Abstract
Across the animal kingdom, males tend to exhibit more behavioural and morphological variability than females, consistent with the ‘greater male variability hypothesis'. This may reflect multiple mechanisms operating at different levels, including selective mechanisms that produce and maintain variation, extended male development, and X chromosome effects. Interestingly, human neuroanatomy shows greater male variability, but this pattern has not been demonstrated in any other species. To address this issue, we investigated sex-specific neuroanatomical variability in chimpanzees by examining relative and absolute surface areas of 23 cortical sulci across 226 individuals (135F/91M), using permutation tests of the male-to-female variance ratio of residuals from MCMC generalized linear mixed models controlling for relatedness. We used these models to estimate sulcal size heritability, simulations to assess the significance of heritability, and Pearson correlations to examine inter-sulcal correlations. Our results show that: (i) male brain structure is relatively more variable; (ii) sulcal surface areas are heritable and therefore potentially subject to selection; (iii) males exhibit lower heritability values, possibly reflecting longer development; and (iv) males exhibit stronger inter-sulcal correlations, providing indirect support for sex chromosome effects. These results provide evidence that greater male neuroanatomical variability extends beyond humans, and suggest both evolutionary and developmental explanations for this phenomenon.
Keywords: sexual selection, neuroanatomy, chimpanzees, variability
1. Introduction
Questions surrounding the mechanisms by which trait variation is produced and maintained over time are at the heart of evolutionary biology. Across the animal kingdom, males tend to exhibit greater behavioural and morphological variability than females, consistent with the ‘greater male variability hypothesis’ [1,2]. Sexually selected traits, in particular, tend to be more variable in males than either (i) the same trait in females or (ii) other, non-sexually selected traits [1]. This phenomenon has puzzled biologists for decades, since directional selection from male–male competition and/or female mate choice is expected to deplete male phenotypic and genotypic variation. However, there are a number of non-mutually exclusive evolutionary and developmental mechanisms that can lead to sex differences in trait variability.
Some of these evolutionary mechanisms include balancing selection, which maintains genetic diversity in populations, and disruptive selection, which favours extreme phenotypes. If these selective processes act on a heritable trait (i.e. a trait for which variation is explained by some genetic, rather than purely environmental, variation) within one sex only, this can lead to multiple morphotypes evolving within that sex. For example, one form of balancing selection, negative frequency-dependent selection, may occur if the relative selective value of a variant is higher when the relative abundance of that variant is lower [3].
Additionally, development can impact sex differences in trait variability, since condition-dependent expression characterizes many sexually selected traits [4]. Accordingly, environmental effects that increase condition variability during development may also increase trait variability. This relationship has been demonstrated in multiple experimental studies (e.g. [5]). The effects of condition on male trait variability may be particularly pronounced in species that exhibit direct male–male competition and, consequently, male-biased body size sexual dimorphism, since this necessitates longer developmental periods for males and leaves them more susceptible to environmental effects on condition.
The influence of sex chromosomes provides an additional developmental explanation for greater male trait variability for species with heterogametic males. In mammals, the Y chromosome contains few genes besides the male sex-determining factors. While males have one set of X-linked alleles that can be expressed, females have two alternative sets of X-linked alleles, which may permit mosaic levels of trait expression and decrease population variability in females relative to males. Consequently, male mammals are expected to show larger variability than females in traits that are influenced by genes located on the X chromosome [6–8].
Interestingly, numerous studies have demonstrated that human males are more variable than females, not only for physical traits (e.g. [9]), but also for aspects of cognition and behaviour [10–15]. Recent work suggests that these differences are likely to reflect greater male variability in brain gene expression and structure [16–21]. Throughout the human literature, the ‘greater male variability hypothesis' [2] has sparked debate regarding its validity and potential causes [11]. For example, while some question the existence of this pattern in cognition (e.g. [22]), others have proposed that these differences reflect extended male development [23], sex chromosome effects [10,24] or sexual selection processes [29]; however, such mechanisms are hard to measure in modern humans. Furthermore, no study to date has examined sex differences in neuroanatomical variability in any non-human taxa, aside from overall brain size [25,26] or cell morphology [27]. Accordingly, it is uncertain whether greater variability in human male neuroanatomy is unusual in nature, or whether it represents a common pattern observed in other species.
To address this question, we investigate sex differences in brain structure variability in chimpanzees, one of the closest living primate relatives of humans, by examining the relative and absolute surface areas of 23 cortical sulci among a sample of 226 individuals (135 females, 91 males; sample sizes vary across analyses—see Material and methods). Such a large sample size of brain structure data from magnetic resonance imaging (MRI) is currently available exclusively in chimpanzees, and not any other non-human primate species. The organization of sulci and gyri across the cortex is likely to reflect other aspects of underlying neural architecture [28], as the folding of the cerebral cortex reflects combined effects of variation in cortical surface area and connectivity [29,30]. Sulcal organization may reflect some aspects of cortical cytoarchitectonic organization [31], since certain functional areas can be consistently located using specific sulci, both across [32] and within species [33–35]; however, this relationship is likely to be limited to certain areas [33–36]. Given that the surface areas of specific cortical areas correlate with certain abilities (e.g. planum temporale and musical skill [37]), larger sulcal surface areas may provide additional surface area for related cortical areas, and therefore the elaboration of certain sensorimotor and cognitive abilities. Cortical volume is the product of thickness and surface area, so it is unclear how much each component contributes to cortical volume variation [38]. Nevertheless, sulcal variation has been linked to inter-individual differences in behaviour (e.g. handedness [39,40]) in primates, and correlates with the severity of some human neurological disorders [41,42]. Additionally, motor and cognitive outcomes in humans are correlated with the surface areas of specific sulci early in development [43]. Previous work has identified sexually dimorphic aspects of sulcal morphology (e.g. sulcal lengths) in multiple primate and non-primate species (e.g. [28,44–46]); however, sex differences in the variability of sulcal morphology have yet to be investigated.
Accordingly, we studied sex differences in variability of sulcal sizes, and predicted that males would exclusively exhibit greater variability (‘Prediction 1’) due to a combination of the evolutionary and developmental mechanisms discussed above. Specifically, male chimpanzees are subject to various sex-biased selective pressures, including direct male–male competition, as demonstrated by intermediate body and canine size dimorphism [47], indirect male–male competition, as demonstrated by large relative testis volume [48], and mechanisms of indirect female mate choice, such as sexual swellings and copulation calls [49]. The attainment of a larger body size necessitates a longer developmental period in male chimpanzees than in females [50], exposing males to additional environmental effects that may influence condition-dependent traits, which include cognition (e.g. [51]). In addition, though dominance rank is positively correlated with male chimpanzee reproductive success, their fission–fusion grouping pattern allows low-ranking males to use alternative mating strategies, such as consortship, to achieve reproductive success [52]. Variation in mating strategies may reflect negative frequency-dependent selection and/or disruptive selection from male aggression biases and/or female preferences [53]. Together, these mechanisms are expected to create multiple routes to male success, producing and maintaining relatively greater variation in male behavioural phenotypes, rather than producing one male phenotype that is under linear directional selection. Finally, there is evidence that X chromosome genes affect sulcal morphology [54], so consistent X chromosome expression across male chimpanzee brain tissues may lead males to exhibit relatively more variable sulcal surface areas.
In order to tease apart whether all of these mechanisms contribute to greater male variability in brain structure, we conducted three additional analyses. Specifically, in order for a trait to be subject to selection, it must be heritable; therefore, if a trait that is more variable in males is not heritable, this pattern cannot reflect selection. Correspondingly, we assessed heritability, and predicted that sulcal surface areas would be heritable (‘Prediction 2’). This is in accordance with previous work on the heritability of sulcal morphology in humans [45] and non-human primates [28,45]. In chimpanzees, sulcal lengths [55], non-sulcal aspects of brain structure [55–58], and aspects of cognition [58–60] and personality [61] are also heritable. Additionally, if greater male trait variation reflects variation in condition due to extended male development, these traits should show relatively more covariation with the environment in males. Heritability measures the degree of phenotypic variation that is due to genetic (versus environmental) variation, so we assessed sex differences in heritability, and predicted that heritability would be lower in males (‘Prediction 3’). Finally, if consistent X chromosome expression across male tissues contributes to greater male variability in sulcal size, we may also expect this expression pattern to produce relatively stronger interregional correlations in male brains [17,19,20]. Accordingly, we examined correlations between sulcal sizes and predicted that more pairs of sulci would exhibit significantly stronger correlations in males (‘Prediction 4’).
2. Material and methods
(a). Subjects
In vivo MRIs from 226 captive chimpanzees (135F/95M) made available through the National Chimpanzee Brain Resource were used in this study. Chimpanzees were housed at the Yerkes National Primate Research Center and the National Center for Chimpanzee Care at The University of Texas MD Anderson Cancer Center. Individuals ranged in age from 6 to 53 years (mean = 26.3, s.d. = 10.6; females: mean = 28.0, s.d. = 11.4; males: mean = 23.8, s.d. = 8.7) at the time of imaging. Studies of humans suggest sex differences in neuroanatomical variability are present across the lifespan [20], the degree of cortical folding reaches adult levels by early childhood and does not decrease with ageing [33] and relative sulcal surface areas do not change substantially with age [62]. Accordingly, all individuals were included in our study to obtain the largest possible sample size.
(b). Sulci labelling
The processing used to extract sulcal measurements from the images derives from BrainVISA, a pipeline initially dedicated to the human brain [63], with some tuning to account for specificities of chimpanzee anatomy. Twenty-three primary and secondary labelled sulci were included in this study. For each sulcus and overall folding area, surface areas from both cerebral hemispheres were combined to obtain total surface area. When the folds bifurcated or comprised several segments, all segments or branches were included in the labelling. Additionally, when delineating certain sulci was difficult, labelling was approached conservatively (see electronic supplementary material, Methods for examples). Details regarding MRI image collection and post-image processing are available in the electronic supplementary material.
(c). Statistical analyses
All statistical analyses were performed in R 3.4.2 [64]. For the total surface area of each sulcus and overall folding area, outliers were identified within each sex as values occurring 1.5 times beyond the interquartile range of boxplots that adjust for skewedness during whisker determination [65,66]. Here, we present, in detail, the results of analyses that excluded identified outliers (correlations: n = 67; variability analyses: n = 164–210). Analyses were re-run including these outliers (correlations: n = 120; variability analyses: n = 177–224), the results of which are available in the electronic supplementary material.
For each sulcus and within each sex, the average surface area range size was calculated across 1000 random samples for every possible sample size (from n = 2 to the actual subset sample size). The average range sizes were plotted against sample size in order to demonstrate that each distribution reached a horizontal asymptote (electronic supplementary material, figures S2–S49).
(i). Sex differences in variability
We measured sex differences in the variability of each sulcus by comparing male and female variances of residuals from generalized linear mixed models (R package MCMCglmm: [67]), controlling for relatedness between individuals. For each sulcus, analyses were conducted on: (i) all individuals for which the sulcus was measured (n = 177–224; mean = 218); and (ii) a subset of these data that excluded outliers for that sulcus (n = 164–210; mean = 199). Results using the latter are presented here in detail (see electronic supplementary material for additional results). All continuous variables were standardized prior to analysis. To test for sex differences in the relative surface area of different sulci, we included remaining folding area (RFA; total folding area − total surface area of sulcus), age, scanner type and rearing condition (wild caught, mother-reared, nursery-reared) as fixed effects. Total sulcal area scales nearly isometrically with total brain volume [68], so including RFA represents a correction for overall brain size. We also ran models excluding RFA to examine sex differences in absolute surface area variability. In all analyses, the pedigree was included as a random effect. For all offspring, the mothers were known, and paternity tests confirmed the fathers of most animals in this study. Following other studies [55], we used the default prior for the mean and variance of fixed effects for Gaussian family models in MCMCglmm and a slightly informative inverse-Wishart prior for the random effects and residual variances (V = 1, nu = 1). Models were run for 1 000 000 iterations, sampling every 100 iterations with a burn-in of 500 000. MCMC diagnostics were run using the R package coda [69]. We ensured proper mixing occurred by visually inspecting trace and density plots. We examined autocorrelation plots to confirm reduced correlation between successive samples (i.e. correlations quickly dropped below the threshold of 0.1) and confirmed effective sample sizes were greater than 1000 for all variables. Finally, we ran each chain twice and confirmed convergence using the Gelman–Rubin statistic (PSRFs < 1.1, [70]). We extracted residuals for each individual and used these values in subsequent analyses.
We tested for significant sex differences in residual variance using permutation tests. In line with previous work [17,20], we calculated the male-to-female variance across the residuals, randomly permuted the sex variable among the residuals 10 000 times, and calculated the proportion of permuted test statistics (absolute value) greater than the observed ratio (absolute value). This proportion is referred to here as ‘pPERM’ and represents a two-sided test of sex differences in variability. We also used the Benjamini–Hochberg procedure to indicate which results remain significant after adjusting for the false discovery rate (i.e. correct for multiple comparisons; adjusted pPERM values).
(ii). Heritability
For each sulcus, we calculated heritability from the MCMCglmm models described above. We estimated the ratio of additive genetic to phenotypic variance for each sample of the posterior distribution, h2 = VA/VP = VA/(VA + VR), where h2 is the heritability, VA the additive genetic variance, VP the phenotypic variance and VR the residual (i.e. non-additive genetic) variation. This was done using the output from MCMCglmm, which provides the additive genetic variance as the posterior distribution of variance for the pedigree random effect (i.e. the ‘animal’ term) in addition to the residual variance (i.e. ‘unit’ term). We extracted mean estimates and 95% highest posterior density intervals from these distributions.
We first tested the significance of heritability estimates by comparing the deviance information criterion (DIC) in models including pedigree information, and in models excluding it, which yielded a DIC differential value (ΔDIC). ΔDIC values from 5 to 10 are considered substantial evidence in favour of the model with the lower DIC, and a difference of 10 effectively rules out the model with the larger DIC [71]. Given that variance components from which heritability is estimated are bound to be positive, even non-significant estimates will not overlap 0. Accordingly, we followed previous studies [55] and also assessed the significance of heritability using a simulation approach consisting of measuring the heritability of random variables using the same population structure and models. ‘pSIM’ values were calculated as the proportion of 1000 simulations yielding lower ΔDIC than each evaluated variable. Due to time and processing constraints, ‘pSIM’ values were only calculated for sulcal measures showing sex differences in variability in our analyses excluding outliers. We used the Benjamini–Hochberg procedure to indicate which results remain significant after adjusting for the false discovery rate (i.e. correct for multiple comparisons; adjusted pSIM values).
(iii). Sex differences in heritability
Sex-specific heritability estimates were calculated for each sulcus as above, using subsets of males or females only. The mean estimates are provided.
(iv). Correlations between sulcal sizes
Correlation analyses were used to assess whether pairs of sulci were correlated, and if there was a difference in the strength of these correlations between males and females. For these analyses only, we limited our sample to individuals that were measured for all 23 sulci (n = 120 (66F/54M)). Here, we present analyses of a subset of these data, which excludes individuals who were outliers for any sulcus or for total folding area (n = 67 (39F/28M)). In line with other work [17,20], Pearson correlation coefficients between every pair of sulci were assessed for males and females separately. The significance of the differences between male and female correlation matrices was assessed by the difference in their Fisher's z-transformed values. p-values were computed by randomly permuting the sex variable among the sulcal measurements 10 000 times, taking the absolute value of the difference between male and female correlation matrices for each permutation, and calculating the proportion of permuted test statistics greater than the absolute value of the observed sex difference in correlation.
3. Results
(a). Sex differences in variability
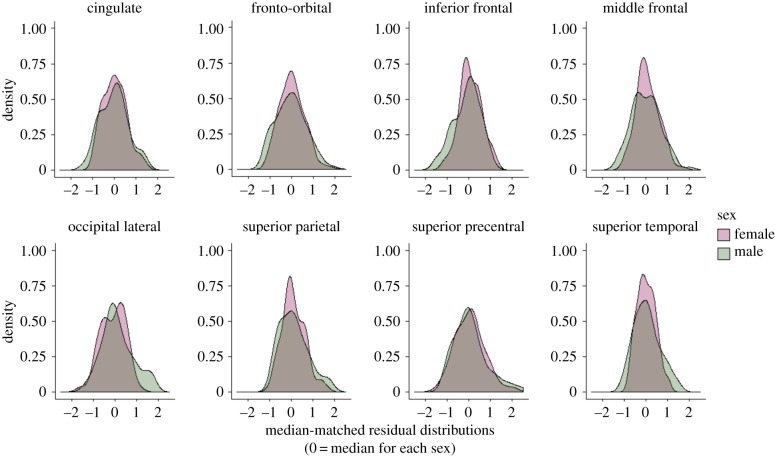
Consistent with Prediction 1, two-sided permutation tests show that males exhibit significantly more variable relative surface areas (SA) at the inferior frontal (pPERM = 0.04), middle frontal (pPERM = 0.03), occipital lateral (pPERM = 0.02), superior parietal (pPERM = 0.04) and superior temporal (pPERM < 0.01) sulci, and more variable absolute SA for the fronto-orbital (pPERM = 0.04), middle frontal (pPERM = 0.03), occipital lateral (pPERM = 0.01), superior parietal (pPERM = 0.02) and superior temporal (pPERM < 0.01) sulci (table 1; electronic supplementary material, table S1; figure 1). This test is significant for the superior temporal sulcus (STS) after adjustment for multiple comparisons (adjusted pPERM < 0.05; table 1; electronic supplementary material, table S1). When evaluated using one-sided tests, a few additional sulci are significantly more variable in males (relative SA: fronto-orbital, superior precentral; absolute SA: cingulate, inferior frontal, superior precentral; figure 1). Males do not exhibit more variability when the middle and superior frontal sulci are combined (electronic supplementary material, table S1; see Material and methods). Females do not exhibit greater variability at any sulcus across all analyses. Results are similar when outliers are included, except that males are more variable at fewer sulci (electronic supplementary material, table S2). Given that measurements are available for more females than males across all sulci, we expect that our estimates of greater male variability are conservative. Resampling procedures confirmed that most distributions approached a horizontal asymptote (electronic supplementary material, figures S1–S48), suggesting our sample sizes are sufficient to capture population-level variation. Coefficient estimates for the covariates included in MCMCglmm models are provided in electronic supplementary material, tables S3 and S4.
Table 1.
Males are more variable in terms of both absolute and relative surface area at multiple cortical sulci. Results for sulci with significant sex differences are shown here (results for all sulci are presented in electronic supplementary material, table S1).
| sulcus | # F | # M | relative surface area |
absolute surface area |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ratioa | pPERMb | pPERM-adjc | heritability | dDICd | pSIMe | pSIM-adjc | M herit | F herit | ratioa | pPERMb | pPERM-adjc | heritability | dDICd | pSIMe | pSIM-adjc | M herit | F herit | |||
| fronto-orbital | 126 | 84 | 0.40 | 0.06 | 0.23 | 0.33 (0.13–0.56) | −19.68 | — | — | 0.38 | 0.44 | 0.40 | 0.04 | 0.21 | 0.33 (0.12−0.56) | −20.80 | 0.09 | 0.09 | 0.41 | 0.44 |
| inferior frontal | 124 | 84 | 0.42 | 0.04 | 0.21 | 0.41 (0.17–0.65) | −38.88 | 0.02 | 0.05 | 0.43 | 0.53 | 0.34 | 0.06 | 0.24 | 0.46 (0.20–0.72) | −52.67 | — | — | 0.45 | 0.54 |
| middle frontalf | 122 | 83 | 0.47 | 0.03 | 0.21 | 0.33 (0.13–0.54) | −19.62 | 0.12 | 0.12 | 0.39 | 0.53 | 0.47 | 0.03 | 0.18 | 0.36 (0.14–0.59) | −25.59 | 0.06 | 0.08 | 0.38 | 0.58 |
| occipital lateral | 117 | 83 | 0.51 | 0.02 | 0.21 | 0.37 (0.14–0.63) | −29.42 | 0.05 | 0.08 | 0.35 | 0.52 | 0.52 | 0.01 | 0.14 | 0.37 (0.13–0.60) | −28.95 | 0.04 | 0.07 | 0.35 | 0.52 |
| superior parietal | 120 | 80 | 0.54 | 0.04 | 0.21 | 0.34 (0.12–0.58) | −24.08 | 0.08 | 0.10 | 0.48 | 0.39 | 0.61 | 0.02 | 0.14 | 0.38 (0.13–0.64) | −30.87 | 0.04 | 0.07 | 0.48 | 0.42 |
| superior temporal | 121 | 83 | 0.76 | <0.01 | 0.01 | 0.58 (0.32–0.84) | −87.79 | <0.01 | <0.01 | 0.54 | 0.36 | 0.70 | <0.01 | 0.02 | 0.48 (0.20–0.77) | −58.24 | <0.01 | 0.02 | 0.51 | 0.61 |
aRatios represent the variance of male residuals divided by the variance of female residuals (positive ratio, greater male variability; negative ratio, greater female variability). Italicized ratios are significantly different between the sexes according to two-sided permutation tests.
bpPERM values were calculated by randomly permuting the sex variable among the residuals 10 000 times and calculating the proportion of permuted test statistics (absolute value) greater than the observed ratio (absolute value). This represents a two-sided permutation test of sex differences in variability. Significant values (p < 0.05) are in bold.
c‘adj’ indicates values adjusted using the Benjamini–Hochberg procedure. Significant values (p < 0.05) are in bold.
dDifference in DIC between models including and excluding the pedigree (negative value indicates that the model including the pedigree has a lower DIC value and therefore provides better model fit).
epSIM values were calculated as the proportion of 1000 simulations yielding a lower ΔDIC than each evaluated variable. Significant values (p < 0.05) are in bold. Dashes indicate instances in which simulations were not run (see Material and methods).
fAs it can be difficult to discern the middle and superior frontal sulci, analyses were repeated with these sulci combined into one measure (mid-superior frontal).
Figure 1.
Males exhibit more variable sulcal surface areas than females. Males exclusively exhibit greater variability than females in the size of multiple cortical sulci, existing more often in both upper and lower extremities of size distributions. Panels show median-matched (0, median for each sex) density plots of male (green) and female (purple) residual distributions from MCMCglmm models of absolute surface areas. Distributions are shown for eight sulci that exhibit significantly greater male variability for this measure when outliers are excluded (in one-sided analyses; males are significantly more variable at five of these sulci in two-sided analyses; see table 1; electronic supplementary material, table S1). (Online version in colour.)
(b). Heritability
Consistent with Prediction 2, DIC indicates that the majority of relative and absolute sulcal measures are heritable (dDIC > 10), with the mean heritability estimates ranging from 0.24 to 0.60 (table 1; electronic supplementary material, tables S1 and S2). DIC simulations indicate that relative SAs are significantly heritable for the inferior frontal (pSIM = 0.02) and superior temporal (pSIM < 0.01) sulci, and absolute SAs are significantly heritable for the occipital lateral (pSIM = 0.04), superior parietal (pSIM = 0.04) and superior temporal (pSIM < 0.1) sulci. Values for the STS remain significant after correcting for multiple comparisons (adjusted pSIM < 0.05).
(c). Sex differences in heritability
Consistent with Prediction 3, males exhibit generally lower heritability estimates than females for both relative and absolute sulcal SAs (table 1; electronic supplementary material, tables S1 and S2). For absolute SAs, males exhibit relatively lower heritability estimates for 14 sulci, while females exhibit relatively lower heritability estimates for 10 sulci. For relative SAs, males exhibit relatively lower heritability estimates for 13 sulci, while females exhibit relatively lower heritability estimates for 11 sulci. Notably, the sulci that are significantly more variable in males also exhibit much lower male heritability estimates, except for the superior parietal sulcus (table 1; electronic supplementary material, tables S1 and S2).
(d). Correlations between sulcal sizes
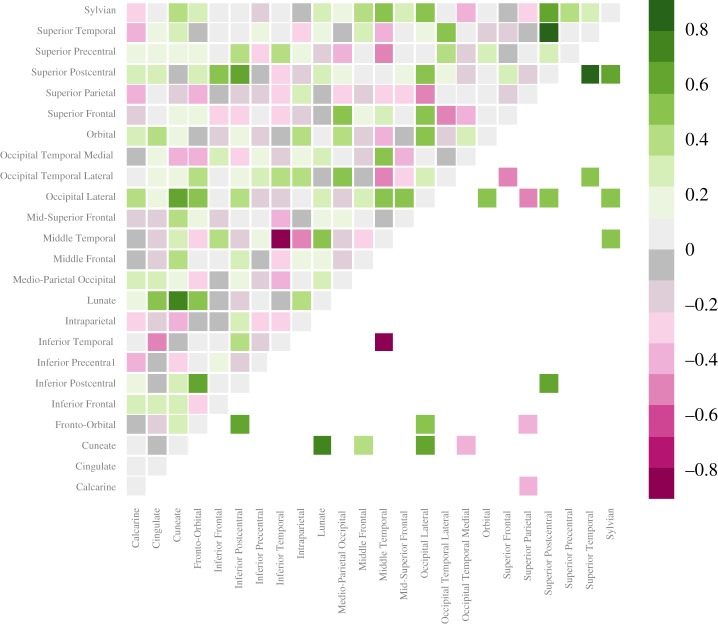
Consistent with Prediction 4, there are more pairs of sulci that exhibit significantly stronger size correlations in male brains than in female brains. Coefficients of correlations between the sizes of pairs of sulci are significantly higher in males for 13 pairs of sulci, while they are significantly higher in females for only six pairs of sulci (figure 2). A similar pattern is found when outliers are included (electronic supplementary material, figure S49).
Figure 2.
There is larger homogeneity across cortical sulci in males (n = 28) and greater diversity in females (n = 39). Anatomical correlation sex difference matrix between all pairs of sulci. Values indicate sex differences in inter-regional correlations (male minus female values). Green colours indicate stronger correlations in males, while purple indicates stronger correlations in females. Darker colours indicate larger sex differences in correlation values. Overall, 19 pairs of sulci show significant sex differences, and these are represented by filled boxes below the diagonal (i.e. unfilled, white boxes represent pairs of sulci that exhibit non-significant sex differences). Of these, males exhibit significantly stronger correlations for 13 pairs, while females exhibit significantly stronger correlations for six pairs. (Online version in colour.)
4. Discussion
To our knowledge, this is the first study to demonstrate greater male neuroanatomical variability in a non-human primate species (in this case one of our closest living relatives, the chimpanzee). Specifically, we find that males exclusively exhibit more variable sulcal surface areas than females (table 1; electronic supplementary material, tables S1 and S2; figure 1). Sulcal measurements were taken consistently across all individuals in this study from MRI data using an automated method; therefore, this intraspecific pattern is not impacted by potential issues surrounding identification of homologous sulci across species. Our results suggest that sex differences in variability may reflect a combination of sexual selection, sex-specific developmental schedules and/or sex chromosome effects. In particular, most of these measures are heritable, so they can be subject to evolutionary selection (table 1; electronic supplementary material, tables S1 and S2). Interestingly, the specific regions that exhibit more variability in males may be associated with cognitive abilities that may facilitate inter- and intrasexual selection. We also find that males exhibit generally lower heritability values than females, suggesting that relatively more male phenotypic variation is explained by the environment (table 1; electronic supplementary material, tables S1 and S2). Finally, males generally exhibit greater correlations than females between sulci, providing indirect support for sex chromosome effects (figure 2; electronic supplementary material, figure S49). Together, these results provide evidence that greater male neuroanatomical variability extends beyond humans and suggest that both developmental and adaptive explanations for this phenomenon may be relevant.
Greater neuroanatomical variability among male chimpanzees may reflect selection, since most of the relevant sulcal measurements are heritable (table 1; electronic supplementary material, tables S1 and S2). If sulcal surface areas represent investment in the size of related cortical areas, and therefore certain cognitive abilities, disruptive/balancing selection on male behaviour may lead males to vary more than females in the size of related sulci. It seems likely that male chimpanzee reproductive tactics have been subject to disruptive/balancing selection, allowing a variety of male behaviours to increase reproductive success and resulting in the production and maintenance of multiple behavioural phenotypes [52]. This may be reflected in our results, since the sulci that are more variable in male chimpanzees may be involved in cognitive abilities that are associated with direct intrasexual competition (i.e. combat), possessive mate guarding and/or consortships.
For example, the STS is involved in social information processing in humans and other primates [72,73]. Interspecific differences in STS morphology are suggested to reflect socio-cognitive differences [74,75], and experimental studies suggest that living in larger groups increases grey matter (GM) in the primate mid-STS [76]. In addition, the orbitofrontal cortex is activated during face-matching tasks in chimpanzees [73], the lateral occipital cortex is activated by faces and other objects in humans and chimpanzees [77,78] and the superior parietal sulcus is associated with visuomotor attention in humans [79,80]. While males using different mating strategies are likely to differ in their socio-cognitive and combat abilities, females may not be expected to show as much variation. Accordingly, greater male variability at the superior temporal, fronto-orbital, occipital lateral and superior parietal sulci may reflect disruptive selection on male social and visual information processing skills, which may be under stabilizing selection in females.
Similarly, different mating strategies may place varying demands on male motor and inhibitory control, since successful male–male combat necessitates movement regulation and subordinate males often need to prevent themselves from feeding and mating in the presence of dominant individuals [81]. By contrast, we may not expect these skills to vary as much among females. Asymmetry of the fronto-orbital sulcus and inferior frontal gyrus predicts handedness for tool use and gesturing, respectively, in chimpanzees [82,83]. Male chimpanzees exhibit more variable asymmetry measures for these regions [83] and tool use performance [65]. Accordingly, greater male variability at the fronto-orbital and inferior frontal sulci may reflect disruptive selection on male motor control. Additionally, the inferior frontal cortex, middle frontal gyrus and superior parietal sulcus facilitate behavioural inhibition [84–86], so greater male variability at the inferior frontal, middle frontal and superior parietal sulci may reflect disruptive selection on male inhibitory control.
In addition to the effects of selection, male chimpanzees may exhibit more neuroanatomical variation since they have longer developmental periods [87]. While female growth slows at around 10 years old, male growth continues until 13 years old [87]. Furthermore, female chimpanzees wean their daughters earlier than their sons [88,89]. This leaves males exposed to additional environmental effects that may influence condition-dependent traits, such as cognition (e.g. [51]). Notably, maternal glucocorticoid concentrations during gestation impact the HPA axis function of male offspring more than that female offspring [90], and males exhibit higher variability for other traits that are likely to be influenced by condition (e.g. body size [87]).
Finally, sex chromosomes may contribute to greater male neuroanatomical variability. In particular, we find that there are more pairs of sulci that exhibit significantly greater size correlations in male brains than in female brains (figure 2; electronic supplementary material, figure S49), in accordance with studies of humans [17,20]. This may reflect that males exhibit consistent X chromosome gene expression across tissues, which is expected to produce both higher inter-regional correlations and greater population variability among males [10,24]. Other studies have also provided indirect evidence for sex chromosome effects on sex differences in trait variability, since in species with homogametic (e.g. ZZ) males, females typically have higher phenotypic variability [8].
Sex differences in morphological and behavioural variability have been identified in numerous taxa, and such differences are likely attributable to developmental effects and/or sexual selection mechanisms that tend to produce and maintain variation. Here, we show that greater male neuroanatomical variability is not only present in humans, but also one of our closest living relatives, chimpanzees. In particular, balancing or disruptive selection on alternative reproductive tactics may maintain cognitive, and therefore neuroanatomical, variation among males, extended developmental periods may leave males more vulnerable to environmental effects, and sex chromosome effects may explain greater inter-regional neuroanatomical correlations among males. Given that the sulcal measurements used here are derived from MRI scans of captive living individuals, the males in our study may not participate in all of the reproductive strategies available to wild chimpanzees; however, our results indicate that this is not necessary to maintain greater male neuroanatomical variability, as this pattern is still evident in the less variable conditions of captivity. Additionally, we acknowledge that some of our results are sensitive to multiple corrections testing; however, we believe this may reflect a lower sample size (due to data availability) compared to human studies, which typically include thousands to tens of thousands of individuals [17–20]. Using this work, future comparative studies on taxa with varying sex chromosome compositions, developmental schedules and mating systems have the potential to tease apart the primary drivers of sex differences in neuroanatomical variability. Altogether, this line of inquiry will contribute to our understanding of intraspecific variation in neuroanatomy and behaviour.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Dr Bill Hopkins for providing access to this dataset, and are grateful to everyone who assisted in its assembly.
Data accessibility
All data used in the analyses presented in this manuscript are provided in the electronic supplementary material.
Authors' contributions
A.R.D. designed the project and performed the analyses with input from J.P.H. S.J.S. assisted in data collection. A.R.D. and J.P.H. wrote the manuscript with input from C.C.S. and S.J.S.
Competing interests
We declare we have no competing interests.
Funding
This work is supported by the NSF Graduate Research Fellowship (grant no. DGE1342536), the NSF Doctoral Dissertation Research Improvement Grant (grant no. BSC1752393) and the National Chimpanzee Brain Resource (supported by NIH grant no. NS092988). The NCCC chimpanzees are supported by NIH Cooperative Agreement U42 OD-011197.
References
- 1.Pomiankowski A, Moller AP. 1995. A resolution of the lek paradox. Proc. R. Soc. B 260, 21–29. ( 10.1098/rspb.1995.0054) [DOI] [Google Scholar]
- 2.Ellis H. 1894. Man and woman. London, UK: Walter Scott. [Google Scholar]
- 3.Wright S. 1939. The distribution of self-sterility alleles in populations. Genetics 24, 538–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jennions MD, Moller AP, Petrie M. 2001. Sexually selected traits and adult survival: a meta-analysis. Q. Rev. Biol. 76, 3–36. ( 10.1086/393743) [DOI] [PubMed] [Google Scholar]
- 5.Cotton S, Fowler K, Pomiankowski A. 2004. Condition dependence of sexual ornament size and variation in the stalk-eyed fly Cyrtodiopsis dalmanni (Diptera: Diopsidae). Evolution 58, 1038–1046. ( 10.1111/j.0014-3820.2004.tb00437.x) [DOI] [PubMed] [Google Scholar]
- 6.James JW. 1973. Covariances between relatives due to sex-linked genes. Biometrics 29, 584–588. ( 10.2307/2529178) [DOI] [PubMed] [Google Scholar]
- 7.Crowley DE, Atchley WR, Rutledge JJ. 1986. Quantitative genetics of Drosophilia melanogaster. I. Sexual dimorphism in genetic parameters of wing traits. Genetics 114, 549–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinhold K, Engqvist L. 2013. The variability is in the sex chromosomes. Evolution 67, 3662–3668. ( 10.1111/evo.12224) [DOI] [PubMed] [Google Scholar]
- 9.Lehre AC, Lehre KP, Laake P, Danbolt NC. 2009. Greater intrasex phenotype variability in males than in females is a fundamental aspect of the gender differences in humans. Dev. Psychobiol. 51, 198–206. ( 10.1002/dev.20358) [DOI] [PubMed] [Google Scholar]
- 10.Johnson W, Carothers A, Deary IJ. 2009. A role for the X chromosome in sex differences in variability in general intelligence? Perspect. Psychol. Sci. 4, 598–611. ( 10.1111/j.1745-6924.2009.01168.x) [DOI] [PubMed] [Google Scholar]
- 11.Feingold A. 1992. Sex differences in variability in intellectual abilities: a new look at an old controversy. Rev. Educ. Res. 62, 61–84. ( 10.3102/00346543062001061) [DOI] [Google Scholar]
- 12.Karwowski M, Jankowska DM, Gralewski J, Gajda A, Wiśniewska E, Lebuda I. 2016. Greater male variability in creativity: a latent variables approach. Think. Skills Creativity 22, 159–166. ( 10.1016/j.tsc.2016.10.005) [DOI] [Google Scholar]
- 13.Else-Quest NM, Hyde JS, Goldsmith HH, Van Hulle CA. 2006. Gender differences in temperament: a meta-analysis. Psychol. Bull. 132, 33 ( 10.1037/0033-2909.132.1.33) [DOI] [PubMed] [Google Scholar]
- 14.Borkenau P, McCrae RR, Terracciano A. 2013. Do men vary more than women in personality? A study in 51 cultures. J. Res. Pers. 47, 135–144. ( 10.1016/j.jrp.2012.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maccoby EE, Jacklin CN. 1974. The psychology of sex differences. Stanford, CA: Stanford University Press. [Google Scholar]
- 16.Lange N, Giedd JN, Castellanos FX, Vaituzis AC, Rapoport JL. 1997. Variability of human brain structure size: ages 4–20 years. Psychiatry Res. 74, 1–12. ( 10.1016/S0925-4927(96)03054-5) [DOI] [PubMed] [Google Scholar]
- 17.Wierenga LM, Sexton JA, Laake P, Giedd JN, Tamnes CK, Pediatric Imaging, Neurocognition, and Genetics Study. 2017. A key characteristic of sex differences in the developing brain: greater variability in brain structure of boys than girls. Cereb. Cortex 28, 2741–2751. ( 10.1093/cercor/bhx154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ritchie SJ, et al. 2018. Sex differences in the adult human brain: evidence from 5216 UK biobank participants. Cereb. Cortex 28, 2959–2975. ( 10.1093/cercor/bhy109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forde NJ, Jeyachandra J, Joseph M, Jacobs GR, Dickie EW, Satterthwaite TD, Shinohara RT, Voineskos AN. 2019. Sex differences in variability of brain structure across the lifespan. bioRxiv, 842567 ( 10.1101/842567) [DOI] [PMC free article] [PubMed]
- 20.Wierenga LM, et al. 2020. Greater male than female variability in regional brain structure across the lifespan. bioRxiv.
- 21.Shi L, Zhang Z, Su B. 2016. Sex biased gene expression profiling of human brains at major developmental stages. Sci. Rep. 6, 21181 ( 10.1038/srep21181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollingworth LS. 1913. The frequency of amentia as related to sex. Med. Rec. 84, 753–756. [Google Scholar]
- 23.Geary DC. 1998. Male, female: the evolution of human sex differences. Washington, DC: American Psychological Association. [Google Scholar]
- 24.Lehrke R. 1972. A theory of X-linkage of major intellectual traits. Am. J. Ment. Defic. 76, 611–619. [PubMed] [Google Scholar]
- 25.Møller AP. 2010. Brain size, head size and behaviour of a passerine bird. J. Evol. Biol. 23, 625–635. ( 10.1111/j.1420-9101.2009.01928.x) [DOI] [PubMed] [Google Scholar]
- 26.Jenkins PD, Albrecht GH. 1991. Sexual dimorphism and sex ratios in Madagascan prosimians. Am. J. Primatol. 24, 1–14. ( 10.1002/ajp.1350240102) [DOI] [PubMed] [Google Scholar]
- 27.Juraska JM. 1984. Sex differences in dendritic response to differential experience in the rat visual cortex. Brain Res. 295, 27–34. ( 10.1016/0006-8993(84)90812-6) [DOI] [PubMed] [Google Scholar]
- 28.Atkinson EG, Rogers J, Mahaney MC, Cox LA, Cheverud JM. 2015. Cortical folding of the primate brain: an interdisciplinary examination of the genetic architecture, modularity, and evolvability of a significant neurological trait in pedigreed baboons (genus Papio). Genetics 200, 651–665. ( 10.1534/genetics.114.173443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mota B, Herculano-Houzel S. 2015. Cortical folding scales universally with surface area and thickness, not number of neurons. Science 349, 74–77. ( 10.1126/science.aaa9101) [DOI] [PubMed] [Google Scholar]
- 30.Van Essen DC. 1997. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 385, 313 ( 10.1038/385313a0) [DOI] [PubMed] [Google Scholar]
- 31.Vogt BA, Vogt L, Farber NB, Bush G. 2005. Architecture and neurocytology of monkey cingulate gyrus. J. Comp. Neurol. 485, 218–239. ( 10.1002/cne.20512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hopkins WD, et al. 2014. Evolution of the central sulcus morphology in primates. Brain Behav. Evol. 84, 19–30. ( 10.1159/000362431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zilles K, et al. 1997. Quantitative analysis of sulci in the human cerebral cortex: development, regional heterogeneity, gender difference, asymmetry, intersubject variability and cortical architecture. Hum. Brain Mapp. 5, 218–221. () [DOI] [PubMed] [Google Scholar]
- 34.Fischl B, Rajendran N, Busa E, Augustinack J, Hinds O, Yeo BT, Mohlberg H, Amunts K, Zilles K. 2008. Cortical folding patterns and predicting cytoarchitecture. Cereb. Cortex 18, 1973–1980. ( 10.1093/cercor/bhm225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiner KS, Zilles K. 2016. The anatomical and functional specialization of the fusiform gyrus. Neuropsychologia 83, 48–62. ( 10.1016/j.neuropsychologia.2015.06.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roland PE, Geyer S, Amunts K, Schormann T, Schleicher A, Malikovic A, Zilles K. 1997. Cytoarchitectural maps of the human brain in standard anatomical space. Hum. Brain Mapp. 5, 222–227. () [DOI] [PubMed] [Google Scholar]
- 37.Elmer S, Hänggi J, Meyer M, Jäncke L. 2013. Increased cortical surface area of the left planum temporale in musicians facilitates the categorization of phonetic and temporal speech sounds. Cortex 49, 2812–2821. ( 10.1016/j.cortex.2013.03.007) [DOI] [PubMed] [Google Scholar]
- 38.Chiarello C, Vazquez D, Felton A, McDowell A. 2016. Structural asymmetry of the human cerebral cortex: regional and between-subject variability of surface area, cortical thickness, and local gyrification. Neuropsychologia 93, 365–379. ( 10.1016/j.neuropsychologia.2016.01.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hopkins WD, Cantalupo C, Taglialatela J. 2006. Handedness is associated with asymmetries in gyrification of the cerebral cortex of chimpanzees. Cereb. Cortex 17, 1750–1756. ( 10.1093/cercor/bhl085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips KA, Sherwood CC. 2005. Primary motor cortex asymmetry is correlated with handedness in capuchin monkeys (Cebus apella). Behav. Neurosci. 119, 1701 ( 10.1037/0735-7044.119.6.1701) [DOI] [PubMed] [Google Scholar]
- 41.Nordahl CW, Dierker D, Mostafavi I, Schumann CM, Rivera SM, Amaral DG, Van Essen DC. 2007. Cortical folding abnormalities in autism revealed by surface-based morphometry. J. Neurosci. 27, 11 725–11 735. ( 10.1523/JNEUROSCI.0777-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garrison JR, Fernyhough C, McCarthy-Jones S, Haggard M, Bank TASR, JS Simons. 2015. Paracingulate sulcus morphology is associated with hallucinations in the human brain. Nat. Commun. 6, 8956 ( 10.1038/ncomms9956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kersbergen KJ, et al. 2016. Relation between clinical risk factors, early cortical changes, and neurodevelopmental outcome in preterm infants. Neuroimage 142, 301–310. ( 10.1016/j.neuroimage.2016.07.010) [DOI] [PubMed] [Google Scholar]
- 44.Imai N, Sawada K, Fukunishi K, Sakata-Haga H, Fukui Y. 2011. Sexual dimorphism of sulcal length asymmetry in the cerebrum of adult cynomolgus monkeys (Macaca fascicularis). Congenit. Anom. 51, 161–166. ( 10.1111/j.1741-4520.2011.00330.x) [DOI] [PubMed] [Google Scholar]
- 45.Rogers J, et al. 2010. On the genetic architecture of cortical folding and brain volume in primates. Neuroimage 53, 1103–1108. ( 10.1016/j.neuroimage.2010.02.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sawada K, Horiuchi-Hirose M, Saito S, Aoki I. 2015. Male prevalent enhancement of leftward asymmetric development of the cerebellar cortex in ferrets (Mustela putorius). Laterality 20, 723–737. ( 10.1080/1357650X.2015.1047379) [DOI] [PubMed] [Google Scholar]
- 47.Plavcan JM. 2012. Sexual size dimorphism, canine dimorphism, and male–male competition in primates. Hum. Nat. 23, 45–67. ( 10.1007/s12110-012-9130-3) [DOI] [PubMed] [Google Scholar]
- 48.Harcourt AH, Harvey PH, Larson SG, Short RV. 1981. Testis weight, body weight and breeding system in primates. Nature 293, 55 ( 10.1038/293055a0) [DOI] [PubMed] [Google Scholar]
- 49.Nunn CL. 1999. The evolution of exaggerated sexual swellings in primates and the graded-signal hypothesis. Anim. Behav. 58, 229–246. ( 10.1006/anbe.1999.1159) [DOI] [PubMed] [Google Scholar]
- 50.Leigh SR, Shea BT. 1995. Ontogeny and the evolution of adult body size dimorphism in apes. Am. J. Primatol. 36, 37–60. ( 10.1002/ajp.1350360104) [DOI] [PubMed] [Google Scholar]
- 51.Pravosudov VV, Lavenex P, Omanska A. 2005. Nutritional deficits during early development affect hippocampal structure and spatial memory later in life. Behav. Neurosci. 119, 1368 ( 10.1037/0735-7044.119.5.1368) [DOI] [PubMed] [Google Scholar]
- 52.Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE. 2009. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Anim. Behav. 77, 873–885. ( 10.1016/j.anbehav.2008.12.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dijkstra PD, Border SE. 2018. How does male–male competition generate negative frequency-dependent selection and disruptive selection during speciation? Curr. Zool. 64, 89–99. ( 10.1093/cz/zox079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raznahan A, et al. 2010. Cortical anatomy in human X monosomy. Neuroimage 49, 2915–2923. ( 10.1016/j.neuroimage.2009.11.057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gómez-Robles A, Hopkins WD, Schapiro SJ, Sherwood CC. 2016. The heritability of chimpanzee and human brain asymmetry. Proc. R. Soc. B 283, 20161319 ( 10.1098/rspb.2016.1319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gómez-Robles A, Hopkins WD, Schapiro SJ, Sherwood CC. 2015. Relaxed genetic control of cortical organization in human brains compared with chimpanzees. Proc. Natl Acad. Sci. USA 112, 14 799–14 804. ( 10.1073/pnas.1512646112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hopkins WD, Latzman RD, Mareno MC, Schapiro SJ, Gómez-Robles A, Sherwood CC. 2018. Heritability of gray matter structural covariation and tool use skills in chimpanzees (Pan troglodytes): a source-based morphometry and quantitative genetic analysis. Cereb. Cortex 29, 3702–3711. ( 10.1093/cercor/bhy250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hopkins WD. 1999. Heritability of hand preference in chimpanzees (Pan troglodytes): evidence from a partial interspecies cross-fostering study. J. Comp. Psychol. 113, 307 ( 10.1037/0735-7036.113.3.307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hopkins WD, Russell JL, Schaeffer J. 2014. Chimpanzee intelligence is heritable. Curr. Biol. 24, 1649–1652. ( 10.1016/j.cub.2014.05.076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hopkins WD, Russell JL, Schaeffer JA, Gardner M, Schapiro SJ. 2009. Handedness for tool use in captive chimpanzees (Pan troglodytes): sex differences, performance, heritability and comparison to the wild. Behaviour 146, 1463–1483. ( 10.1163/156853909X441005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weiss A, King JE, Enns RM. 2002. Subjective well-being is heritable and genetically correlated with dominance in chimpanzees (Pan troglodytes). J. Pers. Soc. Psychol. 83, 1141 ( 10.1037/0022-3514.83.5.1141) [DOI] [PubMed] [Google Scholar]
- 62.Glasel H, Leroy F, Dubois J, Hertz-Pannier L, Mangin JF, Dehaene-Lambertz G. 2011. A robust cerebral asymmetry in the infant brain: the rightward superior temporal sulcus. Neuroimage 58, 716–723. ( 10.1016/j.neuroimage.2011.06.016) [DOI] [PubMed] [Google Scholar]
- 63.Mangin JF, Poupon F, Duchesnay É, Rivière D, Cachia A, Collins DL, Evans AC, Régis J. 2004. Brain morphometry using 3D moment invariants. Med. Image Anal. 8, 187–196. ( 10.1016/j.media.2004.06.016) [DOI] [PubMed] [Google Scholar]
- 64.R Core Team. 2017. R: a language and environment for statistical computing (version 3.4. 2) [computer software] Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 65.Hubert M, Vandervieren E. 2008. An adjusted boxplot for skewed distributions. Comput. Stat. Data Anal. 52, 5186–5201. ( 10.1016/j.csda.2007.11.008) [DOI] [Google Scholar]
- 66.Maechler M, Rousseeuw P, Croux C, Todorov V, Ruckstuhl A, Salibian-Barrera M, Verbeke T, Koller M, Conceicao ELT, di Palma MA. 2018. Package ‘robustbase’.
- 67.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.18637/jss.v033.i02)20808728 [DOI] [Google Scholar]
- 68.Fish AM, et al. 2017. Influences of brain size, sex, and sex chromosome complement on the architecture of human cortical folding. Cereb. Cortex 27, 5557–5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Plummer M, Best N, Cowles K, Vines K. 2006. CODA: convergence diagnosis and output analysis for MCMC. R News 6, 7–11. [Google Scholar]
- 70.Gelman A, Rubin DB. 1992. Inference from iterative simulation using multiple sequences. Stat. Sci. 7, 457–472. ( 10.1214/ss/1177011136) [DOI] [Google Scholar]
- 71.Barnett AG, Koper N, Dobson AJ, Schmiegelow F, Manseau M. 2010. Using information criteria to select the correct variance–covariance structure for longitudinal data in ecology. Methods Ecol. Evol. 1, 15–24. ( 10.1111/j.2041-210X.2009.00009.x) [DOI] [Google Scholar]
- 72.Ghazanfar AA, Chandrasekaran C, Logothetis NK. 2008. Interactions between the superior temporal sulcus and auditory cortex mediate dynamic face/voice integration in rhesus monkeys. J. Neurosci. 28, 4457–4469. ( 10.1523/JNEUROSCI.0541-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parr LA, Hecht E, Barks SK, Preuss TM, Votaw JR. 2009. Face processing in the chimpanzee brain. Curr. Biol. 19, 50–53. ( 10.1016/j.cub.2008.11.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leroy F, et al. 2015. New human-specific brain landmark: the depth asymmetry of superior temporal sulcus. Proc. Natl Acad. Sci. USA 112, 1208–1213. ( 10.1073/pnas.1412389112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rilling JK, Scholz J, Preuss TM, Glasser MF, Errangi BK, Behrens TE. 2012. Differences between chimpanzees and bonobos in neural systems supporting social cognition. Soc. Cogn. Affect. Neurosci. 7, 369–379. ( 10.1093/scan/nsr017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sallet J, et al. 2011. Social network size affects neural circuits in macaques. Science 334, 697–700. ( 10.1126/science.1210027) [DOI] [PubMed] [Google Scholar]
- 77.Grill-Spector K, Kourtzi Z, Kanwisher N. 2001. The lateral occipital complex and its role in object recognition. Vision Res. 41, 1409–1422. ( 10.1016/S0042-6989(01)00073-6) [DOI] [PubMed] [Google Scholar]
- 78.Fukushima H, et al. 2010. Neural correlates of face and object perception in an awake chimpanzee (Pan troglodytes) examined by scalp-surface event-related potentials. PLoS ONE 5, e13366 ( 10.1371/journal.pone.0013366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jackowski AP, Rando K, de Araújo CM, Del Cole CG, Silva I, de Lacerda ALT. 2009. Brain abnormalities in Williams syndrome: a review of structural and functional magnetic resonance imaging findings. Eur. J. Paediatr. Neurol. 13, 305–316. ( 10.1016/j.ejpn.2008.07.002) [DOI] [PubMed] [Google Scholar]
- 80.Vaidya CJ, Foss-Feig J, Shook D, Kaplan L, Kenworthy L, Gaillard WD. 2011. Controlling attention to gaze and arrows in childhood: an fMRI study of typical development and autism spectrum disorders. Dev. Sci. 14, 911–924. ( 10.1111/j.1467-7687.2011.01041.x) [DOI] [PubMed] [Google Scholar]
- 81.Hare B, Call J, Tomasello M. 2001. Do chimpanzees know what conspecifics know? Anim. Behav. 61, 139–151. ( 10.1006/anbe.2000.1518) [DOI] [PubMed] [Google Scholar]
- 82.Schenker NM, Hopkins WD, Spocter MA, Garrison AR, Stimpson CD, Erwin JM, Hof PR, Sherwood CC. 2009. Broca's area homologue in chimpanzees (Pan troglodytes): probabilistic mapping, asymmetry, and comparison to humans. Cereb. Cortex 20, 730–742. ( 10.1093/cercor/bhp138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hopkins WD, Russell JL, Cantalupo C. 2007. Neuroanatomical correlates of handedness for tool use in chimpanzees (Pan troglodytes) implication for theories on the evolution of language. Psychol. Sci. 18, 971–977. ( 10.1111/j.1467-9280.2007.02011.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aron AR, Robbins TW, Poldrack RA. 2014. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn. Sci. 18, 177–185. ( 10.1016/j.tics.2013.12.003) [DOI] [PubMed] [Google Scholar]
- 85.Japee S, Holiday K, Satyshur MD, Mukai I, Ungerleider LG. 2015. A role of right middle frontal gyrus in reorienting of attention: a case study. Front. Syst. Neurosci. 9, 23 ( 10.3389/fnsys.2015.00023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vaidya CJ, Bunge SA, Dudukovic NM, Zalecki CA, Elliott GR, Gabrieli JD. 2005. Altered neural substrates of cognitive control in childhood ADHD: evidence from functional magnetic resonance imaging. Am. J. Psychiatry 162, 1605–1613. ( 10.1176/appi.ajp.162.9.1605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pusey AE, Oehlert GW, Williams JM, Goodall J. 2005. Influence of ecological and social factors on body mass of wild chimpanzees. Int. J. Primatol. 26, 3–31. ( 10.1007/s10764-005-0721-2) [DOI] [Google Scholar]
- 88.Fahy GE, Richards MP, Fuller BT, Deschner T, Hublin JJ, Boesch C. 2014. Stable nitrogen isotope analysis of dentine serial sections elucidate sex differences in weaning patterns of wild chimpanzees (Pan troglodytes). Am. J. Phys. Anthropol. 153, 635–642. ( 10.1002/ajpa.22464) [DOI] [PubMed] [Google Scholar]
- 89.Boesch C. 1997. Evidence for dominant wild female chimpanzees investing more in sons. Anim. Behav. 54, 811–815. ( 10.1006/anbe.1996.0510) [DOI] [PubMed] [Google Scholar]
- 90.Murray CM, Stanton MA, Wellens KR, Santymire RM, Heintz MR, Lonsdorf EV. 2018. Maternal effects on offspring stress physiology in wild chimpanzees. Am. J. Primatol. 80, e22525 ( 10.1002/ajp.22525) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in the analyses presented in this manuscript are provided in the electronic supplementary material.