Abstract
Insular ecosystems share analogous ecological conditions, leading to patterns of convergent evolution that are collectively termed as the ‘island syndrome’. In birds, part of this syndrome is a tendency for a duller plumage, possibly as a result of relaxed sexual selection. Despite this global pattern, some insular species display a more colourful plumage than their mainland relatives, but why this occurs has remained unexplained. Here, we examine the hypothesis that these cases of increased plumage coloration on islands could arise through a relaxation of predation pressure. We used comparative analyses to investigate whether average insular richness of raptors of suitable mass influences the plumage colourfulness and brightness across 110 pairs of insular endemic species and their closest mainland relatives. As predicted, we find a likely negative relationship between insular coloration and insular predation while controlling for mainland predation and coloration, suggesting that species were more likely to become more colourful as the number of insular predators decreased. By contrast, plumage brightness was not influenced by predation pressure. Relaxation from predation, together with drift, might thus be a key mechanism of species phenotypic responses to insularity.
Keywords: insularity, colour volume, comparative analysis, plumage coloration, signal evolution
1. Introduction
Islands are fascinating systems to study biological evolution as they are replicates of geographically isolated systems that share similar ecological characteristics. Their reduced area and simplified biota, including impoverished predator and parasite communities, are usually associated with repeated patterns of convergent evolution [1,2] grouped under the name ‘island syndrome’ [3]. In birds, this syndrome includes changes in coloration, with many insular species exhibiting less colourful and duller (i.e. less bright) ornamentations [4–6]. This decrease in coloration has been proposed to result from a relaxation of two selective forces: selection for species recognition [4–6] and intersexual selection [4,7].
However, not all species respond to insularity in a similar way. Some species living on small islands show a ‘reversed island syndrome’, with increased coloration [4], sexual dimorphism and aggression [8]. Why does coloration increase more often on small islands wherein the decrease should be more marked, given a stronger relaxation of mate choice and need for species recognition? Changes in other selection pressures like predation, as well as changes in the intensity of drift [9], could explain the cases of a reversed island syndrome. Here, we conducted a study to investigate how variation in insular predation relates to island birds' coloration while controlling for the coloration and predation faced by their mainland relatives.
With the exception of aposematism, predation is usually thought to select for inconspicuous signals [10–12, but see 13]. This effect is further supported by the observation that many birds tend to display a conspicuous plumage coloration on their ventral part (hidden to aerial predators) and a cryptic coloration on their back (exposed to predators) [14]. In insular ecosystems, where predator richness is reduced [15], a relaxation from predation pressure could be associated with an increase in conspicuousness. In agreement, the relaxation from predation pressure on small islets influences coloration traits of a lizard species, which evolved towards poorer background matching, hence higher conspicuousness [16].
We studied 110 pairs of endemic insular bird species and their mainland closest relatives and examined whether insular coloration is associated with the number of predator species on islands while controlling for mainland coloration and predation. If predation is an important driver of bird colour evolution on islands, we predict a negative relationship between insular predator richness and insular coloration, so that island species facing more predators are more prone to show a decrease in plumage coloration. Secondly, under the hypothesis that the dorsal part of birds is more likely to be under a predation-driven selection for crypsis [14,17] than the ventral part, we expect the dorsal area of birds to be more influenced by changes in predation.
2. Methods
(a). Data collection
The dataset is composed of 220 bird species (electronic supplementary material, appendix S1), corresponding to 110 endemic insular species from 46 different archipelagos and their closest mainland relatives from a similar latitude [4]. For the present study, we selected only bird species that are not themselves birds of prey. We used spectrometry measurements of plumage coloration for three males and three females of each species (see electronic supplementary material, appendix S2 for details regarding plumage variation) from a previous study by Doutrelant et al. [4] (excepting four new pairs measured in 2018). Reflectance spectra were converted to bird-specific photoreceptor excitations with the Goldsmith model of tetrahedral colour space for a violet sensitive vision [18,19], using the R package pavo [20]. Indeed, predators considered in this study are raptors, which only have limited ultraviolet vision [21,22].
The intensity of coloration was estimated by the brightness, which describes the intensity of the achromatic component of a signal, and the colour volume, defined as the minimal convex volume in the tetrahedral colour space that contains all the patches measured. It quantifies the diversity of colours and is used to describe colourfulness [18].
To estimate predation pressure, we collated data on the average specific richness of sympatric avian predators across the range of each species [23–29] and computed predator–prey mass allometry relationships to exclude some predators based on size mismatch with potential preys [23,30] (electronic supplementary material, appendix S3). Predator densities or species-specific estimates of raptor-driven mortality would provide additional resolution but are not available for such a large-scale study. Predators included were all species from the Accipitriformes and Falconiformes orders, which are known predators of adult birds. Indigenous terrestrial mammals are scarce or lacking on islands, representing a marginal predation pressure on adult birds compared to avian predators [23,31]. Moreover, mammals are mostly blind to ultraviolet and possess a poorer colour vision as they only have two types of photoreceptors (versus four in birds). We did not include snakes because they likely represent a less important predation pressure as only a small number of species specialize on adult birds, even though a few of them are known to be present on some of the islands considered (e.g. Boiga irregularis [32]). Nocturnal avian predators (Strigiformes) were excluded, as their eyes are mostly composed of rod cells, resulting in a poor colour vision [33]. Resident, breeding, and wintering ranges of all bird species considered in this study were retrieved from BirdLife International and NatureServe [34] and HBW [35], using a 10-min resolution grid. For polytypic species, we used only the geographical range of the subspecies that was measured by spectrometry, as subspecies usually differ in coloration. When the range of a subspecies was too complicated to delimitate, the whole range of the species was used. All species range maps from Accipitriformes and Falconiformes (n = 309) were collected at the same spatial resolution, and the number of grid cells shared between each focal species and each raptor species was calculated.
(b). Statistical analyses
We tested the effect of predator richness on the coloration of insular birds while controlling for the coloration and predator richness of their mainland closest relative [7,15,36,37]. The dependent variables were the estimates of coloration of the insular species: colour volume and brightness, which were averaged for males and females of each species. Colour volume was log transformed prior to analyses. Analyses were performed with the R software, v. 3.5.1 [38].
We first investigated how colour volume and brightness of insular species were influenced by predation, considering the whole body of birds, using 107 pairs of species with known colour volumes (three species were excluded as at least four distinct colour patches are needed to compute a volume) and 110 pairs with known brightness. Explanatory variables were sex, insular predation, continental predation, the interaction of both terms with sex, and the absolute value of latitude of the insular species. We also included as covariate the coloration of the continental species of the pair. Island size was not included in the model to avoid collinearity due to its positive correlation with insular predation (r = 0.51; p < 0.001).
We then tested the influence of predation pressure on insular brightness for different body parts separately. The ventral parts and the head are assumed to be more prone to sexual selection compared to other body parts. The dorsal parts (i.e. tail, outer wing and back), more likely to be seen from above by avian predators, are potentially selected for crypsis [14]. The colour volume has not been calculated for different body parts as at least four measurements are needed to compute a volume in a tetrahedral colour space and not all species displayed enough visually distinct colour patches within each body part. Explanatory variables were the same as in previous models, and ‘body part’ was included as a two-level factor (ventral patches/dorsal patches), along with interactions with insular and continental predation.
As species do not represent independent data points due to shared ancestry, all models accounted for the phylogenetic dependence between species using Bayesian phylogenetic mixed models (BPMMs). BPMMs were done using the R package MulTree [39], with a species random effect linked to the phylogeny. All simulations were performed on 100 alternative trees from the most recent known phylogeny [40] instead of a consensus tree, to account for phylogenetic uncertainty [41] (electronic supplementary material, appendix S4).
3. Results
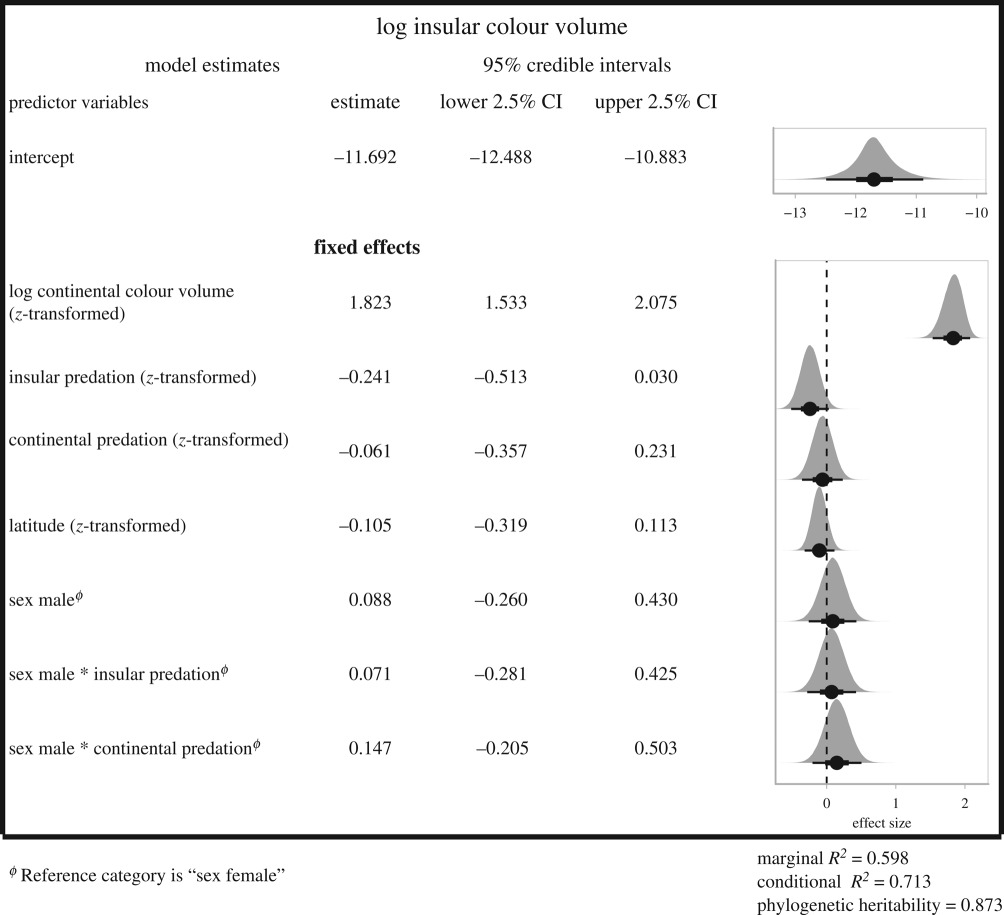
Most islands present decreased average predation richness (electronic supplementary material, appendix S5, figure A1). Following our expectations, we found a likely negative effect of insular predator richness on insular colour volume while accounting for continental colour volume and predation (estimate: −0.241 [95% CI: −0.513; 0.030]; figure 1 and table 1; negative effect of insular predation estimated from the posterior distribution in 95.9% of the cases). Insular species are on average as colourful as their mainland counterparts in the absence of avian predators but are more likely to be less colourful as the number of insular predators increased (figure 1). Similarly, insular species facing a large decrease in predation pressure compared to their mainland counterparts tend to display an increased coloration (electronic supplementary material, appendix S5, table A1 and figure A1). Latitude, sex and the interaction between sex and predator richness did not have any clear effect (table 1).
Figure 1.
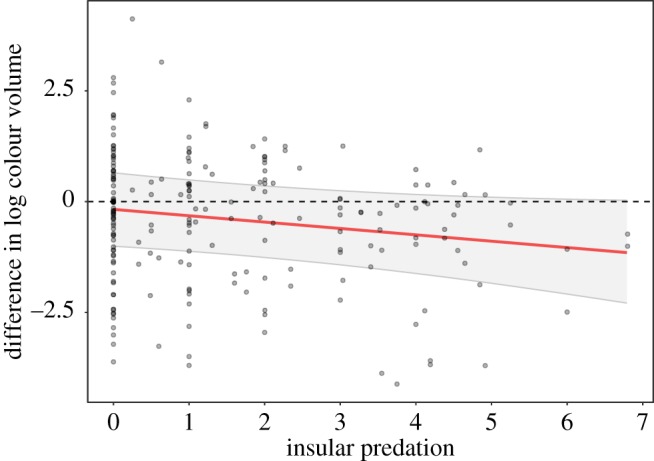
Difference in log colour volume between insular species and their continental counterpart in relation to the number of sympatric predators. A positive value indicates a larger colour volume for the insular species compared to its mainland relative. The regression line and its associated 95% CI are those predicted by the BPMM, accounting for continental predation. The mean log continental colour volume was subtracted to the intercept, and insular predation was transformed back to its original scale. (Online version in colour.)
Table 1.
Results of the BPMM exploring the relation of different factors with the log insular colour volume (n = 107 insular species). All continuous covariates were z-transformed to get standardized effect sizes.
 |
We did not find any clear effect of insular predator richness on insular brightness at the scale of the whole body (estimate: −0.003 [95% CI: −0.011; 0.006]; electronic supplementary material, appendix S5, table A2 and figure A2) or on the dorsal and ventral parts (estimate for the dorsal parts: 0.004 [95% CI: −0.004; 0.013], for the ventral parts: −0.004 [95% CI: −0.013; 0.006]; electronic supplementary material, appendix S5 and table A3).
The phylogenetic heritability was high for both colour volume and brightness of insular birds (respectively, λ = 0.873 and λ = 0.992) [42,43].
4. Discussion
We found a trend suggesting a negative relationship between insular predator richness and insular coloration while accounting for mainland coloration and predation, as well as controlling for a strong phylogenetic signal. These results tend to support the hypothesis that island species facing less predators are more prone to an increase in colour volume. To our knowledge, this is the first comparative study testing the effect of predation on colour evolution on islands. Below, we discuss two non-exclusive hypotheses to explain the response of colour volume to predation.
Species colonizing islands face reduced selection pressures from mate choice and/or the need for efficient species recognition [4], and thus should face a strong effect of genetic drift [9]. The lower predation found on islands represents a reduction of an important selective pressure, thus increasing further stochasticity in plumage colour evolution. Accordingly, about half of the species facing low predation (i.e. left side of figure 1) displayed an increased colour volume, which is consistent with stochastic dynamics. In addition, island size also affected bird coloration (electronic supplementary material, appendix S6). While this effect is likely to be linked to reduced predation pressure, stochasticity is also thought to be stronger on smaller islands due to more pronounced genetic bottlenecks. However, species colonizing islands wherein predation pressure is high are more prone to evolve towards duller coloration because the selective pressure imposed by predation is maintained, while the strength of mate choice and need for efficient species recognition are lowered.
Another possibility, independent of island living, is that predation could act on coloration through colour-dependent predation [44,45], where a decrease in predation pressure is associated with diminished costs of being colourful, and indirectly through changes in sexual selection. Indeed, predation is known to affect prey population size, and the removal of predator species tends to enhance population size and thus prey densities [46]. Higher population densities in birds have been linked to higher rates of extra-pair paternity [47], hence stronger sexual selection [48]. This mechanism would indirectly favour colourfulness when predation is lowered.
Why plumage brightness, either at the scale of the whole body or on different body parts, decreases on islands [4] but does not covary with predation pressure remains unclear. However, even though brightness is expected to be important for long-distance prey detection [49,50], thereby generating a viability cost for brighter individuals by enhancing predation risk, this may not always be the case. Unlike high colour volumes, which make species conspicuous in whichever environment [51], high plumage brightness might render species inconspicuous if it effectively matches the brightness of the environment. A proper evaluation of the link between brightness and predation pressure might thus require a detailed knowledge of the brightness of the visual environment specifically used by each bird species.
Since this study is correlational, additional factors covarying with insularity or predation may influence the results. For instance, availability in dietary resources such as carotenoids at the basis of some pigmental colours may vary between locations inhabited by species within pairs. Furthermore, the use of spectrometry only measures coloration while ignoring colour patterns and ornament sizes that may also be subjected to evolutionary changes, depending on predation pressure and following island colonization.
Islands represent natural laboratories for studying the factors governing evolution at large evolutionary scales [2]. While general patterns of evolution on islands are well documented [1], studies of the underlying mechanisms are still rare. The present study contributes to our understanding of these mechanisms and suggests that predation is one of the factors influencing the evolution of bird plumage coloration on islands worldwide.
Acknowledgements
We thank Paul Sweet and Robert Prys-Jones from the American and British Natural History Museum for their support. Emeline Mourocq helped to build the database. Nathalie Grnac, Romain Guerreiro, Andrea Baquero and Thibaut Powolny helped with data collection at the museums. We thank Jack Thorley, Pietro d'Amelio, Doris Gomez, Pierre de Villemereuil and four anonymous reviewers for their helpful suggestions. This research is a joint research programme conducted under the International Associated Lab CNRS-CIBIO.
Data accessibility
Data are available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.mw6m905tc [52].
Authors' contributions
C.D., R.C., P.-A.C., A.G. and A.R. designed this study. R.C. collated the database. C.D. and A.R. measured plumage coloration. J.P.R. and L.B. performed visual models. P.D. and L.B. collected predation data. L.B. performed analyses with the help of M.P. L.B. wrote the first draft. All authors contributed to revisions of the manuscript, approved its final version and accept to be held accountable for its content.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by ‘Agence Nationale de la Recherche’ (ANR 09-JCJC-0050-0) and Languedoc Roussillon Region (fund ‘chercheur(se) d'avenir 2011) to C.D., European Program Synthesis to R.C. and C.D. and a Collection Study Grant from the AMNH to R.C. R.C. was funded by a Marie Curie Fellowship, programmes ‘Ciência 2008’ and IF (01411/2014/CP1256/CT0007; Portuguese Science and Technology Foundation).
References
- 1.Losos JB, Ricklefs RE. 2009. Adaptation and diversification on islands. Nature 457, 830–836. ( 10.1038/nature07893) [DOI] [PubMed] [Google Scholar]
- 2.Whittaker RJ, Fernández-Palacios JM, Matthews TJ, Borregaard MK, Triantis KA. 2017. Island biogeography: taking the long view of nature's laboratories. Science 357, eaam8326 ( 10.1126/science.aam8326) [DOI] [PubMed] [Google Scholar]
- 3.Adler GH, Levins R. 1994. The island syndrome in rodent populations. Q. Rev. Biol. 69, 473–490. ( 10.1086/418744) [DOI] [PubMed] [Google Scholar]
- 4.Doutrelant C, Paquet M, Renoult JP, Gregoire A, Crochet P-A, Covas R. 2016. Worldwide patterns of bird coloration on islands. Ecol. Lett. 19, 537–545. ( 10.1111/ele.12588) [DOI] [PubMed] [Google Scholar]
- 5.Fitzpatrick S. 1998. Intraspecific variation in wing length and male plumage coloration with migratory behaviour in continental and island populations. J. Avian Biol. 29, 248–256. ( 10.2307/3677107) [DOI] [Google Scholar]
- 6.Grant PR. 1965. Plumage and the evolution of birds on islands. Syst. Zool. 14, 47–52. ( 10.2307/2411902) [DOI] [Google Scholar]
- 7.Griffith SC. 2000. High fidelity on islands: a comparative study of extrapair paternity in passerine birds. Behav. Ecol. 11, 265–273. ( 10.1093/beheco/11.3.265) [DOI] [Google Scholar]
- 8.Raia P, Guarino FM, Turano M, Polese G, Rippa D, Carotenuto F, Monti DM, Cardi M, Fulgione D. 2010. The blue lizard spandrel and the island syndrome. BMC Evol. Biol. 10, 289 ( 10.1186/1471-2148-10-289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Runemark A, Hansson B, Pafilis P, Valakos ED, Svensson EI. 2010. Island biology and morphological divergence of the Skyros wall lizard Podarcis gaigeae: a combined role for local selection and genetic drift on color morph frequency divergence? BMC Evol. Biol. 10 ( 10.1186/1471-2148-10-269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endler J. 1978. A predator's view of animal color patterns. Evol. Biol. 11, 319–364. [Google Scholar]
- 11.Endler J. 1980. Natural selection on color patterns in Poecilia reticulata. Evolution 34, 76–91. ( 10.2307/2408316) [DOI] [PubMed] [Google Scholar]
- 12.Stanger-Hall KF, Lloyd JE. 2015. Flash signal evolution in Photinus fireflies: character displacement and signal exploitation in a visual communication system. Evolution 69, 666–682. ( 10.1111/evo.12606) [DOI] [PubMed] [Google Scholar]
- 13.Cain KE, et al. 2019. Conspicuous plumage does not increase predation risk: a continent-wide test using model songbirds. Am. Nat. 193, 359–372. ( 10.1086/701632) [DOI] [PubMed] [Google Scholar]
- 14.Gomez D, Thery M. 2007. Simultaneous crypsis and conspicuousness in color patterns: comparative analysis of a Neotropical rainforest bird community. Am. Nat. 169, S42–S61. ( 10.1086/510138) [DOI] [PubMed] [Google Scholar]
- 15.Beauchamp G. 2004. Reduced flocking by birds on islands with relaxed predation. Proc. R. Soc. Lond. B 271, 1039–1042. ( 10.1098/rspb.2004.2703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Runemark A, Brydegaard M, Svensson EI. 2014. Does relaxed predation drive phenotypic divergence among insular populations? J. Evol. Biol. 27, 1676–1690. ( 10.1111/jeb.12421) [DOI] [PubMed] [Google Scholar]
- 17.Marshall KLA, Stevens M. 2014. Wall lizards display conspicuous signals to conspecifics and reduce detection by avian predators. Behav. Ecol. 25, 1325–1337. ( 10.1093/beheco/aru126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoddard MC, Prum RO. 2008. Evolution of avian plumage color in a tetrahedral color space: a phylogenetic analysis of New World buntings. Am. Nat. 171, 755–776. ( 10.1086/587526) [DOI] [PubMed] [Google Scholar]
- 19.Goldsmith TH. 1990. Optimization, constraint, and history in the evolution of eyes. Q. Rev. Biol. 65, 281–322. ( 10.1086/393620) [DOI] [PubMed] [Google Scholar]
- 20.Maia R, Gruson H, Endler J, White TE. 2019. pavo 2: new tools for the spectral and spatial analysis of colour in R. Methods Ecol. Evol. 10, 1097–1107. ( 10.1111/2041-210X.13174) [DOI] [Google Scholar]
- 21.Lind O, Mitkus M, Olsson P, Kelber A. 2013. Ultraviolet sensitivity and colour vision in raptor foraging. J. Exp. Biol. 216, 1819–1826. ( 10.1242/jeb.082834) [DOI] [PubMed] [Google Scholar]
- 22.Håstad O, Victorsson J, Ödeen A, Moran NA, Hastad O, Victorsson J, Odeen A. 2005. Differences in color vision make passerines less conspicuous in the eyes of their predators. Proc. Natl. Acad. Sci. USA 102, 6391–6394. ( 10.1073/pnas.0409228102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valcu M, Dale J, Griesser M, Nakagawa S, Kempenaers B. 2014. Global gradients of avian longevity support the classic evolutionary theory of ageing. Ecography (Cop.). 37, 930–938. ( 10.1111/ecog.00929) [DOI] [Google Scholar]
- 24.Kotrschal A, Deacon AE, Magurran AE, Kolm N. 2017. Predation pressure shapes brain anatomy in the wild. Evol. Ecol. 31, 619–633. ( 10.1007/s10682-017-9901-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews G, Goulet CT, Delhey K, Atkins ZS, While GM, Gardner MG, Chapple DG. 2018. Avian predation intensity as a driver of clinal variation in colour morph frequency. J. Anim. Ecol. 60, 1667–1684. ( 10.1111/1365-2656.12894) [DOI] [PubMed] [Google Scholar]
- 26.Slavenko A, Itescu Y, Foufopoulos J, Pafilis P, Meiri S. 2015. Clutch size variability in an ostensibly fix-clutched lizard: effects of insularity on a Mediterranean gecko. Evol. Biol. 42, 129–136. ( 10.1007/s11692-015-9304-0) [DOI] [Google Scholar]
- 27.Ciccotto PJ, Mendelson TC. 2016. The ecological drivers of nuptial color evolution in darters (Percidae: Etheostomatinae). Evolution 70, 745–756. ( 10.1111/evo.12901) [DOI] [PubMed] [Google Scholar]
- 28.Buckley LB, Jetz W. 2007. Insularity and the determinants of lizard population density. Ecol. Lett. 10, 481–489. ( 10.1111/j.1461-0248.2007.01042.x) [DOI] [PubMed] [Google Scholar]
- 29.Donlan JC, Wilcox C. 2008. Diversity, invasive species and extinctions in insular ecosystems. J. Appl. Ecol. 45, 1114–1123. ( 10.1111/j.1365-2664.2008.01482.x) [DOI] [Google Scholar]
- 30.Gravel D, Poisot T, Albouy C, Velez L, Mouillot D. 2013. Inferring food web structure from predator–prey body size relationships. Methods Ecol. Evol. 4, 1083–1090. ( 10.1111/2041-210X.12103) [DOI] [Google Scholar]
- 31.Caro T. 2005. Antipredator defenses in birds and mammals. Chicago, IL: University of Chicago Press. [Google Scholar]
- 32.Savidge JA. 1987. Extinction of an island forest avifauna by an introduced snake. Ecology 68, 660–668. ( 10.2307/1938471) [DOI] [Google Scholar]
- 33.Harmening WM, Wagner H. 2011. From optics to attention: visual perception in barn owls. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 197, 1031–1042. ( 10.1007/s00359-011-0664-3) [DOI] [PubMed] [Google Scholar]
- 34.BirdLife International and NatureServe. 2018. Bird species distribution maps of the world. Version 2.0. Cambridge, UK: /Arlington, IL: BirdLife International/ NatureServe. [Google Scholar]
- 35.del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E (eds). 2019. Handbook of the birds of the world alive. Barcelona, Spain: Lynx Edici. [Google Scholar]
- 36.Covas R. 2012. Evolution of reproductive life histories in island birds worldwide. Proc. R. Soc. B 279, 1531–1537. ( 10.1098/rspb.2011.1785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Møller AP, Birkhead TR. 1992. A pairwise comparative method as illustrated by copulation frequency in birds. Am. Nat. 139, 644–656. ( 10.1086/285348) [DOI] [Google Scholar]
- 38.R Core Team. 2018. R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 39.Guillerme T, Healy K. 2014. mulTree: a package for running MCMCglmm analysis on multiple trees ( 10.5281/zenodo.12902) [DOI]
- 40.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. 2012. The global diversity of birds in space and time. Nature 491, 444–448. ( 10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 41.de Villemereuil P, Wells JA, Edwards RD, Blomberg SP. 2012. Bayesian models for comparative analysis integrating phylogenetic uncertainty. BMC Evol. Biol. 12, 102 ( 10.1186/1471-2148-12-102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401, 877–884. ( 10.1038/44766) [DOI] [PubMed] [Google Scholar]
- 43.Hadfield JD, Nakagawa S. 2010. General quantitative genetic methods for comparative biology: phylogenies, taxonomies and multi-trait models for continuous and categorical characters. J. Evol. Biol. 23, 494–508. ( 10.1111/j.1420-9101.2009.01915.x) [DOI] [PubMed] [Google Scholar]
- 44.Götmark F, Post P, Olsson J, Himmelmann D. 1997. Natural selection and sexual dimorphism: sex-biased sparrowhawk predation favours crypsis in female chaffinches. Oikos 80, 540–548. ( 10.2307/3546627) [DOI] [Google Scholar]
- 45.Slagsvold T, Dale S, Kruszewicz A. 1995. Predation favours cryptic coloration in breeding male pied flycatchers. Anim. Behav. 50, 1109–1121. ( 10.1016/0003-3472(95)80110-3) [DOI] [Google Scholar]
- 46.Salo P, Banks PB, Dickman CR, Korpimäki E. 2010. Predator manipulation experiments: impacts on populations of terrestrial vertebrate prey. Ecol. Monogr. 80, 531–546. ( 10.1890/09-1260.1) [DOI] [Google Scholar]
- 47.Møller AP, Ninni P. 1998. Sperm competition and sexual selection: a meta-analysis of paternity studies of birds. Behav. Ecol. Sociobiol. 43, 345–358. ( 10.1007/s002650050501) [DOI] [Google Scholar]
- 48.Møller AP, Birkhead TR. 1994. The evolution of plumage brightness in birds is related to extrapair paternity. Evolution 48, 1089–1100. ( 10.2307/2410369) [DOI] [PubMed] [Google Scholar]
- 49.Théry M, Casas J. 2002. Predator and prey views of spider camouflage. Nature 415, 133 ( 10.1038/415133a) [DOI] [PubMed] [Google Scholar]
- 50.Spaethe J, Tautz J, Chittka L. 2001. Visual constraints in foraging bumblebees: flower size and color affect search time and flight behavior. Proc. Natl. Acad. Sci. USA 98, 3898–3903. ( 10.1073/pnas.071053098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Renoult JP, Kelber A, Schaefer HM. 2017. Colour spaces in ecology and evolutionary biology. Biol. Rev. 92, 292–315. ( 10.1111/BRV.12230) [DOI] [PubMed] [Google Scholar]
- 52.Bliard L, Paquet M, Robert A, Dufour P, Renoult JP, Grégoire A, Crochet P-A, Covas R, Doutrelant C. 2020. Data from: Examining the link between relaxed predation and bird coloration on islands Dryad Digital Repository. ( 10.5061/dryad.mw6m905tc) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Bliard L, Paquet M, Robert A, Dufour P, Renoult JP, Grégoire A, Crochet P-A, Covas R, Doutrelant C. 2020. Data from: Examining the link between relaxed predation and bird coloration on islands Dryad Digital Repository. ( 10.5061/dryad.mw6m905tc) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data are available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.mw6m905tc [52].


