Abstract
All mammals undergo weaning from milk to solid food. This process requires substantial changes to mammalian oropharyngeal function. The coordination of swallowing and respiration is a crucial component of maintaining airway function throughout feeding and matures over infant development. However, how this coordination is affected by weaning is unknown. In this study, we ask how changes in posture, neural maturation and food properties associated with the weaning affect coordination of respiration and swallowing in a validated infant pig model. We recorded seven piglets feeding before and during the weaning age with liquid milk in a bottle and in a bowl, and solid feed in a bowl. Using videofluoroscopy synchronized with respiration, we found (i) the delay in the onset of inspiration after swallowing does not change with head position, (ii) the delay is different between solid food and bowl drinking at the same age and (iii) the delay increases over time when bottle feeding, suggesting a maturational effect. Significant changes in aerodigestive coordination occur prior to and post-weaning, resulting in distinctive patterns for liquid and solid food. The interplay of maturational timelines of oropharyngeal function at weaning may serve as a locus for behavioural and life-history plasticity.
Keywords: weaning, aerodigestive, maturation, deglutition, mammal
1. Introduction
Suckling is a unique feature in all infant mammals. Similarly, all mammals go through a weaning process, making the transition from suckling milk to independent feeding, including solid food. During this process, changes in the biomechanics of feeding occur. Drinking replaces suckling as a means of ingesting liquids, changing rate and volume of liquid delivery to the oral cavity [1]. Consuming solid food is a multi-step process, including ingestion, stage I and II transport, mastication and swallowing, whereas liquid remains simpler, involving only acquisition, transport and swallowing [2]. Furthermore, the mechanical properties of masticated solid food are different and more variable than those of liquids. Both solid and liquid feeding therefore require a different suite of processes in infants and in adults, but how this transition occurs is less well known.
For mammals of all ages, respiration is nearly continuous and is interrupted briefly for swallowing to protect the airway. Failure to coordinate between swallowing and breathing can result in aspiration pneumonia. In humans, aspiration pneumonia is a clinical concern, especially in the elderly, and in neurologically compromised populations including Parkinson's patients and preterm infants [3,4]. Aerodigestive coordination differs pre- and post-weaning. In healthy adults, swallowing normally occurs during a pause in expiration. There is greater variation in infants [5,6], which often swallow just prior to inhalation [7,8]. How this change occurs across the transition from suckling to eating solid food is unknown.
Swallowing, and its coordination with breathing, is impacted by different mechanisms. Muscle activity and tongue kinematics differ during head-down drinking compared with suckling in infant pigs [1], and food consistency has been found to alter motor patterns and kinematics during feeding [9,10]. Similarly, head posture affects the coordination of swallowing and breathing: the ‘chin tuck’ is a common practice to alter swallowing kinematics and reduce aspiration in adult humans [11–13]. Changes in food texture and food acquisition occur across the weaning process and influence muscle function and kinematics. However, we have little understanding of how infants coordinate deglutition and respiration during the transition from milk to solids and whether those changes occur owing to differences in food properties, differences in head position during food acquisition, or some combination of the two.
In the present study, we use an infant pig model to investigate how different modes of eating (liquid bottle versus liquid bowl, liquid bowl versus solid bowl) impact the timing of the onset of inspiration following a swallow. Infant pigs are an established model for investigating swallowing performance during weaning [14,15] as they represent a generalized mammalian infant condition, but mature quickly from birth to weaning. We analyse two scenarios to measure the different aspects of developmental changes: (i) change in head position (bottle to bowl) and (ii) change in food type (liquid to solid). We integrate these data with previously published results on changes that occur over infancy prior to weaning in bottle feeding [8].
We investigate if, and if so, how, the timing of deglutition changes relative to respiration. We test this with three hypotheses, where timing refers to the delay in seconds between a swallow and the beginning inspiration.
H1––We predict that timing changes with the head position (bottle held in front of the face versus a bowl set on the floor).
H2––We predict that timing changes with different food types (liquid versus solid food).
H3––We predict that timing changes with maturity and that as infant pigs mature, the delay of inspiration relative to the swallow will increase when drinking from a bottle.
2. Material and methods
(a). Data collection
Twelve full term (114 days) piglets were obtained at different times (Shoup Farm, Wooster, OH, USA). Nine were delivered via Caesarean section (C-section) at NEOMED's Comparative Medicine Unit (CMU). Three piglets were purchased at 5 days postnatal age. All animals were housed in the CMU for the duration of the experimental period.
Animals were recorded from 7 to 32 days of age with high speed videofluoroscopy using a fluoroscope (GE9400 C-Arm, 80 kV, 4 MA) and camera at 100 f.p.s. (XC 1 M digital video camera, XCitex, Cambridge, MA, USA). The pigs were filmed drinking milk (Solu-Start swine milk replacer, Land O'Lakes, MN, USA) or pig feed pellets (Ultracare 100, Purina, MN, USA) mixed with barium (E-Z-Paque, E-Z-EM, NY, USA), to visualize the bolus. Data included at least 188 swallows per condition: drinking milk from a bottle/nipple, drinking milk from a bowl, or eating solid pellets. Each animal was filmed eating in multiple conditions. During bowl milk trials, pigs fed from the bowl for approximately 20 s, then transitioned to bottle feeding until satiated. During pellet trials, pigs fed ad libitum.
Weaning started at Day 26 [1], and piglets transitioned from a bottle to milk in a bowl within approximately 1 day. Bottle feeding recordings continued throughout the experiment. Recordings of bowl drinking began at 26 days, while food pellets were introduced at this time. Recording of feeding on pellets began at 29 days, when pigs were capable of sustained eating of solids (figure 1). From day 26 to 32, between one and five recordings were obtained of each condition (bottle, liquid bowl, or chow).
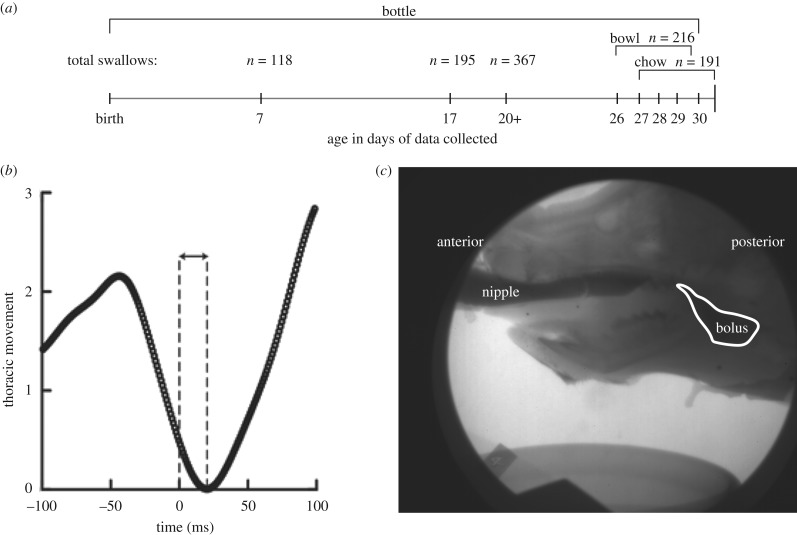
Figure 1.
(a) Timeline of the age of individuals and the ages of introduction of new feeding regimes (milk from bottle, milk from bowl, and chow from bowl) with the number of total swallows per age and treatment. (b) The delay in the onset of inspiration after a swallow where the trace is thoracic movement over time. Time point 0 is the time of the swallow. (c) The bolus at the time of the swallow.
Video data were synchronized using a square wave trigger with simultaneously recorded respiration data obtained with a plethysmograph (TN1132/ST, ADInstruments, Colorado Springs, CO, USA) operated at 10 kHz that was placed around the thorax. Movement of the thorax was recorded in LabChart (PowerLab 16/35, ADInstruments) to determine the patterns of inhalation and expiration.
All procedures were done in accordance with NEOMED IACUC approved protocol no. 17–04-071.
(b). Data extraction
At least 20 swallows were identified in each video sequence using ProAnalyst (XCitex, Woburn, MA, USA). Swallow onset was defined as the first frame of movement of the bolus out of the valleculae. All swallows were identified by one person after a training period in which inter-rater reliability reached 95%. Following Ballester et al. [8], we extracted a 200 ms window of synchronized respiratory data, centred around the swallow, which was set to time 0. We measured delay between the time of the swallow and the onset of inspiration in the plethysmograph trace (figure 1b,c).
(c). Statistical analyses
We fitted a single mixed multi-factorial model to the data and then used post hoc tests for each hypothesis. The dependent/response variable was the delay in inspiration. Individual was included as a standard random factor. We used a single fixed factor, feeding-age, with five levels: bottle 7 days, bottle 17 days, bottle 20+ days, bowl 20+ days and solid food 20+ days. This model assumed normal distributions of the groups, determined visually. For bowl and chow, data were pooled across multiple recordings. The bottle 7 days and bottle 17 days data are from 10 pigs delivered by C-section, previously reported [8]. All other individuals had three levels of the factor. We used traditional two-tailed hypothesis testing (critical significance 0.05), post hoc comparisons using Tukey's honestly significant difference (HSD) test, with a subsequent Bonferroni correction. To test H1 (head position), we compared least-squares means (LSM) of bottle 20+ days with bowl 20+ days. To test H2 (liquid versus solid food), we compared bowl 20+ days with solid 20+ days, and bottle with solid. To test H3 (maturity), we compared bottle 7 days with bottle 17 days and bottle 17 days with bottle 20+ days.
3. Results
The overall model was significant (p < 0.001). Variation among individuals (random factor) was 0.001, while the total error variance was 0.006. Testing for the fixed effect (age–feed) showed that it was significant, with an F-ratio of 12.938, d.f. = 751.
(a). H1: the impact of head position on aerodigestive coordination
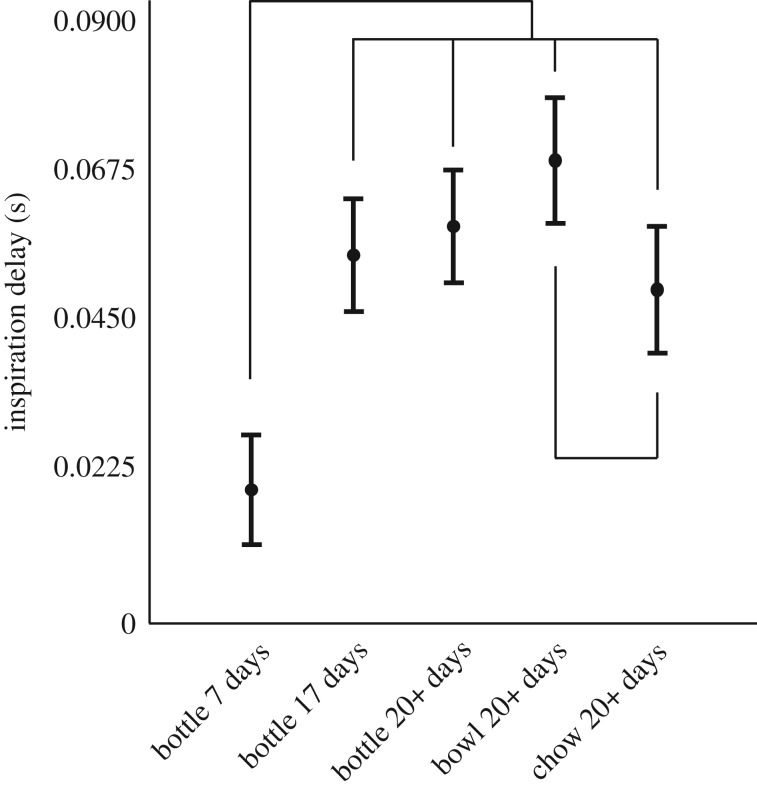
We found no effect of head position on aerodigestive coordination as infants feeding from a bottle at 20+ days did not differ from those drinking from a bowl (bottle 20+ days least square mean (LSM) = 0.057 s ± 0.006, bowl LSM = 0.068 s ± 0.007, table 1 and figure 2).
Table 1.
Pairwise differences between least-square means (LSM) (±s.e.) of group, and p-value for testing with Tukey's test for differences. d, days.
| groups compared |
LSM difference (s.e.) | p-value | |
|---|---|---|---|
| bottle 7 d | bottle 17 d | −0.034 (0.006) | <0.001 |
| bottle 20+ d | −0.037 (0.009) | 0.001 | |
| bowl | −0.047 (0.010) | <0.001 | |
| chow | −0.028 (0.010) | 0.035 | |
| bottle 17 d | bottle 20+ d | −0.003 (0.009) | 0.997 |
| bowl | −0.014 (0.009) | 0.601 | |
| chow | −0.006 (0.010) | 0.972 | |
| bottle 20+ d | bowl | −0.011 (0.005) | 0.187 |
| chow | 0.009 (0.005) | 0.382 | |
| bowl | chow | 0.020 (0.006) | 0.006 |
Figure 2.
Least-squares mean ± s.e. delay of inspiration relative to the swallow (in seconds) for infants feeding on milk from a bottle (at 7, 17 and 20 days of age), milk from a bowl (at Days 20+) and solid food from a bowl (at Days 20+). Brackets show which pairwise comparisons are significantly different using Tukey's HSD. Bottle 7 days is different from all other time points, and bowl and chow at 20+ days are different.
(b). H2: liquid versus solid food
Changing food type (milk to solid) altered the delay of inspiration relative to the swallow. Infants feeding on solid food began inspiration sooner than those drinking from a bowl (bowl LSM = 0.068 s ± 0.007, solid food LSM = 0.048 s ± 0.007, table 1 and figure 2). However, the delay of inspiration relative to the swallow when feeding on chow was not different from feeding on a bottle at 17 or 20+ days old (solid food LSM = 0.048 s ± 0.007, bottle 17 days LSM = 0.054 s ± 0.006, bottle 20+ days LSM = = 0.057 s ± 0.006 table 1 and figure 2).
(c). H3: maturational changes in aerodigestive coordination when bottle feeding
In line with previous work [8], we found substantive changes in aerodigestive coordination associated with postnatal age during bottle feeding (table 1, figure 2, electronic supplementary material and [28]). Day 7 feeding was significantly different from all other treatments. The delay of inspiration relative to the swallow generally increased with postnatal age up to day 17. Days 17 and 20+ had delays that were not statistically different (p = 0.99).
4. Discussion
We found support for some but not all of the three hypotheses. For H1, our results, against our predictions, indicate no significant difference in the timing of inhalation after swallowing when drinking milk from a bowl versus a bottle at 20 days. This result supports the idea that mammals, including humans, separate the acquisition and transport of fluid through the oral cavity and anterior oropharynx from the actual swallow, where liquid transits over or around the laryngeal opening [16,17]. It seems that despite substantive changes in muscle function and kinematics associated with changes in head position and mechanics of drinking from a bottle or a bowl, once the food has been accumulated in the valleculae it is processed similarly [1].
We found partial support for H2, as the inspiration delay during feeding on solid food was shorter than when drinking from a bowl. However, it did not differ compared with the inspiration delay of older pigs feeding on a bottle. A longer delay in inspiration relative to the swallow has been suggested to reduce the incidences of aspiration [8], which suggests that infants experience safer swallows when drinking from a bowl than when eating solids. It is possible that the introduction of a new food consistency, such as solids, requires relearning the delay. Future studies should explore aerodigestive coordination during the consumption of solid foods longitudinally after weaning to determine if this matures similarly to bottle feeding.
In support of H3, we found that the onset of inspiration when feeding from a bottle increased with age [8]. However, by extending our data collection longitudinally, we found that this increase appears to level off, as by the time infants began feeding on solid food, the aerodigestive coordination when feeding from a bottle was similar to Day 17.
(a). Implications for maturation and response to behavioural change
Behavioural changes associated with food consumption at weaning include both sensory (greater variety of textures and flavours, incorporating feedback from mastication) and motor (intercalation of chewing between stage I and stage II transport) components [2]. Furthermore, it is assumed that the risks of airway protection failure for solid food (choking and asphyxiation) are higher than the risks associated with liquid (aspiration pneumonia). Despite this, we found that infants feeding on solid food exhibited a lower, and potentially more dangerous, inspiration delay than those feeding from milk in bowls.
Multiple mechanisms may play a role in this difference. Increasing food viscosity is a common treatment for patients that commonly experience aspiration [18,19], as it is believed to decrease the likelihood of spillage into the airway [20]. Thus, the risk for aspiration may be lower when feeding on viscous masticated solids than when drinking liquids, and infants may be adjusting their aerodigestive coordination accordingly. The decreased delay of inspiration relative to the swallow may represent an immature stage of aerodigestive coordination associated with very early exposure to solid food. Swallowing and respiration are controlled by brainstem networks that interact [21,22].The changes seen in our data may reflect a snapshot of developmental plasticity in that interaction. The shortened delay of inspiration seen with solid food may represent part of a modified swallowing pattern associated with eating solid food. More longitudinal analyses post-weaning along with a comparison of the kinematics and physiology of solid versus liquid food swallowing will help distinguish among these three possibilities.
Weaning changes aerodigestive function. However, the improved efficiency in drinking developed over infancy [23] is retained through weaning. In many mammalian species, including pigs and humans, a period of overlap exists between the onset of solid food intake and the cessation of maternal milk consumption [24–26]. Maintaining efficient aerodigestive function in liquid consumption developed through infancy allows the weanling to extract maximum nutrition from increasingly infrequent milk feeds while it navigates the challenges of eating solid food. Thus, the interplay of maturational timelines of oropharyngeal function at weaning may serve as a locus for behavioural and life-history plasticity, by allowing more time for adult feeding systems to mature functionally and anatomically [27].
5. Conclusion
Aerodigestive coordination during infancy and weaning is complex and multifaceted. Coordination of respiration and swallowing matures over infancy, but does not appear to be impacted by how infants acquire liquids (bottle versus bowl). Instead, the introduction of solid foods alone has an effect on aerodigestive coordination, as the delay of inspiration relative to the swallow differed between infants drinking from a bowl and those consuming solid food. The specific sensorimotor processes affected by the introduction of solid food that underlie these changes are an avenue of future research.
Acknowledgements
The authors thank the NEOMED CMU staff, C. Aiyudu, N.D. Anonuevo, E. Catchpole, J. Irizarry, and K. Wu for their contributions. We also thank three anonymous reviewers for their feedback.
Ethics
All procedures were done in accordance with NEOMED IACUC approved protocol no. 17-04-071.
Data accessibility
All data used in this article can be accessed at Dryad Digital Repository: http://doi.org/10.5061/dryad.2fqz612kw [28].
Authors' contributions
R.Z.G. and F.D.H.G. designed the study. L.E.B., B.M.S., R.Z.G. and F.D.H.G. collected data. L.E.B., B.M.S., R.Z.G. and C.J.M. processed and analysed data. All authors participated in writing and revising the paper and all approved the final version.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by NIH R01 HD088561 to R.Z.G.
References
- 1.Thexton AJ, Crompton AW, German RZ. 1998. Transition from suckling to drinking at weaning: a kinematic and electromyographic study in miniature pigs. J. Exp. Zool. 280, 327–343. () [DOI] [PubMed] [Google Scholar]
- 2.Hiiemae KM, Crompton AW. 1985. Mastication, food transport and swallowing. In Functional vertebrate morphology (eds Hildebrand M, Bramble D, Liem K, Wake D), pp. 262–290. Cambridge, MA: Harvard University Press. [Google Scholar]
- 3.Belo LR, Gomes NAC, Coriolano MdGWdS, de Souza ES, Moura DAA, Asano AG, Lins OG. 2014. The relationship between limit of dysphagia and average volume per swallow in patients with Parkinson's disease. Dysphagia 29, 419–424. ( 10.1007/s00455-013-9512-7) [DOI] [PubMed] [Google Scholar]
- 4.Jadcherla S. 2016. Dysphagia in the high-risk infant: potential factors and mechanisms. Am. J. Clin. Nutr. 103, 622S–628S. ( 10.3945/ajcn.115.110106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gewolb IH, Vice FL. 2006. Maturational changes in the rhythms, patterning, and coordination of respiration and swallow during feeding in preterm and term infants. Dev. Med. Child Neurol. 48, 589–594. ( 10.1017/S001216220600123X) [DOI] [PubMed] [Google Scholar]
- 6.Vice FL, Gewolb IH. 2008. Respiratory patterns and strategies during feeding in preterm infants. Dev. Med. Child Neurol. 50, 467–472. ( 10.1111/j.1469-8749.2008.02065.x) [DOI] [PubMed] [Google Scholar]
- 7.Mayerl CJ, Gould FDH, Bond LE, Stricklen BM, Buddington RK, German RZ. 2019. Preterm birth disrupts the development of feeding and breathing coordination. J. Appl. Physiol. 126, 1681–1686. ( 10.1152/japplphysiol.00101.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballester A, et al. 2018. Maturation of the coordination between respiration and deglutition with and without recurrent laryngeal nerve lesion in an animal model. Dysphagia 33, 627–635. ( 10.1007/s00455-018-9881-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell-Malone R, Crompton AW, Thexton AJ, German RZ. 2011. Ontogenetic changes in mammalian feeding: insights from electromyographic data. Integr. Comp. Biol. 51, 282–288. ( 10.1093/icb/icr026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inamoto Y, et al. 2013. The effect of bolus viscosity on laryngeal closure in swallowing: kinematic analysis using 320-row area detector CT. Dysphagia 28, 33–42. ( 10.1007/s00455-012-9410-4) [DOI] [PubMed] [Google Scholar]
- 11.Ra JY, Hyun JK, Ko KR, Lee SJ. 2014. Chin tuck for prevention of aspiration: effectiveness and appropriate posture. Dysphagia 29, 603–609. ( 10.1007/s00455-014-9551-8) [DOI] [PubMed] [Google Scholar]
- 12.Leigh J-H, Oh B-M, Seo HG, Lee GJ, Min Y, Kim K, Lee JC, Han TR. 2015. Influence of the chin-down and chin-tuck maneuver on the swallowing kinematics of healthy adults. Dysphagia 30, 89–98. ( 10.1007/s00455-014-9580-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saconato M, Chiari BM, Lederman HM, Gonçalves MIR. 2016. Effectiveness of chin-tuck maneuver to facilitate swallowing in neurologic dysphagia. Int. Arch. Otorhinolaryngol. 20, 13–17. ( 10.1055/s-0035-1564721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.German RZ, Crompton AW, Thexton AJ. 1998. The coordination and interaction between respiration and deglutition in young pigs. J. Comp. Physiol. A 182, 539–547. ( 10.1007/s003590050201) [DOI] [PubMed] [Google Scholar]
- 15.German RZ, Crompton AW, Gould FDH, Thexton AJ. 2017. Animal models for dysphagia studies: what have we learnt so far. Dysphagia 32, 73–77. ( 10.1007/s00455-016-9778-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiiemae KM, Palmer JB. 1999. Food transport and bolus formation during complete feeding sequences on foods of different initial consistency. Dysphagia 14, 31–42. ( 10.1007/PL00009582) [DOI] [PubMed] [Google Scholar]
- 17.German RZ, Saxe SA, Crompton AW, Hiiemae KM. 1989. Food transport through the anterior oral cavity in macaques. Am. J. Phys. Anthropol. 80, 369–377. ( 10.1002/ajpa.1330800310) [DOI] [PubMed] [Google Scholar]
- 18.September C, Nicholson TM, Cichero JAY. 2014. Implications of changing the amount of thickener in thickened infant formula for infants with dysphagia. Dysphagia 29, 432–437. ( 10.1007/s00455-014-9523-z) [DOI] [PubMed] [Google Scholar]
- 19.Yoon S-N, Yoo B. 2017. Rheological behaviors of thickened infant formula prepared with xanthan gum-based food thickeners for dysphagic infants. Dysphagia 32, 454–462. ( 10.1007/s00455-017-9786-2) [DOI] [PubMed] [Google Scholar]
- 20.Nicosia MA. 2013. Theoretical estimation of shear rate during the oral phase of swallowing: effect of partial slip. J. Texture Stud. 44, 132–139. ( 10.1111/jtxs.12005) [DOI] [Google Scholar]
- 21.Dick TE, Oku Y, Romaniuk JR, Cherniack NS. 1993. Interaction between central pattern generators for breathing and swallowing in the cat. J. Physiol. 465, 715–730. ( 10.1113/jphysiol.1993.sp019702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishino T, Kohchi T, Honda Y, Shirahata M, Yonezawa T. 1986. Differences in the effects of hypercapnia and hypoxia on the swallowing reflex in cats. Br. J. Anaesth. 58, 903–908. ( 10.1093/bja/58.8.903) [DOI] [PubMed] [Google Scholar]
- 23.Mayerl CJ, Myrla AM, Bond LE, Stricklen BM, German RZ, Gould FD. 2019. Premature birth impacts bolus size and shape through nursing in infant pigs. Pediatr. Res. 87, 656–661. ( 10.1038/s41390-019-0624-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alder EM, Williams FLR, Anderson AS, Forsyth S, Florey C du V, van der Velde P. 2004. What influences the timing of the introduction of solid food to infants? Br. J. Nutr. 92, 527–531. ( 10.1079/BJN20041212) [DOI] [PubMed] [Google Scholar]
- 25.Kuo AA, Inkelas M, Slusser WM, Maidenberg M, Halfon N. 2011. Introduction of solid food to young infants. Matern. Child Health J. 15, 1185–1194. ( 10.1007/s10995-010-0669-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vail B, Prentice P, Dunger DB, Hughes IA, Acerini CL, Ong KK. 2015. Age at weaning and infant growth: primary analysis and systematic review. J. Pediatr. 167, 317–324.e1. ( 10.1016/j.jpeds.2015.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stover KK, Sidote J, Williams SH. 2017. An ontogenetic perspective on symphyseal fusion, occlusion and mandibular loading in alpacas (Vicugna pacos). Zoology 124, 95–105. ( 10.1016/j.zool.2017.06.006) [DOI] [PubMed] [Google Scholar]
- 28.Bond LE, Mayerl CJ, Stricklen BS, German RZ, Gould FDG. 2020. Data from: Changes in the coordination between respiration and swallowing from suckling through weaning Dryad Digital Repository. ( 10.5061/dryad.2fqz612kw) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Bond LE, Mayerl CJ, Stricklen BS, German RZ, Gould FDG. 2020. Data from: Changes in the coordination between respiration and swallowing from suckling through weaning Dryad Digital Repository. ( 10.5061/dryad.2fqz612kw) [DOI] [PMC free article] [PubMed]
Data Availability Statement
All data used in this article can be accessed at Dryad Digital Repository: http://doi.org/10.5061/dryad.2fqz612kw [28].