Abstract
Humans have a long evolutionary relationship with ethanol, pre-dating anthropogenic sources, and possess unusually efficient ethanol metabolism, through a mutation that evolved in our last common ancestor with African great apes. Increased exposure to dietary ethanol through fermenting fruits and nectars is hypothesized to have selected for this in our lineage. Yet, other mammals have frugivorous and nectarivorous diets, raising the possibility of natural ethanol exposure and adaptation in other taxa. We conduct a comparative genetic analysis of alcohol dehydrogenase class IV (ADH IV) across mammals to provide insight into their evolutionary history with ethanol. We find genetic variation and multiple pseudogenization events in ADH IV, indicating the ability to metabolize ethanol is variable. We suggest that ADH enzymes are evolutionarily plastic and show promise for revealing dietary adaptation. We further highlight the derived condition of humans and draw attention to problems with modelling the physiological responses of other mammals on them, a practice that has led to potentially erroneous conclusions about the likelihood of natural intoxication in wild animals. It is a fallacy to assume that other animals share our metabolic adaptations, rather than taking into consideration each species' unique physiology.
Keywords: ethanol metabolism, alcohol dehydrogenase class IV, digestive physiology, dietary adaptation, comparative genetics
1. Introduction
Modern humans have been intentionally producing ethanol for consumption since the Neolithic [1–3]; however, our evolutionary history with ethanol is much deeper. It has been hypothesised that our proclivities for ethanol have evolutionary roots in frugivorous ancestors, who were exposed to naturally occurring alcohol in ripening fruits [4]. Natural fermentation produces ethanol concentrations as high as 3.8% in nectars [5] and 8.1% in fruits [6], and primates may use the olfactory cues from volatilized alcohols to guide their foraging decisions [7]. Indeed, primates are exceptionally sensitive to the odours of aliphatic alcohols, including ethanol [8–10], and at least some prefer to consume solutions with ethanol when they are available [11,12]. Furthermore, evidence from genes underlying ethanol metabolism suggests that humans have deep-rooted adaptations for the consumption of ethanol. Humans (Homo sapiens), chimpanzees (Pan troglodytes), bonobos (Pan paniscus) and gorillas (Gorilla gorilla) share a mutation in the alcohol dehydrogenase class IV gene (annotated as ADH7) that improves the enzyme's efficiency against ethanol 40-fold [13]. The timing of this mutation, circa 10 mya, coincides with increased terrestriality in our lineage, which may have led to more frequent exposure to fermenting fruit on the forest floor [13]. Incidentally, aye-ayes, which are partly nectarivorous [14], have convergently evolved this mutation (figure 1) and have been shown to prefer sugar solutions with higher ethanol contents [11].
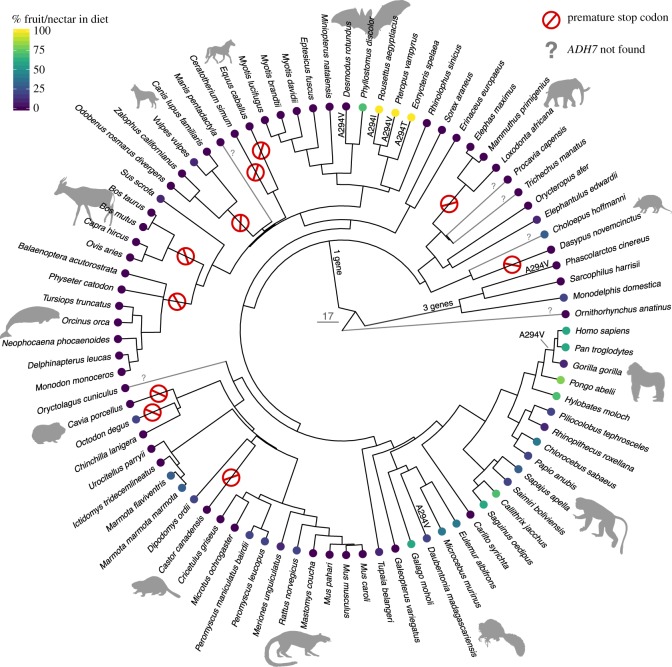
Figure 1.
Protein-altering changes in ADH7 along with evolutionary relationships and diets of species included in our analyses.
Yet other animals have almost certainly also been exposed to dietary ethanol and may actively seek it out [5,11,15]. Chimpanzees routinely fashion leaf sponges to access anthropogenically fermented palm sap [15], the pen-tailed treeshrew consumes fermented nectar that contains enough ethanol to intoxicate a human, yet shows no signs of inebriation [5], and in Sweden, elk are reported to exhibit the signs of intoxication after feeding on rotting apples in the autumn [16]. Many birds consume overwintered berries when they ferment in spring [17] and excessive consumption can be fatal [18]. Similarly, consuming foods containing more than 1% ethanol impairs the flight and echolocation abilities of Egyptian fruit bats (Rousettus aegyptiacus) [19]. Phyllostomid bats, on the other hand, seem to be able to tolerate ethanol and imbibing has no effect on their flying abilities, even at blood alcohol concentrations that qualify a human as legally intoxicated [20].
These anecdotes routinely capture human attention, perhaps because of our enduring fascination with the effects of ethanol on our own species. Possibly the most iconic is the story of African elephants (Loxodonta africana) and marula fruit. According to this widespread lore, elephants across Africa preferentially feed on the fallen, fermenting fruit of the marula tree (Sclerocarya birrea), becoming intoxicated [21–23]. Such accounts have been criticized, however. Researchers have suggested that tales of drunk elephants may be a result of ‘humanizing elephant behavior' [23]. Yet, this argument is based on calculations drawing on human ethanol metabolism, which may be a critical flaw. Ethanol metabolism in humans is derived [13]. An evolutionary change of one alcohol dehydrogenase enzyme in the ancestor of African great apes [13] results in a relatively high alcohol tolerance. Aside from humans, the capacities of other mammals to metabolize ethanol are not well understood. This limits our understanding of mammalian dietary adaptations and the role that ethanol plays in animal diets. A comparative approach is needed to elucidate the evolutionary history of ethanol metabolism and the potential adaptive benefits of ethanol consumption.
Ethanol metabolism in humans begins with oxidation of ethanol to acetaldehyde by the enzyme alcohol dehydrogenase (ADH). Acetaldehyde is further oxidized by aldehyde dehydrogenase (ALDH) to acetate, which can then be excreted or reincorporated as acetyl-CoA [24]. A comprehensive review of the enzymes implicated in ethanol metabolism and the genes coding for them is given by [24]. Here, we assess the functionality of ADH IV, encoded by ADH7, via comparative genetic analyses of 85 mammals to: (1) test the hypothesis that dietary exposure to fruit and/or nectar selects for efficient ethanol metabolism, while lack thereof relaxes selection on ADH7 and (2) identify convergent substitutions at site 294, which strongly impacts enzymatic function. We focus on ADH IV because it is expressed in the mouth, oesophagus and stomach, and thus the first alcohol-metabolizing enzyme to act on ingested ethanol [13]. Further, ADH IV acts on ethanol in fermented fruits and nectars, as well as a variety of other potentially toxic alcohols found in leaves [25], suggesting ADH IV may coevolve with diet.
2. Material and methods
We mined publicly available reference genomes (NCBI) and sequencing data [26] of 85 mammals, spanning 21 orders and feeding on a variety of diets ([27]; figure 1; electronic supplementary material, table S1) for ADH7 gene sequences. To test whether ADH7 was more likely to be retained in frugivorous/nectarivorous lineages or lost in lineages with diets not containing these resources, we performed phylogenetic logistic regression analyses using two different methods in the R package phylolm [28], following [29]. To test for changes in selective pressure acting on ADH7 in lineages with or without a frugivorous/nectarivorous diet, we used the method RELAX from the HyPhy package [30]. To estimate the timing of loss of ADH7 in branches with premature stop codons, we followed the method described in Meredith et al. [31] (electronic supplementary material).
3. Results
We found ADH7 gene sequences for 79 of the 85 mammals we investigated and identified 10 independent pseudogenization events (table 1 and figure 1) evidenced by loss-of-function mutations that introduce one or more premature stop codons. Species with premature stop codons in ADH7 are highly unlikely to express a functional class IV alcohol dehydrogenase because the coding sequence would not translate into a full-length protein containing all domains required for activity. Because ADH7 is a single copy gene in all eutherian species investigated, we classify these as unprocessed, unitary pseudogenes, and the loss is expected to affect the species' gene function repertoire [32,33] (but see [34,35]). Marsupials, unlike eutherian mammals, have three ADH7 paralogs. All three are putatively functional in Monodelphis domestica, while in both Sarcophilus harrisii and Phascolarctos cinereus, one paralog contains independent premature stop codons.
Table 1.
Missense mutations found in ADH7 genes across a diversity of mammals and estimates of timing of pseudogenization
| species/clade | type of mutation | change (inferred ancestral codon → new codon) | location | timing of gene loss (mya) |
|---|---|---|---|---|
| Bovidae | nonsense | tac (Y) → taa (*) | exon 7 | 40.5 |
| frameshift | 1 bp deletion | exon 7 (stop codons in exon 8) | ||
| Cetacea | nonsense | tta (L) → tga (*) | exon 5 | 44.9 |
| Equus | nonsense | tca (S) → tga (*) | exon 2 | 27.5 |
| nonsense | cgc (R) → tga (*) | exon 4 | ||
| nonsense | cca (P) → taa (*) | exon 5 | ||
| nonsense | tgg (W) → tga (*) | exon 7 | ||
| Ceratotherium | frameshift | 1 bp insertion | exon 5 | 27.5 |
| frameshift | 1 bp insertion | exon 8 | ||
| Elephantidae | nonsense | tgc (C) → tga (*) | exon 2 | 54.9 |
| nonsense | ttg (L) → tag (*) | exon 2 | ||
| frameshift | 2 bp insertion | exon 5 | ||
| nonsense | gaa (E) → taa (*) | exon 6 | ||
| nonsense | tat (Y) → tag (*) | exon 7 | ||
| nonsense | tgg (W) → tga (*) | exon 8 | ||
| Castor | frameshift | 11 bp deletion | exon 7 | 32.0 |
| Octodon | nonsense | gag (E) → tag (*) | exon 6 | 16.6 |
| Cavia | frameshift | 1 bp deletion | exon 2 | 18.1 |
| Dasypus | nonsense | tgg (W) → taa (*) | exon 2 | 50.8 |
| frameshift | 1 bp deletion | exon 2 | ||
| Carnivora | frameshift | 1 bp deletion | exon 6 | 48.7 |
The Elephantidae share multiple frameshift-causing indels and point mutations causing numerous premature stop codons, suggesting that they arose in a common ancestor. Bovids (Bos taurus, Bos mutus, Ovis aries, Capra hircus) share two different mutations causing premature stop codons, while carnivores (Canis lupus familiaris, Vulpes vulpes, Odobenus rosmarus, Zalophus californianus) and cetaceans (Delphinopterus leucas, Monodon monoceros, Neophocaena phocaenoides, Physeter catodon) each share one mutation within their respective clades (table 1 and figure 1). Independent pseudogenizations of ADH7 have also arisen separately in the horse (Equus caballus), rhinoceros (Ceratotherium simium), guinea pig (Cavia porcellus), degu (Octodon degu), beaver (Castor canadensis) and armadillo (Dasypus novemcinctus) (table 1 and figure 1). Estimates of the timing of gene losses are summarized in table 1 (details in electronic supplementary material, table S2).
(a). Dietary correlates of gene evolution
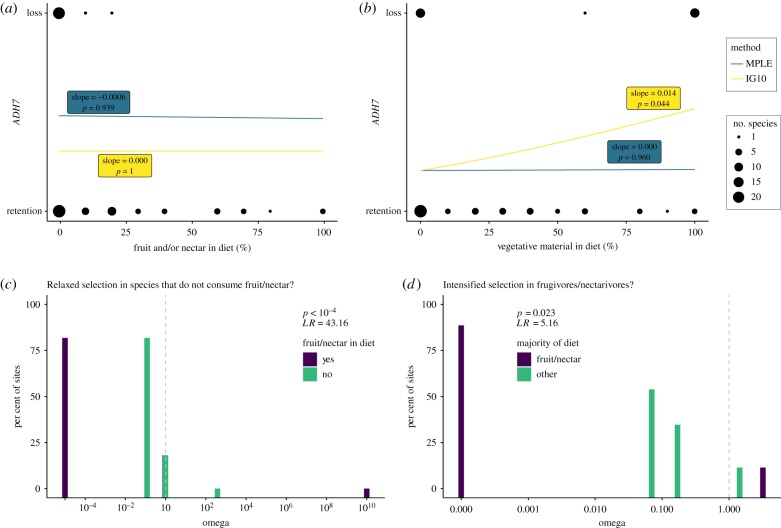
Fruit or nectar inclusion in the diet did not predict ADH7 gene retention (IG10: slope = 0.00, p = 1; MPLE: slope = −0.0006, p = 0.939) in the phylogenetic logistic regression analyses (figure 2a; electronic supplementary material, table S3). There was, however, a weak correlation between the percentage of vegetative plant material in the diet and the loss of ADH7 (IG10: slope = 0.014, p = 0.044; MPLE: slope = 0.00, p = 0.960), suggesting that loss of ADH7 may be correlated with an herbivorous diet (figure 2b; electronic supplementary material, table S3).
Figure 2.
Dietary correlates of gene evolution. Phylogenetic logistic regression analyses show (a) no correlation between amount of fruit/nectar in the diet and gene retention, but a (b) significant correlation between herbivorous diet and ADH7 loss. RELAX analyses suggest selective pressure (c) is relaxed in the absence of fruit/nectar in the diet and (d) intensifies in frugivorous/nectarivorous species.
Results of the RELAX analyses (electronic supplementary material, table S4), on the other hand, show that selective pressure acting on ADH7 is relaxed (K = 0.00) in species that do not consume fruit or nectar as part of their diet (p < 0.0001, LR = 43.16; figure 2c) and is intensified (K = 3.16) in species whose diets contain more than 50% fruit or nectar (p = 0.023, LR = 5.16; figure 2d).
(b). Substitutions at site 294
Intriguingly, we found substitutions at site 294, which is known to impact protein function among putatively functional genes. The large fruit bat (Pteropus vampyrus), common vampire bat (Desmodus rotundus) and koala (Phascolarctos cinereus) have a change from alanine to valine, convergent with the shift observed in African great apes and aye-ayes (Daubentonia madagascariensis), while the Egyptian rousette (Rousettus aegyptiacus) and Cave nectar bat (Eonycteris spelaea) have a shift to isoleucine and threonine, respectively (figure 1).
3. Discussion
(a). Dietary correlates of gene retention and loss
Our results provide strong evidence that the ability to metabolize ethanol is variable across mammals. Our results are partially consistent with the hypothesis that dietary specialization on non-fruit/nectar foods relaxes selection on genes underlying ethanol metabolism, while a diet high in fruit or nectar intensifies it (figure 2c,d). The independent losses of ADH7––evidenced by detection of unitary pseudogenes––in species that specialize on foods other than fruits and nectars suggests decreases in the ability to metabolize ethanol through this ADH pathway [32,33]. Cetaceans and carnivores are highly carnivorous and armadillos insectivorous, while bovids, horses, rhinos, elephants, guinea pigs, degus and beavers are herbivores, so the deterioration of ADH7 in these lineages may be due to a common lack of fruit and nectar foods in their diets. In particular, we find statistical evidence that selection is relaxed in species without fruit or nectar in their diets (figure 2b), but also that herbivory is linked to the loss of ADH7 function (figure 2a; electronic supplementary material, table S3). Convergent, yet distinct, aspects of digestive physiology may account for the homoplasic loss of ADH IV in herbivores. For example, elephantids have relatively small stomachs with rapid transit time to the intestines where the hindgut microbiome may detoxify any large, hydrophobic alcohols (LHAs) produced by plants (e.g. geraniol, cinnamyl alcohol, coniferyl alcohol) [36], thereby reducing the need for LHA detoxification in the upper gastrointestinal tract by ADH IV. To similar effect, bovids possess foregut fermentation where resident bacteria may replace the need for stomach ADH IV detoxification of LHAs. Similar convergence of adaptations to a grass-based diet can be seen in their dentition, including large shearing cusps on molars [37].
However, other species in our sample retain a functional ADH7 despite not consuming fruits or nectar (e.g. insectivorous, sanguivorous bats, shrews; figure 1; electronic supplementary material, table S1), and our logistic regression analysis does not support the hypothesis that proportion of fruit/nectar intake predicts ADH7 gene retention (figure 2a; electronic supplementary material, table S3, but see figure 2d). This curiosity invites consideration of other hypotheses. Generally speaking, mammalian ADH IV enzymes oxidize a wide variety of alcohols, and in vitro ADH IV oxidizes retinol far more efficiently than ethanol, suggesting to some that ADH IV is involved in retinoic acid biosynthesis [38,39]. The expression of ADH IV in the upper GI tract suggests, however, that ADH IV may have evolved to detoxify LHAs and subsequently specialized for ethanol in some frugivorous and nectarivorous lineages (e.g. great apes, aye-ayes, bats), while it was lost in lineages without a need to detoxify dietary alcohols. Future comparative research on alcohol dehydrogenases will shed further light on this mystery.
(b). Substitutions at site 294
Previous research has demonstrated a shift from alanine to valine at residue 294 of ADH IV in the common ancestor of African great apes that improves the efficiency for ethanol metabolism 40-fold relative to the ancestral condition [13]. It is unlikely that other mammals are able to metabolize ethanol in the upper gastrointestinal tract in a way that is comparable to humans and other great apes, as this change is not shared by most of the species in our sample. An exception may be found in bats and koalas. Variation at residue 294, observed in Pteropus vampyrus, Desmodus rotundus and Rousettus aegyptiacus, suggests the potential for convergent changes in ethanol metabolic ability in some fruit bats. Koalas (Phascolarctos cinereus) have a diet specialized on eucalyptus leaves, which are toxic to most other mammals. An improved efficiency of ADH IV may be an adaptation for the detoxification of these leaves, similar to the adaptations in the cytochrome P450 family found in this species [40]. Testing the metabolic efficiency of these enzymes [13] and ADHs expressed in the liver is a priority for future research.
(c). Comment on anthropomorphizing animal physiology
Our results highlight the limitations of extrapolating from one species' physiology to that of another. While it is possible that any of the five lineages in which we identified pseudogenized ADH7 possess alternative mechanisms for ethanol metabolism, it is unlikely that these species approach human-level efficiencies in the absence of diets rich in fermentable sugars. Morris et al. [23] reject the possibility of inebriation in elephants on the basis of extrapolations from modern human physiology. In their calculations they suggest that, based on elephants' large body size and the amount of fruit consumed, elephants could not possibly experience intoxicating effects from the naturally occurring ethanol in fermented marula. Our results show not only a lack of the A294 V mutation that accelerates ethanol metabolism in great apes, but also pseudogenization of ADH7 in African and Asian elephants, suggesting that conclusions about the amount of ethanol required to produce symptoms of inebriation in an African elephant [23] were likely erroneous and the myth of inebriation may well be substantiated. A direct measurement of blood ethanol concentrations following a measured ethanol intake could assess metabolic flux in other mammals relative to that of humans. What is clear, is that the potential alcohol digestome varies widely among mammals to functional effect, and it is almost certainly erroneous to make inferences about one species based on another with divergent physiologies and ecologies.
As Morris et al. [23] point out, humans have a tendency to anthropomorphize animal behaviours. However, it is similarly a mistake to assume that animals share our metabolic and sensory adaptations or limitations. A matador's bull-fighting cape has traditionally been red, supposedly because the red colour angers bulls (or so the story goes). Yet bulls, like most mammals, do not have the ability to distinguish reds from yellows and greens [41]. The trait of trichromatic vision is limited to many day-active primates, including humans [42]. Modern humans are also unique among mammals in their ability to digest lactose, the main sugar in milk, as adults [43], yet we give adult cats milk as a treat. And maybe in (small) part because our own retinas lack cryptochromes, our understanding of how birds use the earth's magnetic field for navigation was delayed until very recently [44,45]. Visually oriented animals like ourselves may also be limited in our ability to imagine the olfactory abilities of other species, such as elephants, which have an exceptional sense of smell [46–50]. Understanding how a study species uses its senses to navigate its environment is crucial when designing cognitive studies.
We must be cautious when extrapolating human (metabolic) functions to other animals, especially model systems for human diseases, including alcoholism. It is crucial to consider each species' physiology, which reflects their ecology and evolutionary history, before making broad assumptions based on humans’ current physiology.
Supplementary Material
Supplementary Material
Data accessibility
Gene sequences used for data analysis are publicly available via NCBI and are included here as a text file in the electronic supplementary material. Code and input files used for dating, PGLS, and RELAX analyses are available via a repository on github (https://github.com/MareikeJaniak/Mammal_ADH_IV).
Authors' contributions
A.D.M. and M.C.J. conceived of the study. M.C.J. led data mining and analyses, G.D. and S.L.P. assisted with data mining and analyses. M.C.J., A.D.M. and M.A.C. led writing of the manuscript and all authors contributed to editing. All authors gave their approval for publication of the final version and agree to be held accountable for the content therein.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Alberta Children's Hospital Research Institute (postdoctoral fellowship to M.C.J.), the Canada Research Chairs Program (A.D.M.) and the Natural Sciences and Engineering Research Council of Canada (A.D.M.).
References
- 1.Katz SH, Voigt MM. 1986. Bread and beer. Expedition 28, 23–34. [Google Scholar]
- 2.McGovern PE, et al. 2004. Fermented beverages of pre- and proto-historic China. Proc. Natl Acad. Sci. USA 101, 17 593–17 598. ( 10.1073/pnas.0407921102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanson D. 2013. Historical evolution of alcohol consumption in society. In Alcohol: science, policy and public health (eds Boyle P, Boffetta P, Lowenfels AB., Burns H, Brawley O), pp. 3–12. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Dudley R. 2000. Evolutionary origins of human alcoholism in primate frugivory. Q. Rev. Biol. 75, 3–15. ( 10.1086/393255) [DOI] [PubMed] [Google Scholar]
- 5.Wiens F, Zitzmann A, Lachance M-A, Yegles M, Pragst F, Wurst FM, von Holst D, Guan SL, Spanagel R.. 2008. Chronic intake of fermented floral nectar by wild treeshrews. Proc. Natl Acad. Sci. USA 105, 10 426–10 431. ( 10.1073/pnas.0801628105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudley R. 2004. Ethanol, fruit ripening, and the historical origins of human alcoholism in primate frugivory. Integr. Comp. Biol. 44, 315–323. ( 10.1093/icb/44.4.315) [DOI] [PubMed] [Google Scholar]
- 7.Melin AD, Veilleux CC. 2020. Primate senses: finding and evaluating food. In Primate diet and nutrition: needing, finding, and using food (eds Lambert JE, Rothman JM). Chicago, IL: University of Chicago Press. [Google Scholar]
- 8.Laska M, Seibt A. 2002. Olfactory sensitivity for aliphatic alcohols in squirrel monkeys and pigtail macaques. J. Exp. Biol. 205, 1633–1643. ( 10.1016/s0166-4328(01)00464-8) [DOI] [PubMed] [Google Scholar]
- 9.Dominy NJ. 2004. Fruits, fingers, and fermentation: the sensory cues available to foraging primates. Integr. Comp. Biol. 44, 295–303. ( 10.1093/icb/44.4.295) [DOI] [PubMed] [Google Scholar]
- 10.Laska M, Rivas Bautista RM, Hernandez Salazar LT. 2006. Olfactory sensitivity for aliphatic alcohols and aldehydes in spider monkeys (Ateles geoffroyi). Am. J. Phys. Anthropol. 129, 112–120. ( 10.1002/ajpa.20252) [DOI] [PubMed] [Google Scholar]
- 11.Gochman SR, Brown MB, Dominy NJ. 2016. Alcohol discrimination and preferences in two species of nectar-feeding primate. R. Soc. Open Sci. 3, 160 217–160 218. ( 10.1098/rsos.160217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dausch ID, Hernandez SLT, Laska M. 2019. Taste responsiveness of spider monkeys to dietary ethanol. Chem. Senses 44, 631–638. ( 10.1093/chemse/bjz049) [DOI] [PubMed] [Google Scholar]
- 13.Carrigan MA, Uryasev O, Frye CB, Eckman BL, Myers CR, Hurley TD, Benner SA. 2015. Hominids adapted to metabolize ethanol long before human-directed fermentation. Proc. Natl Acad. Sci. USA 112, 458–463. ( 10.1073/pnas.1404167111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterling EJ, Dierenfeld ES, Ashbourne CJ, Feistner AT. 1994. Dietary intake, food composition and nutrient intake in wild and captive populations of Daubentonia madagascariensis. Folia Primatol. 62, 115–124. ( 10.1159/000156768) [DOI] [PubMed] [Google Scholar]
- 15.Hockings KJ, et al. 2015. Tools to tipple: ethanol ingestion by wild chimpanzees using leaf-sponges. R. Soc. Open Sci. 2, 150150–150156. ( 10.1098/rsos.150150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooke L. 2018. The truth about animals: stoned sloths, lovelorn hippos, and other tales from the wild side of wildlife. New York, NY: Basic Books. [Google Scholar]
- 17.Dennis JV. 1987. If you drink, don't fly: fermented fruit and sap can inebriate birds. Birder's World 1, 15–19. [Google Scholar]
- 18.Fitzgerald SD, Sullivan JM, Everson RJ. 1990. Suspected ethanol toxicosis in two wild cedar waxwings. Avian Dis. 34, 488–490. ( 10.2307/1591442) [DOI] [PubMed] [Google Scholar]
- 19.Sánchez F, Melcón M, Korine C, Pinshow B. 2010. Ethanol ingestion affects flight performance and echolocation in Egyptian fruit bats. Behav. Process. 84, 555–558. ( 10.1016/j.beproc.2010.02.006) [DOI] [PubMed] [Google Scholar]
- 20.Orbach DN, Veselka N, Dzal Y, Lazure L, Fenton MB. 2010. Drinking and flying: does alcohol consumption affect the flight and echolocation performance of phyllostomid bats? PLoS ONE 5, e8993 ( 10.1371/journal.pone.0008993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sikes SK. 1971. Natural history of the African elephant. London, UK: Weidenfeld & Nicolson. [Google Scholar]
- 22.Siegel RK, Brodie M. 1984. Alcohol self-administration by elephants. Bull. Psychon. Soc. 22, 49–52. ( 10.3758/BF03333758) [DOI] [Google Scholar]
- 23.Morris S, Humphreys D, Reynolds D. 2006. Myth, marula, and elephant: an assessment of voluntary ethanol intoxication of the African elephant (Loxodonta africana) following feeding on the fruit of the marula tree (Sclerocarya birrea). Physiol. Biochem. Zool. 79, 363–369. ( 10.1086/499983) [DOI] [PubMed] [Google Scholar]
- 24.Hurley TD, Edenberg HJ. 2012. Genes encoding enzymes involved in ethanol metabolism. Alcohol Res. 34, 339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakubas WJ, Shah PS, Mason JR, Norman DM. 1992. Avian repellency of coniferyl and cinnamyl derivatives. Ecol. Appl. 2, 147–156. ( 10.2307/1941771) [DOI] [PubMed] [Google Scholar]
- 26.Palkopoulou E, et al. 2018. A comprehensive genomic history of extinct and living elephants. Proc. Natl Acad. Sci. USA 115, E2566–E2574. ( 10.1073/pnas.1720554115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilman H, Belmaker J, Simpson J, de la Rosa C, Rivadeneira MM, Jetz W.. 2014. EltonTraits 1.0: species-level foraging attributes of the world's birds and mammals: ecological archives E095-178. Ecology 95, 2027 ( 10.1890/13-1917.1) [DOI] [Google Scholar]
- 28.Ho L, Si T, Ané C. 2014. A linear-time algorithm for Gaussian and non-Gaussian trait evolution models. Syst. Biol. 63, 397–408. ( 10.1093/sysbio/syu005) [DOI] [PubMed] [Google Scholar]
- 29.Jiao H, Zhang L, Xie H-W, Simmons NB, Liu H, Zhao H. 2019. Trehalase gene as a molecular signature of dietary diversification in mammals. Mol. Biol. Evol. 36, 2171–2183. ( 10.1093/molbev/msz127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wertheim JO, Murrell B, Smith MD, Kosakovsky Pond SL, Scheffler K. 2015. RELAX: detecting relaxed selection in a phylogenetic framework. Mol. Biol. Evol. 32, 820–832. ( 10.1093/molbev/msu400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meredith RW, Gatesy J, Murphy WJ, Ryder OA, Springer MS. 2009. Molecular decay of the tooth gene Enamelin (ENAM) mirrors the loss of enamel in the fossil record of placental mammals. PLoS Genet. 5, e1000634 ( 10.1371/journal.pgen.1000634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tutar Y. 2012. Pseudogenes. Comp. Funct. Genomics 2012, 424526 ( 10.1155/2012/424526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma V, Hecker N, Roscito JG, Foerster L, Langer BE, Hiller M. 2018. A genomics approach reveals insights into the importance of gene losses for mammalian adaptations. Nat. Commun. 9, 1215 ( 10.1038/s41467-018-03667-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheetham SW, Faulkner GJ, Dinger ME. 2020. Overcoming challenges and dogmas to understand the functions of pseudogenes. Nat. Rev. Genet. 21, 191–201. ( 10.1038/s41576-019-0196-1) [DOI] [PubMed] [Google Scholar]
- 35.Goodhead I, Darby AC. 2015. Taking the pseudo out of pseudogenes. Curr. Opin. Microbiol. 23, 102–109. ( 10.1016/j.mib.2014.11.012) [DOI] [PubMed] [Google Scholar]
- 36.McLean S, Duncan AJ. 2006. Pharmacological perspectives on the detoxification of plant secondary metabolites: implications for ingestive behavior of herbivores. J. Chem. Ecol. 32, 1213–1228. ( 10.1007/s10886-006-9081-4) [DOI] [PubMed] [Google Scholar]
- 37.Macfadden BJ. 1997. Origin and evolution of the grazing guild in new world terrestrial mammals. Trends Ecol. Evol. 12, 182–187. ( 10.1016/S0169-5347(97)01049-5) [DOI] [PubMed] [Google Scholar]
- 38.Crosas B, Allali-Hassani A, Martínez SE, Martras S, Persson B, Jörnvall H, Parés X, Farrés J. 2000. Molecular basis for differential substrate specificity in class IV alcohol dehydrogenases: a conserved function in retinoid metabolism but not in ethanol oxidation. J. Biol. Chem. 275, 25180–25187. ( 10.1074/jbc.M910040199) [DOI] [PubMed] [Google Scholar]
- 39.Parés X, Farrés J, Kedishvili N, Duester G. 2008. Medium- and short-chain dehydrogenase/reductase gene and protein families. Cell. Mol. Life Sci. 65, 3936 ( 10.1007/s00018-008-8591-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson RN, et al. 2018. Adaptation and conservation insights from the koala genome. Nat. Genet. 50, 1102–1111. ( 10.1038/s41588-018-0153-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riol JA, Sanchez JM, Eguren VG, Gaudioso VR. 1989. Colour perception in fighting cattle. Appl. Anim. Behav. Sci. 23, 199–206. ( 10.1016/0168-1591(89)90110-X) [DOI] [Google Scholar]
- 42.Carvalho LS, Pessoa DMA, Mountford JK, Davies WIL, Hunt DM. 2017. The genetic and evolutionary drives behind primate color vision. Front. Ecol. Evol. 5, 34 ( 10.3389/fevo.2017.00034) [DOI] [Google Scholar]
- 43.Swallow DM. 2003. Genetics of lactase persistence and lactose intolerance. Annu. Rev. Genet. 37, 197–219. ( 10.1146/annurev.genet.37.110801.143820) [DOI] [PubMed] [Google Scholar]
- 44.Günther A, Einwich A, Sjulstok E, Feederle R, Bolte P, Koch K-W, Solov'yov IA, Mouritsen H. 2018. Double-cone localization and seasonal expression pattern suggest a role in magnetoreception for European robin cryptochrome 4. Curr. Biol. 28, 211–223. ( 10.1016/j.cub.2017.12.003) [DOI] [PubMed] [Google Scholar]
- 45.Pinzon-Rodriguez A, Bensch S, Muheim R. 2018. Expression patterns of cryptochrome genes in avian retina suggest involvement of Cry4 in light-dependent magnetoreception. J. R. Soc. Interface 15, 20180058 ( 10.1098/rsif.2018.0058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niimura Y, Matsui A, Touhara K. 2014. Extreme expansion of the olfactory receptor gene repertoire in African elephants and evolutionary dynamics of orthologous gene groups in 13 placental mammals. Genome Res. 24, 1485–1496. ( 10.1101/gr.169532.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller AK, Hensman MC, Hensman S, Schultz K, Reid P, Shore M, Brown J, Furton KG, Lee S. 2015. African elephants (Loxodonta africana) can detect TNT using olfaction: implications for biosensor application. Appl. Anim. Behav. Sci. 171, 177–183. ( 10.1016/j.applanim.2015.08.003) [DOI] [Google Scholar]
- 48.Bates LA, Sayialel KN, Njiraini NW, Moss CJ, Poole JH, Byrne RW. 2007. Elephants classify human ethnic groups by odor and garment color. Curr. Biol. 17, 1938–1942. ( 10.1016/j.cub.2007.09.060) [DOI] [PubMed] [Google Scholar]
- 49.Schmitt MH, Shuttleworth A, Ward D, Shrader AM. 2018. African elephants use plant odours to make foraging decisions across multiple spatial scales. Anim. Behav. 141, 17–27. ( 10.1016/j.anbehav.2018.04.016) [DOI] [Google Scholar]
- 50.Plotnik JM, Brubaker DL, Dale R, Tiller LN, Mumby HS, Clayton NS. 2019. Elephants have a nose for quantity. Proc. Natl Acad. Sci. USA 116, 12 566–12 571. ( 10.1073/pnas.1818284116) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Gene sequences used for data analysis are publicly available via NCBI and are included here as a text file in the electronic supplementary material. Code and input files used for dating, PGLS, and RELAX analyses are available via a repository on github (https://github.com/MareikeJaniak/Mammal_ADH_IV).