effects on food intake and body weight. Peripherally administered liraglutide in rats acts in part through central GLP-1Rs in both the hypothalamus and the hindbrain. Here, we extend findings supporting a role for hindbrain GLP-1Rs in mediating the anorectic effects of liraglutide in male rats. To dissociate the contribution of GLP-1Rs in the area postrema (AP) and the nucleus tractus solitarius (NTS), we examined the effects of liraglutide in both NTS AAV-shRNA–driven Glp1r knockdown and AP-lesioned animals. Knockdown of NTS GLP-1Rs, but not surgical lesioning of the AP, attenuated the anorectic and body weight–reducing effects of acutely delivered liraglutide. In addition, NTS c-Fos responses were maintained in AP-lesioned animals. Moreover, NTS Glp1r knockdown was sufficient to attenuate the intake- and body weight–reducing effects of chronic daily administered liraglutide over 3 weeks. Development of improved obesity pharmacotherapies requires an understanding of the cellular phenotypes targeted by GLP-1R agonists. Fluorescence in situ hybridization identified Glp1r transcripts in NTS GABAergic neurons, which when inhibited using chemogenetics, attenuated the food intake– and body weight–reducing effects of liraglutide. This work demonstrates the contribution of NTS GLP-1Rs to the anorectic potential of liraglutide and highlights a phenotypically distinct (GABAergic) population of neurons within the NTS that express the GLP-1R and are involved in the mediation of liraglutide signaling.
INTRODUCTION
The ongoing obesity epidemic requires strategies aimed at reducing food intake and sustaining body weight loss. Because lifestyle interventions fail to maintain long-term weight loss (1), pharmacotherapies are used in conjunction with diet and exercise to support clinically meaningful weight loss that persists over time. One such class of drugs includes analogs of the endogenous satiety signal glucagon-like peptide-1 (GLP-1) (2). The long-acting synthetic GLP-1 receptor (GLP-1R) agonist liraglutide shares 97% homology with human GLP-1 and reduces body weight in both humans and preclinical models via a reduction in appetite and energy intake [see (3) for review]. However, despite liraglutide’s clinical implications and U.S. Food and Drug Administration–approved status, the sites of action and mechanisms underlying its anorectic effects are only partially understood.
Similar to native GLP-1 (4, 5), the food intake– and body weight– reducing effects of liraglutide require central GLP-1R activation (6, 7). Because the distribution of GLP-1Rs within the brain is vast (8–11), a systematic analysis of the GLP-1R populations that are functionally relevant for the regulation of feeding behavior is necessary. We chose to examine the involvement of GLP-1Rs within the nucleus tractus solitarius (NTS), a hindbrain hub of energy balance control (12, 13), in the mediation of liraglutide’s anorectic effects. The NTS together with the area postrema (AP) and dorsal motor nucleus of the vagus (DMX) comprise the dorsal vagal complex (DVC). Both endogenous GLP-1 and peripherally administered GLP-1 analogs exert anorectic effects via DVC GLP-1R activation (14–19). Although it has recently been shown that NTS-specific Glp1r knockdown is required for food intake control by endogenous GLP-1 (18), any role of the NTS in mediating the anorectic effects of liraglutide has not yet been examined.
Despite the abundance of GLP-1 research, there is a paucity of studies exploring the cellular substrates that mediate the anorexia induced by both endogenous GLP-1 and GLP-1 analogs. This is not the case for other energy balance signals such as neuropeptide Y/agouti-related protein (NPY/AGRP). The projection patterns of NPY/AGRP neurons and the neural phenotypes of cells expressing the receptors for the NPY/AGRP ligands have been extensively investigated [see (20) for review] despite the fact that this particular energy balance control system has not yet been shown to be a viable target for treating obesity in humans. Only recently have studies begun to comprehensively investigate the cellular phenotypes involved in mediating the intake-reducing effects of liraglutide (21). GLP-1R expression on γ-aminobutyric acid (GABA) neurons has been observed in regions throughout the brain of mice and rats (22–24), including the NTS (24). Extensive work has demonstrated GLP-1R–mediated potentiation of GABA release (22, 25–29); however, few studies have examined the contribution of GABA neurons to the anorectic effects of GLP-1 analogs (21), and no studies have done so specifically within the NTS.
Using chemogenetics [or designer receptors exclusively activated by designer drugs (DREADD)] to silence regionally and cell type– specific populations of neurons in adult rats (30), we examined GABAeric neurons as a distinct physiologically relevant cellular phenotype within the NTS that mediates liraglutide’s anorectic effects. Although there are likely many sites of action for liraglutide across multiple brain nuclei and cell types, our work highlights a behaviorally relevant population of NTS GABAergic neurons that are targeted by liraglutide to reduce food intake and body weight.
RESULTS
NTS GLP-1Rs mediate the food intake– and body weight–reducing effects of acutely delivered liraglutide
The central sites of action that mediate the anorectic effects of liraglutide are not clear. To explore the contribution of the NTS in the food intake– and body weight–reducing effects of liraglutide, we used an AAV-shRNA virus to knock down the GLP-1R within the caudomedial NTS. This strategy (31) has been used to remove the contribution of the GLP-1R within discrete nuclei of the brain (18, 23, 31, 32). After stereotaxic delivery of the Glp1r knockdown virus (AAV-GLP-1R) or a control virus (AAV-control), rats experienced 2 days of postoperative body weight loss followed by body weight recovery and gain (Fig. 1A). Differences in the mean body weight of the Glp1r knockdown and control groups failed to reach statistical significance for the duration of the 26-day experiment (P = 0.07), consistent with previous findings in animals maintained on a chow diet (18). The body weight gain from the time of viral injection to the conclusion of testing (Fig. 1A) was statistically the same between the two tested groups (Fig. 1A, inset). To confirm Glp1r knockdown after experimentation, rats were euthanized, and their brains were extracted. Coronal sections at the caudal extent of the NTS were slide mounted for histological verification of green fluorescent protein (GFP)–labeled viral expression within the NTS (Fig. 1B, inset); see the Supplementary Materials and Methods for inclusion criteria. As a proof of concept, tissue punches from the bilateral NTS were taken for polymerase chain reaction (PCR) analysis of Glp1r mRNA expression. Consistent with previous investigations using the AAV-GLP-1R virus (18, 31, 32), AAV-GLP-1R delivery to the NTS resulted in a 54% reduction in Glp1r mRNA relative to control virus–injected animals (Fig. 1B).
Fig. 1. Knockdown of Glp1r in the NTS attenuates the food intake– and body weight–reducing effects of acutely delivered systemic liraglutide.
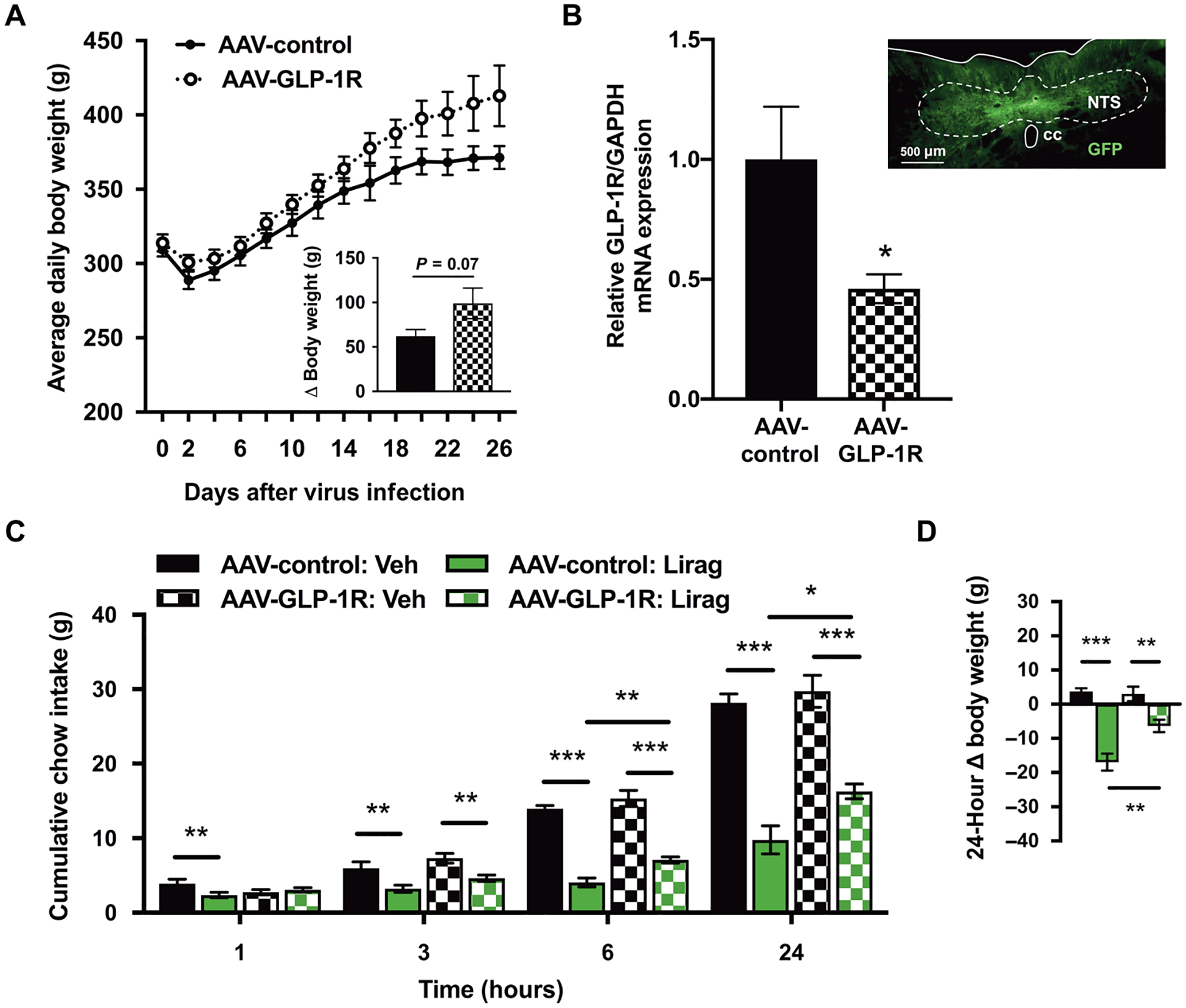
(A) Mean daily body weight for AAV-GLP-1R and AAV-control rats after viral injection (day 0). Inset represents cumulative body weight change over 26 days between viral injection and sacrifice. (B) Reverse transcription PCR analysis of Glp1r expression in the NTS of AAV-GLP-1R– (n = 8) versus AAV-control–treated rats (n = 8). Inset represents histological demonstration of GFP-tagged viral expression at the caudal extent of the micropunch in a representative AAV-GLP-1R–treated rat. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (C and D) Mean cumulative chow intake and body weight change in NTS AAV-GLP-1R and AAV-control rats after vehicle (Veh) or liraglutide (Lirag; 50 μg/kg) treatment. NTS, nucleus tractus solitarius; cc, central canal. Data are expressed as means ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 by unpaired, two-tailed Student’s t tests (A and B) and two-way repeated-measures ANOVA with Sidak’s multiple comparison tests (C and D).
Beginning on day 21, AAV-control and AAV-GLP-1R rats received two counterbalanced drug treatments of either intraperitoneally delivered vehicle or liraglutide, separated by 72 hours. Liraglutide treatment reduced food intake at all measured time points (1, 3, 6, and 24 hours after drug delivery). Post hoc tests showed effects of liraglutide on food intake within the control group at 1, 3, 6, and 24 hours (black versus green solid bars; P < 0.05) and within the Glp1r knockdown group at 3, 6, and 24 hours (black versus green hatched bars; P < 0.05; Fig. 1C). Main effects of Glp1r knockdown were also observed at 6 and 24 hours, with liraglutide reduced feeding more in the AAV-control group than in the AAV-GLP-1R group at both 6 and 24 hours (P < 0.01 and P < 0.05; Fig. 1C). The reduced anorectic effect of liraglutide in the AAV-GLP-1R group relative to the AAV-control group was also observed in the 24-hour body weight reduction, where we observed both main effects and an interaction of knockdown and drug treatment (P < 0.05; Fig. 1D). We observed no differences in cumulative chow intake or body weight between the AAV-control and AAV-GLP-1R groups treated with vehicle (P > 0.05; Fig. 1, C and D).
NTS GLP-1Rs contribute to the food intake– and body weight–reducing effects of chronically delivered liraglutide
As a pharmacotherapy for obesity, liraglutide is administered chronically to overweight individuals. To best model the conditions in which liraglutide is administered in humans, we prepared a group of high-fat diet (HFD)–maintained rats with an NTS-directed AAV-GLP-1R virus or an AAV-control virus to test the effects of once daily subcutaneous liraglutide treatment for 3 weeks. Knockdown of Glp1r did not affect baseline food intake (Fig. 2, A and B). Daily food intake and body weight were measured before (−2 to 0 day or “baseline”) and throughout the 3-week period of liraglutide treatment. In both the AAV-GLP-1R knockdown and control groups, the first day of liraglutide administration resulted in a sudden reduction in food intake relative to the vehicle-injected control groups. Although food intake in the vehicle-treated groups remained similar throughout the duration of the experiment, deviations in food intake emerged between the AAV-control and AAV-GLP-1R liraglutide-treated groups over time such that the reduction in food intake by liraglutide was more pronounced in the AAV-control group (Fig. 2A). Liraglutide significantly reduced food intake in the AAV-control group relative to vehicle-treated rats at weeks 1, 2, and 3 (P < 0.05; Fig. 2B). By contrast, the effects of liraglutide on food intake were less pronounced in the AAV-GLP-1R animals. In this group, liraglutide reduced food intake relative to vehicle-injected rats only during the first, but not second or third, week of drug treatment (P < 0.001; Fig. 2B).
Fig. 2. Knockdown of Glp1r in the NTS attenuates the food intake– and body weight–reducing effects of chronically delivered systemic liraglutide.
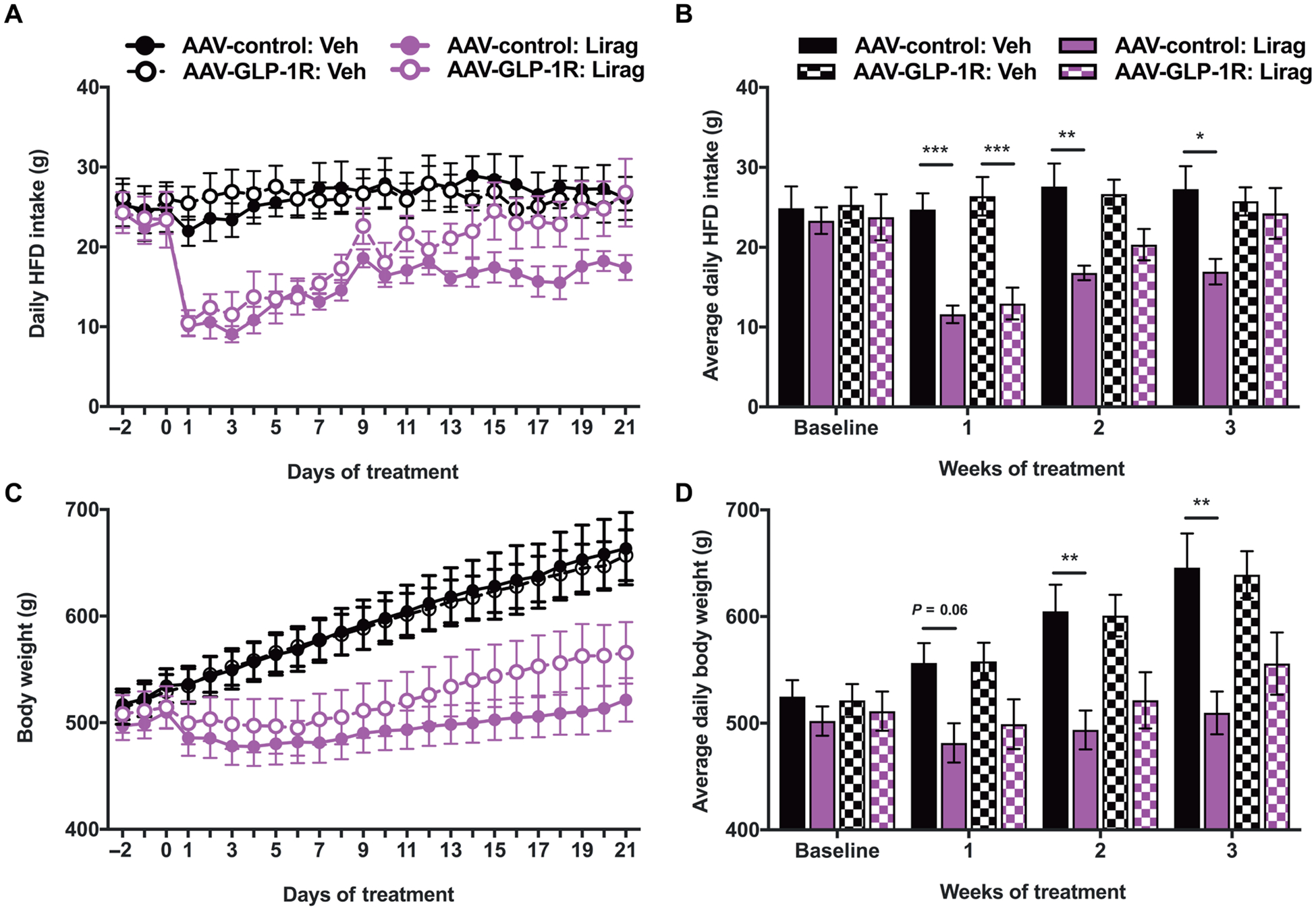
(A) Daily HFD intake for AAV-GLP-1R (n = 10 per group) and AAV-control (n = 9 per group) rats treated with chronic daily vehicle (Veh) or liraglutide (Lirag; 200 μg/kg). (B) Mean daily HFD across 3 weeks of vehicle or liraglutide treatment compared with baseline intake (average of the 3 days before injection). (C) Daily body weight for AAV-GLP-1R and AAV-control rats treated with chronic daily vehicle or liraglutide. (D) Mean body weight across the 3 weeks of vehicle or liraglutide treatment compared with baseline intake (average of the 3 days before injection). Data are expressed as means ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 by two-way ANOVA with Tukey’s multiple comparison tests (B and D).
At the onset of treatment, all rats were obese (514.9 ± 7.8 g). Unlike in standard chow-maintained animals [this work and (18)], there was no trend toward a difference in baseline body weight between the control and Glp1r knockdown groups (Fig. 2, C and D). Similarly to food intake, body weight for the vehicle-treated groups remained similar throughout the duration of the experiment. However, over time, the AAV-control group treated with liraglutide had reduced weight gain relative to the AAV-GLP-1R group treated with liraglutide (Fig. 2C). Liraglutide significantly reduced body weight relative to vehicle treatment only within the AAV-control group at weeks 2 and 3 (P < 0.01; Fig. 2D) and did not significantly reduce body weight in the AAV-GLP-1R knockdown animals at any measured time point (P > 0.05; Fig. 2D).
The AP is not required for the food intake– and body weight–reducing effects of acutely delivered liraglutide
We examined sham and AP-lesioned (APX) animals to test the requirement of the AP in the mediation of liraglutide’s anorectic effects. As a positive control for our APX model, we replicated previously published data showing that salmon calcitonin (sCT; 5 μg/kg) reduces 3-hour food intake only in sham-prepared rats because the AP has been shown to mediate the effects of sCT (33). In sham animals, sCT reduced food intake at 3 hours relative to vehicle-injected rats (7.3 ± 0.35 g versus 4.5 ± 0.37 g; P = 0.006; fig. S1). In contrast, APX, confirmed via histology, prevented sCT–induced hypophagia at 3 hours (7.3 ± 1.06 g for vehicle treatment versus 7.67 ± 0.87 g for sCT treatment; P = 0.90; fig. S1). We observed significant differences in food intake in sham-prepared animals at 3, 6, and 24 hours after liraglutide delivery (P < 0.05; Fig. 3A). Post hoc tests showed liraglutide-induced differences in food intake at 6 and 24 hours in the APX-prepared group (P < 0.05; Fig. 3A). Both sham and APX groups displayed reduced weight loss 24 hours after drug treatment (P < 0.05; Fig. 3B). Although APX induced mild hypophagia, a main effect of surgical manipulation, at 3 and 6 hours (Fig. 3A), as previously reported (34, 35), post hoc testing showed no differences in food intake or body weight between the sham and APX groups at any of the tested drug doses (Fig. 3, A and B), consistent with findings from a previous report (34).
Fig. 3. AP lesion does not attenuate liraglutide-induced food intake and body weight reduction nor c-Fos expression in the NTS.
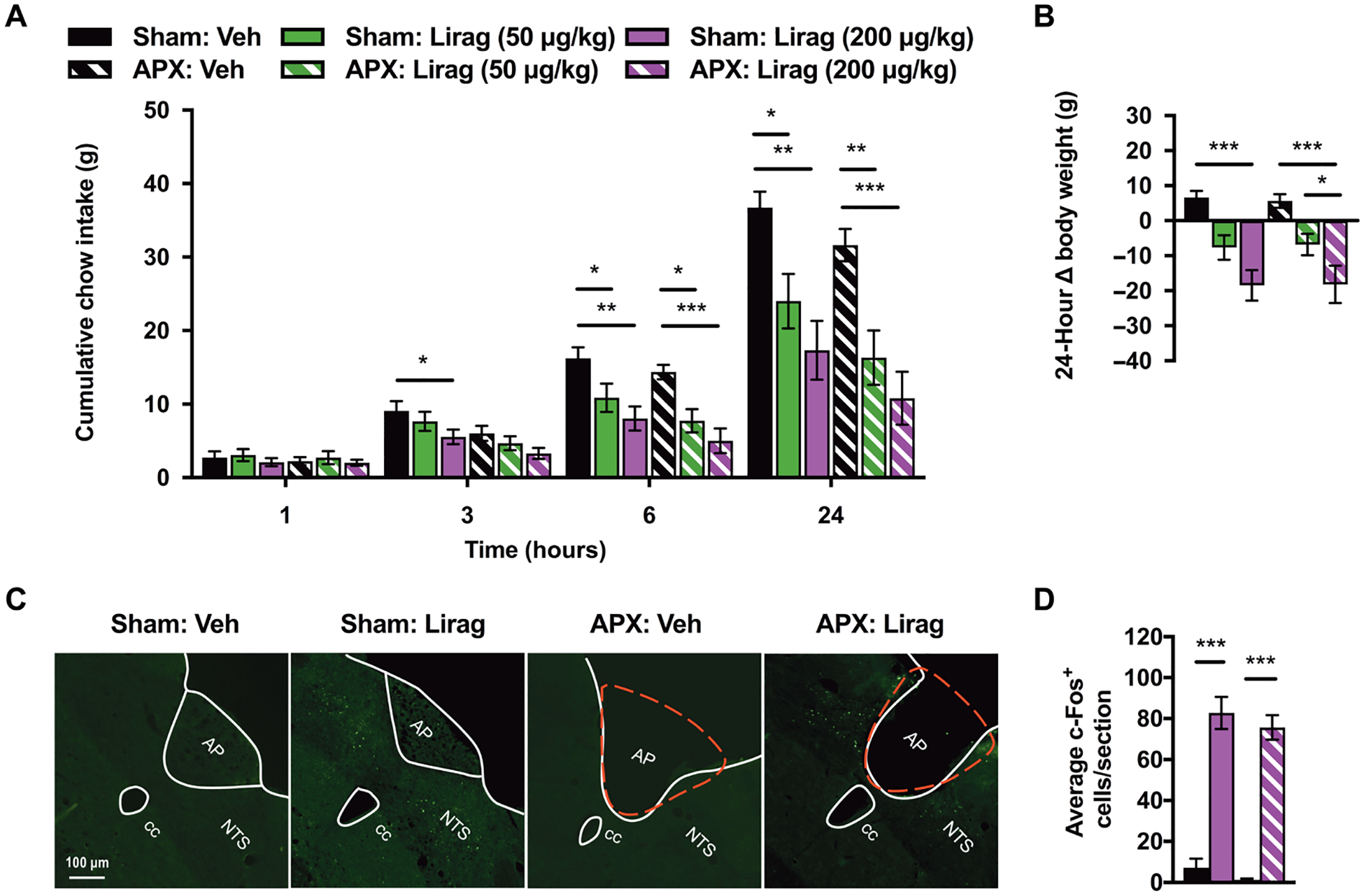
(A and B) Mean cumulative chow intake and body weight change in sham (n = 9) and AP-lesioned (APX; n = 11) rats after vehicle (Veh) or liraglutide (Lirag; 50 or 200 μg/kg) treatment. (C) Representative images of NTS c-Fos expression in sham and APX rats treated 90 min before sacrifice with systemic vehicle (Veh) or liraglutide (Lirag; 200 μg/kg). Red dashed lines represent the removed area postrema in APX rats. (D) Quantification of mean c-Fos+–expressing cells per NTS section in sham and APX rats treated with vehicle (Veh; n = 5 and n = 5, respectively) or liraglutide (Lirag; 200 μg/kg; n = 4 and n = 7, respectively). NTS, nucleus tractus solitarius. AP, area postrema. cc, central canal. Data are expressed as means ± SEM, *P < 0.05, **P < 0.01, and ***P < 0.001 by two-way repeated measures ANOVA with Sidak’s multiple comparison tests (A and B) or by two-way ANOVA with Tukey’s multiple comparison tests (D).
Liraglutide-induced c-Fos activation in the NTS does not require an intact AP
After observing that liraglutide maintained its intake- and body weight–reducing effects in the absence of AP GLP-1Rs, we went on to support our hypothesis that liraglutide acts directly on NTS GLP-1Rs by testing liraglutide-induced c-Fos activation, a molecular marker of neuronal activity, in sham and APX rats. We treated sham and APX-prepared rats at 90 min before sacrifice with either vehicle or liraglutide (200 μg/kg) and then analyzed c-Fos expression within the NTS. We observed a robust c-Fos response within the NTS of both sham and APX rats after liraglutide, but not vehicle, treatment (Fig. 3C). Data were quantified by averaging the c-Fos+ cells per section of the NTS examined. We observed a main effect of drug treatment, and post hoc testing showed that in both sham and APX groups, liraglutide treatment significantly increased the average number of c-Fos–expressing cells within the NTS compared with vehicle-treated animals (P < 0.001; Fig. 3D). There was no main effect of surgical AP manipulation on c-Fos responses (Fig. 3D).
NTS GABAergic cells express GLP-1Rs
Activation of NTS GLP-1Rs by liraglutide requires penetrance of the drug into the DVC. To demonstrate that liraglutide can access NTS GLP-1Rs, we administered a peripheral injection of fluorescently labeled liraglutide (Cy3-Lirag) to a rat and euthanized it 6 hours later for tissue processing. The 6-hour time point was chosen on the basis of previous studies investigating liraglutide penetrance into the brain (34, 36), as well as work in this and other research (7, 37) that shows food intake reduction by liraglutide 6 hours after treatment. Cy3-Lirag (Fig. 4A) was visually confirmed to be within both the AP and NTS. A blue counterstain for 4′,6-diamidino-2-phenylindole (DAPI; Fig. 4A), marking cell nuclei, and optical zoom within the NTS revealed close association of liraglutide with cells in the NTS (Fig. 4A).
Fig. 4. GABAergic neurons in the NTS mediate effects of systemically delivered liraglutide on food intake and body weight.
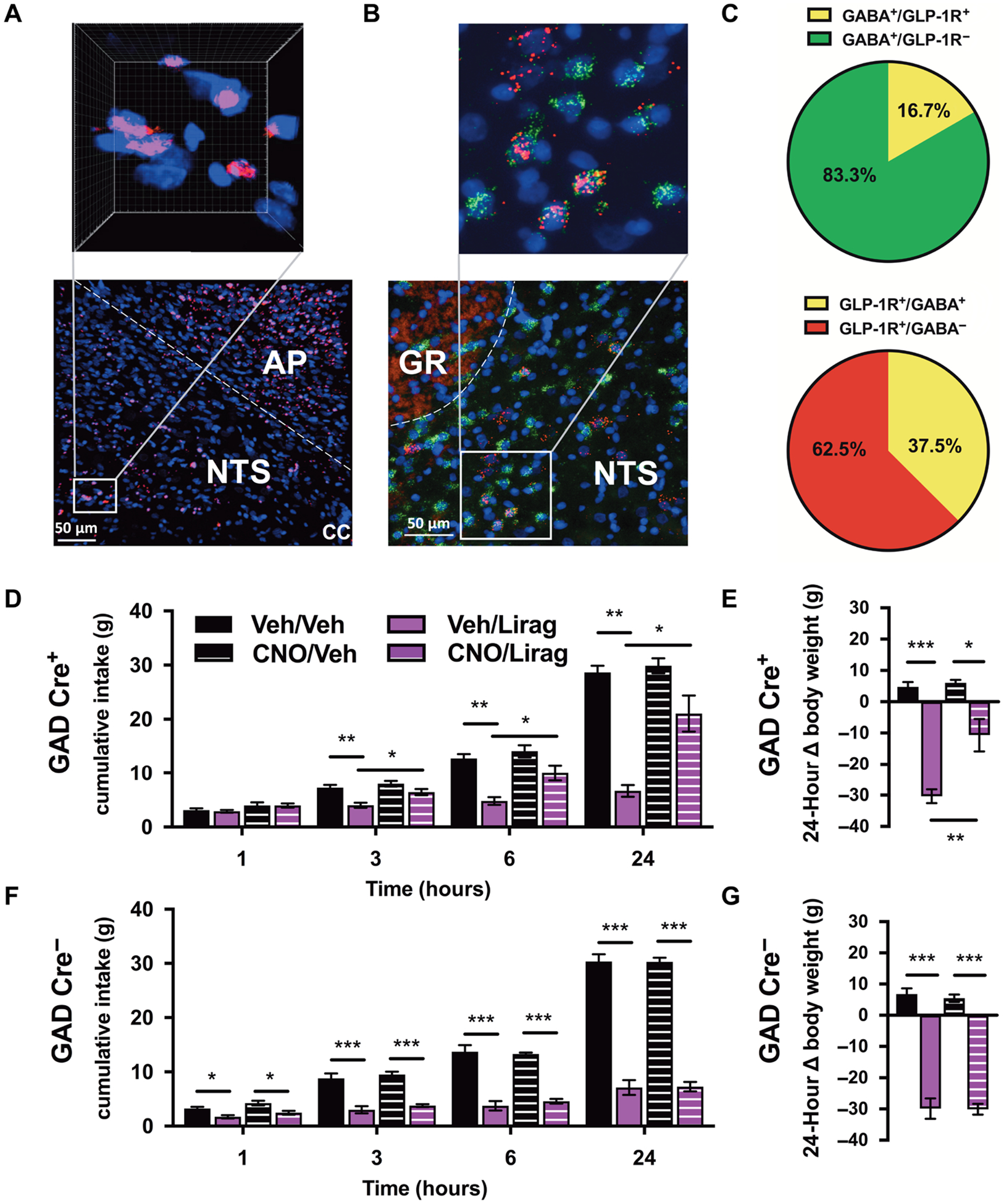
(A) Systemically delivered Cy3-labeled liraglutide (Cy3-Lirag; 200 μg/kg, intraperitoneally; pink) within the DVC. White box within the NTS designates area with increased magnification and three-dimensional reconstruction, pictured above, with Cy3-Lirag and DAPI-stained cell nuclei (blue). (B) FISH showing colocalization of Glp1r (red) and Gad1 (green) mRNA around cell nuclei (DAPI, blue) in the NTS. White box within the NTS designates area with increased magnification pictured above. (C) Percentage of NTS GABAergic neurons that did (GLP-1R+) and did not (GLP-1R−) express the Glp1r (top) and analysis of the percentage of Glp1r mRNA–expressing cells that were (GABA+) or were not (GABA−) GABA cells (bottom). (D and E) Effect of chemogenetic inhibition of GABAergic neuron signaling in the NTS via vehicle or CNO pretreatment (CNO; 1 mg/kg) on food intake and body weight after systemic liraglutide treatment (Lirag; 200 μg/kg) in GAD Cre+ rats (n = 6). (F and G) Effects of DREADD-mediated inhibition of GABAergic neuron signaling in the NTS on food intake and body weight after systemic liraglutide treatment in GAD Cre− rats (n = 7) rats. GR, nucleus gracilis. Scale bar, 50 μm. Data are expressed as means ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 by two-way repeated-measures ANOVA with Sidak’s multiple comparison tests.
To further investigate the phenotype of NTS cells that bind liraglutide, we used fluorescence in situ hybridization (FISH). We analyzed NTS tissue for expression of glutamate decarboxylase (Gad1) mRNA, a commonly used marker of GABAergic neurons, and Glp1r mRNA expression (Fig. 4B). Quantification of colocalization revealed that at the level of the AP, 16.7 ± 1.34% of NTS GABA neurons expressed the Glp1r (GABA+/GLP-1R+), and 37.5 ± 2.71% of NTS GLP-1Rs were expressed on GABA neurons (GLP-1R+/GABA+; Fig. 4C).
NTS GABAergic cells mediate food intake– and body weight–reducing effects of acutely delivered liraglutide
We used chemogenetics to acutely and selectively silence GABAergic neurons within the NTS, via a peripheral injection of clozapine-N-oxide (CNO), to investigate the requirement of this population in mediation of liraglutide’s anorectic effects. In GAD Cre+ animals, effects of both liraglutide and CNO pretreatment were observed at 3, 6, and 24 hours after drug treatment for food intake and also for 24-hour body weight. We also observed an interaction of drug administration and CNO pretreatment at 24 hours for both food intake and body weight in GAD Cre+ rats. Liraglutide reduced food intake only in vehicle-pretreated rats at the above time points (P < 0.01; Fig. 4D). In addition, pretreatment with the CNO significantly attenuated the intake reduction by liraglutide in GAD Cre+ rats such that cumulative food intake was significantly higher in liraglutide-treated animals pretreated with CNO compared with liraglutide-treated animals pretreated with vehicle at the 3-, 6-, and 24-hour time points (P < 0.05; Fig. 4D). Although less pronounced in the CNO-pretreated group, liraglutide did reduce body weight in both vehicle- and CNO-pretreated groups relative to their vehicle-injected control group (P < 0.001 and P < 0.05; Fig. 4E). Similarly to food intake, CNO attenuated the body weight reduction by liraglutide such that liraglutide reduced body weight significantly more in the vehicle-pretreated group than in the CNO-pretreated group (P < 0.01; Fig. 4E).
In GAD Cre− control animals, an effect of liraglutide on both food intake and body weight was observed at all measured time points in both vehicle- and CNO-pretreated rats (P < 0.05; Fig. 4, F and G). CNO pretreatment had no effect on liraglutide-induced food intake or body weight reduction (Fig. 4, F and G). Likewise, CNO alone had no effect on cumulative food intake or body weight in GAD Cre+ or GAD Cre− control animals (Fig. 4, D to G).
DISCUSSION
In both rodent and nonhuman primate models, the caudomedial NTS expresses an abundance of GLP-1Rs (8–11) that regulate feeding behavior by endogenous GLP-1 (18). Here, we demonstrated that NTS GLP-1Rs also mediated the anorectic effects of liraglutide. Although our findings clearly showed that the NTS is a contributing site of action for the acute and chronic effects of systemic liraglutide, Glp1r knockdown did not completely abolish the anorectic effects of liraglutide. Only a partial attenuation of food intake and body weight reduction after liraglutide was observed in our NTS AAV-GLP-1R knockdown rats. Furthermore, under conditions of chronic liraglutide treatment, the attenuation of food intake reduction by Glp1r knockdown was more pronounced, or possibly preceded the effects of knockdown on body weight attenuation. The discrepancies in the onset and magnitude of food intake and body weight reflected in our data are interesting and warrant continued investigation. It is possible that 3 weeks of chronic liraglutide treatment was insufficient for body weight differences to emerge in the liraglutide-treated groups. Because changes in food intake patterns precede consequent changes in body weight, we expect that over time, the body weight difference between knockdown animals treated with vehicle or liraglutide would diminish and be more reflective of food intake patterns. It is also worth noting that animals receiving chronic liraglutide were maintained on an HFD to more accurately model the human population prescribed liraglutide. HFD and resulting obesity may alter the dynamics of body weight loss and regain during chronic liraglutide such that a body weight phenotype requires extensive time to observe. It is also possible that liraglutide-induced reduction of water intake (38, 39) or increases in energy expenditure (14, 40) are challenging the emergence of a body weight phenotype despite the apparent differences in food intake after Glp1r knockdown.
The magnitude of the effect of NTS Glp1r knockdown on food intake and body weight is perhaps limited by methodology, which does not permit complete Glp1r knockdown. Incomplete knockdown of the GLP-1R, consistent with previous findings (18, 31, 32), may contribute to the lack of a strong body weight phenotype before drug treatment and the partial effects of the knockdown on liraglutide-induced anorexia, the intended focus of this work. We would expect to see a more rapid and robust attenuation of liraglutide’s effects with enhanced GLP-1R deletion. Unfortunately, genetic strategies to more thoroughly remove the contribution of the GLP-1R are currently limited to mouse models (6, 21, 41, 42) and present interpretational confounds (for example, developmental compensation) and experimental challenges and limitations such as anatomical restriction of knockdown. It is important to note that the population of cells in the NTS that express the GLP-1R is limited (8). Nevertheless, our studies demonstrate that AAV-assisted knockdown of a mere subset of these limited NTS GLP-1Rs is sufficient to attenuate the intakeand body weight–reducing effects of liraglutide, thereby emphasizing the functional relevance of NTS GLP-1Rs.
It is unlikely that the incomplete knockdown of the NTS GLP-1R entirely accounts for the modest attenuation of liraglutide-induced food intake and body weight reduction. Instead, we speculate that liraglutide acts on a neuroanatomically distributed network of GLP-1Rs and that NTS GLP-1Rs, together with other populations outside of the NTS, mediate the anorectic effects of liraglutide. The NTS is but one of many nuclei that express the GLP-1R (8–11) and has been implicated in orchestrating aspects of energy balance control via the GLP-1R (14–18). Despite high abundance of fluorescently labeled liraglutide in the hypothalamus (34) and anorexia following direct intrahypothalamic GLP-1R agonism (43–46), GLP-1Rs in the hypothalamus are not necessary for liraglutide-induced anorexia (42, 47). Both the acute and chronic anorectic effects of peripheral liraglutide are preserved in mice with hypothalamic Glp1r knockdown (42, 47). These findings are consistent with those showing that hypothalamic GLP-1Rs are also not necessary for the anorectic effects of exendin-4, a GLP-1R–selective agonist (48). Further supporting an extrahypothalamic model for GLP-1R’s anorectic effects are decerebrate rat studies demonstrating a normal reduction in food intake following peripheral GLP-1R agonists (14) and studies that fail to observe hypothalamic neuronal activation following peripheral GLP-1R agonist administration (42, 49, 50). Although mounting evidence is shifting the focus away from the hypothalamus as the primary site of action for the food intake– and body weight–reducing effects of GLP-1 and GLP-1–based drugs, the hypothalamus is undoubtedly a contributing mediator to the effects of GLP-1 and perhaps some aspects of liraglutide signaling (32, 34, 43–46).
Discrepancies in the GLP-1 literature, including the role of hypothalamic GLP-1Rs, are perhaps due to prominent species differences in the GLP-1R system. Site-specific Glp1r knockdown has been used to show the involvement of discrete GLP-1R populations in normal food intake and body weight regulation in rats (18, 32, 51). However, these findings contradict mouse work that fails to produce changes in body weight, fat mass, or hyperphagia with whole-body or CNS-specific GLP-1R knockout (6, 47, 52, 53). In addition to energy balance regulation, the GLP-1 system is required for coordinating visceral illness in response to aversive stimuli in rats, but not in mice (54). Divergent neural circuitry between mice and rats, including differences in the density of GLP-1 axon projections and the central cellular phenotypes expressing preproglucagon or the GLP-1R (8, 54–58), likely accounts for the inconsistent contributions of GLP-1 signaling to behavior. Because species differences are apparent, our findings do not necessarily translate to the mouse or any other species, a noted limitation of our work. Future studies examining the involvement of NTS and hypothalamic GLP-1Rs in liraglutide-induced food intake and body weight reduction should be conducted in both rats and mice. Furthermore, sex differences in the GLP-1 system are largely unexplored, and the field would greatly benefit from parallel studies conducted in female rats. Findings in both sexes should be extended to dissect the contribution of other GLP-1R populations to liraglutide signaling. GLP-1R–expressing nuclei with demonstrated sufficiency for GLP-1R–mediated food intake reduction include the parabrachial nucleus (59, 60), lateral dorsal tegmental nucleus (61), bed nucleus of the stria terminalis (23, 62), hippocampus (63), ventral tegmental area (64, 65), and nucleus accumbens (64, 66, 67).
The NTS is adjacent to and highly interconnected with the AP (68), a circumventricular nucleus with high Glp1r expression (8–11). The position of the AP within the brain supports exposure of GLP-1Rs to circulating liraglutide, and high penetrance of fluorescently labeled liraglutide across the blood-brain barrier and into the AP has been shown in our work and others’ (34, 36). In addition, the AP is one of the most c-Fos–responsive areas of the brain following peripheral liraglutide (21, 36), and it is possible that part of the observed neural activity within the NTS (34, 36) is secondary to AP GLP-1R activation. It is therefore important to disassociate the contributions of the two nuclei to liraglutide-induced neural activation and consequent anorectic effects. We showed that NTS c-Fos following liraglutide persists in the absence of the AP, strongly suggesting that liraglutide has direct action on NTS GLP-1Rs independent of action in the AP. In addition, unlike NTS Glp1r knockdown, APX did not attenuate the food intake– and body weight–reducing effects of liraglutide, suggesting that the NTS, but not the AP, is a functionally relevant site of action for liraglutide’s food intake–reducing effects. Our work is supported by studies in both rats and mice that show preserved short-term anorectic effects of exendin-4 (35) and long-term anorectic effects of liraglutide (34) in APX animals. Collectively, the evidence to date suggests that although peripheral liraglutide is reaching and activating neurons in the AP, the AP is not required for liraglutide’s acute anorectic effects. Further, the observed NTS neural activity after liraglutide is not secondary to AP activation because APX did not attenuate the expression of c-Fos in the NTS after liraglutide. We therefore conclude that the NTS is an independent and essential site of action mediating liraglutide’s anorectic effects.
Our work using fluorescently labeled liraglutide, in addition to other extensive studies of liraglutide penetrance (34, 36), provides visual evidence that peripherally administered liraglutide gains access to NTS GLP-1Rs by crossing the blood-brain barrier. However, the mechanism by which liraglutide is transported across the blood-brain barrier, where it plays a behaviorally relevant role, remains unclear. Potential mechanisms include passage via fenestrated capillaries or ependymal cells such as tanycytes (34, 69–73), both of which have documented roles in the transport of blood-borne molecules (73–76). Of particular relevance, these routes have been proposed for the access of ghrelin and leptin, other circulating appetite-modulating hormones, into the hypothalamus (76, 77). Transcytosis is also conducted by astrocytes (78), and we have demonstrated internalization of fluorescently labeled exendin-4 by cells in the NTS including astrocytes (56), suggesting the potential of astrocyte-mediated transport of GLP-1 analogs. Diffusion of liraglutide from the AP to the NTS via tanycytes or astrocytes is an attractive hypothesis given the abundance of tanycyte-like cells, astrocytes, and astrocyte fibers in the AP and the subpostrema (the border between the NTS and AP) (79–81). Further work will be required to elucidate the mechanism by which liraglutide is penetrating the blood-brain barrier. Once understood, studies should be aimed at how to best exploit these mechanisms for increased access of liraglutide to NTS GLP-1R–expressing cells.
Identification of the phenotypically distinct GLP-1R populations that mediate the anorectic effects of liraglutide is beneficial for the development of more targeted therapeutics for obesity. To this end, neuronal and non-neuronal cells expressing the GLP-1R have been explored for their role in mediating the food intake–reducing effects of GLP-1R agonists including liraglutide and exendin-4. A diverse portfolio of cell types including glutamatergic, proopiomelanocortin (POMC)/cocaine- and amphetamine-regulated transcript (CART)– expressing neurons, and astrocytes have been shown to be involved (21, 34, 56, 65, 82). Our work using FISH confirmed previous data demonstrating the presence of the Glp1r on GABAergic neurons in the NTS (24) and extends these findings by quantifying the percentage of Glp1r mRNA-expressing cells that are GABAergic in the NTS, at the level of the AP. Future work will be necessary to phenotype the remaining 62.5% of GLP-1R–expressing cells in the NTS. We expect that this population includes, but is not limited to, glutamatergic (21) and noradrenergic neurons (24), as well as astrocytes (56). Although the percentage of GABA neurons that expresses the GLP-1R is a small population of GABA neurons in the NTS, it is important to note that NTS GABAergic neurons contribute to the regulation of diverse physiological processes beyond energy balance control including cardiovascular and respiratory function (83–85).
Few studies to date have examined the requirement of distinct cellular phenotypes for liraglutide-induced anorexia. Previous work has initiated these important studies through mice with genetic deletion of GLP-1Rs within glutamatergic and, separately, GABAergic neurons of the entire brain (21). In these studies, regionally distributed glutamate and not GABA neurons were shown to be required for liraglutide-induced food intake and body weight reduction. It is important to realize that the genetic approach used in the prior studies is not ideal for examining the physiology of the GLP-1 system. A whole-brain Glp1r knockdown approach lacks the regional specificity to draw conclusions about the requirement of either glutamate or GABA populations of neurons within discrete nuclei such as the NTS. The redundancy of the GLP-1R–mediated pathways that control food intake presents a scenario where the nuanced contribution of a particular cellular phenotype within a given nucleus may be masked by whole-brain GABAergic Glp1r knockdown. This point is stressed by the failure of whole-body or whole-brain GLP-1R null animals to alter food intake and body weight (6, 86) and suggests that subsets of nuclei must be tested in isolation for their role in GLP-1R–mediated food intake changes. Also, although a common strategy (42, 47, 48), breeding mice with either whole-brain or nucleus-specific Glp1r knockdown presents the opportunity for developmental compensation for the absence of the GLP-1R within other areas of the brain. Ultimately, the GLP-1R distribution pattern in the adult transgenic animal being tested for the effects of liraglutide may not represent that of a normal animal.
To test the requirement of NTS-specific GABAergic neurons for the food intake– and body weight–reducing effects of liraglutide in the rat, we used chemogenetics. Selective acute silencing of NTS GABA neurons in adult rats, via CNO injection in GAD Cre+ rats prepared with NTS injection of an inhibitory DREADD virus, attenuated liraglutide-induced food intake and body weight reduction. Although these studies suggest that liraglutide is acting on NTS GABAergic neurons to mediate food intake and body weight reduction, our chemogenetic manipulation is not specific to NTS GLP-1R–expressing GABAergic neurons. The creation of a GLP-1R–Cre rat would allow for DREADD experiments, which would inform whether the action of liraglutide on GABAergic neurons is first or second order. Although our work indicates that a substantial percentage of Glp1r mRNA transcript–expressing cells are also GABAergic (37.5%), we expect that other populations of GLP-1R–expressing cells, including those on glutamate neurons (21), astrocytes (56), noradrenergic (24), and potentially other nonidentified populations of cells, also contribute to the effects of liraglutide within the NTS. The mechanisms by which these populations of cells suppress feeding may or may not involve the downstream engagement of NTS GABA neurons. To further elucidate the mechanism by which liraglutide reduces feeding via action on NTS GABA neurons, analyses should be extended to identify the projection targets of these cells. Whether the Glp1r-expressing GABAergic cells are local interneurons, known to be heavily expressed with the NTS (87), or projection neurons remains undetermined. It is known that NTS GABA neurons project to preganglionic DMX motor neurons (88–90) for the control of glucose metabolism (91, 92). Recently, chemogenetic activation of NTS GABA neurons has been shown to induce hyperglycemia (30); however, regulation of blood glucose by NTS GABA neurons expressing the GLP-1R has not been explored.
Liraglutide reduces food intake and body weight and has demonstrated success as a pharmacotherapy for the treatment of obesity [see (2) for review]. Clinical trials have shown average weight loss in obese patients without diabetes to be 8.2% (93, 94), within the range (5 to 10%) that supports reductions in the incidence of type 2 diabetes and other obesity-related comorbidities including sleep apnea, osteoarthritis, and hypertension (95–99). However, the potential for improved weight management drugs remains, both in terms of increasing weight loss and reducing side effects (most commonly nausea), which would contribute to drug tolerability and compliance (94, 100, 101). Improving the pharmacological potential and versatility of GLP-1 analogs requires the development of more targeted drugs informed by knowledge of the liraglutide’s site and mechanism of action. An increased understanding of the GLP-1 system can be extended to the treatment of disease beyond obesity because GLP-1 is also implicated in neurodegenerative disease, stroke, depression, anxiety, and addiction (102–108). These data provide evidence that NTS GABAergic neurons are a physiologically relevant population of GLP-1R–expressing cells involved in the meditation of food intake and body weight reduction by liraglutide.
MATERIALS AND METHODS
Study design
This study was designed to explore the site of action of the obesity drug liraglutide within the DVC, specifically the NTS and AP, using male rats. AAV-assisted Glp1r knockdown in the NTS and, separately, surgical lesioning of the AP were used to examine the involvement of each GLP-1R population in mediating the food intake– and body weight–reducing effects of liraglutide. Because visual evidence obtained from rats injected peripherally with fluorescently labeled liraglutide suggested penetrance of the drug into the NTS, we used immunohistochemical comparison of c-Fos responsivity in the NTS in sham and APX rats treated with liraglutide to determine whether the NTS is an independent site of action for liraglutide. We also used FISH to quantify Glp1r mRNA expression on GABAergic neurons and used DREADD technology to demonstrate the involvement of NTS GABA neurons in mediating liraglutide’s effects. Adult male rats were used for all behavioral, immunohistochemical, and in situ hybridization studies. All procedures were performed in accordance with National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Data were excluded only in instances of improperly prepared animals as assessed via postmortem histological verification of viral placement or APX. For all histological analyses, investigators were blinded to experimental groups. Additional materials and methods are available in the Supplementary Materials.
Animals
Male Sprague-Dawley rats (Charles River Laboratories) weighing between 300 and 400 g were used for all studies except those involving chemogenetic suppression of NTS GABAergic neurons. Chemo-genetic experiments used age- and weight-matched Long-Evans rats expressing Cre-recombinase under the Gad1 promoter (GAD Cre+) and GAD Cre− littermate controls (~400 g at testing). Rats were bred by crossing GAD Cre+ rats obtained from the Rat Resource & Research Center (RRRC) at the University of Missouri [LE-Tg(Gad1-iCre)3Ottc rats] to a Long-Evans wild-type rat (Charles River Laboratories). PCR analysis (Transnetyx Inc.) was used to determine offspring genotype. All rats were individually housed in wire-hanging cages in a climate-controlled (22° to 24°C) room with a 12-hour light/12-hour dark cycle. Rats were maintained on an ad libitum diet of standard rat chow (Lab Diet), except where noted (see the Supplementary Materials).
Statistical analysis
All data are expressed as means ± SEM. Statistical analysis including unpaired two-tailed Student’s t tests of normally distributed samples, two-way analysis of variance (ANOVA) with Tukey’s multiple comparison tests, or two-way repeated-measures ANOVA with Sidak’s multiple comparison tests were performed in GraphPad Prism 8. The value of alpha (significance level) was set at 0.05, and all tests of significance were two sided. Figure legends note statistical parameters for each experiment. Immunohistochemical analysis of c-Fos expression was conducted in triplicate coronal sections and quantified in NIH ImageJ. FISH data were quantified from four coronal sections at the level of the AP across four animals using NIH ImageJ. Data are expressed as a summary of the means of the replicates with SEM. Imaris was used to process Cy3-Lirag images captured with confocal microscopy.
Supplementary Material
Acknowledgments:
We would like to thank C. Liberini, L. Stein, R. Leon, N. Hernandez, and B. De Jonghe for technical assistance and experimental advice. We thank Novo Nordisk for supplying the liraglutide used in these studies.
Funding: This work was supported by NIH-DK115762 (M.R.H.), NIH-R01 DA037897 (H.D.S.), and an investigator-initiated sponsored agreement from Novo Nordisk (M.R.H.).
Footnotes
Competing interests: M.R.H. receives research funding from Zealand Pharma, Eli Lilly and Company, and Boehringer Ingelheim that was not used in support of these studies. M.R.H. is the CEO of Cantius Therapeutics LLC that pursues biological work unrelated to the current study. H.D.S. also receives research funding from Novo Nordisk that was not used in support of these studies. All other authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. LE-Tg(Gad1-iCre)3Ottc rats (RRRC#751) were received from the RRRC at the University of Missouri, Columbia, MO.
SUPPLEMENTARY MATERIALS
stm.sciencemag.org/cgi/content/full/12/533/eaay8071/DC1
Materials and Methods
Fig. S1. AP lesioning attenuates the food intake–reducing effects of sCT.
Data file S1. Raw data.
REFERENCES AND NOTES
- 1.Dombrowski SU, Knittle K, Avenell A, Araújo-Soares V, Sniehotta FF, Long term maintenance of weight loss with non-surgical interventions in obese adults: Systematic review and meta-analyses of randomised controlled trials. BMJ 348, g2646 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knudsen LB, Lau J, The discovery and development of liraglutide and semaglutide. Front. Endocrinol 10, 155 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanoski SE, Hayes MR, Skibicka KP, GLP-1 and weight loss: Unraveling the diverse neural circuitry. Am. J. Physiol. Regul. Integr. Comp. Physiol 310, R885–R895 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JPH, Smith DM, Ghatei MA, Herbert J, Bloom SR, A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379, 69–72 (1996). [DOI] [PubMed] [Google Scholar]
- 5.Tang-Christensen M, Larsen PJ, Göke R, Fink-Jensen A, Jessop DS, Møller M, Sheikh SP, Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am. J. Physiol 271, R848–R856 (1996). [DOI] [PubMed] [Google Scholar]
- 6.Sisley S, Gutierrez-Aguilar R, Scott M, D’Alessio DA, Sandoval DA, Seeley RJ, Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J. Clin. Invest 124, 2456–2463 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR, Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 152, 3103–3112 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S, Distributionand characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol. Metab 4, 718–731 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merchenthaler I, Lane M, Shughrue P, Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J. Comp. Neurol 403, 261–280 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Heppner KM, Kirigiti M, Secher A, Paulsen SJ, Buckingham R, Pyke C, Knudsen LB, Vrang N, Grove KL, Expression and distribution of glucagon-like peptide-1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology 156, 255–267 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, Gribble FM, Reimann F, Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes 63, 1224–1233 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grill HJ, Hayes MR, Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab 16, 296–309 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berthoud H-R, Sutton GM, Townsend RL, Patterson LM, Zheng H, Brainstem mechanisms integrating gut-derived satiety signals and descending forebrain information in the control of meal size. Physiol. Behav 89, 517–524 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Hayes MR, Skibicka KP, Grill HJ, Caudal brainstem processing is sufficientfor behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology 149, 4059–4068 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, De Jonghe BC, Kanoski SE, Grill HJ, Bence KK, Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab 13, 320–330 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao S, Kanoski SE, Yan J, Grill HJ, Hayes MR, Hindbrain leptin and glucagon-like-peptide-1 receptor signaling interact to suppress food intake in an additive manner. Int. J. Obes 36, 1522–1528 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes MR, Bradley L, Grill HJ, Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 150, 2654–2659 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alhadeff AL, Mergler BD, Zimmer DJ, Turner CA, Reiner DJ, Schmidt HD, Grill HJ, Hayes MR, Endogenous glucagon-like peptide-1 receptor signaling in the nucleus tractus solitarius is required for food intake control. Neuropsychopharmacology 42, 1471–1479 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Punjabi M, Arnold M, Rüttimann E, Graber M, Geary N, Pacheco-López G, Langhans W, Circulating glucagon-like peptide-1 (GLP-1) inhibits eating in male rats by actingin the hindbrain and without inducing avoidance. Endocrinology 155, 1690–1699 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Parker JA, Bloom SR, Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology 63, 18–30 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Adams JM, Pei H, Sandoval DA, Seeley RJ, Chang RB, Liberles SD, Olson DP, Liraglutide modulates appetite and body weight through glucagon-like peptide 1 receptor-expressing glutamatergic neurons. Diabetes 67, 1538–1548 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rebosio C, Balbi M, Passalacqua M, Ricciarelli R, Fedele E, Presynaptic GLP-1 receptors enhance the depolarization-evoked release of glutamate and GABA in the mouse cortex and hippocampus. Biofactors 44, 148–157 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Zheng H, Reiner DJ, Hayes MR, Rinaman L, Chronic suppression of glucagon-like peptide-1 receptor (GLP1R) mRNA translation in the rat bed nucleus of the stria terminalis reduces anxiety-like behavior and stress-induced hypophagia, but prolongs stress-induced elevation of plasma corticosterone. J. Neurosci 39, 2649–2663 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Card JP, Johnson AL, Llewellyn-Smith IJ, Zheng H, Anand R, Brierley DI, Trapp S, Rinaman L, GLP-1 neurons form a local synaptic circuit within the rodent nucleus of the solitary tract. J. Comp. Neurol 526, 2149–2164 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wan S, Coleman FH, Travagli RA, Glucagon-like peptide-1 excites pancreas-projecting preganglionic vagal motoneurons. Am. J. Physiol. Gastrointest. Liver Physiol 292, G1474–G1482 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Farkas I, Vastagh C, Farkas E, Bálint F, Skrapits K, Hrabovszky E, Fekete C, Liposits Z, Glucagon-like peptide-1 excites firing and increases GABAergic miniature postsynaptic currents (mPSCs) in gonadotropin-releasing hormone (GnRH) neurons of the male mice via activation of nitric oxide (NO) and suppression of endocannabinoid signaling pathways. Front. Cell. Neurosci 10, 214 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korol SV, Jin Z, Birnir B, The GLP-1 receptor agonist exendin-4 and diazepam differentially regulate GABAA receptor-mediated tonic currents in rat hippocampal CA3 pyramidal neurons. PLOS ONE 10, e0124765 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korol SV, Jin Z, Babateen O, Birnir B, GLP-1 and exendin-4 transiently enhance GABAA receptor-mediated synaptic and tonic currents in rat hippocampal CA3 pyramidal neurons. Diabetes 64, 79–89 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Gupta G, Dahiya R, Dua K, Chellappan DK, Tiwari J, Sharma GN, Singh SK, Mishra A, Sharma RK, Agrawal M, Anticonvulsant effect of liraglutide, GLP-1 agonist by avertinga change in GABA and brain glutathione level on picrotoxin-induced seizures. EXCLI J 16, 752–754 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boychuk CR, Smith KC, Peterson LE, Boychuk JA, Butler CR, Derera ID, McCarthy JJ, Smith BN, A hindbrain inhibitory microcircuit mediates vagally-coordinated glucose regulation. Sci. Rep 9, 2722 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, Van Nest DS, Guercio LA, Wimmer ME, Olivos DR, De Jonghe BC, Hayes MR, Glucagon-like peptide-1 receptor activation in the ventral tegmental area decreases the reinforcing efficacy of cocaine. Neuropsychopharmacology 41, 1917–1928 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.López-Ferreras L, Richard JE, Noble EE, Eerola K, Anderberg RH, Olandersson K, Taing L, Kanoski SE, Hayes MR, Skibicka KP, Lateral hypothalamic GLP-1 receptors are critical for the control of food reinforcement, ingestive behavior and body weight. Mol. Psychiatry 23, 1157–1168 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braegger FE, Asarian L, Dahl K, Lutz TA, Boyle CN, The role of the area postrema in the anorectic effects of amylin and salmon calcitonin: Behavioral and neuronal phenotyping. Eur. J. Neurosci 40, 3055–3066 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Secher A, Jelsing J, Baquero AF, Hecksher-Sørensen J, Cowley MA, Dalbøge LS, Hansen G, Grove KL, Pyke C, Raun K, Schäffer L, Tang-Christensen M, Verma S, Witgen BM, Vrang N, Bjerre Knudsen L, The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Invest 124, 4473–4488 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baraboi E-D, Smith P, Ferguson AV, Richard D, Lesions of area postremaand subfornical organ alter exendin-4-induced brain activation without preventing the hypophagic effect of the GLP-1 receptor agonist. Am. J. Physiol. Regul. Integr. Comp. Physiol 298, R1098–R1110 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Salinas CBG, Lu TT-H, Gabery S, Marstal K, Alanentalo T, Mercer AJ, Cornea A, Conradsen K, Hecksher-Sørensen J, Dahl AB, Knudsen LB, Secher A, Integrated brain atlas for unbiased mapping of nervous system effects following liraglutide treatment. Sci. Rep 8, 10310 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayes MR, Kanoski SE, Alhadeff AL, Grill HJ, Comparative effects of the long-acting GLP-1 receptor ligands, liraglutide and exendin-4, on food intake and body weight suppression in rats. Obesity 19, 1342–1349 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Larsen PJ, Fledelius C, Knudsen LB, Tang-Christensen M, Systemic administration of the long-acting GLP-1 derivative NN2211 induces lasting and reversible weight loss in both normal and obese rats. Diabetes 50, 2530–2539 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Mckay NJ, Kanoski SE, Hayes MR, Daniels D, Glucagon-like peptide-1 receptor agonists suppress water intake independent of effects on food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol 301, R1755–R1764 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK, Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J. Clin. Invest 110, 43–52 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Conde K, Zhang P, Lilascharoen V, Xu Z, Lim BK, Seeley RJ, Zhu JJ, Scott MM, Pang ZP, Enhanced AMPA receptor trafficking mediates the anorexigenic effectof endogenous glucagon-like peptide-1 in the paraventricular hypothalamus. Neuron 96, 897–909.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burmeister MA, Ayala JE, Smouse H, Landivar-Rocha A, Brown JD, Drucker DJ, Stoffers DA, Sandoval DA, Seeley RJ, Ayala JE, The hypothalamic glucagon-like peptide 1 receptor is sufficient but not necessary for the regulation of energy balance and glucose homeostasis in mice. Diabetes 66, 372–384 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandoval DA, Bagnol D, Woods SC, D’Alessio DA, Seeley RJ, Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes 57, 2046–2054 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, Serrano M, Fernø J, Salvador J, Escalada J, Dieguez C, Lopez M, Frühbeck G, Nogueiras R, GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 63, 3346–3358 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Kinzig KP, D’Alessio DA, Seeley RJ, The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J. Neurosci 22, 10470–10476 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schick RR, Zimmermann JP, vorm Walde T, Schusdziarra V, Peptides that regulate food intake: Glucagon-like peptide 1-(7–36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 284, R1427–R1435 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Burmeister MA, Brown JD, Ayala JE, Stoffers DA, Sandoval DA, Seeley RJ, Ayala JE, The glucagon-like peptide-1 receptor in the ventromedial hypothalamus reduces short-term food intake in male mice by regulating nutrient sensor activity. Am. J. Physiol. Endocrinol. Metab 313, E651–E662 (2017).28811293 [Google Scholar]
- 48.Ghosal S, Packard AEB, Mahbod P, McKlveen JM, Seeley RJ, Myers B, Ulrich-Lai Y, Smith EP, D’Alessio DA, Herman JP, Disruption of glucagon-like peptide 1 signaling in sim1 neurons reduces physiological and behavioral reactivity to acute and chronic stress. J. Neurosci 37, 184–193 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baraboi E-D, St-Pierre DH, Shooner J, Timofeeva E, Richard D, Brain activation following peripheral administration of the GLP-1 receptor agonist exendin-4. Am. J. Physiol. Regul. Integr. Comp. Physiol 301, R1011–R1024 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Parker JA, McCullough KA, Field BCT, Minnion JS, Martin NM, Ghatei MA, Bloom SR, Glucagon and GLP-1 inhibit food intake and increase c-fos expressionin similar appetite regulating centres in the brainstem and amygdala. Int. J. Obes 37, 1391–1398 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Krieger J-P, Arnold M, Pettersen KG, Lossel P, Langhans W, Lee SJ, Knockdownof GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes 65, 34–43 (2016). [DOI] [PubMed] [Google Scholar]
- 52.Finan B, Yang B, Ottaway N, Stemmer K, Müller TD, Yi C-X, Habegger K, Schriever SC, García-Cáceres C, Kabra DG, Hembree J, Holland J, Raver C, Seeley RJ, Hans W, Irmler M, Beckers J, de Angelis MH, Tiano JP, Mauvais-Jarvis F, Perez-Tilve D, Pfluger P, Zhang L, Gelfanov V, DiMarchi RD, Tschöp MH, Targeted estrogen delivery reverses the metabolic syndrome. Nat. Med 18, 1847–1856 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scrocchi LA, Hill ME, Saleh J, Perkins B, Drucker DJ, Elimination of glucagon-like peptide 1R signaling does not modify weight gain and islet adaptation in micewith combined disruption of leptin and GLP-1 action. Diabetes 49, 1552–1560 (2000). [DOI] [PubMed] [Google Scholar]
- 54.Lachey JL, D’Alessio DA, Rinaman L, Elmquist JK, Drucker DJ, Seeley RJ, The role of central glucagon-like peptide-1 in mediating the effects of visceral illness: Differential effects in rats and mice. Endocrinology 146, 458–462 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Trapp S, Cork SC, PPG neurons of the lower brain stem and their role in brain GLP-1 receptor activation. Am. J. Physiol. Regul. Integr. Comp. Physiol 309, R795–R804 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reiner DJ, Mietlicki-Baase EG, McGrath LE, Zimmer DJ, Bence KK, Sousa GL, Konanur VR, Krawczyk J, Burk DH, Kanoski SE, Hermann GE, Rogers RC, Hayes MR, Astrocytes regulate GLP-1 receptor-mediated effects on energy balance. J. Neurosci 36, 3531–3540 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huo L, Gamber KM, Grill HJ, Bjørbæk C, Divergent leptin signaling in proglucagon neurons of the nucleus of the solitary tract in mice and rats. Endocrinology 149, 492–497 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marina N, Turovsky E, Christie IN, Hosford PS, Hadjihambi A, Korsak A, Ang R, Mastitskaya S, Sheikhbahaei S, Theparambil SM, Gourine AV, Brain metabolic sensing and metabolic signaling at the level of an astrocyte. Glia 66, 1185–1199 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swick JC, Alhadeff AL, Grill HJ, Urrea P, Lee SM, Roh H, Baird J-P, Parabrachial nucleus contributions to glucagon-like peptide-1 receptor agonist-induced hypophagia. Neuropsychopharmacology 40, 2001–2014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alhadeff AL, Baird J-P, Swick JC, Hayes MR, Grill HJ, Glucagon-like peptide-1 receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake and motivation to feed. Neuropsychopharmacology 39, 2233–2243 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reiner DJ, Leon RM, McGrath LE, Koch-Laskowski K, Hahn JD, Kanoski SE, Mietlicki-Baase EG, Hayes MR, Glucagon-like peptide-1 receptor signaling in the lateral dorsal tegmental nucleus regulates energy balance. Neuropsychopharmacology 43, 627–637 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams DL, Lilly NA, Edwards IJ, Yao P, Richards JE, Trapp S, GLP-1 action in the mouse bed nucleus of the stria terminalis. Neuropharmacology 131, 83–95 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsu TM, Hahn JD, Konanur VR, Lam A, Kanoski SE, Hippocampal GLP-1 receptors influence food intake, meal size and effort-based responding for food through volume transmission. Neuropsychopharmacology 40, 327–337 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alhadeff AL, Rupprecht LE, Hayes MR, GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153, 647–658 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, Hayes MR, The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am. J. Physiol. Endocrinol. Metab 305, E1367–E1374 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP, The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: A new role for mesolimbic GLP-1 receptors. J. Neurosci 32, 4812–4820 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dossat AM, Lilly N, Kay K, Williams DL, Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J. Neurosci 31, 14453–14457 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shapiro RE, Miselis RR, The central neural connections of the area postrema of the rat. J. Comp. Neurol 234, 344–364 (1985). [DOI] [PubMed] [Google Scholar]
- 69.McClean PL, Parthsarathy V, Faivre E, Holscher C, The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J. Neurosci 31, 6587–6594 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kastin AJ, Akerstrom V, Pan W, Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J. Mol. Neurosci 18, 7–14 (2002). [DOI] [PubMed] [Google Scholar]
- 71.Abernethy WB, Bell MA, Morris M, Moody DM, Microvascular density of the human paraventricular nucleus decreases with aging but not hypertension. Exp. Neurol 121, 270–274 (1993). [DOI] [PubMed] [Google Scholar]
- 72.Rodríguez EM, Blázquez JL, Guerra M, The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: The former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides 31, 757–776 (2010). [DOI] [PubMed] [Google Scholar]
- 73.Langlet F, Mullier A, Bouret SG, Prevot V, Dehouck B, Tanycyte-like cells forma blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. J. Comp. Neurol 521, 3389–3405 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peruzzo B, Pastor F, Blázquez J, Amat P, Rodríguez E, Polarized endocytosisand transcytosis in the hypothalamic tanycytes of the rat. Cell Tissue Res 317, 147–164 (2004). [DOI] [PubMed] [Google Scholar]
- 75.Mullier A, Bouret SG, Prevot V, Dehouck B, Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulationin the adult mouse brain. J. Comp. Neurol 518, 943–962 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schaeffer M, Langlet F, Lafont C, Molino F, Hodson DJ, Roux T, Lamarque L, Verdié P, Bourrier E, Dehouck B, Banères J-L, Martinez J, Méry P-F, Marie J, Trinquet E, Fehrentz J-A, Prévot V, Mollard P, Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc. Natl. Acad. Sci. U.S.A 110, 1512–1517 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Balland E, Dam J, Langlet F, Caron E, Steculorum S, Messina A, Rasika S, Falluel-Morel A, Anouar Y, Dehouck B, Trinquet E, Jockers R, Bouret SG, Prévot V, Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab 19, 293–301 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohen-Kashi-Malina K, Cooper I, Teichberg VI, Mechanisms of glutamate efflux at the blood-brain barrier: Involvement of glial cells. J. Cereb. Blood Flow Metab 32, 177–189 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guillebaud F, Girardet C, Abysique A, Gaigé S, Barbouche R, Verneuil J, Jean A, Leprince J, Tonon M-C, Dallaporta M, Lebrun B, Troadec J-D, Glial endozepines inhibit feeding-related autonomic functions by acting at the brainstem level. Front. Neurosci 11, 308 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maolood N, Meister B, Protein components of the blood–brain barrier (BBB)in the brainstem area postrema–nucleus tractus solitarius region. J. Chem. Neuroanat 37, 182–195 (2009). [DOI] [PubMed] [Google Scholar]
- 81.Fodor M, Palkovits M, Gallatz K, Fine structure of the area subpostrema in rat. Open gate for the medullary autonomic centers. Ideggyogy. Sz 60, 83–88 (2007). [PubMed] [Google Scholar]
- 82.Mietlicki-Baase EG, Ortinski PI, Reiner DJ, Sinon CG, McCutcheon JE, Pierce RC, Roitman MF, Hayes MR, Glucagon-like peptide-1 receptor activation in the nucleus accumbens core suppresses feeding by increasing glutamatergic AMPA/kainate signaling. J. Neurosci 34, 6985–6992 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Andresen MC, Mendelowitz D, Sensory afferent neurotransmission in caudal nucleus tractus solitarius—Common denominators. Chem. Senses 21, 387–395 (1996). [DOI] [PubMed] [Google Scholar]
- 84.Travagli RA, Hermann GE, Browning KN, Rogers RC, Brainstem circuits regulating gastric function. Annu. Rev. Physiol 68, 279–305 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR, Central pathways of pulmonary and lower airway vagal afferents. J. Appl. Physiol 101, 618–627 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scrocchi LA, Brown TJ, MaClusky N, Brubaker PL, Auerbach AB, Joyner AL, Drucker DJ, Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat. Med 2, 1254–1258 (1996). [DOI] [PubMed] [Google Scholar]
- 87.Kawai Y, Senba E, Organization of excitatory and inhibitory local networks in the caudal nucleus of tractus solitarius of rats revealed in in vitro slice preparation. J. Comp. Neurol 373, 309–321 (1996). [DOI] [PubMed] [Google Scholar]
- 88.Davis SF, Derbenev AV, Williams KW, Glatzer NR, Smith BN, Excitatoryand inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res 1017, 208–217 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Babic T, Browning KN, Travagli RA, Differential organization of excitatoryand inhibitory synapses within the rat dorsal vagal complex. Am. J. Physiol. Gastrointest. Liver Physiol 300, G21–G32 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Travagli RA, Gillis RA, Rossiter CD, Vicini S, Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am. J. Physiol 260, G531–G536 (1991). [DOI] [PubMed] [Google Scholar]
- 91.Shapiro RE, Miselis RR, The central organization of the vagus nerve innervating the stomach of the rat. J. Comp. Neurol 238, 473–488 (1985). [DOI] [PubMed] [Google Scholar]
- 92.Berthoud HR, Carlson NR, Powley TL, Topography of efferent vagal innervation of the rat gastrointestinal tract. Am. J. Physiol 260, R200–R207 (1991). [DOI] [PubMed] [Google Scholar]
- 93.le Roux C, Aroda V, Hemmingsson J, Cancino AP, Christensen R, Pi-Sunyer X, Comparison of efficacy and safety of liraglutide 3.0 mg in individuals with BMI above and below 35 kg/m2: A post-hoc analysis. Obes. Facts 10, 531–544 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mehta A, Marso SP, Neeland IJ, Liraglutide for weight management: A critical review of the evidence. Obes. Sci. Pract 3, 3–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, Brancati FL, Peters A, Wagenknecht L; Look AHEAD Research Group, Benefitsof modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 34, 1481–1486 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM, Influence of weight reduction on blood pressure: A meta-analysis of randomized controlled trials. Hypertension 42, 878–884 (2003). [DOI] [PubMed] [Google Scholar]
- 97.Aucott LS, Influences of weight loss on long-term diabetes outcomes. Proc. Nutr. Soc 67, 54–59 (2008). [DOI] [PubMed] [Google Scholar]
- 98.Fontaine KR, Barofsky I, Bartlett SJ, Franckowiak SC, Andersen RE, Weight loss and health-related quality of life: Results at 1-year follow-up. Eat. Behav 5, 85–88 (2004). [DOI] [PubMed] [Google Scholar]
- 99.Aucott L, Poobalan A, Smith WCS, Avenell A, Jung R, Broom J, Effects of weight loss in overweight/obese individuals and long-term hypertension outcomes: A systematic review. Hypertension 45, 1035–1041 (2005). [DOI] [PubMed] [Google Scholar]
- 100.Lean MEJ, Carraro R, Finer N, Hartvig H, Lindegaard ML, Rössner S, Van Gaal L, Astrup A; NN8022–1807 Investigators, Tolerability of nausea and vomitingand associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. Int. J. Obes 38, 689–697 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WHM, Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int. J. Obes 38, 784–793 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perry T, Lahiri DK, Chen D, Zhou J, Shaw KTY, Egan JM, Greig NH, A novel neurotrophic property of glucagon-like peptide 1: A promoter of nerve growth factor-mediated differentiation in PC12 cells. J. Pharmacol. Exp. Ther 300, 958–966 (2002). [DOI] [PubMed] [Google Scholar]
- 103.During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN, Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat. Med 9, 1173–1179 (2003). [DOI] [PubMed] [Google Scholar]
- 104.Gault VA, Hölscher C, GLP-1 receptor agonists show neuroprotective effects in animal models of diabetes. Peptides 100, 101–107 (2018). [DOI] [PubMed] [Google Scholar]
- 105.Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Mattson MP, Hoffer BJ, Wang Y, Greig NH, GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc. Natl. Acad. Sci 106, 1285–1290 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Noyan-Ashraf MH, Momen MA, Ban K, Sadi A-M, Zhou Y-Q, Riazi AM, Baggio LL, Henkelman RM, Husain M, Drucker DJ, GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 58, 975–983 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yun SP, Kam T-I, Panicker N, Kim S, Oh Y, Park J-S, Kwon S-H, Park YJ, Karuppagounder SS, Park H, Kim S, Oh N, Kim NA, Lee S, Brahmachari S, Mao X, Lee JH, Kumar M, An D, Kang S-U, Lee Y, Lee KC, Na DH, Kim D, Lee SH, Roschke VV, Liddelow SA, Mari Z, Barres BA, Dawson VL, Lee S, Dawson TM, Ko HS, Block of A1 astrocyte conversion by microglia is neuroprotective in modelsof Parkinson’s disease. Nat. Med 24, 931–938 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hernandez NS, Schmidt HD, Central GLP-1 receptors: Novel molecular targetsfor cocaine use disorder. Physiol. Behav 206, 93–105 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Paxinos G, Watson C, The rat brain in stereotaxic coordinates (2007); http://searchworks.stanford.edu/view/5790940. [DOI] [PubMed]
- 110.Borner T, Arnold M, Ruud J, Breit SN, Langhans W, Lutz TA, Blomqvist A, Riediger T, Anorexia-cachexia syndrome in hepatoma tumour-bearing rats requires the area postrema but not vagal afferents and is paralleled by increased MIC-1/GDF15. J. Cachexia. Sarcopenia Muscle 8, 417–427 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mul JD, Begg DP, Barrera JG, Li B, Matter EK, D’Alessio DA, Woods SC, Seeley RJ, Sandoval DA, High-fat diet changes the temporal profile of GLP-1 receptor-mediated hypophagia in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 305, R68–R77 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME; NN8022–1807 Study Group, Effects of liraglutidein the treatment of obesity: A randomised, double-blind, placebo-controlled study. Lancet 374, 1606–1616 (2009). [DOI] [PubMed] [Google Scholar]
- 113.Fortin SM, Roitman MF, Challenges to body fluid homeostasis differentially recruit phasic dopamine signaling in a taste-selective manner. J. Neurosci 38, 6841–6853 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pyke C, Knudsen LB, The glucagon-like peptide-1 receptor—Or not? Endocrinology 154, 4–8 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


