Abstract
Sex chromosomes in birds have long been considered to be extremely stable. However, this notion has lately been challenged by findings of independent autosome–sex chromosome fusions within songbirds, several of which occur within a single clade, the superfamily Sylvioidea. To understand what ecological and evolutionary processes drive changes in sex chromosome systems, we need complete descriptions of sex chromosome diversity across taxonomic groups. Here, we characterize the sex chromosome systems across Sylvioidea using whole-genome data of species representatives of 10 different families, including two published and eight new genomes. We describe a novel fusion in the family Cisticolidae (represented by Cisticola juncidis) involving a part of chromosome 4. We also confirm the previously identified fusion between chromosome Z and a part of chromosome 4A in all 10 families and show that fusions involving parts of chromosomes 3 and 5 are not found outside the families where they were first discovered (Alaudidae and Panuridae). These findings add to the complexity of the sex chromosome system in Sylvioidea, where four independent autosome–sex chromosome fusions have now been identified.
Keywords: sex chromosome, birds, autosome–sex chromosome fusion, genomics, Sylvioidea
1. Introduction
Chromosomal sex determination is common in animals and has evolved independently from different autosomes many times [1–3]. A steadily increasing number of sex chromosome formations and turnovers are now being identified through the use of genomic and cytogenetic approaches, revealing that sex chromosome stability varies greatly across animal clades [3]. Frequent transitions between sex chromosome systems (switches between XY and ZW, and between genetic and environmental sex determination) have occurred in some insects, fish, amphibians and reptiles [4–6]. In other clades, such as eutherian mammals [7] and birds [8], the central component of the sex chromosome systems formed in the common ancestor of each group and is shared by all members. Despite our increasing awareness of the variation in sex chromosome diversity and stability among taxa, unanswered questions remain regarding its underlying ecological and evolutionary causes and consequences [9]. To shed more light on these processes, additional empirical evidence from independently evolved sex chromosomes from a wide range of taxonomic groups, including inferences of homology between the sex chromosomes and chromosomes in other species, is critically needed [10].
Birds have long been viewed as examples of extreme sex chromosome stability, where species from widely different clades share synteny across the entire Z chromosome [11]. However, new evidence of independently occurring autosome–sex chromosome fusions reveals that sex chromosomes in birds are more variable than was previously believed [12–15]. The most taxonomically widespread autosome–sex chromosome fusion that is currently known is that of the members of Sylvioidea [12,13], a superfamily of songbirds consisting of more than 1200 species in approximately 22 families that have diverged from other species around 25 million years ago [16,17]. In all Sylvioidea species studied so far, a part of chromosome 4A (based on chromosome naming from the zebra finch) has fused to the ancestral sex chromosomes [12,13]. Moreover, within Sylvioidea, members of the family Alaudidae (larks) were recently reported as having two additional autosome–sex chromosome fusions involving chromosomes 3 and 5 [15,18], where the fusion with chromosome 3 is also shared by their sister lineage Panuridae (bearded reedlings) [15]. Further studies on sex chromosome variation within this clade of birds will add important information for understanding the processes underlying the dynamics of sex chromosome evolution.
Here, we characterize the sex chromosome systems across the Sylvioidea clade, using whole-genome sequencing data from males and females of 10 species belonging to 10 different families (electronic supplementary material, figure S1). We screened these species for signatures of sex linkage across their entire genomes using two measurements: sex-specific differences in sequence read coverage and single-nucleotide variations (SNVs). These two measurements have the capability of identifying sex chromosome systems of varying degree of W (or Y) degeneration, a feature that is often but not always correlated with time since recombination suppression between Z and W (or X and Y) [3]. Genome coverage reveals a clearer signature for old, highly degraded sex chromosome systems, while sex-specific SNV is more distinctive for sex-linked genomic regions of more recent origin [9]. Using these methods, we aim to scan the Sylvioidea clade for any additional and so far undetected changes to the sex chromosome system.
2. Material and methods
(a). Data
DNA extracted from blood samples of one female and one male for each of the 10 studied species was paired-end sequenced (2 × 150 bp) with Illumina HiSeqX technology. Two of these species, Alauda arvensis (Eurasian skylark) and Panurus biarmicus (bearded reedling), were included in a previous study [15]. The other eight species are as follows: Acrocephalus schoenobaenus (sedge warbler), Aegithalos caudatus (long-tailed tit), Cettia cetti (Cetti's warbler), Cisticola juncidis (zitting cisticola), Cecropis daurica (red-rumped swallow), Locustella luscinioides (Savi's warbler), Phylloscopus collybita (common chiffchaff) and Sylvia atricapilla (Eurasian blackcap).
(b). Identification of sex-linked genomic regions
We used the methodology from Sigeman et al. [15] (‘the homogametic-reference method’), and a recently modified approach with improved features particularly for highly heterozygotic species (‘the consensus-reference method’), to identify sex-linked regions in the genomes of the 10 species. The homogametic-reference method included creating a reference genome based on the male (i.e. the homogametic sex, ZZ) from each species, followed by alignment of the female and male sample of each species to that reference genome, variant calling and genome coverage calculations (using increasingly stringent mismatches criteria). Next, we generated chromosome coordinates for each 5 kbp window across all scaffolds based on synteny to the Taeniopygia guttata (zebra finch) genome assembly, using the methodology from ref. [15]. See electronic supplementary material, methods for details and table S2 for reference genome statistics. The consensus-reference method included (i) aligning the reads from the male and the female samples to the previously generated male reference genome, (ii) scoring alleles in both individuals and (iii) creating a new consensus-reference genome by exchanging the reference allele with the non-reference allele for all biallelic variants with a non-reference allele count ≥2 in the two samples (see electronic supplementary material, method for details). The new consensus genome assembly was then used as input to the same pipeline as for the homogametic-reference method, which resulted in more equal genome-wide mapping success of both sexes in most species (electronic supplementary material, table S3), while retaining the sex-specific SNV signature across sex-linked regions. Here, we present results based on the consensus-reference method, while results based on both methods are presented in electronic supplementary material, figures S3 to S12. However, as we only compare one male and one female, independent verification of any putative sex-linked regions (e.g. by molecular validation; see below) is recommended in order to distinguish these from potential diverged autosomal haplotypes that may differ between samples (e.g. due to inversion polymorphisms).
Statistics and plotting were done in R v. 3.6.1 [19]. Genome coverage values were normalized between the female and male sample by dividing the female-to-male ratio for each 5 kbp window with the median female-to-male ratio for all windows across the dataset. To remove extreme values, boxplots were made for all chromosomes and outliers (i.e. outside 1.5 times the interquartile range above the upper quartile and below the lower quartile) were removed from the dataset. Mean values for the normalized and filtered genome coverage values were then calculated across 1 Mbp and 100 kbp genomic regions. Here, we present genome coverage values allowing two mismatches to the reference genome, while electronic supplementary material, figures S3a,b–S12a,b present results from all settings. SNVs were counted for each sex in 1 Mbp and 100 kbp genomic regions. Standard error for the coverage and SNV values was calculated for each chromosome based on the 1 Mbp bins.
(c). Molecular validation of novel sex-linked regions
To independently verify sex linkage for the novel finding in C. juncidis (see Results section), we genotyped seven individuals for five loci within the putative sex-linked region on chromosome 4. One of these individuals was the female used for whole-genome sequencing. The same samples were also sexed using the primer pairs P2 and P8 [20]. The polymerase chain reaction (PCR) products were run on agarose gels. Primer sequences are provided in electronic supplementary material, table S5 and full PCR protocol in electronic supplementary material, methods.
3. Results
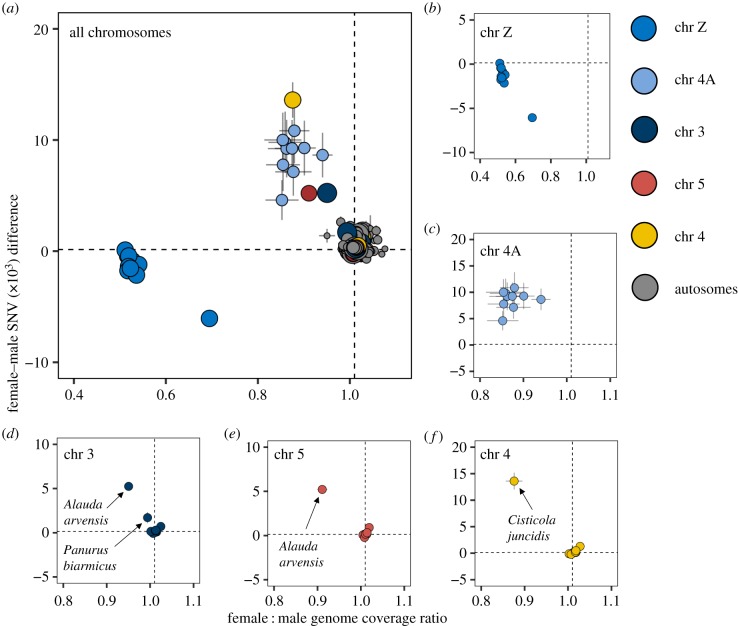
The median genome-wide coverage ratio between females and males across all species and all chromosomes was 1.010 (±s.e. 0.001) and the median genome-wide SNV difference between females and males was 0.144 × 103 (±s.e. 0.029 × 103). Five chromosomes (Z, 4A, 3, 5 and 4) deviated strongly from these values in at least one of the 10 species (figure 1; electronic supplementary material, figure S2). As expected of old and heavily degenerated sex chromosomes, the females of all 10 species had drastically lower read coverage at the ancestral sex chromosome Z than males, while the number of SNVs was similar between the sexes or lower in the female than in the male (figure 1a,b; electronic supplementary material, table S4,S6). Moreover, all species showed low female-to-male read coverage ratio and a high female skew in number of SNVs at chromosome 4A (figure 1a,c; electronic supplementary material, table S4,S6), which is expected for less degenerated sex-linked regions. A more detailed scan showed sex linkage over the first 9.6 Mbps of chromosome 4A (electronic supplementary material, figures S3–S12), a result that confirms sex linkage of this exact genome region across Sylvioidea [12,13,15,21]. The sex linkage of chromosomes 3 and 5 in Alaudidae, and chromosome 3 in Panuridae, which has previously been reported [15], was not present in any of the other eight families (figure 1a,d,e). However, a novel finding was that Cisticola juncidis (zitting cisticola) in the family Cisticolidae, in contrast with all other species, had a sex-linked read coverage and SNV pattern for yet another chromosome, chromosome 4 (figure 1a,f; electronic supplementary material, table S4,S6; figure 3–12).
Figure 1.
Mean (±s.e.) female-to-male read coverage ratio and female-to-male difference in number of SNVs for each chromosome in 10 species belonging to 10 different Sylvioidea families. Chromosomes identified as sex-linked in any of the studied species (chromosomes Z, 4A, 3, 5 and 4) are coloured differently from the other chromosomes. The sizes of the dots correspond to chromosome length, and the dashed lines mark the median based on all chromosomes in all species. (a) All chromosomes in all 10 species (i.e. 10 dots for each chromosome). (b–f) Chromosomes identified as sex-linked in at least one species shown separately. (b) Chromosome Z and (c) chromosome 4A are sex-linked in all studied species. (d) Chromosome 3 is sex-linked in Alauda arvensis (Alaudidae) and Panurus biarmicus (Panuridae) and (e) chromosome 5 in A. arvensis, as reported previously [15]. (f) Chromosome 4 is sex-linked in Cisticola juncidis (Cisticolidae) but not the other species, which is a novel finding for this study.
A detailed analysis of the sex-linked chromosomes in C. juncidis (i.e. Z, 4A and 4) across 100 kbp windows showed that chromosome Z is sex-linked over its entire length (72.9 Mbp), chromosome 4A up to position 9.6 Mbp and chromosome 4 between positions 13.8 and 49.1 Mbp, i.e. over a 35.3 Mbp region (figure 2a). The chromosome 4 region between 13.8 and approximately 35 Mbp shows a more pronounced differentiation (especially for SNVs) between the sexes than the region between approximately 35 and 49.1 Mbp (figure 2a), possibly owing to recombination becoming suppressed at different time points. As additional evidence of sex linkage of chromosome 4 in C. juncidis, we confirmed the occurrence of female-specific polymorphism by genotyping two females and five males at five loci located in the sex-linked region of which all showed sex-specific differences, but most clearly so at two of these loci (electronic supplementary material, figure S13). The finding of a unique autosome–sex chromosome fusion in Cisticolidae further adds to the complexity of the sex chromosome systems within Sylvioidea, with a minimum of four fusion events involving five chromosomes (Z, 4A, 3, 5 and 4; figure 2b).
Figure 2.
(a) Female-to-male difference in number of SNVs, and read coverage ratio, along the chromosomes showing signatures of sex linkage in Cisticola juncidis (i.e. Z, 4A and 4) across 100 kbp windows (SNV outlier values in blue and genome coverage outlier values in red; see electronic supplementary material, Methods for details). (b) A phylogeny of the studied species (based on ref. [17]) and inferences of the appearances of the autosome–sex chromosome fusions. All species share the ancestral sex chromosome (Z) as well as a fusion involving the first 9.6 Mbp of chromosome 4A. As reported previously, a part of chromosome 3 is sex-linked in both Panurus biarmicus (Panuridae) and Alauda arvensis (Alaudidae), and a part of chromosome 5 in A. arvensis [15]. A novel finding is that a part of chromosome 4 is sex-linked in C. juncidis (Cisticolidae). Bird illustrations from HBW (© Lynx Edicions).
4. Discussion
Using whole-genome analyses of species across a large proportion of the Sylvioidea superfamily (electronic supplementary material, figure S1), we have confirmed sex linkage of the first 9.6 Mbp of chromosome 4A across Sylvioidea [12,13,21,22] and found sex linkage of parts of chromosomes 3 and 5 to be present only in the families where they were previously discovered, i.e. Alaudidae (chromosomes 3 and 5) and Panuridae (chromosome 3) [15]. In addition to these three already identified autosome–sex chromosome fusions, we found a novel fusion in Cisticolidae involving chromosome 4, which was also independently confirmed through genotyping of five loci in additional C. juncidis females and males. Genome sequencing of additional species and genera within Cisticolidae will reveal more precisely when this region evolved sex linkage. With the current data, we can conclude that this event occurred less than 22 million years ago defined by the split between Cisticolidae and other lineages in our dataset [17]. Also, our data allow us to infer homology to other species and identify convergence across independently evolved sex chromosome systems [23]. Interestingly, this shows that the sex chromosomes of several clades of frogs and toads share homology to the fused Cisticolidae chromosome 4 [24,25]. This means that all chromosomes involved in the autosome–sex chromosome fusions in Sylvioidea (i.e. Z, 4A, 3, 5 and 4) have been recruited as sex chromosomes repeatedly across different vertebrate clades (see ref. [15]).
Sylvioidea is a large clade consisting of over 1200 species [16]. Still, the discovery of four independent changes to the sex chromosomes within the group paints a contrasting picture to the notion of extreme sex chromosome stability within birds [11,26]. Two trails of logic follow from these findings. The first is that members of Sylvioidea may be especially prone to the emergence and fixation of these kinds of fusions. The second is that bird sex chromosomes are much more varied than is currently assumed and that the contrasting pattern of Sylvioidea birds compared to other birds arises due to study-bias. If the latter is true, we expect to find much greater variation in bird sex chromosomes during the coming years due to the exponential rate of bird genomes being sequenced, for example, through initiatives such as the Bird 10 000 genomes (B10K) Project [27]. We recommend that sequencing efforts in additional species will, whenever possible, include individuals of both sexes in order to ensure the advancement of our understanding of sex chromosome diversity in birds.
Supplementary Material
Acknowledgements
We wish to thank S. Bensch, F. García Castellanos and J. Neto for DNA samples.
Ethics
Samples were collected non-destructively and with permission from the relevant authorities (CEMPA, Instituto de Conservação da Natureza e Florestas, Portugal; Oficina de Impulso Socioeconómico del Medio Ambiente, Consejería de Agua, Agricultura y Medio Ambiente, Región de Murcia, Spain; Ringmärkningscentralen, Naturhistoriska Riksmuseet, Sweden; Malmö/Lund Djurförsöksetiska Nämnd, Sweden, no. 17277-18).
Data accessibility
Sequence data used in this study are deposited in NCBI Sequence Read Archive under accession PRJNA578893. Electronic supplementary materials, methods, tables, figures and code are available from the Dryad Digital Repository (doi:10.5061/dryad.dbrv15dxk) [28].
Authors' contributions
H.S., S.P. and B.H. conceived the study. H.S. performed analyses with input from B.H. H.S. and B.H. wrote the paper with input from S.P. All authors approved of the final version of this manuscript to be published and agreed to be held accountable for the content therein.
Competing interests
We declare we have no competing interests.
Funding
Sequencing was performed by the SNP&SEQ Technology Platform in Uppsala, which is part of the NGI Sweden and SciLife-Lab, supported by the Swedish Research Council and the Knut and Alice Wallenberg Foundation. Bioinformatics analyses were performed on resources provided by SNIC at UPPMAX. The research was funded by a grant from the Swedish Research Council (to B.H., grant no. 621-2016-689).
References
- 1.Ohno S. 1967. Sex chromosomes and sex-linked genes, vol. 1 Berlin, Germany: Springer Berlin Heidelberg. [Google Scholar]
- 2.Bull JJ. 1983. Evolution of sex determining mechanisms Menlo Park, CA: Benjamin Cummings. [Google Scholar]
- 3.Bachtrog D, et al. 2014. Sex determination: why so many ways of doing it? PLoS Biol. 12, e1001899.4 ( 10.1371/journal.pbio.1001899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pennell MW, Mank JE, Peichel CL. 2018. Transitions in sex determination and sex chromosomes across vertebrate species. Mol. Ecol. 27, 3950–3963. ( 10.1111/mec.14540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackmon H, Demuth JP. 2014. Estimating tempo and mode of Y chromosome turnover: explaining Y chromosome loss with the fragile Y hypothesis. Genetics 197, 561–572. ( 10.1534/genetics.114.164269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vicoso B, Bachtrog D. 2015. Numerous transitions of sex chromosomes in Diptera. PLoS Biol. 13, e1002078 ( 10.1371/journal.pbio.1002078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortez D, Marin R, Toledo-Flores D, Froidevaux L, Liechti A, Waters PD, Gruetzner F, Kaessmann H. 2014. Origins and functional evolution of Y chromosomes across mammals. Nature 508, 488–493. ( 10.1038/nature13151) [DOI] [PubMed] [Google Scholar]
- 8.Zhou Q, Zhang J, Bachtrog D, An N, Huang Q, Jarvis ED, Gilbert MT, Zhang G. 2014. Complex evolutionary trajectories of sex chromosomes across bird taxa. Science 346, e246338 ( 10.1126/science.1246338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer DH, Rogers TF, Dean R, Wright AE. 2019. How to identify sex chromosomes and their turnover. Mol. Ecol. 28, 4709–4724. ( 10.1111/mec.15245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Tree of Sex Consortium. 2014. Tree of Sex: a database of sexual systems. Sci. Data 1, 140015 ( 10.1038/sdata.2014.15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nanda I, Schlegelmilch K, Haaf T, Schartl M, Schmid M. 2008. Synteny conservation of the Z chromosome in 14 avian species (11 families) supports a role for Z dosage in avian sex determination. Cytogenet Genome Res. 122, 150–156. ( 10.1159/000163092) [DOI] [PubMed] [Google Scholar]
- 12.Pala I, Naurin S, Stervander M, Hasselquist D, Bensch S, Hansson B. 2012. Evidence of a neo-sex chromosome in birds. Heredity 108, 264–272. ( 10.1038/hdy.2011.70) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pala I, Hasselquist D, Bensch S, Hansson B. 2012. Patterns of molecular evolution of an avian neo-sex chromosome. Mol. Biol. Evol. 29, 3741–3754. ( 10.1093/molbev/mss177) [DOI] [PubMed] [Google Scholar]
- 14.Gan HM, Falk S, Morales HE, Austin CM. 2019. Genomic evidence of neo-sex chromosomes in the eastern yellow robin. Gigascience 8, giz111 ( 10.1093/gigascience/giz111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sigeman H, Ponnikas S, Chauhan P, Dierickx E, de L Brooke M, Hansson B. 2019. Repeated sex chromosome evolution in vertebrates supported by expanded avian sex chromosomes. Proc. R. Soc. B 286, 20192051 ( 10.1098/rspb.2019.2051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fregin S, Haase M, Olsson U, Alström P. 2012. New insights into family relationships within the avian superfamily Sylvioidea (Passeriformes) based on seven molecular markers. BMC Evol. Biol. 12, 1–12. ( 10.1186/1471-2148-12-157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliveros CH, et al. 2019. Earth history and the passerine superradiation. Proc. Natl Acad. Sci. USA. 116, 7916–7925. ( 10.1073/pnas.1813206116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dierickx EG, Sin SYW, van Veelen HPJ, de L Brooke M, Liu Y, Edwards SV, Martin SH. 2020. Genetic diversity, demographic history and neo-sex chromosomes in the critically endangered Raso lark. Proc. R. Soc. B 287, 20192613 ( 10.1098/rspb.2019.2613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R Core Team. 2019. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 20.Griffiths R, Double MC, Orr K, Dawson RJG. 1988. A DNA test to sex most birds. Mol. Ecol. 7, 1071–1075. ( 10.1046/j.1365-294x.1998.00389.x) [DOI] [PubMed] [Google Scholar]
- 21.Leroy T, et al. 2019. A bird's white-eye view on neo-sex chromosome evolution. bioRxiv26 505610 ( 10.1101/505610) [DOI]
- 22.Sigeman H, Ponnikas S, Videvall E, Zhang H, Chauhan P, Naurin S, Hansson B. 2018. Insights into avian incomplete dosage compensation: sex-biased gene expression coevolves with sex chromosome degeneration in the common whitethroat. Genes 9, 373 ( 10.3390/genes9080373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlesworth D, Mank JE. 2010. The birds and the bees and the flowers and the trees: lessons from genetic mapping of sex determination in plants and animals. Genetics 186, 9–31. ( 10.1534/genetics.110.117697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brelsford A, et al. 2013. Homologous sex chromosomes in three deeply divergent anuran species. Evolution 67, 2434–2440. ( 10.1111/evo.12151) [DOI] [PubMed] [Google Scholar]
- 25.Jeffries DL, et al. 2018. A rapid rate of sex-chromosome turnover and non-random transitions in true frogs. Nat. Comms. 9, 11 ( 10.1038/s41467-018-06517-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellegren H. 2010. Evolutionary stasis: the stable chromosomes of birds. Trends Ecol. Evol. 25, 283–291. ( 10.1016/j.tree.2009.12.004) [DOI] [PubMed] [Google Scholar]
- 27.Zhang G. 2015. Bird sequencing project takes off. Nature 522, 34 ( 10.1038/522034d) [DOI] [PubMed] [Google Scholar]
- 28.Sigeman H, Ponnikas S, Hansson B. 2020. Whole-genome analysis across 10 songbird families within Sylvioidea reveals a novel autosome-sex chromosome fusion Dryad Digital Repository. ( 10.5061/dryad.dbrv15dxk) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Sigeman H, Ponnikas S, Hansson B. 2020. Whole-genome analysis across 10 songbird families within Sylvioidea reveals a novel autosome-sex chromosome fusion Dryad Digital Repository. ( 10.5061/dryad.dbrv15dxk) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Sequence data used in this study are deposited in NCBI Sequence Read Archive under accession PRJNA578893. Electronic supplementary materials, methods, tables, figures and code are available from the Dryad Digital Repository (doi:10.5061/dryad.dbrv15dxk) [28].