Abstract
While non-human primate studies have long been conducted in laboratories, and more recently at zoological parks, sanctuaries are increasingly considered a viable setting for research. Accredited sanctuaries in non-range countries house thousands of primates formerly used as subjects of medical research, trained performers or personal pets. In range countries, however, sanctuaries typically house orphaned primates confiscated from illegal poaching and the bushmeat and pet trafficking trades. Although the primary mission of these sanctuaries is to rescue and rehabilitate residents, many of these organizations are increasingly willing to participate in non-invasive research. Notably, from a scientific standpoint, most sanctuaries provide potential advantages over traditional settings, such as large, naturalistic physical and social environments which may result in more relevant models of primates' free-ranging wild counterparts than other captive settings. As a result, an impressive scope of research in the fields of primate behaviour, cognition, veterinary science, genetics and physiology have been studied in sanctuaries. In this review, we examine the range and form of research that has been conducted at accredited sanctuaries around the world. We also describe the potential challenges of sanctuary-based work and the considerations that external researchers may face when deciding to collaborate with primate sanctuaries on their research projects.
Keywords: primate, sanctuary, research, chimpanzee, review
1. Introduction
For most of the past century, the vast majority of captive non-human primate (hereafter simply ‘primate’) research was concentrated in laboratory settings where academic and human health advancement was effectively the core impetus for housing those species in captivity. In 2010, United States research facilities were home to over 70 000 primates used in biomedical research [1], while even more were subjects of behavioural and cognitive studies in universities and other academic centres. But, increasingly over the past 40 years, the sites of captive primate research have begun to diversify. For instance, research in zoo settings continues to rise, and primates are often the most-studied taxa among the many species housed in these settings [2,3]. Hopper [4] provided a review of zoo-based cognitive research, finding the same high prevalence of primate-focused studies. In these settings, research typically falls alongside other institutional mission-based priorities such as education, leisure and conservation. As a result, researchers are just one group of stakeholders among many in zoos.
While primates have been housed in laboratories and zoos for over a century in many parts of the world, a more recent housing category is sanctuaries, where primates are rehomed from situations where they are orphaned or unable to survive on their own, no longer needed, wanted, or from which they require protection. In general, these organizations have categorically prioritized the care and wellbeing of resident primates above other potential interests, including research. Indeed, over a decade ago, Brent [5] stated ‘the scientific study of non-human primates in sanctuaries in the United States is virtually non-existent,' but nonetheless had the foresight to advocate for the growth in this area that has transpired recently. Brent [5] recognized the potential value that non-invasive studies could bring in terms of improving the care and management of sanctuary-housed primates, and advocated for collaborations between researchers and sanctuary staff. Today, many primate sanctuaries around the world are increasingly participating in non-invasive research [6], which contributes to new understanding about these animals, while preserving their commitment to the wellbeing of these residents.
In this review paper, we will examine the range of peer-reviewed research that has been conducted in primate sanctuary settings or with biosamples taken from sanctuary-housed primates. As with any review, we begin by deliberately drawing the boundaries of the scope of our search, which begins with consideration of what constitutes a sanctuary. Like the word zoo, the term sanctuary likely evokes a particular reaction from people; however, in practice, these organizations can vary broadly in mission, resources, sustainability and effectiveness. Here, we restrict our review, with only a few exceptions, to publications from captive research conducted in accredited sanctuary organizations: those that have met some particular set of guidelines for administrative functioning and are part of a community of like-minded organizations. There are several accrediting bodies for sanctuaries worldwide, including the Pan African Sanctuary Alliance (PASA) for African primate sanctuaries and the Global Federation of Animal Sanctuaries (GFAS) which accredits sanctuaries for a range of taxa around the world. In rarer circumstances, some sanctuaries are accredited by bodies typically associated with zoos, such as the Association of Zoos and Aquariums (AZA) in North America. The primary exceptions we made to the accreditation restriction are for organizations that broadly meet the criteria of sanctuaries, including a lack of breeding and a focus on rehoming primates in need (for example, Kumamoto Sanctuary in Japan and Fundación Mona in Spain).
The result of our selection criteria means that a comparatively small number of publications from reputable sanctuary organizations are not reported in this review. We chose not to include organizations such as Duke Lemur Center in this review as its primary mission is focused much more so on research than providing sanctuary for primates in need of rehoming. Similarly, we excluded sanctuaries at which most primates were only temporarily housed prior to being reintroduced back to the wild. For instance, the Orangutan Care Center and Quarantine (OCCQ) is excluded from this review for those reasons, despite past involvement in a variety of research initiatives. While these selection criteria exclude a small proportion of sanctuaries engaged in captive primate research, they do provide both a means by which to consistently frame the scope of research we review and ensure a high level of care is provided to the resident primates under study. Those organizations that do meet our criteria tend to be largely skewed towards research with great apes, in particular chimpanzees (Pan troglodytes), which to some degree also reflects the species distribution of sanctuary residents themselves. Specific interest in Pan spp. is not unlike that seen in other research settings and may be perpetuated by the fact that a robust set of scientific background information is readily available to support future studies [3].
The vast majority of captive primate research publications coming from sanctuary-based work are either from western-based scientists working in African sanctuaries or from a small handful of sanctuaries with heavy investment in behavioural sciences (e.g. Kumamoto Sanctuary and Fundación Mona). This uneven distribution could be influenced by the predetermined scope of our literature search (focusing only on captive research from sanctuaries that have attained accreditation status) or from our methodology (it is possible that there are non-English language reports that were not counted). A more parsimonious explanation is that our findings reflect the skewed distribution of current research interests and capacity in the sanctuary world. As most sanctuaries around the world are focused almost entirely on maintaining the rescue and rehabilitation operations of their organization, any potential investment in research activities may be seen as competing with those aims. While we describe several advantages to sanctuary research, we also acknowledge the historical, cultural and logistical barriers to these organizations reaching their research potential.
(a). Consideration of sanctuary-based research
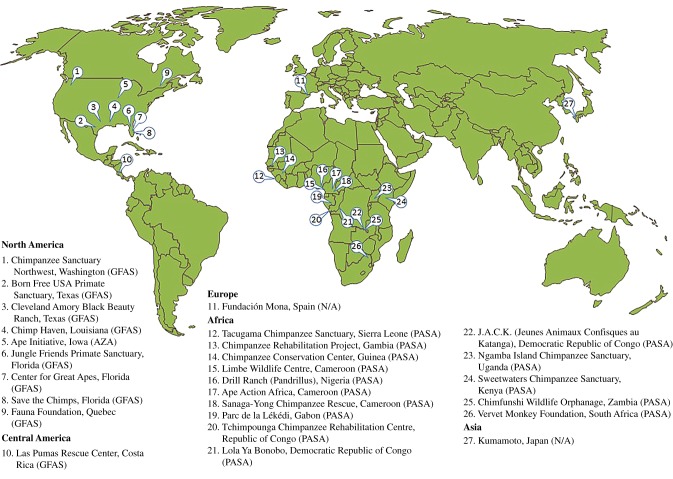
Accredited primate sanctuaries exist around the world (figure 1), though there are important regional differences in terms of the organizational missions and how research is conducted therein. Range country primate sanctuaries in Africa, Asia and South and Central America are often in place to receive formerly wild primates displaced by illegal human activities, such as poaching, the bushmeat trade, pet trade trafficking, and via confiscation by a third party. Conversely, non-range country sanctuaries, located in North America and Europe, primarily receive primates who are voluntarily provided or legally facilitated from laboratories, owned as personal pets, or used in entertainment. For the purposes of this review, we, therefore, consider Kumamoto Sanctuary in Japan (a primate range country) as categorized with non-range country organizations because of the nature of their population.
Figure 1.
Locations and primary accreditors of the sanctuaries where research described in this review took place. AZA, Association of Zoos and Aquariums; GFAS, Global Federation of Animal Sanctuaries; PASA, Pan African Sanctuary Alliance.
(b). Housing and types of research
As we have described, the degree to which research opportunities compete alongside other aspects of an organization's mission varies both between and within housing categories. While animal care is an important aspect of research centres, the fundamental purpose of the primates living there is to participate in scientific studies. Accordingly, in laboratories, we see a greater range of studies that include not only non-invasive behavioural investigations but also invasive biomedical studies (those that may include non-care-related insertions, injections or incisions) that use primates as models for human health conditions. Indeed, in a review of primate research published in 2001, Carlsson et al. [7] found that the most common areas of research were those in which primates were used as models for human conditions, as well as neuroscience and biochemistry research, concluding that 14% of primate studies could be classified as non-invasive. Research based at zoological parks also varies considerably, though it is very rarely considered invasive in nature and generally focuses on determining positive applications for animals rather than humans. Stoinski et al. [2] surveyed AZA-accredited zoos and reported that the most commonly studied topics included reproduction, behaviour, physiology, conservation, husbandry, cognition, pathology, genetics and animal nutrition.
Primate sanctuaries are typically the most restrictive in terms of allowable research. For instance, the Global Federation of Animal Sanctuaries' Operations Standards [8] only allows research that does not negatively impact animal welfare, interfere with normal daily activities, or cause pain or distress (terms that are left undefined in this context). However, they also point out that exceptions may be made with the approval of an appropriate decision-making body if it is determined that the health and welfare interests of the animal(s) are best served by participating in the study, or if there will be a tangible benefit for the individual animal(s) involved. They also allow samples to be collected during routine examinations, enclosure cleaning and post-mortem. Some sanctuaries may insist that samples collected from their residents are neither used solely for human medical benefit nor to infect animals housed elsewhere. As such, while many sanctuaries will likely have a narrower scope of allowable research compared to zoos and laboratories, opportunities to conduct research at sanctuaries do indeed exist.
(c). Sanctuary research capacity
In addition to the type of research being conducted at the sanctuary, there are other considerations in play when considering sanctuary-based research. The first is the research capacity of the organization. With a few exceptions, most primate sanctuaries do not have full-time research staff and the burden of conducting research is, therefore, often carried by external researchers or non-research sanctuary staff or volunteers. In either case, this presents logistical challenges ranging from the time and effort involved in hosting external personnel on site, to the degree to which research activities reduce staff capacity for other core functions. Moreover, studies of complex cognitive abilities or involving the collection of certain biosamples may require greater expertise than some sanctuaries are able to provide, often resulting in a greater focus on observational behavioural research. Secondly, the degree to which animals are accessible for research may differ from more traditional settings. Enclosures at sanctuaries often tend to be larger than in laboratories (e.g. [9]) and, compared to many zoo exhibits, are not necessarily designed to facilitate behavioural observations. Some sanctuaries have attempted to overcome these challenges by installing elevated observation decks or a system of remote cameras. Hansen et al. [10] conducted an evaluation of such a camera system in a chimpanzee sanctuary and found that, although cameras provided narrower perspectives on animal subjects, the observer was able to view large enclosures at a much faster rate compared to in-person observations. Much larger multi-acre enclosures, such as those found in range-country sanctuaries, may, therefore, be even more difficult settings to conduct behavioural observations.
(d). Sanctuary populations
As discussed earlier, the nature of sanctuary populations may differ depending on the region in which the sanctuary is located (table 1). Broadly speaking, many range-country sanctuaries have a positive population flow in which the influx of primates (rescued from circumstances related to illegal poaching, pet trafficking and the bushmeat trade) is generally greater than the outflux, with recently placed younger individuals often outnumbering older residents. Such circumstances may create opportunities for developmental and longitudinal research, though many of the residents may have atypical early histories involving little parent-rearing or traumatic experiences. On the other hand, with most non-range sanctuaries, there is a population bias towards older individuals. Here, sanctuaries function primarily as end-of-life facilities in which there is no breeding and mortality rates reflect an aged population. As a result, research on ageing or studies leveraging post-mortem biosampling may be well suited to these facilities should they meet the research criteria of the organization. Furthermore, the source of incoming primates in non-range sanctuaries may be more varied, as individuals may arrive with histories as laboratory research subjects, personal pets, or commercial entertainers. While some research may focus specifically on the effects of these past experiences [11–13], the atypical social histories of many of these individuals may be an important consideration. While some studies report long-term impacts of atypical and traumatic early histories on sanctuary-housed chimpanzees [11,12,14,15], other findings suggest that (range-country) sanctuary apes are as mentally healthy as apes in other settings (e.g. zoos) and can be seen as a valuable resource for non-invasive research [16]. In range country sanctuaries, such developmental improvements are likely related to the young age of arrival as well as the institutional rehabilitation and care programmes in place for these individuals.
Table 1.
Primate species housed at each of the sanctuaries where research described in this review took place.
| sanctuary | primate species housed |
|---|---|
| 1. Chimpanzee Sanctuary Northwest | Pan troglodytes |
| 2. Born Free USA Primate Sanctuary | Chlorocebus pygerthyrus; Macaca spp.; Papio spp. |
| 3. Cleveland Amory Black Beauty Ranch | Cebus spp.; Hylobates spp.; Macaca spp. |
| 4. Chimp Haven | Pan troglodytes |
| 5. Ape Initiative | Pan paniscus |
| 6. Jungle Friends Primate Sanctuary | Ateles spp.; Callithrix spp.; Cebus spp.; Saguinus spp.; Saimiri spp. |
| 7. Center For Great Apes | Pan troglodytes; Pongo pygmaeus |
| 8. Save the Chimps | Pan troglodytes |
| 9. Fauna Foundation | Macaca mulatta; Pan troglodytes |
| 10. Las Pumas Rescue Center | Ateles geoffroyi; Cebus capucinus |
| 11. Fundación Mona | Macaca mulatta; Pan troglodytes |
| 12. Tacugama Chimpanzee Sanctuary | Pan troglodytes |
| 13. Chimpanzee Rehabilitation Project | Pan troglodytes |
| 14. Chimpanzee Conservation Center | Pan troglodytes |
| 15. Limbe Wildlife Centre | Cercocebus spp.; Cercopithecus spp.; Gorilla gorilla gorilla; Mandrillus spp.; Pan troglodytes; Papio anubis |
| 16. Drill Ranch (Pandrillus) | Mandrillus leucophaeus; Pan troglodytes |
| 17. Ape Action Africa | Cercocebus spp.; Cercopithecus spp.; Chlorocebus tantalus; Gorilla gorilla gorilla; Mandrillus sphinx; Pan troglodytes; Papio anubis |
| 18. Sanaga-Yong Chimpanzee Rescue | Pan troglodytes |
| 19. Parc de la Lékédi | Cercocebus spp.; Cercopithecus spp.; Colobus satanas; Erythrocebus patas; Gorilla gorilla gorilla; Lophocebus albigena; Mandrillus sphinx; Miopithecus ogouensis; Pan troglodytes |
| 20. Tchimpounga Chimpanzee Rehabilitation Centre | Pan troglodytes |
| 21. Lola Ya Bonobo | Pan paniscus |
| 22. J.A.C.K. (Jeunes Animaux Confisques au Katanga) | Pan troglodytes |
| 23. Ngamba Island Chimpanzee Sanctuary | Pan troglodytes |
| 24. Sweetwaters Chimpanzee Sanctuary | Pan troglodytes |
| 25. Chimfunshi Wildlife Orphanage | Chlorocebus pygerythrus; Pan troglodytes; Papio cynocephalus |
| 26. Vervet Monkey Foundation | Chlorocebus pygerythrus |
| 27. Kumamoto Sanctuary | Pan paniscus; Pan troglodytes |
Note. Listed species are based on published research, sanctuary websites and personal communication. As sanctuary populations are variable, this table should be viewed as a guide rather than a conclusive list.
(e). Sanctuary affiliations
Research in sanctuary settings is not evenly distributed across organizations. Some sanctuaries are heavily invested in conducting research, and this is best illustrated by a trio of sanctuaries housing chimpanzees around the world. Kumamoto Sanctuary is unusual in that it is a chimpanzee sanctuary organization that maintained the facility, care staff and animals of a former biomedical research site. The sanctuary maintains an affiliation with a major university and has staff scientists on site to facilitate a wide range of biological, behavioural and cognitive research with the resident chimpanzees and bonobos (Pan paniscus) [17]. As such, the research productivity of this organization is quite high. In the United States, Chimp Haven was founded by scientists from the laboratory research community and continues to maintain a research department focused primarily on applied behavioural and welfare studies [18]. Here, additional research resources are facilitated though a collaboration with the research-oriented Lincoln Park Zoo in Chicago, IL [19]. In the central African country of Zambia, Chimfunshi Wildlife Orphanage (hereafter Chimfunshi) has conducted research for many years through collaborations with the Max Planck Institute for Evolutionary Anthropology in Germany and a core team of researchers primarily focused on cultural variation between the different chimpanzee communities.
2. Advantages of sanctuary-based research
While there are several factors requiring consideration in sanctuary-based primate research, there are also advantages relative to the more traditional laboratory settings and zoological parks. Investigations that prioritize subjects as models of free-ranging primates will often find the largest and most naturalistic enclosures in sanctuary settings—especially those in range countries [9]. In many of these environments, the primates themselves may well be considered attractive research models as the benefits of a free-ranging, naturalistic lifestyle are combined with some degree of control typical of captive settings. Likewise, large and species-typical social groups are important for the validity of much behavioural research [15], and larger sanctuary enclosures may facilitate such groupings of research subjects.
Additionally, while zoo settings may provide some degree of naturalism for some primates, the potential impact of visitors on behaviour and biology is always a consideration. Unfamiliar humans are a rarity in sanctuary settings, and thus may be more attractive to researchers interested in visitor effects (note [20] for potential impact of visitors in a sanctuary setting). Importantly, sanctuaries stand to benefit from research as well. The influx of expertise and academic support associated with research has the potential to bolster a sanctuary organization through greater visibility to global audiences, which could result in broader public support. Moreover, partnerships with sanctuary organizations in range countries can also help support their conservation initiatives as many organizations are involved in the reintroduction of primates to their natural habitats [21,22].
3. Review of research
To perform this review, we conducted a literature search with Google Scholar, using the name of each accredited sanctuary as search criteria to identify peer-reviewed articles as well as academic theses and dissertations. We also conducted broader searches using combinations of the words ‘sanctuary,’ ‘primate,’ ‘monkey’ and ‘ape,’ and then reviewed the methods section of each of these publications to confirm they included at least one sanctuary-housed primate or biosample (usually hair, saliva, urine or faeces) from a sanctuary-housed primate. Publications meeting these criteria were also classified based on subject matter under one of the following categories: behaviour and welfare, cognition, and, collectively, veterinary, genetic and physiology. Notably, many studies that include sanctuary-housed primates also include wild, zoo- or laboratory-housed subjects. Accordingly, while many of these studies occurred exclusively at sanctuaries or with samples from sanctuary-housed primates, a significant portion were collaborative efforts involving multiple institutions and/or field sites, with sanctuary-housed primates making up a subset of the subjects. Finally, we note our overt decision to avoid detailed descriptions of methodologies and research conclusions in this review to better emphasize the breadth of topics that have been explored with sanctuary-housed primates.
(a). Behaviour and welfare
Among the most obvious categories of research suited to sanctuaries are behavioural studies and those focused on welfare applications. Observational studies, in which resident primates are watched from afar without any disruption to their daily routine, are classically non-invasive in nature and the scientific investment to procure these data is relatively modest. Great ape (particularly chimpanzee) behavioural studies are typically the most prevalent in sanctuary settings, with studies focusing on activity budgets [23–25], grooming [26–29], locomotion [30,31] and space-use [32], sexual behaviour [33,34], social networks [35], play behaviour [36,37], behavioural flexibility [38], social interactions [39–41] and dominance hierarchies [42,43]. Specific behaviours may also be of particular interest in sanctuary settings given their relationship to care and management. At Chimpanzee Sanctuary Northwest in the United States, researchers examined the chimpanzees' acclimation to a novel outdoor environment [44], and although sanctuaries are typically not open to the public, there have nonetheless been several evaluations of the potential influence of visitors [20,45,46]. At both Chimp Haven [47] and Chimpanzee Sanctuary Uto (now Kumamoto Sanctuary) [48], nesting behaviour has been prioritized as a behaviour to be promoted with their resident chimpanzees, and studied intensively as a result.
Research on chimpanzee and bonobo communicative behaviour has also received significant attention, with studies of great ape communication conducted at Kumamoto [49,50], Ape Initiative in the United States [51–53] and Chimfunshi [54]. At Lola Ya Bonobo in the Democratic Republic of Congo (hereafter Lola), studies have focused on bonobos' copulation calls [55–58], gestures [59,60], vocal–gestural combinations in infants [61], sex signals [62], communication signals [63] and contest hoots [64]. Chimpanzee facial expressions have also been studied at both Ngamba Island in Uganda (hereafter Ngamba) [65] and Chimfunshi [66,67]. On the other hand, research on the behaviour of other sanctuary-housed primates, such as drills (Mandrillus leucophaeus) [68], capuchins (Cebus apella, Cebus capucinus) [69], and macaques (Macaca mulatta, Macaca fuscata) and olive baboons (Papio anubis) [70] is evident but was much rarer to find published in our search.
Measures of the welfare of sanctuary-housed primates have also received considerable research attention. Broad chimpanzee welfare assessments [71–73], as well as studies focusing on both positive [37] and negative [74,75] indicators of wellbeing, have been studied in sanctuary settings. Leveraging the diverse backgrounds of sanctuary primates, researchers have further explored the welfare effects of atypical early life histories, such as the sudden loss of parents by orphaned wild chimpanzees [76], or atypical exposure to humans on former pets or performers [11–13,77]. Given the often traumatic lives of primates prior to arriving at a sanctuary, the psychological health of sanctuary-housed great apes has also received attention. Studies at Save the Chimps [78] as well as at Lola and the Tchimpounga Chimpanzee Rehabilitation Center (hereafter Tchimpounga) in the Republic of Congo [16] broadly explored psychological health, while the effects of the bushmeat trade on chimpanzees have also been documented [79]. Other studies involving sanctuary chimpanzees have focused on mood disorders, such as signs of generalized anxiety and compulsive disorders [80–82] and post-traumatic stress disorder in chimpanzees at Fauna Foundation in Canada (hereafter Fauna) [83,84].
Associated with welfare studies are those investigations that overtly acknowledge and focus on anthropogenic impacts on apes. For instance, a study at Lola focused on the outcomes of human interactions with the resident bonobos [85], and another at Sweetwaters Chimpanzee Sanctuary in Kenya (hereafter Sweetwaters), examined the effect of being watched by humans on chimpanzee resource acquisition [86]. Similarly, at Fauna, chimpanzees' responses to sound [87] and caregiver interactions [70] have been studied to determine their effects on the chimpanzees.
In contrast to studies that focus on elements that are unique to captive conditions, other studies focus on the very naturalistic state of some sanctuary environments, especially those in range-country facilities that provide expansive, naturally forested areas and species-typical foraging opportunities. In these cases, resident primates may represent suitable proxies for behaviours typically studied with wild populations. Ecological studies, such as those focused on fruit-opening behaviours by chimpanzees at Chimfunshi [88] and feeding decisions when presented with contaminated foods by bonobos at Lola [89], leverage natural foraging conditions at these sanctuaries for research opportunities. Other naturalistic studies have looked at primate food preferences [90], reactions to novel foods [91], interactions with wildlife [92,93] and responses to deaths of groupmates [94–96].
Some behavioural studies, while still generally considered non-invasive in nature, nonetheless involve some manipulation of the primates themselves or their environment. Animals may be temporarily separated from their group, shifted to other areas of their housing, or provided with resources or objects that would otherwise not be present. For instance, the study of cooperative behaviours among great apes has been widespread in sanctuary settings, with studies involving chimpanzees at Ngamba [97–104], Tchimpounga [105–107] and Sweetwaters [108], as well as bonobos at Lola [105,109]. In addition to cooperation, chimpanzee resource competition [110,111], helping behaviour [112–114] and altruism [115] have also been studied. Such studies, as well as extensive research on prosociality with both chimpanzees [116–119] and bonobos [97,120–124], use behavioural measures to assess apes' cognitive abilities. For example, yawn contagion, frequently used as a measure of empathy, has been studied in chimpanzees at Tacugama Chimpanzee Sanctuary in Sierra Leone (hereafter Tacugama) [125] and bonobos at Lola [126].
(b). Cognition
Though behavioural studies are often considered best-suited for sanctuary settings because of their unobtrusive nature, the depth and range of cognitive studies conducted in primate sanctuaries, particularly in range countries, is impressive. An array of innovative and easy to implement methods to assess primate thinking, learning, perception and emotion have been used in sanctuaries, with many studies buoyed by access to large sample sizes typically unavailable in zoos or research centres.
Among the first sanctuaries to embrace collaborations with external researchers interested in primate cognition was Ngamba. Researchers from several European and American universities have travelled there to work alongside sanctuary staff and resident chimpanzees on a wide range of topics; including, social learning in ecological contexts [127,128], problem-solving of a trap-tube task [129], deception [130], quantitative abilities [131], reasoning and inference abilities [132–134], perspective taking [135], inhibitory control compared across the great apes and human children [136], imitation and emulation [137,138], and one study finding that dogs (Canis familiaris) but not chimpanzees comprehend imperative human pointing [139]. Work at other chimpanzee sanctuaries in Africa followed suit, emphasizing individual [140] and social learning [141] in addition to comparative cognition research involving multiple species [142–144]. Specifically, researchers have also investigated topics such as reciprocity of ‘favours' by chimpanzees [145], development of spatial memory [146], auditory sequence learning [147] and joint attention [148–150].
Studies of the ways in which sanctuary-housed primates, primarily chimpanzees, make decisions and solve problems have yielded numerous publications over the previous two decades. Cognitive development [41,125,146,151,152], decision-making [153–157] and tool-use [94,158–163] are some of the most commonly studied topics involving great apes. However, researchers have also investigated several more specific topics with sanctuary-housed chimpanzees, such as the influence of both social presence [164] and early rearing history [165] on problem-solving. Other problem-solving research has looked at chimpanzees' use of causal and arbitrary cues [166] as well as the role of visual feedback in the floating peanut task [167]. The performance of chimpanzees at Ngamba on the floating peanut task has also been studied comparatively with human children and non-sanctuary-housed gorillas (Gorilla gorilla gorilla) and orangutans (Pongo pygmaeus) [168]. Though cognitive research has typically focused on chimpanzees, the cognition of sanctuary-housed bonobos is also well studied. Lola, in particular, is responsible for a number of studies on wild-born bonobo cognition [169,170], frequently in collaboration with other institutions to compare performance across multiple species [144,151–155,158,171–175]. Captive-born bonobo cognition has also received attention at both Ape Initiative [148,176,177] and Kumamoto [178–180].
As in other settings, the methods to study cognition in sanctuaries are highly varied, ranging from puzzles [129,138,167,168] to video playbacks of conspecifics [181]. Among the most common studies, in both range- and non-range-country sanctuaries, are those focused on handedness and laterality [177,182–192], in part because of methodology that can be as simple as providing small PVC tubes from which the apes pick out sticky foods with their finger. On the opposite end of the technology spectrum, the utilization of infrared eye-tracking devices continues to grow. Researchers at Kumamoto, often in collaboration with the Wolfgang Kohler Primate Research Center in Leipzig, Germany, have used these devices to investigate anticipatory looking and false beliefs [193–197] to explore topics such as joint attention and theory of mind.
(c). Veterinary, genetic and physiology
Primate sanctuaries, particularly those in range countries, have also been used for myriad studies on veterinary topics, as well as genetic and physiological research. Procuring biosamples from wild-born, but sanctuary-housed, primates is often far simpler in these managed settings than from their counterparts in the wild, making sanctuaries a logistically attractive option for many researchers in this field.
A wide range of clinical conditions specific to primates have thus been studied in sanctuary settings. Cardiac function [198] and disease [199,200] have been investigated in both chimpanzees and bonobos, as well as improvements to cardiac monitoring [201] and heart rate responses to anaesthetic protocols [202]. At Kumamoto, clinical cases of maxillary sarcoma [203], leprosy [204] and Down syndrome [205] have all been reported in chimpanzees, while researchers have also investigated a non-invasive test for tuberculosis in PASA sanctuaries [206]. Parasitology research has been conducted at a number of PASA sanctuaries, including a multi-institutional investigation of Trypanosoma brucei, the parasite responsible for sleeping sickness [207], which used samples from sanctuary-housed gorillas at Limbe Wildlife Center in Cameroon (hereafter Limbe) and chimpanzees from several facilities. Predictably, Plasmodium falciparum, the parasite causing malaria, has been studied extensively in African sanctuaries, with researchers collaborating to analyse samples obtained from all four great ape species [208,209], while similar research has been conducted at Kumamoto [210]. Parasites responsible for schistosomiasis have also been studied at Ngamba [211–215], while other intestinal parasites have been researched in sanctuary-housed chimpanzees at Kumamoto [216,217], Limbe and Sweetwaters [218], as well as in chimpanzees and mandrills (Mandrillus sphinx) at Tchimpounga [219] and bonobos at Lola [220].
Sanctuary research has also contributed to virology, including the potentially worrisome confirmed transfer of a pathogen between sanctuary workers and great apes [221,222]. Further research has looked at samples from both living and deceased great apes to better understand adenoviruses [223], polyomaviruses [224], enterovirus [225–227], Epstein–Barr virus [228], cytomegaloviruses [229], herpesvirus [230], poliovirus [231], retroviruses [232], sapovirus [233] and pneumonia [234]. Hepatitis B has also been studied with sanctuary-housed chimpanzees and gorillas [235–237], while other studies have looked at viral infections in chimpanzees at Ngamba [238] and inflammatory disease risk in chimpanzees at Sweetwaters [239].
Sanctuary-based research on the primate microbiome has used samples from Limbe [218,240], Ngamba [241,242], Sweetwaters [218,243–245], Tacugama [246,247] and Lola [247]. One noteworthy finding from this microbiome research has been the extent to which captivity humanizes the microbiome of numerous primate species housed at a variety of institutions, including sanctuaries [248]. Researchers have also studied various bacteria using samples from chimpanzees at several African sanctuaries [221,249–252], as well as biomarkers [253] and blood groups [254] in sanctuary-housed great apes. One case study even describes blood transfusions to treat an anaemic chimpanzee at Tchimpounga [255]. Post-mortem reports from chimpanzees who died at GFAS-accredited sanctuaries have been reviewed [256], while longevity and mortality of captive chimpanzees in Japan, including at Kumamoto, has also been studied [257].
Hormones, in particular cortisol and testosterone, have also been studied with sanctuary-housed primates. Cortisol has been measured in chimpanzees as an indicator of stress at Ngamba [258], while several studies at Kumamoto have further explored cortisol levels obtained from hair samples and its associated effects in chimpanzees [259–262]. Testosterone has been assessed in chimpanzees at Ngamba [263,264] and Kumamoto [265], as well as in chimpanzees at Tchimpounga and bonobos at Lola [266], where the effects of testosterone on cognitive performance were also tested [267]. Additionally, testosterone and cortisol have been studied in bonobos at Lola in the context of food competition [268]. Other hormonal relationships, such as the effects of castration on macaque social behaviour [269] and the effect of ageing on chimpanzee fertility [270], have been conducted at American and African sanctuaries, respectively.
At Kumamoto, researchers have looked at chimpanzee morphology [271,272], chimpanzee and bonobo finger length proportions [273], and the skeletal material of deceased primates from several sanctuaries [274]. Other topics of study at Kumamoto include chimpanzee colour vision [275,276], taste receptors [277,278], growth [279,280] and reproductive biology [281]. There are relatively few biological studies of non-ape species, though researchers at the Vervet Monkey Foundation in South Africa have used subcutaneous thermal sensors to examine internal temperature variation in vervet monkeys (Chlorocebus pygerythrus) [282]. Thermal imaging has also been used by researchers to examine psycho-physiological responses of resident chimpanzees [181,283].
A number of studies have also explored the genetics of sanctuary-housed primates. Research involving the genetics of chimpanzees has been conducted at Kumamoto [284–288], Limbe [289,290] and Ngamba [291,292]. Several other studies have further explored great ape genetics at multiple PASA-accredited sanctuaries [247,293–297], while one study looked at the genetics of chimpanzee neuroticism at sanctuaries in Africa and Japan [298]. At Kumamoto, DNA was also recently used to estimate chimpanzee ages [299], while even the mitochondrial DNA of the chimpanzee louse has been studied at Tacugama [300].
4. The future of sanctuary-based research
Though captive primate research in sanctuary settings has been conducted for far shorter a time period than in laboratories and zoological parks, this review demonstrates the impressive growth and scope of those efforts. Importantly, many of the studies mentioned in this review are highly collaborative in nature. Relatively few sanctuaries employ staff scientists, and many of the studies described here are initiated ‘from the outside' by scientists and students seeking novel and appropriate subjects for their research. Such inter-institutional and collaborative investigations may be particularly powerful and demonstrate increased variation for the factors under study. Indeed, it is increasingly the case that different types of institutions are willing and able to work together to increase sample sizes and research relevancy. For instance, much of the research focused on using large samples of primates to assess influences on personality has successfully combined populations living across zoos, laboratories, sanctuaries and even the wild [301–307]. Other collaborative efforts, such as the research partnership between Lincoln Park Zoo and Chimp Haven [19], work to leverage the respective institutional strengths of two organizations. Most broadly, the ManyPrimates initiative [308] aims to facilitate collaboration among primate researchers working in a variety of settings, including sanctuaries, by pooling resources, standardizing study methods, and growing sample sizes to augment research potency. Collaborations in which investment burdens are shared, information is openly exchanged, and participants invest in the success of their partners and the project, are those that are most likely to succeed. Such partnerships begin with the consideration of a sanctuary organization as more than simply a research resource housing potential research subjects, and an acceptance that organizational priorities may differ from those of traditional research venues.
Along with the aforementioned benefits of conducting research in a sanctuary setting, there comes a number of potential challenges in considering the availability of study opportunities. Unlike in laboratory and zoo settings, where self-sustaining populations of primates remain available for research purposes, the primates replenishing sanctuary facilities for the future are usually by-products of other activities, such as medical research, the bushmeat or pet trafficking trades, or use in the entertainment business. As those activities wane, likely so too will the influx of primates into sanctuaries. For example, the vast majority of chimpanzees housed in accredited sanctuaries in the United States have been transferred into the sanctuary community after their usefulness as subjects of medical research or as pets and entertainers ended. The dissolution of these activities resulted in a huge growth in the sanctuary chimpanzee population over the past two decades, but projections point to a steady decline in that population as those chimpanzees age and eventually die out. With no breeding programmes in accredited sanctuary settings, it is reasonable to expect that those chimpanzee populations, and any associated research with them, will conclude in about 40 years' time (Faust, unpublished data). However, this is not necessarily the case for other regions or other species. In range countries, unfortunately, the illegal bushmeat and pet trades continue to produce orphaned primates, among which the most fortunate funnel into nearby accredited sanctuaries. In non-range countries, biomedical research with monkeys remains common; as such, one might expect a continued influx of monkeys to sanctuary settings [309] and growth in organizations that house those species. Given that their very existence depends on the decisions and activities of other organizations and industries, the long-term sustainability of sanctuaries, and their associated research programmes, is thus difficult to predict. Indeed, many sanctuaries are overt about their organization's philosophy to ‘be in business to be out of business.’
Regardless of the long-term outlook for sanctuary organizations, the idea that sanctuaries can aid research is no longer a niche concept [310]. While in some cases they may serve as a replacement for research opportunities that are no longer available in other settings, there may also be lines of scientific inquiry that are actually best suited for sanctuaries. For instance, without breeding programmes, many sanctuaries have an increasingly aged population of primates in residence and studies of ageing may, therefore, be particularly well-suited for these populations. This review demonstrates the perhaps surprising breadth and depth of research being conducted in sanctuary settings around the world. While relatively narrow in taxonomic scope, existing and emerging partnerships with sanctuaries have produced high-quality science with important application potential. Continued development of collaborations between scientists and sanctuaries is likely to be of benefit to both the academic and animal care communities alike.
Data accessibility
This article has no additional data.
Authors' contributions
S.R. conceived and wrote the review. J.L. wrote the review and created the figures.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by the Women's Board of Lincoln Park Zoo.
References
- 1.Lankau EW, Turner PV, Mullan RJ, Galland GG. 2014. Use of nonhuman primates in research in North America. J. Am. Assoc. Lab. Anim. Sci. 53, 278–282. [PMC free article] [PubMed] [Google Scholar]
- 2.Stoinski TS, Lukas KE, Maple TL. 1998. A survey of research in North American zoos and aquariums. Zoo Biol. 17, 167–180. () [DOI] [Google Scholar]
- 3.Rose PE, Brereton JE, Rowden LJ, de Figueiredo RL, Riley LM. 2019. What's new from the zoo? An analysis of ten years of zoo-themed research output. Palgrave Commun. 5, 1–10. ( 10.1057/s41599-019-0345-3) [DOI] [Google Scholar]
- 4.Hopper LM. 2017. Cognitive research in zoos. Curr. Opin. Behav. Sci. 16, 100–110. ( 10.1016/j.cobeha.2017.04.006) [DOI] [Google Scholar]
- 5.Brent L. 2007. Life-long well being: applying animal welfare science to nonhuman primates in sanctuaries. J. Appl. Anim. Welf. Sci. 10, 55–61. ( 10.1080/10888700701277626) [DOI] [PubMed] [Google Scholar]
- 6.Grimm D. 2016. Chimpanzee sanctuaries open door to more research. Science 353, 433–434. ( 10.1126/science.353.6298.433) [DOI] [PubMed] [Google Scholar]
- 7.Carlsson H-E, Schapiro SJ, Farah I, Hau J. 2004. Use of primates in research: a global overview. Am. J. Primatol. Off. J. Am. Soc. Primatol. 63, 225–237. ( 10.1002/ajp.20054) [DOI] [PubMed] [Google Scholar]
- 8.Global Federation of Animal Sanctuaries. 2019. Operations Standards See https://www.sanctuaryfederation.org/wp-content/uploads/2019/07/Revised-Operations-Standards-2019.pdf
- 9.Ross SR. 2019. Chimpanzee welfare in the context of science, policy, and practice. In Chimpanzees in context (eds Ross SR, Hopper LM). Chicago, IL: University of Chicago Press. [Google Scholar]
- 10.Hansen BK, Fultz AL, Hopper LM, Ross SR. 2018. An evaluation of video cameras for collecting observational data on sanctuary-housed chimpanzees (Pan troglodytes). Zoo Biol. 37, 156–161. ( 10.1002/zoo.21410) [DOI] [PubMed] [Google Scholar]
- 11.Freeman HD, Ross SR. 2014. The impact of atypical early histories on pet or performer chimpanzees. PeerJ 2, e579 ( 10.7717/peerj.579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman HD, Weiss A, Ross SR. 2016. Atypical early histories predict lower extraversion in captive chimpanzees. Dev. Psychobiol. 58, 519–527. ( 10.1002/dev.21395) [DOI] [PubMed] [Google Scholar]
- 13.Crailsheim D, Stüger HP, Kalcher-Sommersguter E, Llorente M. 2020. Early life experience and alterations of group composition shape the social grooming networks of former pet and entertainment chimpanzees (Pan troglodytes). PLoS ONE 15, e0226947 ( 10.1371/journal.pone.0226947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopresti-Goodman SM, Kameka M, Dube A. 2013. Stereotypical behaviors in chimpanzees rescued from the African bushmeat and pet trade. Behav. Sci. 3, 1–20. ( 10.3390/bs3010001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cronin KA, Jacobson SL, Bonnie KE, Hopper LM. 2017. Studying primate cognition in a social setting to improve validity and welfare: a literature review highlighting successful approaches. PeerJ 5, e3649 ( 10.7717/peerj.3649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wobber V, Hare B. 2011. Psychological health of orphan bonobos and chimpanzees in African sanctuaries. PLoS ONE 6, e17147 ( 10.1371/journal.pone.0017147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirata S, Morimura N, Watanuki K, Ross SR. 2020. The establishment of sanctuaries for former laboratory chimpanzees: challenges, successes, and cross-cultural context. In Chimpanzees in context (eds Hopper LM, Ross SR). Chicago, IL: University of Chicago Press. [Google Scholar]
- 18.Fultz A. 2017. A guide for modern sanctuaries with examples from a captive chimpanzee sanctuary. Anim. Stud. J. 6, 9–29. [Google Scholar]
- 19.Ross SR, Hansen BK, Hopper LM, Fultz A. 2019. A unique zoo-sanctuary collaboration for chimpanzees. Am. J. Primatol. 81, e22941 ( 10.1002/ajp.22941) [DOI] [PubMed] [Google Scholar]
- 20.Hansen BK, Hopper LM, Fultz AL, Ross SR. In press. Understanding the behavior of sanctuary-housed chimpanzees during public programs. Anthrozoos [Google Scholar]
- 21.Farmer KH. 2002. Pan-African sanctuary alliance: status and range of activities for great ape conservation. Am. J. Primatol. Off. J. Am. Soc. Primatol. 58, 117–132. ( 10.1002/ajp.10054) [DOI] [PubMed] [Google Scholar]
- 22.Ferrie GM, Farmer KH, Kuhar CW, Grand AP, Sherman J, Bettinger TL. 2014. The social, economic, and environmental contributions of Pan African Sanctuary Alliance primate sanctuaries in Africa. Biodivers. Conserv. 23, 187–201. ( 10.1007/s10531-013-0592-3) [DOI] [Google Scholar]
- 23.Persad-Clem RA. 2009. Adaptation of captive chimpanzees (Pan troglodytes) to free ranging in a natural temperate environment. PhD thesis, Miami University, Oxford, OH: See https://search.proquest.com/openview/d102fe6c5c8b455cc6ce0251e8aef96e/1?pq-origsite=gscholar&cbl=51922&diss=y. [Google Scholar]
- 24.Sullins K. 2019. Effect of group size on the activity budget of two captive chimpanzees (Pan troglodytes). Master's thesis, Central Washington University, Ellensburg, WA: See https://digitalcommons.cwu.edu/cgi/viewcontent.cgi?article=2116&context=etd. [Google Scholar]
- 25.Emelue GU, Dododawa Z. 2017. Behaviour of chimpanzees (Blumenbach, 1775) at Pandrillus drill ranch, Boki, Cross River State, Nigeria. Niger. J. Wildl. Manage. 1, 73–79. [Google Scholar]
- 26.van Leeuwen EJC, Cronin KA, Haun DBM, Mundry R, Bodamer MD. 2012. Neighbouring chimpanzee communities show different preferences in social grooming behaviour. Proc. R. Soc. B 279, 4362–4367. ( 10.1098/rspb.2012.1543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Leeuwen EJC, Mundry R, Cronin KA, Bodamer M, Haun DBM. 2017. Chimpanzee culture extends beyond matrilineal family units. Curr. Biol. 27, R588–R590. ( 10.1016/j.cub.2017.05.003) [DOI] [PubMed] [Google Scholar]
- 28.Wrangham RW, et al. 2016. Distribution of a chimpanzee social custom is explained by matrilineal relationship rather than conformity. Curr. Biol. 26, 3033–3037. ( 10.1016/j.cub.2016.09.005) [DOI] [PubMed] [Google Scholar]
- 29.Schroepfer-Walker K, Wobber V, Hare B. 2015. Experimental evidence that grooming and play are social currency in bonobos and chimpanzees. Behaviour 152, 545–562. ( 10.1163/1568539X-00003258) [DOI] [Google Scholar]
- 30.Neufuss J, Robbins MM, Baeumer J, Humle T, Kivell TL. 2017. Comparison of hand use and forelimb posture during vertical climbing in mountain gorillas (Gorilla beringei beringei) and chimpanzees (Pan troglodytes). Am. J. Phys. Anthropol. 164, 651–664. ( 10.1002/ajpa.23303) [DOI] [PubMed] [Google Scholar]
- 31.Neufuss J, Robbins MM, Baeumer J, Humle T, Kivell TL. 2018. Gait characteristics of vertical climbing in mountain gorillas and chimpanzees. J. Zool. 306, 129–138. ( 10.1111/jzo.12577) [DOI] [PubMed] [Google Scholar]
- 32.Soubiea H. 2017. Chimpanzee (Pan troglodytes) space use in a sanctuary setting. Master's thesis, Central Washington University, Ellensburg, WA: See https://digitalcommons.cwu.edu/etd/628/. [Google Scholar]
- 33.Clay Z, de Waal FBM. 2015. Sex and strife: post-conflict sexual contacts in bonobos. Behaviour 152, 313–334. ( 10.1163/1568539X-00003155) [DOI] [Google Scholar]
- 34.Woods V, Hare B. 2011. Bonobo but not chimpanzee infants use socio-sexual contact with peers. Primates 52, 111–116. ( 10.1007/s10329-010-0229-z) [DOI] [PubMed] [Google Scholar]
- 35.van Leeuwen EJC, Cronin KA, Haun DBM. 2018. Population-specific social dynamics in chimpanzees. Proc. Natl Acad. Sci. USA 115, 11 393–11 400. ( 10.1073/pnas.1722614115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bauer EB. 2005. Dyadic play interactions and social relationships in three species of complex social mammals: domestic dogs (Canis familiaris), bonobos (Pan paniscus) and chimpanzees (Pan troglodytes). PhD thesis, University of Michigan, Ann Arbor, MI: See https://search.proquest.com/openview/a9f44c1b4b26ebfd999914bcb9ef4e1f/1?pq-origsite=gscholar&cbl=18750&diss=y. [Google Scholar]
- 37.Yamanashi Y, Nogami E, Teramoto M, Morimura N, Hirata S. 2018. Adult–adult social play in captive chimpanzees: is it indicative of positive animal welfare? Appl. Anim. Behav. Sci. 199, 75–83. ( 10.1016/j.applanim.2017.10.006) [DOI] [Google Scholar]
- 38.Leeuwen EJCV, Cronin KA, Schütte S, Call J, Haun DBM. 2013. Chimpanzees (Pan troglodytes) flexibly adjust their behaviour in order to maximize payoffs, not to conform to majorities. PLoS ONE 8, e80945 ( 10.1371/journal.pone.0080945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cronin KA, van Leeuwen EJC, Vreeman V, Haun DBM. 2014. Population-level variability in the social climates of four chimpanzee societies. Evol. Hum. Behav. 35, 389–396. ( 10.1016/j.evolhumbehav.2014.05.004) [DOI] [Google Scholar]
- 40.Milne SC. 2015. An empirical and theoretical comparison of the socio-ecological behaviors of captive chimpanzees (Pan troglodytes), bonobos (Pan paniscus), and western lowland gorillas (Gorilla gorilla): social tolerance and behavioral responses to changes in food quality and distribution. Master's thesis, Kennesaw State University, Kennesaw, GA: See http://digitalcommons.kennesaw.edu/cgi/viewcontent.cgi?article=1008&context=integrbiol_etd. [Google Scholar]
- 41.Clay Z, de Waal FB. 2013. Development of socio-emotional competence in bonobos. Proc. Natl Acad. Sci. USA 110, 18 121–18 126. ( 10.1073/pnas.1316449110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Funkhouser JA. 2018. Re-evaluating captive chimpanzee ‘dominance’: dominance hierarchy and chimpanzee–caregiver relationships at Chimpanzee Sanctuary Northwest. Master's thesis, Central Washington University, Ellensburg, WA: See https://digitalcommons.cwu.edu/etd/887/. [Google Scholar]
- 43.Funkhouser JA, Mayhew JA, Mulcahy JB. 2018. Social network and dominance hierarchy analyses at Chimpanzee Sanctuary Northwest. PLoS ONE 13, e0191898 ( 10.1371/journal.pone.0191898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heggs L, Matheson M, Ross SR, Mulcahy JB. 2012. The effect a novel outdoor environment on the behavior of chimpanzees in a sanctuary setting. Am. J. Primatol., 74(Suppl. 1), 36. [Google Scholar]
- 45.Farley AA.2016. Comparison of chimpanzee (Pan Troglodytes) behavior on tour and non-tour days at Chimpanzee Sanctuary Northwest. Masters thesis, Central Washington University, Washington.
- 46.López-Álvarez J, Sanjorge Y, Soloaga S, Crailsheim D, Llorente M. 2019. Looking for visitor's effect in sanctuaries: implications of guided visitor groups on the behavior of the chimpanzees at Fundació Mona. Animals 9, 347 ( 10.3390/ani9060347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fultz A, Brent L, Breaux SD, Grand AP. 2013. An evaluation of nest-building behavior by sanctuary chimpanzees with access to forested habitats. Folia Primatol. (Basel) 84, 405–420. ( 10.1159/000353900) [DOI] [PubMed] [Google Scholar]
- 48.Morimura N, Fujisawa M, Mori Y, Teramoto M. 2012. Environmental influences on sleep behavior in captive male chimpanzees (Pan troglodytes). Int. J. Primatol. 33, 822–829. ( 10.1007/s10764-012-9612-5) [DOI] [Google Scholar]
- 49.Kano F, Moore R, Krupenye C, Hirata S, Tomonaga M, Call J. 2018. Human ostensive signals do not enhance gaze following in chimpanzees, but do enhance object-oriented attention. Anim. Cogn. 21, 715–728. ( 10.1007/s10071-018-1205-z) [DOI] [PubMed] [Google Scholar]
- 50.Kutsukake N, Teramoto M, Homma S, Mori Y, Matsudaira K, Kobayashi H, Ishida T, Okanoya K, Hasegawa T. 2012. Individual variation in behavioural reactions to unfamiliar conspecific vocalisation and hormonal underpinnings in male chimpanzees. Ethology 118, 269–280. ( 10.1111/j.1439-0310.2011.02009.x) [DOI] [Google Scholar]
- 51.Lyn H, Russell JL, Leavens DA, Bard KA, Boysen ST, Schaeffer JA, Hopkins WD. 2014. Apes communicate about absent and displaced objects: methodology matters. Anim. Cogn. 17, 85–94. ( 10.1007/s10071-013-0640-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rabinowitz A. 2016. Linguistic competency of bonobos (Pan paniscus) raised in a language-enriched environment. Master's thesis, Iowa State University, Ames, IA: See https://lib.dr.iastate.edu/etd/15794. [Google Scholar]
- 53.Schwob N. 2017. Evidence of language prerequisites in Pan: orofacial-motor and breath control in chimpanzees and bonobos. Master's thesis, Kennesaw State University, Kennesaw, GA: See https://digitalcommons.kennesaw.edu/integrbiol_etd/25. [Google Scholar]
- 54.Oña LS, Sandler W, Liebal K. 2019. A stepping stone to compositionality in chimpanzee communication. PeerJ 7, e7623 ( 10.7717/peerj.7623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clay Z, Zuberbühler K. 2011. The structure of bonobo copulation calls during reproductive and non-reproductive sex: structure of bonobo copulation calls. Ethology 117, 1158–1169. ( 10.1111/j.1439-0310.2011.01975.x) [DOI] [Google Scholar]
- 56.Clay Z, Zuberbühler K. 2012. Communication during sex among female bonobos: effects of dominance, solicitation and audience. Sci. Rep. 2, 291 ( 10.1038/srep00291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clay Z, Pika S, Gruber T, Zuberbühler K. 2011. Female bonobos use copulation calls as social signals. Biol. Lett. 7, 513–516. ( 10.1098/rsbl.2010.1227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clay Z. 2011. Vocal communication in bonobos (Pan paniscus): studies in the contexts of feeding and sex. PhD thesis, University of St Andrews, St Andrews, Scotland: See https://research-repository.st-andrews.ac.uk/handle/10023/1842. [Google Scholar]
- 59.Genty E, Zuberbühler K. 2014. Spatial reference in a bonobo gesture. Curr. Biol. 24, 1601–1605. ( 10.1016/j.cub.2014.05.065) [DOI] [PubMed] [Google Scholar]
- 60.MacLean EL, Hare BA. 2015. Bonobos and chimpanzees exploit helpful but not prohibitive gestures. Behaviour 152, 493–520. ( 10.1163/1568539x-00003203) [DOI] [Google Scholar]
- 61.Genty E. 2019. Vocal–gestural combinations in infant bonobos: new insights into signal functional specificity. Anim. Cogn. 22, 505–518. ( 10.1007/s10071-019-01267-0) [DOI] [PubMed] [Google Scholar]
- 62.Genty E, Neumann C, Zuberbühler K. 2015. Complex patterns of signalling to convey different social goals of sex in bonobos, Pan paniscus. Sci. Rep. 5, 16135 ( 10.1038/srep16135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Genty E, Neumann C, Zuberbühler K. 2015. Bonobos modify communication signals according to recipient familiarity. Sci. Rep. 5, 16442 ( 10.1038/srep16442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Genty E, Clay Z, Hobaiter C, Zuberbühler K. 2014. Multi-modal use of a socially directed call in bonobos. PLoS ONE 9, e84738 ( 10.1371/journal.pone.0084738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Waller BM, Misch A, Whitehouse J, Herrmann E. 2014. Children, but not chimpanzees, have facial correlates of determination. Biol. Lett. 10, 20130974 ( 10.1098/rsbl.2013.0974) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davila-Ross M, Jesus G, Osborne J, Bard KA. 2015. Chimpanzees (Pan troglodytes) produce the same types of ‘laugh faces’ when they emit laughter and when they are silent. PLoS ONE 10, e0127337 ( 10.1371/journal.pone.0127337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davila-Ross M, Allcock B, Thomas C, Bard KA. 2011. Aping expressions? Chimpanzees produce distinct laugh types when responding to laughter of others. Emotion 11, 1013–1020. ( 10.1037/a0022594) [DOI] [PubMed] [Google Scholar]
- 68.Maurice ME, Fuashi NA, Lengha TK. 2017. The influence of some ecological factors on drill monkeys Mandrillus leucophaeus (Cuvier)—in Limbe Wildlife Center (LWC), Southwest Region, Cameroon. Int. J. Biodivers. Conserv. 9, 256–264. ( 10.5897/ijbc2017.1097) [DOI] [Google Scholar]
- 69.Weldon PJ, Aldrich JR, Klun JA, Oliver JE, Debboun M. 2003. Benzoquinones from millipedes deter mosquitoes and elicit self-anointing in capuchin monkeys (Cebus spp.). Naturwissenschaften 90, 301–304. ( 10.1007/s00114-003-0427-2) [DOI] [PubMed] [Google Scholar]
- 70.Stolar L. 2018. Response of three species of monkeys to caregiver use of species-typical behavior. Master's thesis, Central Washington University, Ellensburg, WA: See https://digitalcommons.cwu.edu/etd/896. [Google Scholar]
- 71.Bateson M, Nettle D. 2015. Development of a cognitive bias methodology for measuring low mood in chimpanzees. PeerJ 3, e998 ( 10.7717/peerj.998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cronin KA, West V, Ross SR. 2016. Investigating the relationship between welfare and rearing young in captive chimpanzees (Pan troglodytes). Appl. Anim. Behav. Sci. 181, 166–172. ( 10.1016/j.applanim.2016.05.014) [DOI] [Google Scholar]
- 73.Robinson LM, et al. 2017. Chimpanzees with positive welfare are happier, extraverted, and emotionally stable. Appl. Anim. Behav. Sci. 191, 90–97. ( 10.1016/j.applanim.2017.02.008) [DOI] [Google Scholar]
- 74.Fultz A, Brent L, Loeser E. 2010. Abnormal behaviors in sanctuary chimpanzees (Pan troglodytes). Am. J. Primatol. 72, 28–29. [Google Scholar]
- 75.Case L, Yanagi A, Loeser E, Fultz A. 2015. Human–animal relationships: the use of species-typical food calls and chimpanzee (Pan troglodytes) names: welfare-oriented tools to manage sanctuary chimpanzees. Anim. Behav. Cogn. 2, 254–266. ( 10.12966/abc.08.05.2015) [DOI] [Google Scholar]
- 76.van Leeuwen EJC, Mulenga IC, Chidester DL. 2014. Early social deprivation negatively affects social skill acquisition in chimpanzees (Pan troglodytes). Anim. Cogn. 17, 407–414. ( 10.1007/s10071-013-0672-5) [DOI] [PubMed] [Google Scholar]
- 77.Llorente M, Riba D, Ballesta S, Feliu O, Rostán C. 2015. Rehabilitation and socialization of chimpanzees (Pan troglodytes) used for entertainment and as pets: an 8-year study at Fundació Mona. Int. J. Primatol. 36, 605–624. ( 10.1007/s10764-015-9842-4) [DOI] [Google Scholar]
- 78.Lopresti-Goodman SM, Bezner J, Ritter C. 2015. Psychological distress in chimpanzees rescued from laboratories. J. Trauma Dissociation 16, 349–366. ( 10.1080/15299732.2014.1003673) [DOI] [PubMed] [Google Scholar]
- 79.Ortín S, Úbeda Y, Garriga RM, Llorente M. 2019. Bushmeat trade consequences predict higher anxiety, restraint, and dominance in chimpanzees. Dev. Psychobiol. 61, 874–887. ( 10.1002/dev.21853) [DOI] [PubMed] [Google Scholar]
- 80.Ferdowsian HR, Durham DL, Kimwele C, Kranendonk G, Otali E, Akugizibwe T, Mulcahy JB, Ajarova L, Johnson CM. 2011. Signs of mood and anxiety disorders in chimpanzees. PLoS ONE 6, e19855 ( 10.1371/journal.pone.0019855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ferdowsian HR, et al. 2012. Signs of generalized anxiety and compulsive disorders in chimpanzees. J. Vet. Behav. 7, 353–361. ( 10.1016/j.jveb.2012.01.006) [DOI] [Google Scholar]
- 82.Rosati AG, et al. 2013. Assessing the psychological health of captive and wild apes: a response to Ferdowsian et al. (2011). J. Comp. Psychol. 127, 329–336. ( 10.1037/a0029144) [DOI] [PubMed] [Google Scholar]
- 83.Bradshaw GA, Capaldo T, Lindner L, Grow G. 2008. Building an inner sanctuary: complex PTSD in chimpanzees. J. Trauma Dissociation 9, 9–34. ( 10.1080/15299730802073619) [DOI] [PubMed] [Google Scholar]
- 84.Bradshaw GA, Capaldo T, Lindner L, Grow G. 2009. Developmental context effects on bicultural posttrauma self repair in chimpanzees. Dev. Psychol. 45, 1376–1388. ( 10.1037/a0015860) [DOI] [PubMed] [Google Scholar]
- 85.Pika S, Zuberbühler K. 2008. Social games between bonobos and humans: evidence for shared intentionality? Am. J. Primatol. 70, 207–210. ( 10.1002/ajp.20469) [DOI] [PubMed] [Google Scholar]
- 86.Engelmann JM, Herrmann E, Tomasello M. 2016. The effects of being watched on resource acquisition in chimpanzees and human children. Anim. Cogn. 19, 147–151. ( 10.1007/s10071-015-0920-y) [DOI] [PubMed] [Google Scholar]
- 87.Coffman G. 2019. The effect of sound on captive chimpanzees (Pan troglodytes). Thesis, Central Washington University, Ellensburg, WA: See https://digitalcommons.cwu.edu/etd/1199. [Google Scholar]
- 88.Rawlings B, Davila-Ross M, Boysen ST. 2014. Semi-wild chimpanzees open hard-shelled fruits differently across communities. Anim. Cogn. 17, 891–899. ( 10.1007/s10071-013-0722-z) [DOI] [PubMed] [Google Scholar]
- 89.Sarabian C, Belais R, MacIntosh AJJ. 2018. Feeding decisions under contamination risk in bonobos. Phil. Trans. R. Soc. B 373, 20170195 ( 10.1098/rstb.2017.0195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wobber V, Hare B, Wrangham R. 2008. Great apes prefer cooked food. J. Hum. Evol. 55, 340–348. ( 10.1016/j.jhevol.2008.03.003) [DOI] [PubMed] [Google Scholar]
- 91.Forss SIF, Motes-Rodrigo A, Hrubesch C, Tennie C. 2019. Differences in novel food response between Pongo and Pan. Am. J. Primatol. 81, e22945 ( 10.1002/ajp.22945) [DOI] [PubMed] [Google Scholar]
- 92.Ross SR, Holmes AN, Lonsdorf EV. 2009. Interactions between zoo-housed great apes and local wildlife. Am. J. Primatol. Off. J. Am. Soc. Primatol. 71, 458–465. ( 10.1002/ajp.20675) [DOI] [PubMed] [Google Scholar]
- 93.Llorente M, Riba D, Mosquera M, Ventura M, Feliu O. 2012. Hunting activity among naturalistically housed chimpanzees (Pan troglodytes) at the Fundació Mona (Girona, Spain). Predation, occasional consumption and strategies in rehabilitated animals. Animals 2, 363–376. ( 10.3390/ani2030363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Leeuwen EJC, Cronin KA, Haun DBM. 2017. Tool use for corpse cleaning in chimpanzees. Sci. Rep. 7, 44091 ( 10.1038/srep44091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cronin KA, van Leeuwen EJC, Mulenga IC, Bodamer MD. 2011. Behavioral response of a chimpanzee mother toward her dead infant. Am. J. Primatol. 73, 415–421. ( 10.1002/ajp.20927) [DOI] [PubMed] [Google Scholar]
- 96.Leeuwen EJC, Mulenga IC, Bodamer MD, Cronin KA. 2016. Chimpanzees' responses to the dead body of a 9-year-old group member. Am. J. Primatol. 78, 914–922. ( 10.1002/ajp.22560) [DOI] [PubMed] [Google Scholar]
- 97.Krupenye C, Hare B. 2018. Bonobos prefer individuals that hinder others over those that help. Curr. Biol. 28, 280–286. ( 10.1016/j.cub.2017.11.061) [DOI] [PubMed] [Google Scholar]
- 98.Schneider A-C, Melis AP, Tomasello M. 2012. How chimpanzees solve collective action problems. Proc. R. Soc. B 279, 4946–4954. ( 10.1098/rspb.2012.1948) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.John M, Duguid S, Tomasello M, Melis AP. 2019. How chimpanzees (Pan troglodytes) share the spoils with collaborators and bystanders. PLoS ONE 14, e0222795 ( 10.1371/journal.pone.0222795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bullinger AF, Melis AP, Tomasello M. 2014. Chimpanzees (Pan troglodytes) instrumentally help but do not communicate in a mutualistic cooperative task. J. Comp. Psychol. 128, 251–260. ( 10.1037/a0035645) [DOI] [PubMed] [Google Scholar]
- 101.Bullinger AF, Melis AP, Tomasello M. 2011. Chimpanzees, Pan troglodytes, prefer individual over collaborative strategies towards goals. Anim. Behav. 82, 1135–1141. ( 10.1016/j.anbehav.2011.08.008) [DOI] [Google Scholar]
- 102.Melis AP, Hare B, Tomasello M. 2006. Chimpanzees recruit the best collaborators. Science 311, 1297–1300. ( 10.1126/science.1123007) [DOI] [PubMed] [Google Scholar]
- 103.Melis AP, Hare B, Tomasello M. 2006. Engineering cooperation in chimpanzees: tolerance constraints on cooperation. Anim. Behav. 72, 275–286. ( 10.1016/j.anbehav.2005.09.018) [DOI] [Google Scholar]
- 104.Melis AP, Hare B, Tomasello M. 2009. Chimpanzees coordinate in a negotiation game. Evol. Hum. Behav. 30, 381–392. ( 10.1016/j.evolhumbehav.2009.05.003) [DOI] [Google Scholar]
- 105.Hare B, Melis AP, Woods V, Hastings S, Wrangham R. 2007. Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Curr. Biol. 17, 619–623. ( 10.1016/j.cub.2007.02.040) [DOI] [PubMed] [Google Scholar]
- 106.Rekers Y, Haun DBM, Tomasello M. 2011. Children, but not chimpanzees, prefer to collaborate. Curr. Biol. 21, 1756–1758. ( 10.1016/j.cub.2011.08.066) [DOI] [PubMed] [Google Scholar]
- 107.Rosati AG, DiNicola LM, Buckholtz JW. 2018. Chimpanzee cooperation is fast and independent from self-control. Psychol. Sci. 29, 1832–1845. ( 10.1177/0956797618800042) [DOI] [PubMed] [Google Scholar]
- 108.Melis AP, Tomasello M. 2019. Chimpanzees (Pan troglodytes) coordinate by communicating in a collaborative problem-solving task. Proc. R. Soc. B 286, 20190408 ( 10.1098/rspb.2019.0408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.MacLean E, Hare B. 2013. Spontaneous triadic engagement in bonobos (Pan paniscus) and chimpanzees (Pan troglodytes). J. Comp. Psychol. 127, 245–255. ( 10.1037/a0030935) [DOI] [PubMed] [Google Scholar]
- 110.Koomen R, Herrmann E. 2018. The effects of social context and food abundance on chimpanzee feeding competition. Am. J. Primatol. 80, e22734 ( 10.1002/ajp.22734) [DOI] [PubMed] [Google Scholar]
- 111.Koomen R, Herrmann E. 2018. Chimpanzees overcome the tragedy of the commons with dominance. Sci. Rep. 8, 1–12. ( 10.1038/s41598-018-28416-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Engelmann JM, Haux LM, Herrmann E. 2019. Helping in young children and chimpanzees shows partiality towards friends. Evol. Hum. Behav. 40, 292–300. ( 10.1016/j.evolhumbehav.2019.01.003) [DOI] [Google Scholar]
- 113.Melis AP, Tomasello M. 2013. Chimpanzees’ (Pan troglodytes) strategic helping in a collaborative task. Biol. Lett. 9, 20130009 ( 10.1098/rsbl.2013.0009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Melis AP, Warneken F, Jensen K, Schneider A-C, Call J, Tomasello M. 2011. Chimpanzees help conspecifics obtain food and non-food items. Proc. R. Soc. B 278, 1405–1413. ( 10.1098/rspb.2010.1735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Warneken F, Hare B, Melis AP, Hanus D, Tomasello M. 2007. Spontaneous altruism by chimpanzees and young children. PLoS Biol. 5, e184 ( 10.1371/journal.pbio.0050184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liebal K, Vaish A, Haun D, Tomasello M. 2014. Does sympathy motivate prosocial behaviour in great apes? PLoS ONE 9, e84299 ( 10.1371/journal.pone.0084299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Melis AP, Schneider A-C, Tomasello M. 2011. Chimpanzees, Pan troglodytes, share food in the same way after collaborative and individual food acquisition. Anim. Behav. 82, 485–493. ( 10.1016/j.anbehav.2011.05.024) [DOI] [Google Scholar]
- 118.Tennie C, Jensen K, Call J. 2016. The nature of prosociality in chimpanzees. Nat. Commun. 7, 13915 ( 10.1038/ncomms13915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Celli ML, Tomonaga M, Udono T, Teramoto M, Nagano K. 2006. Spontaneous object sharing in captive chimpanzees (Pan troglodytes). Int. J. Comp. Psychol. 19, 439–446. [Google Scholar]
- 120.Clay Z, de Waal FBM. 2013. Bonobos respond to distress in others: consolation across the age spectrum. PLoS ONE 8, e55206 ( 10.1371/journal.pone.0055206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hare B, Kwetuenda S. 2010. Bonobos voluntarily share their own food with others. Curr. Biol. 20, R230–R231. ( 10.1016/j.cub.2009.12.038) [DOI] [PubMed] [Google Scholar]
- 122.Krupenye C, Tan J, Hare B. 2018. Bonobos voluntarily hand food to others but not toys or tools. Proc. R. Soc. B 285, 20181536 ( 10.1098/rspb.2018.1536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tan J, Kwetuenda S, Hare B. 2015. Preference or paradigm? Bonobos show no evidence of other-regard in the standard prosocial choice task. Behaviour 152, 521–544. ( 10.1163/1568539X-00003230) [DOI] [Google Scholar]
- 124.Tan J, Hare B. 2013. Bonobos share with strangers. PLoS ONE 8, e51922 ( 10.1371/journal.pone.0051922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Madsen EA, Persson T, Sayehli S, Lenninger S, Sonesson G. 2013. Chimpanzees show a developmental increase in susceptibility to contagious yawning: a test of the effect of ontogeny and emotional closeness on yawn contagion. PLoS ONE 8, e76266 ( 10.1371/journal.pone.0076266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tan J, Ariely D, Hare B. 2017. Bonobos respond prosocially toward members of other groups. Sci. Rep. 7, 1–11. ( 10.1038/s41598-017-15320-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gustafsson E, Jalme MS, Kamoga D, Mugisha L, Snounou G, Bomsel M-C, Krief S. 2016. Food acceptance and social learning opportunities in semi-free eastern chimpanzees (Pan troglodytes schweinfurthii). Ethology 122, 158–170. ( 10.1111/eth.12458) [DOI] [Google Scholar]
- 128.Marshall-Pescini S, Whiten A. 2008. Social learning of nut-cracking behavior in East African sanctuary-living chimpanzees (Pan troglodytes schweinfurthii). J. Comp. Psychol. 122, 186 ( 10.1037/0735-7036.122.2.186) [DOI] [PubMed] [Google Scholar]
- 129.Horner V, Whiten A. 2007. Learning from others' mistakes? Limits on understanding a trap-tube task by young chimpanzees (Pan troglodytes) and children (Homo sapiens). J. Comp. Psychol. 121, 12–21. ( 10.1037/0735-7036.121.1.12) [DOI] [PubMed] [Google Scholar]
- 130.Karg K, Schmelz M, Call J, Tomasello M. 2015. Chimpanzees strategically manipulate what others can see. Anim. Cogn. 18, 1069–1076. ( 10.1007/s10071-015-0875-z) [DOI] [PubMed] [Google Scholar]
- 131.Hanus D, Call J. 2014. When maths trumps logic: probabilistic judgements in chimpanzees. Biol. Lett. 10, 20140892 ( 10.1098/rsbl.2014.0892) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Eckert J, Rakoczy H, Call J, Herrmann E, Hanus D. 2018. Chimpanzees consider humans’ psychological states when drawing statistical inferences. Curr. Biol. 28, 1959–1963. ( 10.1016/j.cub.2018.04.077) [DOI] [PubMed] [Google Scholar]
- 133.Eckert J, Call J, Hermes J, Herrmann E, Rakoczy H. 2018. Intuitive statistical inferences in chimpanzees and humans follow Weber's law. Cognition 180, 99–107. ( 10.1016/j.cognition.2018.07.004) [DOI] [PubMed] [Google Scholar]
- 134.Karg K, Schmelz M, Call J, Tomasello M. 2015. The goggles experiment: can chimpanzees use self-experience to infer what a competitor can see? Anim. Behav. 105, 211–221. ( 10.1016/j.anbehav.2015.04.028) [DOI] [Google Scholar]
- 135.Karg K, Schmelz M, Call J, Tomasello M. 2016. Differing views: can chimpanzees do Level 2 perspective-taking? Anim. Cogn. 19, 555–564. ( 10.1007/s10071-016-0956-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vlamings PHJM, Hare B, Call J. 2010. Reaching around barriers: the performance of the great apes and 3–5-year-old children. Anim. Cogn. 13, 273–285. ( 10.1007/s10071-009-0265-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Horner V, Whiten A. 2005. Causal knowledge and imitation/emulation switching in chimpanzees (Pan troglodytes) and children (Homo sapiens). Anim. Cogn. 8, 164–181. ( 10.1007/s10071-004-0239-6) [DOI] [PubMed] [Google Scholar]
- 138.Tennie C, Call J, Tomasello M. 2010. Evidence for emulation in chimpanzees in social settings using the floating peanut task. PLoS ONE 5, e10544 ( 10.1371/journal.pone.0010544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kirchhofer KC, Zimmermann F, Kaminski J, Tomasello M. 2012. Dogs (Canis familiaris), but not chimpanzees (Pan troglodytes), understand imperative pointing. PLoS ONE 7, e30913 ( 10.1371/journal.pone.0030913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Warneken F, Rosati AG. 2015. Cognitive capacities for cooking in chimpanzees. Proc. R. Soc. B 282, 20150229 ( 10.1098/rspb.2015.0229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.van Leeuwen EJC, Cronin KA, Haun DBM. 2014. A group-specific arbitrary tradition in chimpanzees (Pan troglodytes). Anim. Cogn. 17, 1421–1425. ( 10.1007/s10071-014-0766-8) [DOI] [PubMed] [Google Scholar]
- 142.Haun DBM, Rekers Y, Tomasello M. 2012. Majority-biased transmission in chimpanzees and human children, but not orangutans. Curr. Biol. 22, 727–731. ( 10.1016/j.cub.2012.03.006) [DOI] [PubMed] [Google Scholar]
- 143.Wobber V, Hare B. 2009. Testing the social dog hypothesis: are dogs also more skilled than chimpanzees in non-communicative social tasks? Behav. Processes 81, 423–428. ( 10.1016/j.beproc.2009.04.003) [DOI] [PubMed] [Google Scholar]
- 144.Herrmann E, Hare B, Call J, Tomasello M. 2010. Differences in the cognitive skills of bonobos and chimpanzees. PLoS ONE 5, e12438 ( 10.1371/journal.pone.0012438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Melis AP, Hare B, Tomasello M. 2008. Do chimpanzees reciprocate received favours? Anim. Behav. 76, 951–962. ( 10.1016/j.anbehav.2008.05.014) [DOI] [Google Scholar]
- 146.Rosati AG. 2019. Heterochrony in chimpanzee and bonobo spatial memory development. Am. J. Phys. Anthropol. 169, 302–321. ( 10.1002/ajpa.23833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Endress AD, Carden S, Versace E, Hauser MD. 2010. The apes' edge: positional learning in chimpanzees and humans. Anim. Cogn. 13, 483–495. ( 10.1007/s10071-009-0299-8) [DOI] [PubMed] [Google Scholar]
- 148.Pitman CA, Shumaker RW. 2009. Does early care affect joint attention in great apes (Pan troglodytes, Pan paniscus, Pongo abelii, Pongo pygmaeus, Gorilla gorilla)? J. Comp. Psychol. 123, 334–341. ( 10.1037/a0015840) [DOI] [PubMed] [Google Scholar]
- 149.Wolf W, Tomasello M. 2019. Visually attending to a video together facilitates great ape social closeness. Proc. R. Soc. B 286, 20190488 ( 10.1098/rspb.2019.0488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Herrmann E, Tomasello M. 2015. Focusing and shifting attention in human children (Homo sapiens) and chimpanzees (Pan troglodytes). J. Comp. Psychol. 129, 268–274. ( 10.1037/a0039384) [DOI] [PubMed] [Google Scholar]
- 151.Wobber V, Herrmann E, Hare B, Wrangham R, Tomasello M. 2014. Differences in the early cognitive development of children and great apes. Dev. Psychobiol. 56, 547–573. ( 10.1002/dev.21125) [DOI] [PubMed] [Google Scholar]
- 152.Wobber V, Wrangham R, Hare B. 2010. Bonobos exhibit delayed development of social behavior and cognition relative to chimpanzees. Curr. Biol. 20, 226–230. ( 10.1016/j.cub.2009.11.070) [DOI] [PubMed] [Google Scholar]
- 153.Rosati AG, Hare B. 2011. Chimpanzees and bonobos distinguish between risk and ambiguity. Biol. Lett. 7, 15–18. ( 10.1098/rsbl.2010.0927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rosati AG, Hare B. 2012. Decision making across social contexts: competition increases preferences for risk in chimpanzees and bonobos. Anim. Behav. 84, 869–879. ( 10.1016/j.anbehav.2012.07.010) [DOI] [Google Scholar]
- 155.Rosati AG, Hare B. 2013. Chimpanzees and bonobos exhibit emotional responses to decision outcomes. PLoS ONE 8, e63058 ( 10.1371/journal.pone.0063058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Herrmann E, Haux LM, Zeidler H, Engelmann JM. 2019. Human children but not chimpanzees make irrational decisions driven by social comparison. Proc. R. Soc. B 286, 20182228 ( 10.1098/rspb.2018.2228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nettle D, Cronin KA, Bateson M. 2013. Responses of chimpanzees to cues of conspecific observation. Anim. Behav. 86, 595–602. ( 10.1016/j.anbehav.2013.06.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gruber T, Clay Z, Zuberbühler K. 2010. A comparison of bonobo and chimpanzee tool use: evidence for a female bias in the Pan lineage. Anim. Behav. 80, 1023–1033. ( 10.1016/j.anbehav.2010.09.005) [DOI] [Google Scholar]
- 159.Schweinfurth MK, DeTroy SE, van Leeuwen EJC, Call J, Haun DBM. 2018. Spontaneous social tool use in chimpanzees (Pan troglodytes). J. Comp. Psychol. 132, 455–463. ( 10.1037/com0000127) [DOI] [PubMed] [Google Scholar]
- 160.Bandini E, Tennie C. 2019. Individual acquisition of ‘stick pounding’ behavior by naïve chimpanzees. Am. J. Primatol. 81, e22987 ( 10.1002/ajp.22987) [DOI] [PubMed] [Google Scholar]
- 161.Arroyo A, Hirata S, Matsuzawa T, de la Torre I.. 2016. Nut cracking tools used by captive chimpanzees (Pan troglodytes) and their comparison with early stone age percussive artefacts from Olduvai gorge. PLoS ONE 11, e0166788 ( 10.1371/journal.pone.0166788) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Herrmann E, Wobber V, Call J. 2008. Great apes' (Pan troglodytes, Pan paniscus, Gorilla gorilla, Pongo pygmaeus) understanding of tool functional properties after limited experience. J. Comp. Psychol. 122, 220–230. ( 10.1037/0735-7036.122.2.220) [DOI] [PubMed] [Google Scholar]
- 163.Bandini E. 2018. On the individual learning of primate material culture. PhD thesis, Eberhard Karls University of Tübingen, Tübingen, Germany: See https://publikationen.uni-tuebingen.de/xmlui/bitstream/handle/10900/83653/Bandini%20PhD%20Thesis%20(library%20copy)%20(1).pdf?sequence=1. [Google Scholar]
- 164.Cronin KA, Pieper BA, van Leeuwen EJC, Mundry R, Haun DBM. 2014. Problem solving in the presence of others: how rank and relationship quality impact resource acquisition in chimpanzees (Pan troglodytes). PLoS ONE 9, e93204 ( 10.1371/journal.pone.0093204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Morimura N, Mori Y. 2010. Effects of early rearing conditions on problem-solving skill in captive male chimpanzees (Pan troglodytes). Am. J. Primatol. Off. J. Am. Soc. Primatol. 72, 626–633. ( 10.1002/ajp.20819) [DOI] [PubMed] [Google Scholar]
- 166.Hanus D, Call J. 2011. Chimpanzee problem-solving: contrasting the use of causal and arbitrary cues. Anim. Cogn. 14, 871–878. ( 10.1007/s10071-011-0421-6) [DOI] [PubMed] [Google Scholar]
- 167.Ebel SJ, Schmelz M, Herrmann E, Call J. 2019. Innovative problem solving in great apes: the role of visual feedback in the floating peanut task. Anim. Cogn. 22, 791–805. ( 10.1007/s10071-019-01275-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hanus D, Mendes N, Tennie C, Call J. 2011. Comparing the performances of apes (Gorilla gorilla, Pan troglodytes, Pongo pygmaeus) and human children (Homo sapiens) in the floating peanut task. PLoS ONE 6, e19555 ( 10.1371/journal.pone.0019555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Clay Z, Ravaux L, de Waal FBM, Zuberbühler K. 2016. Bonobos (Pan paniscus) vocally protest against violations of social expectations. J. Comp. Psychol. 130, 44–54. ( 10.1037/a0040088) [DOI] [PubMed] [Google Scholar]
- 170.Rosati AG. 2015. Context influences spatial frames of reference in bonobos (Pan paniscus). Behaviour 152, 375–406. ( 10.1163/1568539X-00003189) [DOI] [Google Scholar]
- 171.Clay Z, Tennie C. 2018. Is overimitation a uniquely human phenomenon? Insights from human children as compared to bonobos. Child Dev. 89, 1535–1544. ( 10.1111/cdev.12857) [DOI] [PubMed] [Google Scholar]
- 172.Rosati AG, Hare B. 2012. Chimpanzees and bonobos exhibit divergent spatial memory development. Dev. Sci. 15, 840–853. ( 10.1111/j.1467-7687.2012.01182.x) [DOI] [PubMed] [Google Scholar]
- 173.Lucca K, MacLean EL, Hare B. 2018. The development and flexibility of gaze alternations in bonobos and chimpanzees. Dev. Sci. 21, e12598 ( 10.1111/desc.12598) [DOI] [PubMed] [Google Scholar]
- 174.Krupenye C, Rosati AG, Hare B. 2015. Bonobos and chimpanzees exhibit human-like framing effects. Biol. Lett. 11, 20140527 ( 10.1098/rsbl.2014.0527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Herrmann E, Keupp S, Hare B, Vaish A, Tomasello M. 2013. Direct and indirect reputation formation in nonhuman great apes (Pan paniscus, Pan troglodytes, Gorilla gorilla, Pongo pygmaeus) and human children (Homo sapiens). J. Comp. Psychol. 127, 63–75. ( 10.1037/a0028929) [DOI] [PubMed] [Google Scholar]
- 176.Dolins FL, Schweller K, Milne S. 2017. Technology advancing the study of animal cognition: using virtual reality to present virtually simulated environments to investigate nonhuman primate spatial cognition. Curr. Zool. 63, 97–108. ( 10.1093/cz/zow121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hopkins WD, Schaeffer J, Russell JL, Bogart SL, Meguerditchian A, Coulon O. 2015. A comparative assessment of handedness and its potential neuroanatomical correlates in chimpanzees (Pan troglodytes) and bonobos (Pan paniscus). Behaviour 152, 461–492. ( 10.1163/1568539X-00003204) [DOI] [Google Scholar]
- 178.Itakura S. 1994. Differentiated responses to different human conditions by chimpanzees. Percept. Mot. Skills 79, 1288–1290. ( 10.2466/pms.1994.79.3.1288) [DOI] [PubMed] [Google Scholar]
- 179.Kano F, Hirata S, Call J. 2015. Social attention in the two species of pan: bonobos make more eye contact than chimpanzees. PLoS ONE 10, e0129684 ( 10.1371/journal.pone.0129684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kano F, Shepherd SV, Hirata S, Call J. 2018. Primate social attention: species differences and effects of individual experience in humans, great apes, and macaques. PLoS ONE 13, e0193283 ( 10.1371/journal.pone.0193283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kano F, Hirata S, Deschner T, Behringer V, Call J. 2016. Nasal temperature drop in response to a playback of conspecific fights in chimpanzees: a thermo-imaging study. Physiol. Behav. 155, 83–94. ( 10.1016/j.physbeh.2015.11.029) [DOI] [PubMed] [Google Scholar]
- 182.Brandenburg M. 2013. Handedness during feeding in chimpanzees (Pan troglodytes) at Chimfunshi Wildlife Orphanage, Zambia. Master's thesis, Iowa State University, Ames, IA: See https://lib.dr.iastate.edu/etd/13210. [Google Scholar]
- 183.Chapelain AS, Hogervorst E, Mbonzo P, Hopkins WD. 2011. Hand preferences for bimanual coordination in 77 bonobos (Pan paniscus): replication and extension. Int. J. Primatol. 32, 491–510. ( 10.1007/s10764-010-9484-5) [DOI] [Google Scholar]
- 184.Fitch WT, Braccini SN. 2013. Primate laterality and the biology and evolution of human handedness: a review and synthesis: primate laterality and the biology and evolution of human handedness. Ann. N. Y. Acad. Sci. 1288, 70–85. ( 10.1111/nyas.12071) [DOI] [PubMed] [Google Scholar]
- 185.Llorente M, Riba D, Palou L, Carrasco L, Mosquera M, Colell M, Feliu O. 2011. Population-level right-handedness for a coordinated bimanual task in naturalistic housed chimpanzees: replication and extension in 114 animals from Zambia and Spain. Am. J. Primatol. 73, 281–290. ( 10.1002/ajp.20895) [DOI] [PubMed] [Google Scholar]
- 186.Forrester GS, Rawlings B, Davila-Ross M. 2016. An analysis of bimanual actions in natural feeding of semi-wild chimpanzees. Am. J. Phys. Anthropol. 159, 85–92. ( 10.1002/ajpa.22845) [DOI] [PubMed] [Google Scholar]
- 187.Meguerditchian A, et al. 2015. Handedness for unimanual grasping in 564 great apes: the effect on grip morphology and a comparison with hand use for a bimanual coordinated task. Front. Psychol. 6, 1794 ( 10.3389/fpsyg.2015.01794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mosquera M, Geribàs N, Bargalló A, Llorente M, Riba D. 2012. Complex tasks force hand laterality and technological behaviour in naturalistically housed chimpanzees: inferences in hominin evolution. Sci. World J. 2012, 1–12. ( 10.1100/2012/514809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Neufuss J, Humle T, Cremaschi A, Kivell TL. 2017. Nut-cracking behaviour in wild-born, rehabilitated bonobos (Pan paniscus): a comprehensive study of hand-preference, hand grips and efficiency. Am. J. Primatol. 79, e22589 ( 10.1002/ajp.22589) [DOI] [PubMed] [Google Scholar]
- 190.Padrell M, Gómez-Martínez C, Llorente M. 2019. Short and long-term temporal consistency of hand preference in sanctuary chimpanzees (Pan troglodytes) for unimanual and bimanual coordinated tasks. Behav. Processes 167, 103911 ( 10.1016/j.beproc.2019.103911) [DOI] [PubMed] [Google Scholar]
- 191.Llorente M, Mosquera M, Fabré M. 2009. Manual laterality for simple reaching and bimanual coordinated task in naturalistic housed Pan troglodytes. Int. J. Primatol. 30, 183–197. ( 10.1007/s10764-009-9338-1) [DOI] [Google Scholar]
- 192.Mosquera M, et al. 2007. Ethological study of manual laterality in naturalistic housed chimpanzees (Pan troglodytes) from the Mona Foundation Sanctuary (Girona, Spain). Laterality Asymmetries Body Brain Cogn. 12, 19–30. ( 10.1080/13576500600886754) [DOI] [PubMed] [Google Scholar]
- 193.Kano F, Hirata S. 2015. Great apes make anticipatory looks based on long-term memory of single events. Curr. Biol. 25, 2513–2517. ( 10.1016/j.cub.2015.08.004) [DOI] [PubMed] [Google Scholar]
- 194.Kano F, Krupenye C, Hirata S, Call J. 2017. Eye tracking uncovered great apes' ability to anticipate that other individuals will act according to false beliefs. Commun. Integr. Biol. 10, e1299836 ( 10.1080/19420889.2017.1299836) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kano F, Krupenye C, Hirata S, Tomonaga M, Call J. 2019. Great apes use self-experience to anticipate an agent's action in a false-belief test. Proc. Natl Acad. Sci. USA 116, 20 904–20 909. ( 10.1073/pnas.1910095116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Krupenye C, Kano F, Hirata S, Call J, Tomasello M. 2016. Great apes anticipate that other individuals will act according to false beliefs. Science 354, 110–114. ( 10.1126/science.aaf8110) [DOI] [PubMed] [Google Scholar]
- 197.Krupenye C, Kano F, Hirata S, Call J, Tomasello M. 2017. A test of the submentalizing hypothesis: apes' performance in a false belief task inanimate control. Commun. Integr. Biol. 10, e1343771 ( 10.1080/19420889.2017.1343771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Drane A, et al. 2019. Cardiac structure and function characterized across age groups and between sexes in healthy wild-born captive chimpanzees (Pan troglodytes) living in sanctuaries. Am. J. Vet. Res. 80, 547–557. ( 10.2460/ajvr.80.6.547) [DOI] [PubMed] [Google Scholar]
- 199.Jones P, Mahamba C, Rest J, André C. 2005. Fatal inflammatory heart disease in a bonobo (Pan paniscus). J. Med. Primatol. 34, 45–49. ( 10.1111/j.1600-0684.2004.00091.x) [DOI] [PubMed] [Google Scholar]
- 200.Strong V, Moittié S, Sheppard MN, Liptovszky M, White K, Redrobe S, Cobb M, Baiker K. 2019. Idiopathic myocardial fibrosis in captive chimpanzees (Pan troglodytes). Vet. Pathol. 57, 183–191. ( 10.1177/0300985819879442) [DOI] [PubMed] [Google Scholar]
- 201.Atencia R, Revuelta L, Somauroo JD, Shave RE. 2015. Electrocardiogram reference intervals for clinically normal wild-born chimpanzees (Pan troglodytes). Am. J. Vet. Res. 76, 688–693. ( 10.2460/ajvr.76.8.688) [DOI] [PubMed] [Google Scholar]
- 202.Atencia R, et al. 2017. Heart rate and indirect blood pressure responses to four different field anesthetic protocols in wild-born captive chimpanzees (Pan troglodytes). J. Zoo Wildl. Med. 48, 636–644. ( 10.1638/2016-0181.1) [DOI] [PubMed] [Google Scholar]
- 203.Fujisawa M, et al. 2014. A case of maxillary sarcoma in a chimpanzee (Pan troglodytes). J. Med. Primatol. 43, 111–114. ( 10.1111/jmp.12086) [DOI] [PubMed] [Google Scholar]
- 204.Suzuki K, Udono T, Fujisawa M, Tanigawa K, Idani G, Ishii N. 2010. Infection during infancy and long incubation period of leprosy suggested in a case of a chimpanzee used for medical research. J. Clin. Microbiol. 48, 3432–3434. ( 10.1128/JCM.00017-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hirata S, Hirai H, Nogami E, Morimura N, Udono T. 2017. Chimpanzee down syndrome: a case study of trisomy 22 in a captive chimpanzee. Primates 58, 267–273. ( 10.1007/s10329-017-0597-8) [DOI] [PubMed] [Google Scholar]
- 206.Wolf TM, Mugisha L, Shoyama FM, O'Malley MJ, Flynn JL, Asiimwe B, Travis DA, Singer RS, Sreevatsan S. 2015. Noninvasive test for tuberculosis detection among primates. Emerg. Infect. Dis. 21, 468–470. ( 10.3201/eid2103.140052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jirků M, et al. 2015. Wild chimpanzees are infected by Trypanosoma brucei. Int. J. Parasitol. Parasites Wildl. 4, 277–282. ( 10.1016/j.ijppaw.2015.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Liu W, et al. 2014. African origin of the malaria parasite Plasmodium vivax. Nat. Commun. 5, 1–10. ( 10.1038/ncomms4346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Duval L, Nerrienet E, Rousset D, Mba SAS, Houze S, Fourment M, Bras JL, Robert V, Ariey F. 2009. Chimpanzee malaria parasites related to Plasmodium ovale in Africa. PLoS ONE 4, e5520 ( 10.1371/journal.pone.0005520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hayakawa T, Arisue N, Udono T, Hirai H, Sattabongkot J, Toyama T, Tsuboi T, Horii T, Tanabe K. 2009. Identification of Plasmodium malariae, a human malaria parasite, in imported chimpanzees. PLoS ONE 4, e7412 ( 10.1371/journal.pone.0007412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Stothard JR, Mugisha L, Standley CJ. 2012. Stopping schistosomes from ‘monkeying-around’ in chimpanzees. Trends Parasitol. 28, 320–326. ( 10.1016/j.pt.2012.05.007) [DOI] [PubMed] [Google Scholar]
- 212.Standley CJ, et al. 2011. Confirmed infection with intestinal schistosomiasis in semi-captive wild-born chimpanzees on Ngamba Island, Uganda. Vector-Borne Zoonotic Dis. 11, 169–176. ( 10.1089/vbz.2010.0156) [DOI] [PubMed] [Google Scholar]
- 213.Standley CJ, Stothard JR. 2012. DNA barcoding of schistosome cercariae reveals a novel sub-lineage within Schistosoma rodhaini from Ngamba Island Chimpanzee Sanctuary, Lake Victoria. J. Parasitol. 98, 1049–1051. ( 10.1645/GE-3091.1) [DOI] [PubMed] [Google Scholar]
- 214.Standley CJ, Mugisha L, Dobson AP, Stothard JR. 2012. Zoonotic schistosomiasis in non-human primates: past, present and future activities at the human–wildlife interface in Africa. J. Helminthol. 86, 131–140. ( 10.1017/S0022149X12000028) [DOI] [PubMed] [Google Scholar]
- 215.Standley CJ, et al. 2013. Intestinal schistosomiasis in chimpanzees on Ngamba Island, Uganda: observations on liver fibrosis, schistosome genetic diversity and praziquantel treatment. Parasitology 140, 285–295. ( 10.1017/S0031182012001576) [DOI] [PubMed] [Google Scholar]
- 216.Hasegawa H, Udono T. 2007. Chimpanzee pinworm, Enterobius anthropopitheci (Nematoda: Oxyuridae), maintained for more than twenty years in captive chimpanzees in Japan. J. Parasitol. 93, 850–853. ( 10.1645/GE-1039R.1) [DOI] [PubMed] [Google Scholar]
- 217.Tachibana H, Cheng X-J, Kobayashi S, Fujita Y, Udono T. 2000. Entamoeba dispar, but not E. histolytica, detected in a colony of chimpanzees in Japan. Parasitol. Res. 86, 537–541. ( 10.1007/s004360000205) [DOI] [PubMed] [Google Scholar]
- 218.Sak B, Kvac M, Petrzelková K, Kvetonová D, Pomajbikova K, Mulama M, Kiyang J, Modrỳ D. 2011. Diversity of microsporidia (Fungi: Microsporidia) among captive great apes in European zoos and African sanctuaries: evidence for zoonotic transmission? Folia Parasitol. (Praha) 58, 81 ( 10.14411/fp.2011.008) [DOI] [PubMed] [Google Scholar]
- 219.Debenham JJ, Atencia R, Midtgaard F, Robertson LJ. 2015. Occurrence of Giardia and Cryptosporidium in captive chimpanzees (Pan troglodytes), mandrills (Mandrillus sphinx) and wild Zanzibar red colobus monkeys (Procolobus kirkii). J. Med. Primatol. 44, 60–65. ( 10.1111/jmp.12158) [DOI] [PubMed] [Google Scholar]
- 220.Ngbolua K-N, Gbatea AK, Ashande CM, Djolu RD, Kamienge MK, Lompoko RB. 2018. A parasitological survey on the feces of Pan paniscus Schwartz (1929) in semi-liberty at ‘Lola ya Bonobo’ sanctuary (Kinshasa city, DR Congo). J. Adv. Bot. Zool. 6, 1–4. [Google Scholar]
- 221.Schaumburg F, Mugisha L, Peck B, Becker K, Gillespie TR, Peters G, Leendertz FH. 2012. Drug-resistant human Staphylococcus aureus in sanctuary apes pose a threat to endangered wild ape populations. Am. J. Primatol. 74, 1071–1075. ( 10.1002/ajp.22067) [DOI] [PubMed] [Google Scholar]
- 222.Unwin S, Robinson I, Schmidt V, Colin C, Ford L, Humle T. 2012. Does confirmed pathogen transfer between sanctuary workers and great apes mean that reintroduction should not occur? Commentary on ‘Drug-resistant human Staphylococcus aureus findings in sanctuary apes and its threat to wild ape populations'. Am. J. Primatol. 74, 1076–1083. ( 10.1002/ajp.22069) [DOI] [PubMed] [Google Scholar]
- 223.Wevers D, et al. 2011. Novel adenoviruses in wild primates: a high level of genetic diversity and evidence of zoonotic transmissions. J. Virol. 85, 10 774–10 784. ( 10.1128/JVI.00810-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Scuda N, et al. 2013. Novel polyomaviruses of nonhuman primates: genetic and serological predictors for the existence of multiple unknown polyomaviruses within the human population. PLoS Pathog. 9, e1003429 ( 10.1371/journal.ppat.1003429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Harvala H, et al. 2012. High seroprevalence of enterovirus infections in apes and old world monkeys. Emerg. Infect. Dis. 18, 283–286. ( 10.3201/eid1802.111363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mombo IM, et al. 2015. First detection of an enterovirus C99 in a captive chimpanzee with acute flaccid paralysis, from the Tchimpounga Chimpanzee Rehabilitation Center, Republic of Congo. PLoS ONE 10, e0136700 ( 10.1371/journal.pone.0136700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sadeuh-Mba SA, et al. 2014. Characterization of enteroviruses from non-human primates in Cameroon revealed virus types widespread in humans along with candidate new types and species. PLoS Negl. Trop. Dis. 8, e3052 ( 10.1371/journal.pntd.0003052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yamamoto R, Teramoto M, Hayasaka I, Ikeda K, Hasegawa T, Ishida T. 2010. Reactivation of lymphocryptovirus (Epstein–Barr virus chimpanzee) and dominance in chimpanzees. J. Gen. Virol. 91, 2049–2053. ( 10.1099/vir.0.022376-0) [DOI] [PubMed] [Google Scholar]
- 229.Leendertz FH, et al. 2009. Novel cytomegaloviruses in free-ranging and captive great apes: phylogenetic evidence for bidirectional horizontal transmission. J. Gen. Virol. 90, 2386–2394. ( 10.1099/vir.0.011866-0) [DOI] [PubMed] [Google Scholar]
- 230.Mugisha L, Leendertz FH, Opuda-Asibo J, Olobo JO, Ehlers B. 2010. A novel herpesvirus in the sanctuary chimpanzees on Ngamba Island in Uganda. J. Med. Primatol. 39, 71–76. ( 10.1111/j.1600-0684.2009.00396.x) [DOI] [PubMed] [Google Scholar]
- 231.Mugisha L, Pauli G, Opuda-Asibo J, Joseph OO, Leendertz FH, Diedrich S. 2010. Evaluation of poliovirus antibody titers in orally vaccinated semi-captive chimpanzees in Uganda. J. Med. Primatol. 39, 123–128. ( 10.1111/j.1600-0684.2010.00400.x) [DOI] [PubMed] [Google Scholar]
- 232.Mugisha L, Kucherer C, Ellerbrok H, Junglen S, Opuda-Asibo J, Joseph OO, Pauli G, Leendertz FH. 2010. Retroviruses in wild-born semi-captive east African sanctuary chimpanzees (Pan troglodytes schweinfurthii). Open Vet. Sci. J. 4, 6–10. ( 10.2174/1874318801004010006) [DOI] [Google Scholar]
- 233.Mombo IM, Berthet N, Bouchier C, Fair JN, Schneider BS, Renaud F, Leroy EM, Rougeron V. 2014. Characterization of a genogroup I sapovirus isolated from chimpanzees in the Republic of Congo. Genome Announc. 2, pii. e00680-14.. ( 10.1128/genomeA.00680-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Köndgen S, et al. 2017. Evidence for human Streptococcus pneumoniae in wild and captive chimpanzees: a potential threat to wild populations. Sci. Rep. 7, 1–8. ( 10.1038/s41598-017-14769-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lyons S, et al. 2012. Species association of hepatitis B virus (HBV) in non-human apes; evidence for recombination between gorilla and chimpanzee variants. PLoS ONE 7, e33430 ( 10.1371/journal.pone.0033430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Starkman SE, MacDonald DM, Lewis JCM, Holmes EC, Simmonds P. 2003. Geographic and species association of hepatitis B virus genotypes in non-human primates. Virology 314, 381–393. ( 10.1016/S0042-6822(03)00430-6) [DOI] [PubMed] [Google Scholar]
- 237.Mugisha L, Kaiser M, Ellerbrok H, Pauli G, Opuda-Asibo J, Joseph OO, Leendertz FH. 2011. The ‘original’ hepatitis B virus of eastern chimpanzees (Pan trogrodytes schweinfurthii). Virus Res. 155, 372–375. ( 10.1016/j.virusres.2010.10.017) [DOI] [PubMed] [Google Scholar]
- 238.Mugisha L, Kücherer C, Ellerbrok H, Junglen S, Opuda-Asibo J, Joseph OO, Pauli G, Ehlers B, Leendertz FH. 2011. Multiple viral infections in confiscated wild born semi-captive chimpanzees (Pan troglodytes schweinfurthii) in a sanctuary in Uganda: implications for sanctuary management and conservation. In Proc. AAZV Conf., 190–195. [Google Scholar]
- 239.Obanda V, Omondi GP, Chiyo PI. 2014. The influence of body mass index, age and sex on inflammatory disease risk in semi-captive chimpanzees. PLoS ONE 9, e104602 ( 10.1371/journal.pone.0104602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Modrý D, Petrželková KJ, Pomajbíková K, Tokiwa T, Křížek J, Imai S, Vallo P, Profousová I, Šlapeta J. 2009. The occurrence and ape-to-ape transmission of the entodiniomorphid ciliate Troglodytella abrassarti in captive gorillas. J. Eukaryot. Microbiol. 56, 83–87. ( 10.1111/j.1550-7408.2008.00369.x) [DOI] [PubMed] [Google Scholar]
- 241.Ellis RJ, Bruce KD, Jenkins C, Stothard JR, Ajarova L, Mugisha L, Viney ME. 2013. Comparison of the distal gut microbiota from people and animals in Africa. PLoS ONE 8, e54783 ( 10.1371/journal.pone.0054783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Schaumburg F, et al. 2013. Evaluation of non-invasive biological samples to monitor Staphylococcus aureus colonization in great apes and lemurs. PLoS ONE 8, e78046 ( 10.1371/journal.pone.0078046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Pomajbíková K, Petrželková KJ, Petrášová J, Profousová I, Kalousová B, Jirků M, Sá RM, Modrý D. 2012. Distribution of the entodiniomorphid ciliate Troglocorys cava Tokiwa, Modrý, Ito, Pomajbíková, Petrželková, & Imai, (Entodiniomorphida: Blepharocorythidae) in wild and captive chimpanzees. J. Eukaryot. Microbiol. 59, 97–99. ( 10.1111/j.1550-7408.2011.00586.x) [DOI] [PubMed] [Google Scholar]
- 244.Vincent O, Stephen C, Patrick C. 2012. Anti-helminth-induced changes in the prevalence of entodiniomorphid ciliates in semi-captive chimpanzees. Afr. J. Ecol. 50, 60–65. ( 10.1111/j.1365-2028.2011.01294.x) [DOI] [Google Scholar]
- 245.Doležalová J, et al. 2015. Molecular phylogeny of anoplocephalid tapeworms (Cestoda: Anoplocephalidae) infecting humans and non-human primates. Parasitology 142, 1278–1289. ( 10.1017/S003118201500058X) [DOI] [PubMed] [Google Scholar]
- 246.Irbis C, Garriga R, Kabasawa A, Ushida K. 2008. Phylogenetic analysis of Troglodytella abrassarti isolated from Chimpanzees (Pan troglodytes verus) in the wild and in captivity. J. Gen. Appl. Microbiol. 54, 409–413. ( 10.2323/jgam.54.409) [DOI] [PubMed] [Google Scholar]
- 247.Li J, et al. 2013. The saliva microbiome of Pan and Homo. BMC Microbiol. 13, 204 ( 10.1186/1471-2180-13-204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Clayton JB, et al. 2016. Captivity humanizes the primate microbiome. Proc. Natl Acad. Sci. USA 113, 10 376–10 381. ( 10.1073/pnas.1521835113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Moodley Y, et al. 2012. Age of the association between Helicobacter pylori and man. PLoS Pathog. 8, e1002693 ( 10.1371/journal.ppat.1002693) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mugisha L, Köndgen S, Kaddu-Mulindwa D, Gaffikin L, Leendertz FH. 2014. Nasopharyngeal colonization by potentially pathogenic bacteria found in healthy semi-captive wild-born chimpanzees in Uganda: Chimpanzee Nasopharyngeal Colonization. Am. J. Primatol. 76, 103–110. ( 10.1002/ajp.22212) [DOI] [PubMed] [Google Scholar]
- 251.Verhulst NO, Umanets A, Weldegergis BT, Maas JPA, Visser TM, Dicke M, Smidt H, Takken W. 2018. Do apes smell like humans? The role of skin bacteria and volatiles of primates in mosquito host selection. J. Exp. Biol. 221, jeb185959 ( 10.1242/jeb.185959) [DOI] [PubMed] [Google Scholar]
- 252.Flahou B, et al. 2014. Diversity of zoonotic enterohepatic Helicobacter species and detection of a putative novel gastric Helicobacter species in wild and wild-born captive chimpanzees and western lowland gorillas. Vet. Microbiol. 174, 186–194. ( 10.1016/j.vetmic.2014.08.032) [DOI] [PubMed] [Google Scholar]
- 253.Ronke C, et al. 2015. Lineage-specific changes in biomarkers in great apes and humans. PLoS ONE 10, e0134548 ( 10.1371/journal.pone.0134548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Gamble KC, Moyse JA, Lovstad JN, Ober CB, Thompson EE. 2011. Blood groups in the species survival plan®, European endangered species program, and managed in situ populations of bonobo (Pan paniscus), common chimpanzee (Pan troglodytes), gorilla (Gorilla ssp.), and orangutan (Pongo pygmaeus ssp.). Zoo Biol. 30, 427–444. ( 10.1002/zoo.20348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Debenham JJ, Atencia R. 2014. Homologous whole blood transfusion during treatment of severe anemia in a chimpanzee (Pan troglodytes). J. Zoo Wildl. Med. 45, 654–657. ( 10.1638/2013-0091R2.1) [DOI] [PubMed] [Google Scholar]
- 256.Capaldo T, Peppercorn M. 2012. A review of autopsy reports on chimpanzees in or from US laboratories. Altern. Lab. Anim. 40, 259–269. ( 10.1177/026119291204000505) [DOI] [PubMed] [Google Scholar]
- 257.Havercamp K, Watanuki K, Tomonaga M, Matsuzawa T, Hirata S. 2019. Longevity and mortality of captive chimpanzees in Japan from 1921 to 2018. Primates 60, 525–535. ( 10.1007/s10329-019-00755-8) [DOI] [PubMed] [Google Scholar]
- 258.Carlitz EHD, Kirschbaum C, Miller R, Rukundo J, van Schaik CP. 2015. Effects of body region and time on hair cortisol concentrations in chimpanzees (Pan troglodytes). Gen. Comp. Endocrinol. 223, 9–15. ( 10.1016/j.ygcen.2015.09.022) [DOI] [PubMed] [Google Scholar]
- 259.Yamanashi Y, Teramoto M, Morimura N, Hirata S, Suzuki J, Hayashi M, Kinoshita K, Murayama M, Idani G. 2016. Analysis of hair cortisol levels in captive chimpanzees: effect of various methods on cortisol stability and variability. MethodsX 3, 110–117. ( 10.1016/j.mex.2016.01.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yamanashi Y, Morimura N, Mori Y, Hayashi M, Suzuki J. 2013. Cortisol analysis of hair of captive chimpanzees (Pan troglodytes). Gen. Comp. Endocrinol. 194, 55–63. ( 10.1016/j.ygcen.2013.08.013) [DOI] [PubMed] [Google Scholar]
- 261.Yamanashi Y, Teramoto M, Morimura N, Nogami E, Hirata S. 2018. Social relationship and hair cortisol level in captive male chimpanzees (Pan troglodytes). Primates 59, 145–152. ( 10.1007/s10329-017-0641-8) [DOI] [PubMed] [Google Scholar]
- 262.Yamanashi Y, Teramoto M, Morimura N, Hirata S, Inoue-Murayama M, Idani G. 2016. Effects of relocation and individual and environmental factors on the long-term stress levels in captive chimpanzees (Pan troglodytes): monitoring hair cortisol and behaviors. PLoS ONE 11, e0160029 ( 10.1371/journal.pone.0160029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hauser B, Mugisha L, Preis A, Deschner T. 2011. LC–MS analysis of androgen metabolites in serum and urine from east African chimpanzees (Pan troglodytes schweinfurthii). Gen. Comp. Endocrinol. 170, 92–98. ( 10.1016/j.ygcen.2010.09.012) [DOI] [PubMed] [Google Scholar]
- 264.Preis A, Mugisha L, Hauser B, Weltring A, Deschner T. 2011. Androgen and androgen metabolite levels in serum and urine of East African chimpanzees (Pan troglodytes schweinfurthii): comparison of EIA and LC–MS analyses. Gen. Comp. Endocrinol. 174, 335–343. ( 10.1016/j.ygcen.2011.09.010) [DOI] [PubMed] [Google Scholar]
- 265.Kutsukake N, et al. 2009. Validation of salivary cortisol and testosterone assays in chimpanzees by liquid chromatography-tandem mass spectrometry. Am. J. Primatol. 71, 696–706. ( 10.1002/ajp.20708) [DOI] [PubMed] [Google Scholar]
- 266.Wobber V, Hare B, Lipson S, Wrangham R, Ellison P. 2013. Different ontogenetic patterns of testosterone production reflect divergent male reproductive strategies in chimpanzees and bonobos. Physiol. Behav. 116-117, 44–53. ( 10.1016/j.physbeh.2013.03.003) [DOI] [PubMed] [Google Scholar]
- 267.Wobber V, Herrmann E. 2015. The influence of testosterone on cognitive performance in bonobos and chimpanzees. Behaviour 152, 407–423. ( 10.1163/1568539X-00003202) [DOI] [Google Scholar]
- 268.Wobber V, Hare B, Maboto J, Lipson S, Wrangham R, Ellison PT. 2010. Differential changes in steroid hormones before competition in bonobos and chimpanzees. Proc. Natl Acad. Sci. USA 107, 12 457–12 462. ( 10.1073/pnas.1007411107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Takeshita RSC, Huffman MA, Kinoshita K, Bercovitch FB. 2017. Effect of castration on social behavior and hormones in male Japanese macaques (Macaca fuscata). Physiol. Behav. 181, 43–50. ( 10.1016/j.physbeh.2017.09.006) [DOI] [PubMed] [Google Scholar]
- 270.Thompson ME, et al. 2007. Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Curr. Biol. 17, 2150–2156. ( 10.1016/j.cub.2007.11.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kikuchi Y. 2010. Comparative analysis of muscle architecture in primate arm and forearm. Anat. Histol. Embryol. 39, 93–106. ( 10.1111/j.1439-0264.2009.00986.x) [DOI] [PubMed] [Google Scholar]
- 272.Kikuchi Y, Takemoto H, Kuraoka A. 2012. Relationship between humeral geometry and shoulder muscle power among suspensory, knuckle-walking, and digitigrade/palmigrade quadrupedal primates. J. Anat. 220, 29–41. ( 10.1111/j.1469-7580.2011.01451.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.McIntyre MH, Herrmann E, Wobber V, Halbwax M, Mohamba C, de Sousa N, Atencia R, Cox D, Hare B. 2009. Bonobos have a more human-like second-to-fourth finger length ratio (2D:4D) than chimpanzees: a hypothesized indication of lower prenatal androgens. J. Hum. Evol. 56, 361–365. ( 10.1016/j.jhevol.2008.12.004) [DOI] [PubMed] [Google Scholar]
- 274.Garrod B, Roberts AM, Duhig C, Cox D, McGrew W. 2015. Burial, excavation, and preparation of primate skeletal material for morphological study. Primates 56, 311–316. ( 10.1007/s10329-015-0480-4) [DOI] [PubMed] [Google Scholar]
- 275.Saito A, et al. 2003. Behavioral evidence of color vision deficiency in a protanomalia chimpanzee (Pan troglodytes). Primates 44, 171–176. ( 10.1007/s10329-002-0017-5) [DOI] [PubMed] [Google Scholar]
- 276.Terao K, et al. 2005. Identification of a protanomalous chimpanzee by molecular genetic and electroretinogram analyses. Vision Res. 45, 1225–1235. ( 10.1016/j.visres.2004.11.016) [DOI] [PubMed] [Google Scholar]
- 277.Hayakawa T, Sugawara T, Go Y, Udono T, Hirai H, Imai H. 2012. Eco-geographical diversification of bitter taste receptor genes (TAS2Rs) among subspecies of chimpanzees (Pan troglodytes). PLoS ONE 7, e43277 ( 10.1371/journal.pone.0043277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Sugawara T, Go Y, Udono T, Morimura N, Tomonaga M, Hirai H, Imai H. 2011. Diversification of bitter taste receptor gene family in western chimpanzees. Mol. Biol. Evol. 28, 921–931. ( 10.1093/molbev/msq279) [DOI] [PubMed] [Google Scholar]
- 279.Hamada Y, Udono T, Teramoto M, Sugawara T. 1996. The growth pattern of chimpanzees: somatic growth and reproductive maturation in Pan troglodytes. Primates 37, 279–295. ( 10.1007/BF02381860) [DOI] [Google Scholar]
- 280.Hamada Y, Udono T. 2002. Longitudinal analysis of length growth in the chimpanzee (Pan troglodytes). Am. J. Phys. Anthropol. 118, 268–284. ( 10.1002/ajpa.10078) [DOI] [PubMed] [Google Scholar]
- 281.Suzuki K, Yoshimoto N, Shimoda K, Sakamoto W, Ide Y, Kaneko T, Nakashima T, Hayasaka I, Nakagata N. 2004. Cytoplasmic dysmorphisms in metaphase II chimpanzee oocytes. Reprod. Biomed. Online 9, 54–58. ( 10.1016/S1472-6483(10)62110-4) [DOI] [PubMed] [Google Scholar]
- 282.Navarro-Serra A, Sanz-Cabañes H. 2019. Subcutaneous thermal sensor microchip validation in vervet monkeys (Chlorocebus pygerythrus) during cnormothermic and hypothermic situations. J. Med. Primatol. 48, 77–81. ( 10.1111/jmp.12398) [DOI] [PubMed] [Google Scholar]
- 283.Sato Y, Hirata S, Kano F. 2019. Spontaneous attention and psycho-physiological responses to others' injury in chimpanzees. Anim. Cogn. 22, 807–823. ( 10.1007/s10071-019-01276-z) [DOI] [PubMed] [Google Scholar]
- 284.Inoue-Murayama M, Weiss A, Morimura N, Tanaka M, Yamagiwa J, Idani G. 2011. Molecular behavioral research in great apes. In From genes to animal behavior, pp. 239–253. Berlin, Germany: Springer. [Google Scholar]
- 285.Fukuda K, et al. 2013. Regional DNA methylation differences between humans and chimpanzees are associated with genetic changes, transcriptional divergence and disease genes. J. Hum. Genet. 58, 446–454. ( 10.1038/jhg.2013.55) [DOI] [PubMed] [Google Scholar]
- 286.Sakate R, et al. 2003. Analysis of 5′-end sequences of chimpanzee cDNAs. Genome Res. 13, 1022–1026. ( 10.1101/gr.783103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Takenaka A, Nakamura S, Mitsunaga F, Inoue-Murayama M, Udono T, Suryobroto B. 2012. Human-specific SNP in obesity genes, adrenergic receptor beta2 (ADRB2), Beta3 (ADRB3), and PPAR γ2 (PPARG), during primate evolution. PLoS ONE 7, e43461 ( 10.1371/journal.pone.0043461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Uesaka M, Agata K, Oishi T, Nakashima K, Imamura T. 2017. Evolutionary acquisition of promoter-associated non-coding RNA (pancRNA) repertoires diversifies species-dependent gene activation mechanisms in mammals. BMC Genomics 18, 285 ( 10.1186/s12864-017-3662-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Ghobrial L, Lankester F, Kiyang JA, Akih AE, de Vries S, Fotso R, Gadsby EL, Jenkins PD, Gonder MK. 2010. Tracing the origins of rescued chimpanzees reveals widespread chimpanzee hunting in Cameroon. BMC Ecol. 10, 2 ( 10.1186/1472-6785-10-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Gonder MK, Locatelli S, Ghobrial L, Mitchell MW, Kujawski JT, Lankester FJ, Stewart C-B, Tishkoff SA. 2011. Evidence from Cameroon reveals differences in the genetic structure and histories of chimpanzee populations. Proc. Natl Acad. Sci. USA 108, 4766–4771. ( 10.1073/pnas.1015422108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Jin X, et al. 2012. An effort to use human-based exome capture methods to analyze chimpanzee and macaque exomes. PLoS ONE 7, e40637 ( 10.1371/journal.pone.0040637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Johnson KP, Allen JM, Olds BP, Mugisha L, Reed DL, Paige KN, Pittendrigh BR. 2014. Rates of genomic divergence in humans, chimpanzees and their lice. Proc. R. Soc. B 281, 20132174 ( 10.1098/rspb.2013.2174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.de Manuel M, et al. 2016. Chimpanzee genomic diversity reveals ancient admixture with bonobos. Science 354, 477–481. ( 10.1126/science.aag2602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Prado-Martinez J, et al. 2013. Great ape genetic diversity and population history. Nature 499, 471–475. ( 10.1038/nature12228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Good JM, et al. 2013. Comparative population genomics of the ejaculate in humans and the great apes. Mol. Biol. Evol. 30, 964–976. ( 10.1093/molbev/mst005) [DOI] [PubMed] [Google Scholar]
- 296.Maibach V, Hans JB, Hvilsom C, Marques-Bonet T, Vigilant L. 2017. MHC class I diversity in chimpanzees and bonobos. Immunogenetics 69, 661–676. ( 10.1007/s00251-017-0990-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Teixeira JC, et al. 2015. Long-term balancing selection in lad1 maintains a missense trans-species polymorphism in humans, chimpanzees, and bonobos. Mol. Biol. Evol. 32, 1186–1196. ( 10.1093/molbev/msv007) [DOI] [PubMed] [Google Scholar]
- 298.Hong K-W, Weiss A, Morimura N, Udono T, Hayasaka I, Humle T, Murayama Y, Ito S, Inoue-Murayama M. 2011. Polymorphism of the tryptophan hydroxylase 2 (TPH2) gene is associated with chimpanzee neuroticism. PLoS ONE 6, e22144 ( 10.1371/journal.pone.0022144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Ito H, Udono T, Hirata S, Inoue-Murayama M. 2018. Estimation of chimpanzee age based on DNA methylation. Sci. Rep. 8, 1–5. ( 10.1038/s41598-018-28318-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Herd KE, Barker SC, Shao R. 2015. The mitochondrial genome of the chimpanzee louse, Pediculus schaeffi: insights into the process of mitochondrial genome fragmentation in the blood-sucking lice of great apes. BMC Genomics 16, 661 ( 10.1186/s12864-015-1843-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Weiss A, King JE, Inoue-Murayama M, Matsuzawa T, Oswald AJ. 2012. Evidence for a midlife crisis in great apes consistent with the U-shape in human well-being. Proc. Natl Acad. Sci. USA 109, 19 949–19 952. ( 10.1073/pnas.1212592109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Altschul DM, Hopkins WD, Herrelko ES, Inoue-Murayama M, Matsuzawa T, King JE, Ross SR, Weiss A. 2018. Personality links with lifespan in chimpanzees. eLife 7, e33781 ( 10.7554/eLife.33781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Brittain RS, Corr JA.2009. Determining personality in sanctuary chimpanzees (Pan troglodytes). Student Summer Scholars. 21. See http://scholarworks.gvsu.edu/sss/21 .
- 304.Herrmann E, Hare B, Cissewski J, Tomasello M. 2011. A comparison of temperament in nonhuman apes and human infants. Dev. Sci. 14, 1393–1405. ( 10.1111/j.1467-7687.2011.01082.x) [DOI] [PubMed] [Google Scholar]
- 305.Weiss A, Inoue-Murayama M, Hong K-W, Inoue E, Udono T, Ochiai T, Matsuzawa T, Hirata S, King JE. 2009. Assessing chimpanzee personality and subjective well-being in Japan. Am. J. Primatol. Off. J. Am. Soc. Primatol. 71, 283–292. ( 10.1002/ajp.20649) [DOI] [PubMed] [Google Scholar]
- 306.Wilson VA, Weiss A, Humle T, Morimura N, Udono T, Idani G, Matsuzawa T, Hirata S, Inoue-Murayama M. 2017. Chimpanzee personality and the arginine vasopressin receptor 1A genotype. Behav. Genet. 47, 215–226. ( 10.1007/s10519-016-9822-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Úbeda Y, Llorente M. 2015. Personality in sanctuary-housed chimpanzees: a comparative approach of psychobiological and penta-factorial human models. Evol. Psychol. 13, 182–196. ( 10.1177/147470491501300111) [DOI] [PubMed] [Google Scholar]
- 308.ManyPrimates, Altschul DM, et al. 2019. Establishing an infrastructure for collaboration in primate cognition research. PLoS ONE 14, e0223675 ( 10.1371/journal.pone.0223675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Grimm D. 2019. Ready to retire? Science 366, 1182–1185. ( 10.1126/science.366.6470.1182) [DOI] [PubMed] [Google Scholar]
- 310.Miller G. 2007. Sanctuaries aid research and vice versa. Science 317, 1339 ( 10.1126/science.317.5843.1339) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.