Abstract
Purpose of Review
Short-chain fatty acids (SCFAs), the main bacterial fermentation products in the hindgut of hindgut fermenters, are also present in the foregut lumen. We discuss the impact of SCFAs in the duodenal defense mechanisms and in the gastrointestinal (GI) pathogenesis.
Recent Findings
Luminal SCFAs augment the duodenal mucosal defenses via release of serotonin (5-HT) and glucagon-like peptide-2 (GLP-2) from enteroendocrine cells. Released GLP-2 protects the small intestinal mucosa from nonsteroidal anti-inflammatory drug-induced enteropathy. SCFAs are also rapidly absorbed via SCFA transporters and interact with afferent and myenteric nerves. Excessive SCFA signals with 5-HT3 receptor overactivation may be implicated in the pathogenesis of irritable bowel syndrome symptoms. SCFA production exhibits diurnal rhythms with host physiological responses, suggesting that oral SCFA treatment may adjust the GI clocks.
Summary
SCFAs are not only a source of energy but also signaling molecules for the local regulation of the GI tract and systemic regulation via release of gut hormones. Targeting SCFA signals may be a novel therapeutic for GI diseases and metabolic syndrome.
Keywords: Short-chain fatty acid receptors, Glucagon-like peptide-2, Serotonin, Mucosal defense, Nutrition sensing
Introduction
Short-chain fatty acids (SCFAs) are volatile free fatty acids, consisting of C2–C6 carboxylic acids, including acetate (C2), propionate (C3), butyrate (C4), valerate (C5), and caproate (C6); C2–C4 are key microbial metabolites that interact with host cells. SCFAs not only are a source of energy but also serve as signaling ligands to cell surface G protein-coupled receptors (GPCRs) and can also activate intracellular signaling through transmembrane transport via monocarboxylate transporters (MCTs) [1, 2•]. SCFAs are primarily synthesized by bacterial fermentation of dietary fiber and carbohydrates in the gastrointestinal (GI) lumen, including the oral cavity, the rumen of ruminant, and the cecum and proximal colon of hindgut fermenters. In hindgut fermenters including humans, SCFAs are also present in the lumen of the foregut, derived from the fermentation of nutrients by oral flora and oral intake of vinegar and of fermented/preserved foods, even though their concentration is significantly lower than in the hindgut [3, 4].
During the past decade, the significant progress in deorphanization and characterization of nutrient-sensing GPCRs including SCFA receptors, free fatty acid receptor (FFA) 2 and FFA3, as well as the SCFA transporters, MCT, and Na+-coupled MCT (SMCT) families, has contributed to clarifying the mechanism of physiological responses to luminal SCFAs in the upper GI tract [1, 5]. Luminal SCFAs stimulate the release of 5-hydroxytryptamine (5-HT, serotonin) and proglucagon products such as glucagon-like peptide-2 (GLP-2) in the duodenum [6••,7••, 8], bioactive molecules that are related to the GI pathophysiology. We here summarize the recent findings of the contributions of luminal SCFAs in the duodenum towards mucosal defense mechanisms and gastrointestinal pathophysiology.
SCFA Content in the Gut Lumen
SCFAs are products of fermentation of dietary fiber and other carbohydrates resistant to mammalian enzymes by the microbiota in the cecum and the proximal colon of hindgut fermenters. Most studies for luminal SCFAs have been performed in the colon, where total SCFA concentration can reach ~ 100 mM [9, 10]. Despite lower concentration, SCFAs are physiologically present in the upper GI tract lumen. The concentration of acetate in saliva is ~ 6 mM, derived from oral flora, and is ~ 0.4 mM in the duodenal juice in humans [3, 4], suggesting that the foregut mucosa is generally exposed to low concentrations of SCFAs. Furthermore, some food contains acetate, since common table vinegar consists of ~ 800 mM (4–7%) acetate, and the vinegared rice for sushi contains ~ 60 mM acetate. Therefore, SCFAs are likely present at ~millimolar concentrations in the duodenal lumen. Even though foregut concentrations of total SCFAs decrease to ~ 0.5 mM in the duodenum, their luminal concentrations remain sufficient to activate SCFA signaling pathways. In contrast, rodents are coprophagic, suggesting that SCFA ingestion is more common in rodents than in humans. In ruminants, the ruminal concentrations of SCFAs are ~ 120 mM in bovines and ovines [11], all of which are absorbed from the rumen as the principal energy source.
SCFAs serve as an energy source not only for ruminants but also for hindgut fermenters [11]; percentages of an organism’s energy requirements that are fulfilled by SCFAs are 63% from the rumen and 9% from the hindgut of cattle; even in humans, the large intestine provides 6–10% of overall energy requirements from SCFAs depending on dietary intake of fermentable substrates. Absorbed SCFAs generate ATP through the TCA cycle; acetate, propionate, and butyrate produce 10, 18 and 27 net ATP/mol, in comparison with glucose that generates 38 net ATP/mol. Therefore, SCFAs are considered a metabolic fuel [12].
In human clinical studies, the supplementation of vinegar to a carbohydrate-rich diet improves glycemic control [13, 14]. Luminal SCFAs are rapidly absorbed by the duodenal mucosa through the electrogenic, high-affinity transporter termed SMCT-1 [2•] and slowly by the jejunal or ileal low-affinity SMCT-2 [5]. SMCT-1 is expressed in the enterocyte apical membrane whereas MCT-1 and MCT-4 are expressed in the basolateral membrane, which facilitate transmucosal SCFA absorption in the proximal duodenum. Furthermore, colonic SCFA absorption can provide up to 540 kcal per day in humans, contributing to nutritional needs, especially for patients with short bowel syndrome and those consuming high-fiber diets [15].
Rapid absorption of acetate in the duodenum is also utilized to measure gastric emptying in clinical studies, known as 13C-acetate breath test [16]. Ingested 13C-acetate is present in the exhaled breath as 13CO2, the amount over time that correlates with gastric emptying time, with peak excretion at ~ 40 min following oral ingestion and at ~ 10 min following intraduodenal administration [16], supporting the rapid absorption of acetate from the duodenal mucosa after exit from the stomach.
Duodenal Mucosal Defense Factors
The duodenal mucosa is regularly exposed to endogenous and exogenous chemicals, including gastric acid H+, bile acid, and digestive enzymes, and nutrients from food, alcohol, and drugs, respectively. Since the duodenum is the first part of the small intestine where absorption of nutrients primarily occurs, the duodenal mucosa needs to sense luminal conditions, such as pH and presence of nutrients or toxic chemicals. In the most recent decade, luminal chemosensors in the GI tract have been identified in accordance with the deorphanization and characterization of nutrient-sensing GPCRs. In response to luminal substances, the GI mucosa secretes gut hormones or neurotransmitters by luminal chemosensing, in order to not only regulate local gut function but also affect systemic metabolism, energy balance, and food intake. Since the duodenal mucosa contains the most abundant number and kinds of enteroendocrine cells [17], the duodenal mucosa is the first part responding to luminal chemicals and nutrients in order to enhance mucosal defenses as well as local and remote control of digestive organs, including the lower part of the small intestine, pancreas, and biliary tract.
Duodenal mucosal chemosensing is classified into three pathways [18]. (1) luminal chemicals are passively or actively transported through the epithelial cells into the subepithelial space and stimulate subepithelial afferent nerves by activation of the corresponding receptors. Luminal H+/CO2 sensing and capsaicin sensing use this pathway [19, 20]. (2) Luminal chemicals activate receptors expressed on the brush border membrane of epithelial cells, either directly or after interaction with ecto-enzymes on the brush border membranes. This pathway includes luminal purinergic signaling [21]. (3) Luminal chemicals activate apical or basolateral membrane receptors expressed on enteroendocrine cells, followed by the release of gut hormones or mediators. Nutrient sensing belongs to this pathway [22]. We here focus on this latter pattern that is related to SCFA sensing via SCFA receptors as described below.
Duodenal defense factors for luminal H+ consist of multilayered defenses; “pre-epithelial” HCO3− and mucus secretion, “epithelial” intracellular pH (pHi) regulation with ion transporters and ecto- and cytosolic enzyme activities, and “subepithelial” blood flow response regulated via afferent nerves and mediator releases. Simultaneous measurements of these factors such as mucosal blood flow, mucus secretion, and enterocyte pHi, or HCO3− secretion through duodenal loop perfusion with measurement of mediator release into the portal vein, enables us to assess the presence of luminal chemosensing via the pathways as mentioned above.
SCFA Sensing and Duodenal Mucosal Defenses
The nutrient GPCRs for SCFAs are identified as FFA2 and FFA3 [1]. FFA2 and FFA3 are expressed in L cells in the colonic epithelium, colocalized with peptide YY (PYY) and GLP-1 [23, 24], suggesting that PYY and GLP-1 are secreted by SCFAs in the colon. In contrast, FFA2 is localized in enterochromaffin (EC) cells, which contain 5-HT, while FFA3 is colocalized with GLP-1 in L cells in rat duodenal mucosa [25]. Luminal perfusion of the SCFAs acetate and propionate at physiological concentrations (10–1000 μM) [3] dose dependently stimulates mucosal protective HCO3− secretion in rat duodenum [6••], suggesting that luminal SCFAs augment duodenal mucosal defense mechanisms. Luminal SCFAs also stimulate duodenal HCO3− secretion accompanied by decreased paracellular permeability and net fluid movement [26••], confirming the mucosal protective effects of SCFAs in the duodenum. Interestingly, IV injection of SCFA suppresses duodenal HCO3− secretion but decreases paracellular permeability, suggesting that the mechanisms underlying the effects of SCFA on HCO3− secretion and paracellular permeability are differentially regulated. Luminal perfusion of the selective FFA2 agonist phenylacetamide-1 increases the release of 5-HT from EC cells and stimulates duodenal HCO3− secretion via activation of 5-HT4 and muscarinic receptors [7••, 25]. In contrast, luminal perfusion of the selective FFA3 agonist AR420626 increases the release of GLP-2 from L cells and stimulates HCO3− secretion via GLP-2 receptor activation [8]. Exogenous GLP-2 or taste receptor activation which releases GLP-2 stimulates HCO3− secretion via release of vasoactive intestinal peptide (VIP) and nitric oxide (NO) [27], consistent with GLP-2 receptors colocalized with VIP and NO synthase in the myenteric plexus [28]. The endogenous SCFAs acetate and propionate also stimulate HCO3− secretion via GLP-2 receptor activation, enhanced by the dipeptidyl peptidase (DPP) 4 inhibitor NVP-728 [25]. Furthermore, capsaicin-sensitive afferent nerve activation is also involved in luminal acetate-induced HCO3− secretion [29], suggesting that the transmucosally absorbed SCFAs activate subepithelial afferent nerves.
In contrast to the luminal effects of SCFAs, serosal administration of SCFAs or the selective FFA3 agonist AR420626 suppresses nicotine-induced electrogenic anion secretion via inhibition of the post-ganglionic neurons in rat colon [30], consistent with the suppressive effect of IV-injected SCFA on duodenal HCO3− secretion [26••]. Involvement of GLP-2 signaling downstream of luminal SCFA sensing is also confirmed by the observation that IV infusion of GLP-2 stimulates duodenal HCO3− secretion accompanied by decreased paracellular permeability [26••].
SCFA Sensing and Pathophysiology
Release of the potent bioactive molecules 5-HT and GLP-2 from EC cells and L cells, respectively (at least in the rat), in response to luminal SCFA perfusion suggests luminal SCFAs are likely to initiate and sustain important pathophysiological responses.
FFA3-GLP-2 and Nonsteroidal Anti-inflammatory Drug (NSAID)-Induced Enteropathy
GLP-2 is an intestinotrophic factor that has pro-proliferative effects on intestinal crypt cells via the release of insulin-like growth factor 1 (IGF1), epidermal growth factor (EGF), and other bioactive factors that promote stem cell proliferation and differentiation [31]. The stable human GLP-2 analog teduglutide is therefore clinically used to treat short bowel syndrome, in which it increases small intestinal mass and improves the nutritional state of patients dependent on parenteral nutritional support [32].
GLP-2, similar to GLP-1, is rapidly degraded and inactivated by DPP4. DPP4 inhibitors are clinically used to treat diabetes, since DPP4 inhibition enhances the insulinotropic effect of GLP-1 [33]. The DPP4 inhibitor NVP-728 enhances amino acid- and bile acid-augmented HCO3− secretion in rat duodenum via prolonging the t1/2 of GLP-2 [34], suggesting that enhanced endogenous GLP-2 signaling by the luminal GPCR agonist such as amino acids, bile acids, and FFA3 ligands [8, 34], acting at L cells, combined with DPP4 inhibition augments duodenal defense mechanisms.
NSAIDs not only injure the gastric mucosa but also induce small intestinal ulcers, termed NSAID-induced enteropathy, which is the cause of obscure GI bleeding in 10% of patients [35]. Exogenous GLP-2 prevents NSAID-induced enteropathy and promotes healing of small intestinal ulcers [36]. Furthermore, the DPP4 inhibitor alone significantly inhibits NSAID-induced enteropathy, and an oral amino acid combined with the DPP4 inhibitor accelerates small intestinal ulcer healing via the GLP-2 pathway [36]. These results suggest that exogenous GLP-2 or luminal nutrients that enhance endogenous GLP-2 release combined with DPP4 inhibition may be therapeutic for NSAID-induced enteropathy.
Since one of SCFA receptors, FFA3, is expressed on L cells and SCFA-augmented duodenal HCO3- secretion is enhanced by the DPP4 inhibitor [6••], activation of FFA3 expressed on L cells releases GLP-2, which reduces the magnitude of NSAID-induced enteropathy. Luminal perfusion of the selective synthetic FFA3 agonist AR420626 stimulates duodenal HCO3− secretion, accompanied by the increased release of GLP-2, effects inhibited by the FFA3 antagonist CF3-MQC or the GLP-2 receptor antagonist/partial agonist GLP-2 (3–33) [8], suggesting that selective activation of FFA3 expressed on L cells releases GLP-2. Furthermore, oral administration of AR420626 prevents the formation of NSAID-induced small intestinal ulcers [8]. FFA3 is also expressed on myenteric neurons. Intraperitoneal treatment or serosal application of AR420626 inhibits nicotinic acetylcholine receptor-mediated colonic motility and ion secretion [30, 37]. Nevertheless, intraperitoneal AR420626 has no effect on NSAID-induced enteropathy [8], suggesting that enhanced release of GLP-2 via luminal FFA3 activation may be a novel therapeutic for prevention of NSAID-induced enteropathy.
FFA2, 5-HT3, and IBS
A variety of stimulants trigger 5-HT release from EC cells, including mechanical stimulation (intraluminal pressure [38], mucosal stroking [39]), diet or food poisoning such as bitter tastants [40], gastric acid [41], bacterial metabolites such as SCFAs [42], infectious diseases (cholera toxin [43], rotavirus [44]), and chemotherapeutic agents (cisplatin [45]). Furthermore, intraduodenal infusion of acid or lipid triggers dyspeptic symptoms in patients with functional dyspepsia [46], suggesting that the duodenum can be the origin of dyspeptic symptoms. Since dyspeptic symptoms including fullness, bloating, nausea, satiety, epigastric burning sensation, and epigastric pain are alleviated with 5-HT3 receptor antagonists [47], the generation of these symptoms are implicated in 5-HT release, although these symptoms are linked to the release of several gut hormones such as cholecystokinin (CCK), GLPs, and PYY [48, 49]. Moreover, 5-HT is rich in the duodenal mucosa. The number of EC cells and mucosal 5-HT content is highest in the duodenum in humans and second highest in rats [17, 50]. About 95% of 5-HT is synthesized and released from EC cells in the GI tract, also suggesting that 5-HT-associated dyspeptic symptoms originate from the GI tract. Therefore, duodenal 5-HT release is likely involved not only in physiological mucosal defense but also in pathological symptom generation.
Irritable bowel syndrome (IBS) is a functional disorder involving the GI tract. IBS symptoms including nausea, epigastric pain, and diarrhea are clinically treated with 5-HT3 receptor antagonists [51, 52], implicated in the endogenous release of 5-HT. Small intestinal bacterial overgrowth (SIBO) is implicated in the pathogenesis of IBS, since IBS patients often have breath hydrogen production shortly after ingestion of a fermentable substrate and since nonabsorbable antibiotics improve IBS symptoms [53, 54]. Diets low in fermentable oligo-, di-, and monosaccharides and polyols (FODMAPs) also improve IBS symptoms [55]. SCFAs derived from fermentation by gut microbiota are the common factors underlying SIBO and FODMAPS.
In contrast to the FFA3/GLP-2 pathway and to the aforementioned luminal FFA2 agonist-induced duodenal HCO3− secretion, oral administration of the selective FFA2 agonist phenylacetamide-1, which increases 5-HT release from EC cells, dose dependently induces duodenal mucosal injury, manifest as massive villous erosions in response to treatment with ulcerogenic doses of NSAID, although the duodenal mucosa is normally spared from NSAID-induced enteropathy [7••]. Duodenal mucosal injury induced by the FFA2 activation with NSAID treatment involves stimulation of gastric acid secretion and 5-HT3 receptor activation, accompanied by increased 5-HT release into the portal vein and impaired acid-induced hyperemia via 5-HT3 receptor activation [7••]. Since the pathogenesis of NSAID-induced enteropathy includes reduction of mesenteric blood flow, mucus secretion, and HCO3− secretion, these results suggest that NSAID treatment at ulcerogenic doses with concomitant FFA2 activation is accompanied by excessive release of 5-HT that reduces duodenal mucosal blood flow, disrupting duodenal mucosal defenses to luminal acid, followed by duodenal mucosal injury. Intra-aortic administration of 5-HT at low doses increases duodenal blood flow and decreases blood flow at high doses [7••], confirming that blood flow is one of important factors in duodenal mucosal defenses.
These results suggest that increased luminal SCFAs by SIBO and FODMAP diet may induce excessive 5-HT release from EC cells via FFA2 activation, followed by overactivation of 5-HT3 receptors, implicating IBS symptom generation, such as hyperperistalsis, luminal ion secretion, and pain.
SCFAs and Gastric Mucosal Defenses
The effects of luminal SCFA on the gastric mucosal defenses are not fully understood. Although 1 M acetate reduces ethanol-induced gastric mucosal injury, the protective effects of acetate are possibly due to hyperosmolality, since NaCl at the same luminal concentration is also mucosally protective [56] due to the well-described “adaptive cytoprotection” of hypertonic solutions [57]. Nevertheless, 5-HT suppresses stimulated gastric acid secretion [58, 59], and since FFA2 is expressed on EC cells located in the gastric fundus [60], luminal SCFA may reduce acid-related gastric mucosal injury through 5-HT release from gastric EC cells. Luminal perfusion of the selective FFA2 agonist phenylacetamide-1 reduces basal and stimulated gastric acid secretion via 5-HT3 receptor activation [61], consistent with a previous study reporting that the 5-HT3 agonist 2-methyl-5-HT suppresses stimulated gastric acid secretion in rat isolated stomach [62]. Furthermore, pretreatment with oral SCFAs (acetate and propionate, 1 mmol/kg) reduces ethanol-induced gastric mucosal hemorrhagic injury, whereas NaCl (1 mmol/kg) has no effect [61].
The protective effects of FFA2 activation are reversed by pretreatment with the 5-HT3 receptor antagonist ondansetron, suggesting that luminal SCFA protects the gastric mucosa from ethanol-induced injury by reduction of acid secretion via the FFA2-5-HT-5HT3 receptor pathway.
SCFAs and Circadian Rhythm
There are several recent studies indicating that the gut microbiota also exhibit diurnal rhythms. In mammals, clock genes such as interlocked transcription-translation feedback loop (TTFL), transcriptional activators such as brain and muscle ARNT-like protein 1 (BMAL1), and the circadian locomotor output cycles kaput (CLOCK) have been reported [63•, 64••]. In BMAL1 genetic knockouts, disruption of diurnal food intake patterns and SCFA level rhythmicity, as well as inhibition of colonic smooth muscle contractility via SCFA signaling, was observed [63•]. In the separate study, antibiotic disruption of the gut microbiota in mice led to the loss of diurnal oscillations in the SCFA level [64••]. Subsequent oral administration of SCFAs or prebiotic dietary fiber supplementation facilitated the adjustment of the peripheral clocks with stimulation timing dependency. This study also suggests that the rhythm of cecal SCFA production might be influenced by altered feeding rhythms. These results suggest that the close association of the diurnal oscillations between the host and the microbiota through their symbiotic relationships is possibly influenced by feeding rhythm, which is implicated in the cephalic phase, gastric phase, and small intestinal phase of digestion via vagal activity and gut hormone release, emphasizing the promise of therapeutic oral administration of SCFAs with the goal of adjusting circadian rhythm.
Conclusions
The relation of SCFA production by fermentative gut bacteria; the release of potent bioactive molecules such as 5-HT, GLP-1, and GLP-2 from enteroendocrine cells in response to luminal SCFAs; the presence of SCFAs receptors on enteric nerves and on enteroendocrine cells; and the response of IBS patients to low-FODMAP diets and 5-HT3 receptor antagonists combined with observations that loss of fermentative bacteria from the gut microbiome is associated with a disease [51–55] strongly support the nexus linking diet, the gut microbiome, and human disease (Fig. 1). It is hoped that this mechanistic framework will inform future studies of the gut microbiome and its metabolites.
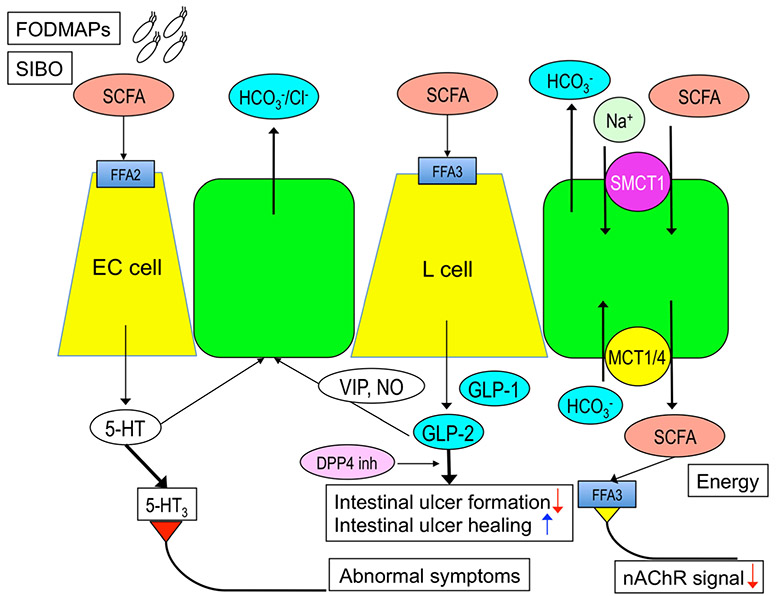
Fig. 1.
The roles of luminal SCFAs in the duodenum. Luminal SCFAs release 5-hydroxytryptamine (5-HT) from enterochromaffin (EC) cells via free fatty acid receptor (FFA) 2 activation and glucagon-like peptide (GLP)-1 and GLP-2 from L cells via FFA3 activation. Released 5-HT and GLP-2 stimulate the mucosal protective HCO3− secretion from the epithelial cells, the latter mediated by vasoactive intestinal peptide (VIP) and nitric oxide (NO) released from enteric neurons. Luminal SCFAs are also absorbed via apical sodium-dependent monocarboxylate transporter (SMCT)-1 and basolateral monocarboxylate transporter (MCT)-1 and 4. Absorbed SCFAs are a part of energy source for the body and also suppress nicotinic acetylcholine receptor (nAChR) signal for secretion and motility. Released GLP-2 enhanced by dipeptidyl peptidase 4 (DPP4) inhibitor (DPP4 inh) inhibits small intestinal ulcer formation and promotes intestinal ulcer healing. Although physiological amount of 5-HT is mucosal protective, excessive release of 5-HT by higher SCFA-FFA2 signal induced by fermentable oligo-, di-, and monosaccharide and polyol (FODMAP) diet followed by small intestinal bacterial overgrowth (SIBO) overactivates 5-HT3 receptors on afferent nerves, which produces abnormal symptoms observed in irritable bowel syndrome patients. There are two sides of the effects of luminal SCFAs in the duodenum
Acknowledgments
Funding Information This work was supported by a Department of Veterans Affairs Merit Review Award and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-54221.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–9. [DOI] [PubMed] [Google Scholar]
- 2.•.Kaji I, Iwanaga T, Watanabe M, Guth PH, Engel E, Kaunitz JD, et al. SCFA transport in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2015;308:G188–97This study provides the evidence of rapid, electrogenic SCFA absorption via apical SMCT-1 in the duodenal mucosa.
- 3.Hoverstad T, Bjorneklett A, Midtvedt T, Fausa O, Bohmer T. Short-chain fatty acids in the proximal gastrointestinal tract of healthy subjects. Scand J Gastroenterol. 1984;19:1053–8. [PubMed] [Google Scholar]
- 4.Botta GA, Radin L, Costa A, Schito G, Blasi G. Gas-liquid chromatography of the gingival fluid as an aid in periodontal diagnosis. J Periodontal Res. 1985;20:450–7. [DOI] [PubMed] [Google Scholar]
- 5.Iwanaga T, Kishimoto A. Cellular distributions of monocarboxylate transporters: a review. Biomed Res. 2015;36:279–301. [DOI] [PubMed] [Google Scholar]
- 6.••.Akiba Y, Inoue T, Kaji I, Higashiyama M, Narimatsu K, Iwamoto K, et al. Short-chain fatty acid sensing in rat duodenum. J Physiol. 2015;593:585–99This study provides for the first time that luminal SCFAs stimulate duodenal mucosa to enhance mucosal defense via distinct 5-HT and GLP-2 pathways via SCFA receptors.
- 7.••.Akiba Y, Maruta K, Narimatsu K, Said H, Kaji I, Kuri A, et al. FFA2 activation combined with ulcerogenic COX inhibition induces duodenal mucosal injury via the 5-HT pathway in rats. Am J Physiol Gastrointest Liver Physiol. 2017;313:G117–28This study links 5-HT release via FFA2 activation to duodenal mucosal injury with indomethacin treatment, implicating in 5-HT-related IBS symptom generation.
- 8.Said H, Akiba Y, Narimatsu K, Maruta K, Kuri A, Iwamoto K, et al. FFA3 activation stimulates duodenal bicarbonate secretion and prevents NSAID-induced enteropathy via the GLP-2 pathway in rats. Dig Dis Sci. 2017;62:1944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Illman RJ, Topping DL, Trimble RP. Effects of food restriction and starvation-refeeding on volatile fatty acid concentrations in the rat. J Nutr. 1986;116:1694–700. [DOI] [PubMed] [Google Scholar]
- 10.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70:567–90. [DOI] [PubMed] [Google Scholar]
- 12.Skutches CL, Holroyde CP, Myers RN, Paul P, Reichard GA. Plasma acetate turnover and oxidation. J Clin Invest. 1979;64:708–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostman E, Granfeldt Y, Persson L, Bjorck I. Vinegar supplementation lowers glucose and insulin responses and increases satiety after a bread meal in healthy subjects. Eur J Clin Nutr. 2005;59:983–8. [DOI] [PubMed] [Google Scholar]
- 14.Brighenti F, Castellani G, Benini L, Casiraghi MC, Leopardi E, Crovetti R, et al. Effect of neutralized and native vinegar on blood glucose and acetate responses to a mixed meal in healthy subjects. Eur J Clin Nutr. 1995;49:242–7. [PubMed] [Google Scholar]
- 15.Ruppin H, Bar-Meir S, Soergel KH, Wood CM, Schmitt MG Jr. Absorption of short-chain fatty acids by the colon. Gastroenterology. 1980;78:1500–7. [PubMed] [Google Scholar]
- 16.Braden B, Adams S, Duan LP, Orth KH, Maul FD, Lembcke B, et al. The [13C]acetate breath test accurately reflects gastric emptying of liquids in both liquid and semisolid test meals. Gastroenterology. 1995;108:1048–55. [DOI] [PubMed] [Google Scholar]
- 17.Sjölund K, Sandén G, Håkanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120–30. [PubMed] [Google Scholar]
- 18.Akiba Y, Kaunitz JD. Duodenal luminal chemosensing; acid, ATP, and nutrients. Curr Pharm Des. 2014;20:2760–5. [DOI] [PubMed] [Google Scholar]
- 19.Akiba Y, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Acid-sensing pathways of rat duodenum. Am J Physiol Gastrointest Liver Physiol. 1999;277:G268–74. [DOI] [PubMed] [Google Scholar]
- 20.Akiba Y, Ghayouri S, Takeuchi T, Mizumori M, Guth PH, Engel E, et al. Carbonic anhydrases and mucosal vanilloid receptors help mediate the hyperemic response to luminal CO2 in rat duodenum. Gastroenterology. 2006;131:142–52. [DOI] [PubMed] [Google Scholar]
- 21.Mizumori M, Ham M, Guth PH, Engel E, Kaunitz JD, Akiba Y. Intestinal alkaline phosphatase regulates protective surface microclimate pH in rat duodenum. J Physiol. 2009;587:3651–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelstoft MS, Egerod KL, Holst B, Schwartz TW. A gut feeling for obesity: 7TM sensors on enteroendocrine cells. Cell Metab. 2008;8:447–9. [DOI] [PubMed] [Google Scholar]
- 23.Tazoe H, Otomo Y, Karaki S, Kato I, Fukami Y, Terasaki M, et al. Expression of short-chain fatty acid receptor GPR41 in the human colon. BiomedRes. 2009;30:149–56. [DOI] [PubMed] [Google Scholar]
- 24.Karaki S, Tazoe H, Hayashi H, Kashiwabara H, Tooyama K, Suzuki Y, et al. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J Mol Histol. 2008;39:135–42. [DOI] [PubMed] [Google Scholar]
- 25.Akiba Y, Inoue T, Kaji I, Higashiyama M, Guth PH, Engel E, et al. Short-chain fatty acid sensing in rat duodenum. J Physiol. 2014;593:585–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.••.Wan Saudi WS, Sjöblom M. Short-chain fatty acids augment rat duodenal mucosal barrier function. Exp Physiol. 2017;102:791–803This study provides that luminal SCFAs rather than IV SCFAs enhance duodenal defense mechanisms.
- 27.Wang JH, Inoue T, Higashiyama M, Guth PH, Engel E, Kaunitz JD, et al. Umami receptor activation increases duodenal bicarbonate secretion via glucagon-like peptide-2 release in rats. J Pharmacol Exp Ther. 2011;339:464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, Zhang G, et al. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130:150–64. [DOI] [PubMed] [Google Scholar]
- 29.Kaji I, Iwanaga T, Watanabe M, Guth PH, Engel E, Kaunitz JD, et al. SCFA transport in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2014;308:G188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaji I, Akiba Y, Konno K, Watanabe M, Kimura S, Iwanaga T, et al. Neural FFA3 activation inversely regulates anion secretion evoked by nicotinic ACh receptor activation in rat proximal colon. J Physiol. 2016;594:3339–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowland KJ, Brubaker PL. The “cryptic” mechanism of action of glucagon-like peptide-2. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1–8. [DOI] [PubMed] [Google Scholar]
- 32.Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O'Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60:902–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drucker DJ, Nauck MA. The incretin system: glucagon-like pep-tide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705. [DOI] [PubMed] [Google Scholar]
- 34.Inoue T, Wang JH, Higashiyama M, Rudenkyy S, Higuchi K, Guth PH, et al. Dipeptidyl peptidase IV inhibition potentiates amino acid- and bile acid-induced bicarbonate secretion in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2012;303:G810–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higuchi K, Umegaki E, Watanabe T, Yoda Y, Morita E, Murano M, et al. Present status and strategy of NSAIDs-induced small bowel injury. J Gastroenterol. 2009;44:879–88. [DOI] [PubMed] [Google Scholar]
- 36.Inoue T, Higashiyama M, Kaji I, Rudenkyy S, Higuchi K, Guth PH, et al. Dipeptidyl peptidase IV inhibition prevents the formation and promotes the healing of indomethacin-induced intestinal ulcers in rats. Dig Dis Sci. 2014;59:1286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaji I, Akiba Y, Furuyama T, Adelson DW, Iwamoto K, Watanabe M, et al. Free fatty acid receptor 3 activation suppresses neurogenic motility in rat proximal colon. Neurogastroenterol Motil, in press. 2017. [DOI] [PMC free article] [PubMed]
- 38.Fujimiya M, Okumiya K, Kuwahara A. Immunoelectron microscopic study of the luminal release of serotonin from rat enterochromaffin cells induced by high intraluminal pressure. Histochem Cell Biol. 1997;108:105–13. [DOI] [PubMed] [Google Scholar]
- 39.Kellum JM, Albuquerque FC, Stoner MC, Harris RP. Stroking human jejunal mucosa induces 5-HT release and Cl− secretion via afferent neurons and 5-HT4 receptors. Am J Phys. 1999;277:G515–20. [DOI] [PubMed] [Google Scholar]
- 40.Braun T, Voland P, Kunz L, Prinz C, Gratzl M. Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology. 2007;132:1890–901. [DOI] [PubMed] [Google Scholar]
- 41.Kellum JM, Donowitz M, Cerel A, Wu J. Acid and isoproterenol cause serotonin release by acting on opposite surfaces of duodenal mucosa. J Surg Res. 1984;36:172–6. [DOI] [PubMed] [Google Scholar]
- 42.Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269–76. [DOI] [PubMed] [Google Scholar]
- 43.Turvill JL, Connor P, Farthing MJ. The inhibition of cholera toxin-induced 5-HT release by the 5-HT3 receptor antagonist, granisetron, in the rat. Br J Pharmacol. 2000;130:1031–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hagbom M, Istrate C, Engblom D, Karlsson T, Rodriguez-Diaz J, Buesa J, et al. Rotavirus stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting. PLoS Pathog. 2011;7:e1002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cubeddu LX. Serotonin mechanisms in chemotherapy-induced emesis in cancer patients. Oncology. 1996;53(Suppl 1):18–25. [DOI] [PubMed] [Google Scholar]
- 46.Lee KJ, Tack J. Duodenal implications in the pathophysiology of functional dyspepsia. J Neurogastroenterol Motil. 2010;16:251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beattie DT, Smith JA. Serotonin pharmacology in the gastrointestinal tract: a review. Naunyn Schmiedeberg's Arch Pharmacol. 2008;377:181–203. [DOI] [PubMed] [Google Scholar]
- 48.Bharucha AE, Camilleri M, Burton DD, Thieke SL, Feuerhak KJ, Basu A, et al. Increased nutrient sensitivity and plasma concentrations of enteral hormones during duodenal nutrient infusion in functional dyspepsia. Am J Gastroenterol. 2014;109:1910–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Boxel OS, ter Linde JJ, Siersema PD, Smout AJ. Role of chemical stimulation of the duodenum in dyspeptic symptom generation. Am J Gastroenterol. 2010;105:803–11. [DOI] [PubMed] [Google Scholar]
- 50.Glisic R, Koko V, Todorovic V, Drndarevic N, Cvijic G. Serotonin-producing enterochromaffin (EC) cells of gastrointestinal mucosa in dexamethasone-treated rats. Regul Pept. 2006;136:30–9. [DOI] [PubMed] [Google Scholar]
- 51.Fukudo S, Kinoshita Y, Okumura T, Ida M, Akiho H, Nakashima Y, et al. Ramosetron reduces symptoms of irritable bowel syndrome with diarrhea and improves quality of life in women. Gastroenterology. 2016;150:358–66. [DOI] [PubMed] [Google Scholar]
- 52.Garsed K, Chernova J, Hastings M, Lam C, Marciani L, Singh G, et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut. 2014;63:1617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghoshal UC, Srivastava D, Misra A, Ghoshal U. A proof-of-concept study showing antibiotics to be more effective in irritable bowel syndrome with than without small-intestinal bacterial over-growth: a randomized, double-blind, placebo-controlled trial. Eur J Gastroenterol Hepatol. 2016;28:281–9. [DOI] [PubMed] [Google Scholar]
- 54.Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503–6. [DOI] [PubMed] [Google Scholar]
- 55.Bohn L, Storsrud S, Liljebo T, Collin L, Lindfors P, Tornblom H, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149:1399–407. [DOI] [PubMed] [Google Scholar]
- 56.Barreto JC, Smith GS, Tornwall MS, Miller TA. Protective action of oral N-acetylcysteine against gastric injury: role of hypertonic sodium. Am J Physiol. 1993;264:G422–6. [DOI] [PubMed] [Google Scholar]
- 57.Aihara E, Sasaki Y, Ise F, Kita K, Nomura Y, Takeuchi K. Distinct mechanisms of acid-induced HCO3− secretion in normal and slightly permeable stomachs. Am J Physiol Gastrointest Liver Physiol. 2006;291:G464–71. [DOI] [PubMed] [Google Scholar]
- 58.Black JW, Fisher EW, Smith AN. The effects of 5-hydroxytryptamine on gastric secretion in anaesthetized dogs. J Physiol. 1958;141:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Canfield SP, Spencer JE. The inhibitory effects of 5-hydroxytryptamine on gastric acid secretion by the rat isolated stomach. Br J Pharmacol. 1983;78:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaji I, Akiba Y, Kaunitz JD, Karaki S, Kuwahara A. Differential expression of short-chain fatty acid receptor FFA2 and FFA3 in foregut. Gastroenterology. 2012;142:S494. [Google Scholar]
- 61.Said HM, Akiba Y, Kaji I, Narimatsu K, Kaunitz JD. FFA2 activation suppresses basal and stimulated gastric acid secretion via 5-HT3 receptor activation in rats. Gastroenterology. 2015;148:S–315. [Google Scholar]
- 62.Lai YC, Ho Y, Huang KH, Tsai LH. Effects of serotonin on acid secretion in isolated rat stomach: the role of 5-HT3 receptors. Chin J Physiol. 2009;52:395–405. [DOI] [PubMed] [Google Scholar]
- 63.•.Segers A, Desmet L, Thijs T, Verbeke K, Tack J, Depoortere I. The circadian clock regulates the diurnal levels of microbial short-chain fatty acids and their rhythmic effects on colon contractility in mice. Acta Physiol (Oxf). 2018:e13193.This study reports diurnal fluctuation of fecal SCFAs synchronized with colonic myenteric neural FFA3 expression, suggesting that luminal SCFA production regulates SCFA receptor expression.
- 64.••.Tahara Y, Yamazaki M, Sukigara H, Motohashi H, Sasaki H, Miyakawa H, et al. Gut microbiota-derived short chain fatty acids induce circadian clock entrainment in mouse peripheral tissue. Sci Rep. 2018;8:1395.This study provides a new concept that oral SCFA treatment facilitates peripheral clock adjustment, suggesting that the microbiome and host organs are communicating with circadian rhythmicity.