Abstract
For many animals and insects that are experiencing dramatic population declines, the only recourse for conservationists is captive rearing. To ensure success, reared individuals should be biologically indistinct from those in the wild. We tested if this is true with monarch butterflies, Danaus plexippus, which are increasingly being reared for release by citizens and commercial breeders. Since late-summer monarchs should be as migration capable as possible for surviving the arduous long-distance migration, we evaluated four migration-relevant traits across two groups of captive-reared monarchs (n = 41 and 42) and one group of wild-caught migrants (n = 41). Monarchs (descendants of wild individuals) were reared from eggs to adulthood either in a warm indoor room next to a window, or in an incubator that mimicked late-summer conditions. Using an apparatus consisting of a perch mounted to an electronic force gauge, we assessed ‘grip strength' of all groups, then used image analysis to measure forewing size, pigmentation and elongation. In three of the four traits, reared monarchs underperformed compared to wild ones, even those reared under conditions that should have produced migration-ready individuals. The average strength of reared monarchs combined was 56% less than the wild group, even when accounting for size. Their orange wing colour was paler (an indicator of poor condition and flight ability) and their forewings were less elongated (elongation is associated with migration propensity) than wild monarchs. The reason(s) behind these effects is unknown but could stem from the frequent disturbance and/or handling of reared monarchs, or the fact that rearing removes the element of natural selection from all stages. Regardless, these results explain prior tagging studies that showed reared monarchs have lower migratory success compared to wild.
Keywords: monarch butterflies, Danaus plexippus, conservation, captive rearing, functional morphology, grip strength
1. Introduction
Rearing young animals in captivity for release into the wild is sometimes a necessary approach to conserve species in extreme peril. In these cases, every effort should be made to ensure that reared individuals end up being biologically identical to their wild counterparts. This issue is especially critical if the species has a uniquely demanding life stage, and which requires fully functional and robust individuals. The monarch butterfly (Danaus plexippus) in eastern North America typifies this scenario. Despite warnings from monarch conservation groups [1], a growing number of citizens have taken it upon themselves to rear monarchs in captivity, sometimes in large numbers, in an effort to ‘boost' the monarch population. Monarchs are also commercially reared for release at weddings and festive events, and these releases are typically promoted as helping the population. While these efforts may be well intentioned, new research shows how monarchs reared in artificial conditions have trouble orienting properly during their autumn migration [2]. Other new work showed bouts of handling leads to physiological stress in developing monarchs [3], which may occur repeatedly during rearing activities. These surprising results highlight how little is known about the effects of captive rearing on monarch development, and given the growing popularity of rearing by hobbyists and commercial breeders, speak to the immediate need for further study.
The monarch in North America undertakes an extremely arduous migration each autumn, attempting to reach distant overwintering colonies, either in central Mexico or on the California coast, in a journey lasting two months. A plethora of evidence shows that only the hardiest of individuals complete this journey. For example, small-winged monarchs are less likely to complete the journey [4–6], leading to larger body sizes in migratory populations compared to resident [7]. Moreover, monarchs with deeper orange pigmentation (an indicator of individual quality in monarchs) are also more successful than paler monarchs [8,9]. Thus far, evidence from tagging studies shows that captive-reared monarchs are less successful in reaching their winter colonies than wild migrants [10,11]. The exact cause of this lower success is not known. Here, we directly measured four key fitness traits of monarchs (physical strength, wing size, wing colour and shape) that had been reared in captivity and compared them against monarchs collected in the wild, in an effort to understand why captive-reared monarchs have lower migration success.
2. Material and methods
(a). Monarch sources
Detailed methods for this project are presented in electronic supplementary material, file S1. Captive monarchs were reared as part of unrelated research projects at the University of Georgia during September 2019 and were descendants of wild-caught monarchs captured within the last 12 months. There were two rearing scenarios. One group (n = 42) had been reared from eggs to adulthood in a warm (28°C) indoor laboratory room next to a large window. Additional overhead lights in the room maintained a long (15 h) day length. This environment was intended to approximate conditions that monarchs would experience in the summer. Our other group (n = 41) was reared in an environmental chamber that simulated late-summer conditions (declining day length, low temperatures, [23°C day, 18°C night]), which would be experienced by the migratory generation in the wild. In both scenarios, larvae were reared individually in pint-size plastic containers and fed cuttings of greenhouse-grown milkweed, while also undergoing standard daily cleanings of their containers. After pupation, the adult monarchs were stored in glassine envelopes at 13°C until testing (below). For comparison, we collected wild adult monarchs (n = 41) during early-October while they were migrating through this region. Those monarchs were also held in glassine envelopes at 13°C until testing.
(b). Grip strength measurement
Here we assessed the ‘grip strength' of adult monarchs as a proxy for their overall strength. This approach is commonly used to assess whole-organism performance in studies of beetles (e.g. [12]) but to our knowledge, this has never been tested in butterflies. This measurement should be relevant for monarchs, as migrants would need to be able to hold fast to roost trees or vegetation during storms and in high winds. We devised a simple apparatus (figure 1) consisting of a wooden perch mounted to an electronic force gauge. A plastic screen wrapped around the perch to provide surface texture (grip holds). On the day of testing (22 October 2019), one of us (F.M.S.) assessed all monarchs in each group. Importantly, the observer was blind to the groupings and all monarchs were arranged in a randomized manner prior to testing. The observer held each monarch above the perch, allowing it to grab with its true legs. Then, the monarch was gently pulled upward until it released the perch. The amount of force required to release was registered by the gauge (in Newtons) and shown on a computer monitor. This was done five times for each monarch, and we retained the maximum reading for each individual. Videos of this procedure are provided in electronic supplementary material.
Figure 1.
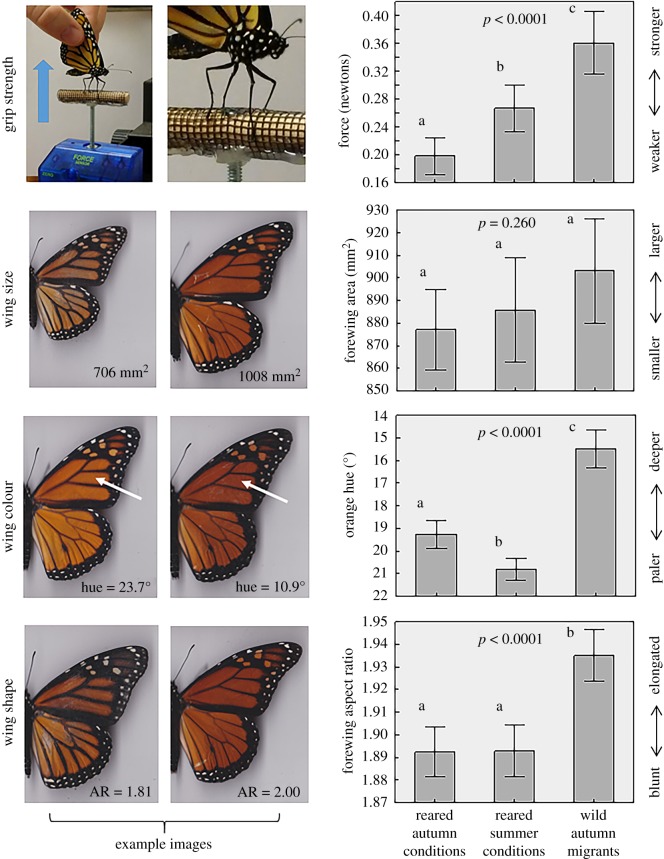
Collective results of comparisons of captive-reared and wild-caught (migrant) monarch butterflies. Captive monarchs were descended from wild-caught individuals and were reared either in an environmental chamber set to autumn-like conditions (n = 41), or a warm, indoor room with ambient and artificial lighting (approximating summer conditions, n = 42). Wild monarchs (n = 41) were collected while migrating through Athens, GA during autumn 2019. Grip strength was measured using an electronic force gauge that registered how much force it required to pull a monarch off of a perch (see electronic supplementary material videos). Wing area, orange colour and aspect ratio were measured from digital scans of monarchs with image analysis software. Arrows indicate wing cell used to measure orange hue. P-values reflect significance of the ‘monarch group' parameter in ANCOVA or ANOVA models that also included monarch sex (and wing size in the strength analysis). Letters above bars denote homogeneous subsets. Whiskers around means are 95% confidence intervals.
(c). Scanning and image analysis
After completion of the strength tests, we obtained a digital image of each monarch with a standard computer scanner. Monarchs were briefly chilled to prevent movement, then held in place (on their back, with wings spread) on the scanner using weights. Using image analysis software (FoveaPro, www.reindeergraphics.com), we measured three parameters on the right forewing: wing area (in mm2), wing elongation (aspect ratio, or the length/width) and the shade (hue) of orange pigmentation. Orange hue was assessed by selecting the central wing cell on the forewing and measuring the average hue score (in degrees) of all pixels in that selection [9]. In monarchs, lower hue scores reflect deeper, reddish-orange, colours (figure 1), and this ‘redness' is associated with improved flight performance and migration success [8,9].
(d). Data analyses
The full dataset from this project is available in the electronic supplementary material. All parameters of interest (grip strength, wing size, wing colour and wing aspect ratio) were normally distributed (see electronic supplementary material, file S1). We used general linear models to evaluate if any of these parameters differed between the three monarch groups (two rearing treatments and wild). The models also included monarch sex, and in the case of grip strength, we included wing size to control for allometric scaling of strength. Thus, grip strength was examined using ANCOVA, while forewing area, orange hue and aspect ratio were examined using fixed-effects ANOVA. Analyses were performed using Statistica 13.3 (www.tibco.com).
3. Results
The three monarch groups differed significantly in grip strength (p < 0.0001; electronic supplementary material, table S2); wild monarchs were stronger than both groups of captive-reared monarchs (figure 1). Note that our statistical model had accounted for body size (wing area), which was a significant predictor of strength (p = 0.005). In terms of magnitude, the average force required to release the perch in wild monarchs was 0.36 N (± 0.14 s.d.), which was approximately 38% greater than the average for monarchs in our indoor room (0.27 N ± 0.10 s.d.), and 80% greater than those reared in our incubator (0.20 N ± 0.07 s.d.). The average strength of both reared groups combined (0.23 N ± 0.09 s.d.) was 56% less than the wild group.
As a follow-up to these strength results, we randomly selected 12 of the reared monarchs and housed them in a flight cage (with a food source) near a window for one week, then retested them using the same procedure (and same observer; see electronic supplementary material, file S1). Their second strength scores were not different to the first (paired t-test, t = 0.27, p = 0.7899).
Forewing size did not differ across groups (p = 0.260; electronic supplementary material, table S2), though wild monarchs were slightly larger than reared ones (figure 1). Orange hue scores differed significantly (p < 0.0001; electronic supplementary material, table S2); monarchs in both reared groups were paler than wild migrants (figure 1). Last, wild monarchs had significantly more elongated forewings (higher aspect ratio) than in both reared groups (p < 0.0001; electronic supplementary material, table S2; figure 1). We noted the average aspect ratio of the wild monarchs (mean = 1.94) was consistent with previous estimates from migratory populations in North America, while those of both reared groups (mean = 1.89) were similar to observed aspect ratios in non-migratory monarch populations in Costa Rica and Puerto Rico [7].
4. Discussion
Here, we assessed four different physical traits of monarchs that are either presumed or known to be important for migration across captive-reared and wild migrants, and in three of these tests, reared monarchs scored significantly lower than their wild counterparts. On average, they were weaker in our novel grip strength tests, even when accounting for size, their wings were paler (an indicator of condition and flight ability), and their wings were less elongated (elongation is associated with migration propensity) than wild monarchs (figure 1). Reared monarchs also tended to be smaller than wild, but not significantly so. Surprisingly, these patterns were found in both rearing treatments, even within monarchs reared under late-summer conditions (declining day length, lower temperature). These findings all underscore the fact that captive rearing does not sufficiently replicate nature in the case of the monarch. Specifically, captive-reared monarchs appear to be functionally different to their wild counterparts in key traits important for migration.
The exact reasons for the differences between reared and wild monarchs here require further study. It is possible that the rearing process itself somehow contributes to the effect. Indeed, monarchs from both of our (very different) rearing environments showed similar deficiencies (figure 1), which may implicate the rearing procedures. Monarchs from both groups underwent the same daily routines, involving cleaning out their containers and replenishing their milkweed. While these activities were brief, it is possible that these repeated bouts of disturbance could stress the monarchs at this early developmental stage [3], leading to re-allocation of resources away from development of muscle or wing tissue.
Another possible explanation is the fact that rearing removes the element of natural selection. In the wild, fewer than 5% of all eggs and immature monarchs survive the larval stage [13]. Consider that the wild monarchs we tested were collected midway along the migratory journey (in northeast Georgia, in October) and had likely been migrating for at least one month already. Thus, these monarchs had not only survived the larval stage, but then again survived this far in the migratory journey. In essence, wild monarchs are the products of a series of selection events that presumably allow only the most robust and hardy individuals to survive. By contrast, rearing monarchs in captivity from the egg stage removes all natural deterrents (milkweed latex, predators and weather extremes), and larvae are typically fed ad libidum. Thus, rearing results in almost all monarchs making it to the adult stage, even those that would not have succeeded in the wild.
From a conservation perspective, our findings raise an important concern over the practice of captive rearing (regardless of the species), which is that individuals raised in captivity may be functionally different than those in the wild. This would be especially worrisome if those deficiencies compromise the success of the animals or insects in question. In the case of the monarchs, our results help to explain why reared monarchs have low (migratory) tag-recovery rates in eastern [10] and western [11] North America. Furthermore, these results, combined with prior evidence showing reductions in navigational ability of reared monarchs [2], argue that conservation of monarchs should not include captive-rearing approaches, even if small numbers of reared monarchs are indeed recovered at wintering destinations. It is those that do not succeed that would be more problematic; if these individuals survive to reproduce (such as by overwintering in the southern US, [14]), over time this could lead to a gradual erosion of migration potential of the entire population.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the members of the Altizer laboratory at the University of Georgia for assistance with rearing monarchs. The manuscript was improved after helpful feedback from two anonymous reviewers.
Ethics
All insect collecting was conducted on land owned by the University of Georgia, or the authors' personal residence, and no ethical approval or permission was required.
Data accessibility
All data for the experiments conducted in this project are available in the electronic supplementary material files associated with this manuscript.
Authors' contributions
A.K.D. contributed to the conception and design of the experiments, data collection, data analysis and writing the manuscript. F.M.S. and A.M.B. contributed to the data collection and logistics of the experiments and reviewed early drafts of the manuscript. All authors approved the final version of the manuscript and agree to be held accountable for the content.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Pelton E. 2018. Keep monarchs wild: why captive rearing isn't the way to help monarchs. Portland, OR: Xerces; Society Blog; See https://xerces.org/blog/keep-monarchs-wild. [Google Scholar]
- 2.Tenger-Trolander A, Lu W, Noyes M, Kronforst MR. 2019. Contemporary loss of migration in monarch butterflies. Proc. Natl Acad. Sci. USA 116, 14 671–14 676. ( 10.1073/pnas.1904690116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis AK. 2020. Evaluating cardiac reactions of monarch butterflies to human handling across three life stages. J. Lepid. Soc. 74, 43–50. ( 10.18473/lepi.74i1.a1) [DOI] [Google Scholar]
- 4.Freedman MG, Dingle H. 2018. Wing morphology in migratory North American monarchs: characterizing sources of variation and understanding changes through time. Anim. Migr. 5, 61–73. ( 10.1515/ami-2018-0003) [DOI] [Google Scholar]
- 5.Yang LH, Ostrovsky D, Rogers MC, Welker JM. 2016. Intra-population variation in the natal origins and wing morphology of overwintering western monarch butterflies Danaus plexippus. Ecography 39, 998–1007. ( 10.1111/ecog.01994) [DOI] [Google Scholar]
- 6.Flockhart DTT, Fitz-gerald B, Brower LP, Derbyshire R, Altizer S, Hobson KA, Wassenaar LI, Ryan Norris D. 2017. Migration distance as a selective episode for wing morphology in a migratory insect. Mov. Ecol. 5, 9 ( 10.1186/s40462-017-0102-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altizer S, Davis AK. 2010. Populations of monarch butterflies with different migratory behaviors show divergence in wing morphology. Evolution 64, 1018–1028. ( 10.1111/j.1558-5646.2010.00946.x) [DOI] [PubMed] [Google Scholar]
- 8.Hanley D, Miller NG, Flockhart DT, Norris DR. 2013. Forewing pigmentation predicts migration distance in wild-caught migratory monarch butterflies. Behav. Ecol. 24, 1108–1113. ( 10.1093/beheco/art037) [DOI] [Google Scholar]
- 9.Davis AK, Chi J, Bradley CA, Altizer S. 2012. The redder the better: wing color predicts flight performance in monarch butterflies. PloS ONE 7, e41323 ( 10.1371/journal.pone.0041323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steffy G. 2015. Trends observed in fall migrant monarch butterflies (Lepidoptera: Nymphalidae) east of the Appalachian Mountains at an inland stopover in southern Pennsylvania over an eighteen year period. Ann. Entomol. Soc. Am. 108, 718–728. ( 10.1093/aesa/sav046) [DOI] [Google Scholar]
- 11.Morris GM, Kline C, Morris SM. 2015. Status of Danaus plexippus population in Arizona. J. Lepid. Soc. 69, 91–107. ( 10.18473/lepi.69i2.a10) [DOI] [Google Scholar]
- 12.Lailvaux SP, Hathway J, Pomfret J, Knell RJ. 2005. Horn size predicts physical performance in the beetle Euoniticellus intermedius (Coleoptera: Scarabaeidae). Funct. Ecol. 19, 632–639. ( 10.1111/j.1365-2435.2005.01024.x) [DOI] [Google Scholar]
- 13.De Anda A, Oberhauser K. 2015. Invertebrate natural enemies and stage-specific mortality rates of monarch eggs and larvae. In Monarchs in a changing world: biology and conservation of an iconic butterfly (eds Oberhauser K, Nail KR, Altizer S), pp. 60–70. Ithaca, NY: Cornell University Press. [Google Scholar]
- 14.Howard E, Aschen H, Davis AK. 2010. Citizen science observations of monarch butterfly overwintering in the southern United States. Psyche 2010, 689301 ( 10.1155/2010/689301) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data for the experiments conducted in this project are available in the electronic supplementary material files associated with this manuscript.