Abstract
Theories of human cognition prominently feature Broca’s area, which causally contributes to a myriad of mental functions. However, Broca’s area is not a monolithic, multi-purpose unit; it is structurally and functionally heterogeneous. Some functions engaging (subsets of) this area share neuro-cognitive resources, whereas others rely on separable circuits. A decade of converging evidence has now illuminated a fundamental distinction between two sub-regions of Broca’s area that likely play computationally distinct roles in cognition: one belongs to the domain-specific “language network”, the other to the domain-general “multiple-demand network”. Claims about Broca’s area should be (re)cast in terms of these (and other, as yet undetermined) functional components, to establish a cumulative research enterprise where empirical findings could be replicated, and theoretical proposals could be meaningfully compared and falsified.
Keywords: Broca’s area, LIFG, language, executive functions, domain-specificity, articulation
The Construct of Broca’s Area
The modern enterprise of human cognitive neuroscience commenced with the landmark proposal by Paul Broca [1] linking speech articulation mechanisms to the posterior portion of the left inferior frontal gyrus (LIFG), a region hence known as “Broca’s area”. Since this original observation, Broca’s area has been implicated in diverse cognitive functions, extending well beyond articulation. Most proposals fall under one of two general hypotheses: according to one prominent hypothesis, Broca’s area implements syntactic/combinatorial processing either in language [2–6] or across multiple domains [7–10]. According to another hypothesis, Broca’s area supports executive functions, like working memory, selection, or cognitive control [11–13]. Thus, despite its advanced scientific age, this region continues to play a central role in current theorizing about high-level cognition, including some allegedly human-specific capacities (cf. Box 1). We here argue that these and other hypotheses construed as referring to “Broca’s area” cannot be meaningfully evaluated because this region consists of multiple functionally distinct components.
Box 1: Possible homologies between human and non-human left frontal brain areas.
With our genome largely shared with other primates [123], it is perhaps unsurprising that many of our perceptual, motor, and cognitive abilities—and their neural substrates—appear similar. Whereas a major neural difference is the expansion of the human frontal cortex [124], most cytoarchitectonic areas, including those in Broca’s area, appear to have homologs in non-human primates [125; cf. 126]. Similarly, based on functional correlations in fMRI, at least some frontal areas and their associated functional networks appear to be conserved between humans and macaques [127]. Of most relevance here are two networks that were recently identified in macaque brains that appear homologous to (i) the MD network [128], and (ii) the human articulation network [129].
From single-cell physiology, we have long known that frontal and parietal areas in non- human primates exhibit MD-like properties: flexible coding of task-relevant information [17,130–132]. However, such recordings are necessarily limited in their coverage. A recent study [128] used data from resting-state fMRI to map a putative MD network in macaques, guided by the topography of the human MD network and known human-macaque brain correspondences. They successfully recovered a set of functionally inter-connected areas, including three lateral frontal areas, one in the vicinity of the “Broca’s area” homolog [125].
Although human language has no equal in the animal kingdom, communication systems of our primate relatives can importantly inform our understanding of human communication [133]. A recent fMRI investigation of social interactions in macaques [129] reported that generating lipsmacks (canonical primate emotional expression) recruits a set of brain areas that strongly resemble the human articulatory control network [107,134] (Box 2), including parts of ventrolateral premotor cortex.
In summary, non-human primate lateral frontal cortex contains areas that are likely homologous with the human MD areas, and areas that control vocal production. Less clear is whether any frontal areas correspond to the human high-level language areas. Although language is uncontroversially more rich and flexible than non-human communication systems, recent research suggests that even properties that have long been argued to be unique to human language, like compositionality, are robustly present and can be fruitfully studied in non-human primates [135], and that non-human communication exhibits information-theoretic optimization [136] similar to human languages [119]. In fact, the brain areas of macaque monkeys that appear to be sensitive to the interpretation of socially-relevant signals bear a striking similarity to the language network in humans ([129]; see also 137–138]).
Empirical and theoretical papers still commonly use the term “Broca’s area” [14] or refer to one of its anatomical sub-divisions (the opercular or the triangular portion of the LIFG, corresponding to Brodmann Areas (BA) 44 and 45, respectively, according to some atlases [15]). However, in this piece we argue that the construct of Broca’s area, or its macro-anatomic sub-regions, is problematic and has led to empirical confusion and misguided theorizing. Our argument is organized as follows: first, we discuss the problem of inter-individual macro- and micro-anatomical variability in the part of the brain that houses Broca’s area. We then summarize recent work that has established the existence of two distinct functional regions within Broca’s area: one that belongs to the fronto-temporal language- selective network [16], and one that belongs to the domain-general fronto-parietal multiple-demand (MD) network [17–18]. We proceed to show how failing to account for inter-individual variability in the precise locations of these and other functional components has led to inaccurate inferences about Broca’s area in prior studies. We conclude by highlighting recent findings that are beginning to illuminate the role of the language-selective and the domain-general components of Broca’s area, and their associated networks, in language processing.
Inter-Individual Variability of Broca’s Area
Broca’s area is characterized by striking individual differences in both macro- and micro-anatomy, which has likely muddled empirical data and theoretical frameworks over the years. Macro-anatomic variability concerns differences in the size, shape, and location of gyri and sulci, the presence/absence and depth of sulci, the number of sulcus segments, and the number and position of side branches. Variability in these features has been reported for the left frontal cortex, including specifically Broca’s area [19–24]. For example, one study [23] used MRI to examine the pars opercularis in 50 individuals and found considerable variability in its shape: in some brains, almost the entire structure was visible on the surface, appearing as a single convolution or two convolutions, whereas in other brains, the region was partially or completely hidden in the inferior precentral sulcus. Another study [24] found that on the lateral frontal surface, even the relatively stable sulcal landmarks exhibited variability of up to ~6mm (i.e., ~3 voxels, or more in higher-resolution sequences).
Furthermore, finer sub-divisions within the LIFG also exhibit high inter-individual variability. Such sub-divisions may be defined based on differences in cellular properties (cyto-architecture), the level of axon myelination (myelo-architecture), and the density and distribution of receptor types (recepto-architecture) [15,25–29], and plausibly correspond to different functional components. Importantly, these micro-architectonic areas are not accessible to current in-vivo human brain imaging techniques and do not bear a consistent relation to gyri and sulci [15,22]. For example, one study [22] reported that the BA44/BA6 border varies in its location between the rostral and caudal walls of the precentral sulcus, which means that, across individuals, it differs with respect to the fundus of the sulcus by 1–2cm.
Inter-individual variability adversely affects all analyses that assume a stable relationship between a location in a common coordinate system (like the Talairach or the MNI template; [30]) and function. Such analyses include traditional random-effects analyses [31] as well as analyses of functional correlations performed at the group level [32], voxel-based morphometry/lesion-symptom mapping approaches common in neuropsychological patient research [33], and meta-analyses of activation peaks [34]. The problem is that any given coordinate in a common space may correspond to one functional region in one individual, but a different functional region in another [30,35–36] (Figure 1c). Indeed, functional activations in the association cortices, including lateral frontal cortex, do not align well across individuals [37–39]. Even identifying macro-anatomical landmarks in the native brain space of each individual [40] is problematic because—at least for higher-order association areas— these landmarks cannot be used as reliable proxies for micro-anatomical areas and, therefore, for function.
Figure 1. Responses to language vs. executive demands in LIFG.
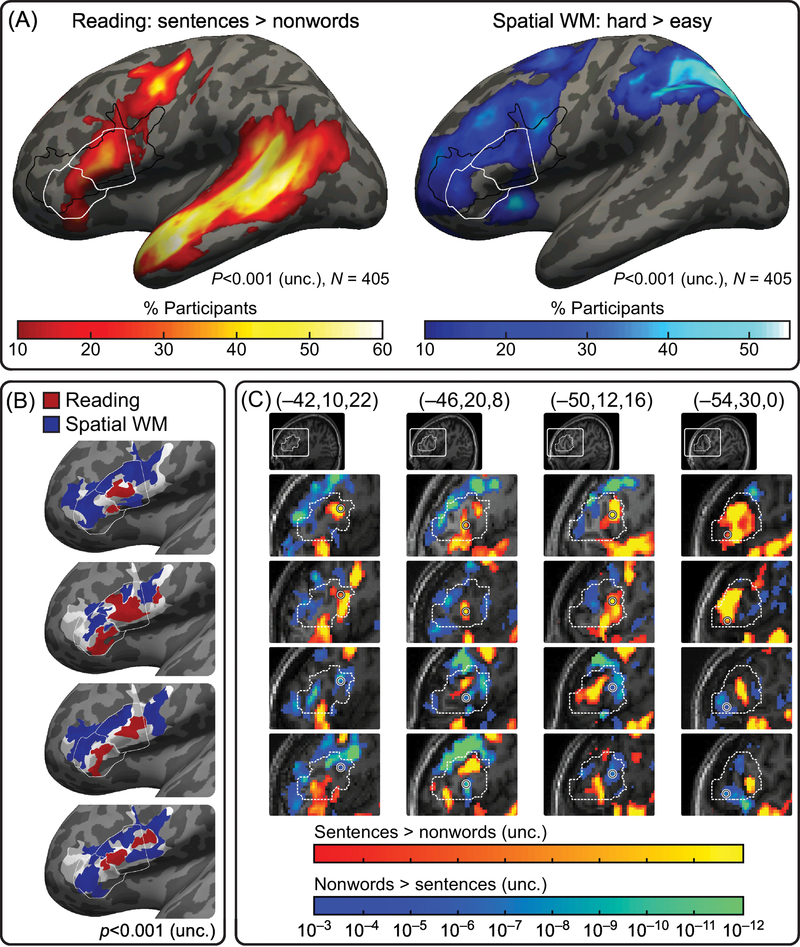
(a) Probabilistic overlap maps across a sample of 405 participants showing, for each voxel, the percentage of participants who show significant (p<0.001, uncorrected) effects for a contrast between passive reading of sentences and nonword lists (left), and a contrast between hard and easy versions of a spatial working memory task (right). (The data are projected onto a lateral view of an inflated left hemisphere of an average brain template.) These two contrasts robustly and reliably identify the language network and the multiple-demand (MD) network, respectively. The boundaries of the opercular and triangular dub-divisions of the IFG are outlined in white [148]. The boundaries within which BA44 and BA45 could fall are outlined in black [149]. The IFG contains regions responsive to the sentences>nonwords contrast, as well as distinct regions responsive to the hard>easy working memory contrast. (b) Functionally distinct regions within the IFG in individual participants. Significant effects (p<0.001, uncorrected) for the sentences>nonwords contrast (red) and hard>easy working memory contrast (blue) in the IFG of four sample brains. Activations are only shown if they fall within the probabilistic borders of BA44/BA45 (light patch) and/or the boundaries of the opercular and triangular sub-divisions of the IFG (white outlines). (c) Variability in the locations of language and MD regions in the IFG. Each column shows a different coordinate in the MNI space (circled); the top two panels show activation maps in subjects with language-selective activity in that coordinate, and the bottom two panels show activation maps in subjects with domain-general MD activity in that coordinate. The white dashed contour shows the boundaries of the opercular and triangular sub-divisions of the IFG based on [150].
Given these challenges, how can we make progress toward characterizing the functional organization of Broca’s area and understanding its consequences for the cognitive architecture of the human mind? Some researchers—including us—have argued that inter-individual variability in the precise locations of functional areas must be taken into consideration. Currently, this can be achieved with one of two procedures: (1) examining functional responses at the level of individual brains and then pooling data from the ‘same’ functional areas (cf. the same location in a common space or the same macro- anatomic area) to draw population-level inferences [30,35–36,41]; or (2) interpreting locations in common coordinate systems in relation to probabilistic activation atlases [42] (Figure 1a) that can help estimate the probability of a given coordinate falling within a particular functional area. Indeed, these kinds of approaches have revealed a robust dissociation within Broca’s area: a distinction between a language-selective region and a domain-general region. We describe this dissociation in the following section.
Two Distinct Functional Regions Within Broca’s Area
A number of prior studies have reported functional dissociations within Broca’s area/LIFG [43–48]. Here, we focus on the dissociation that has most consistently and robustly emerged across diverse methodological approaches—between a language-selective region and a domain-general region (see Box 2 for another region not discussed here). We present four kinds of data in support of this dissociation: functional responses in task-based paradigms [46,49], functional correlations during naturalistic cognition [50–51], correlations across individuals in effect sizes from task-based paradigms [49], and cognitive deficits following brain damage [52].
Box 2: An articulation-responsive region within Broca’s area.
The initial attention that Broca’s area received was when a severe deficit in speech articulation was observed in a patient with damage to posterior LIFG, who exhibited apparently preserved comprehension and non-linguistic abilities [1]. Later patient work and brain imaging studies in healthy adults have implicated a number of additional areas in articulation, including regions within the precentral gyrus, supplementary motor area, the insula, superior temporal cortex, and cerebellum [107,134,139–141]. Some have even questioned the role of Broca’s area in articulation [141]. However, much recent evidence aligns with Broca’s original claim [142–143]. In fact, [107] found that a region within Broca’s area, but not other frontal areas that are active during articulation, appears to exhibit some selectivity for speech production relative to non-articulation tasks that share features with speech production, like the production of non-speech oral-motor movements. In line with this selectivity, most current proposals link this region with speech-specific functions, such as implementing motor syllable programs [144] or containing portions of the speech sound map [134], but the precise contribution of this region to articulation remains debated. For example, using intracranial recording data, [145] argued that sites within left posterior IFG mediate information flowing from the temporal cortex (where phonological planning likely occurs [146]) to motor regions. Given that during actual speech, only the motor regions were active, and sites in posterior LIFG were silent, they hypothesized that the latter prepare an articulatory code that is sent to the motor cortex where it is implemented. Relatedly, using intraoperative cortical cooling, [147] found that interfering with neural activity in posterior LIFG affected the timing of speech, but not its quality (which was instead affected by cooling the speech motor cortex). The authors suggested that posterior LIFG may support sequence generation for speech production. Importantly, and regardless of its precise computations, the articulation-responsive region within posterior LIFG is distinct from both the high- level language-selective region and the domain-general MD region within Broca’s area [107].
The first three sets of findings come from studies that have relied on the functional localization approach [30,35,41]. In this approach, a robust and well-normed “localizer” task is used to identify some brain areas that show a particular functional signature. For example, one can find the language-responsive portion of Broca’s area by contrasting responses to sentences vs. nonwords, or speech vs. foreign/acoustically degraded speech [41,53], and its domain-general portion by contrasting responses to a more vs. less demanding executive-function task [58] (Figure 1). (Although localizer contrasts are sometimes criticized for the choice of particular stimuli/tasks [54], much evidence now suggests that well-validated localizers parcellate the brain in much the same way as data-driven, whole-brain clustering of voxel time-courses during naturalistic paradigms [55–56], or patterns of white-matter connections among areas [57]. As a result, a functional localizer simply provides a quick and efficient way to pick out the relevant functional subset of the brain, absent the ability to rely on visible anatomy as a guide.) The fourth finding comes from a study that has interpreted lesion data with respect to large-N probabilistic activation atlases for the language and the MD networks.
Distinct Responses in Task-Based Paradigms
Two very different functional response profiles co-exist within Broca’s area [46,49]. One region appears to be selective for linguistic input (both visual and auditory [41,53,59]) (Figure 2) over performing a variety of non-linguistic cognitive tasks. These tasks include music perception [16], arithmetic processing [16,60–61], executive function tasks [16,49], thinking about others’ mental states [62], and action/gesture observation [63–64] (Figure 2). This region is similar in its response profile to several other regions, which jointly form the left-lateralized fronto-temporal “core language network” [41,65–66] (left side of Figure 1a, brain insets with red parcels in Figure 2). This network includes at least two other areas in the left frontal cortex—one in the orbital portion of the LIFG and one in the posterior portion of the left middle frontal gyrus—as well as areas in the left temporal lobe. The regions of this network have been linked to “high-level” (i.e., a-modal) language processing, including lexical and combinatorial operations [41,67], but excluding lower-level perceptual and motor components [66,68].
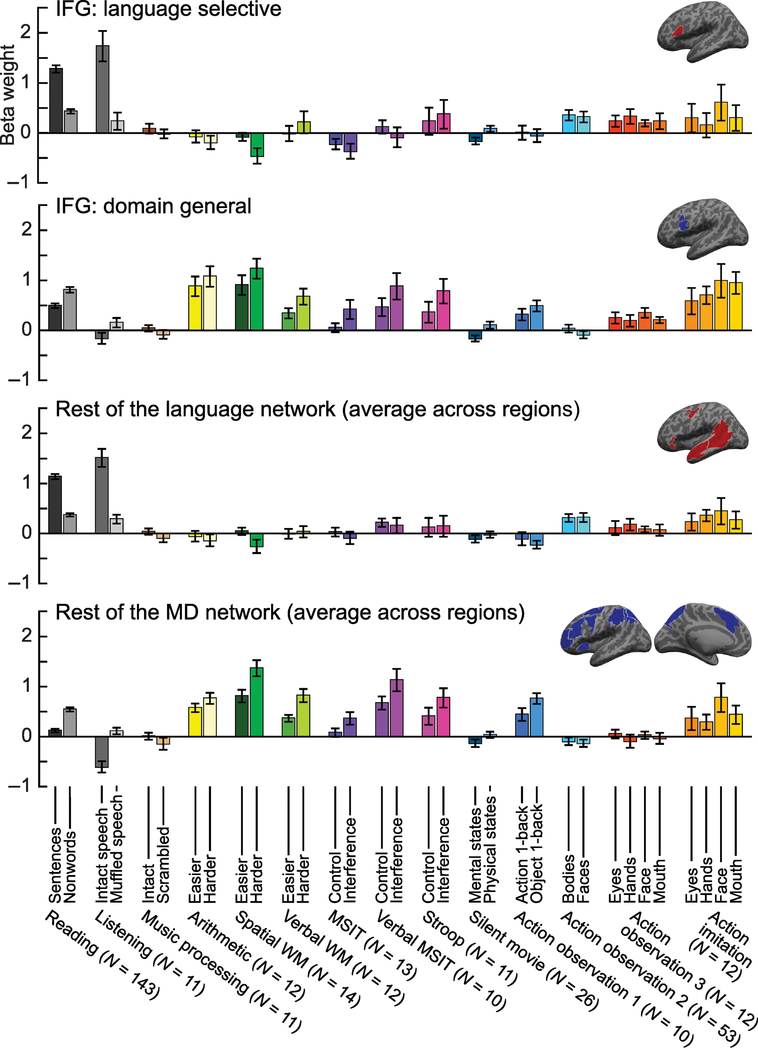
Figure 2. Two regions within Broca’s area and their associated networks.
Response profiles of functionally-defined regions within Broca’s area (top two bar graphs), separately for its language-selective and domain-general MD regions (sources of the data: [16,41,53,58,62–63]). The former region responds selectively to language across modalities (reading and listening) but shows low responses to linguistically/acoustically degraded controls as well as a wide array of non- linguistic stimuli and tasks (music, arithmetic, spatial and verbal working memory, cognitive control tasks, physical and mental events, and action observation). The latter region, in contrast, scales its response with processing demands in both the linguistic domain (where degraded control materials are harder to process than intact input) and multiple, non-linguistic domains. The bottom two bar graphs show the response profiles across the rest of the language network (averaged across 4 regions) and the rest of the MD network (averaged across 18 regions). Note the similarity between the response profile of the language-selective IFG region and the language network, on the one hand, and the domain-general IFG region and the MD network, on the other hand. All regions within and outside the IFG were functionally defined in individual participants using the corresponding contrasts (Figure 1a). The locations of these functional regions were constrained to fall within large areas of cortex that contain the activations of most participants in prior studies (these areas are colored on the inflated brain images on the top-right corner of each panel).
This language-selective region is abutted by highly domain-general regions (right side of Figure 1a, brain insets with blue parcels in Figure 2). Here, we focus on the region that falls posteriorly to the language-selective region (albeit with considerable inter-individual variability in its precise location; Figure 1b-c). We note, however, that functionally similar regions also lie superiorly to it, along the left inferior frontal sulcus/middle frontal gyrus, and inferiorly, within the left anterior insula (Figure 1a-b), sometimes forming a contiguous activation cluster with the region falling within the LIFG. These regions respond to diverse demanding perceptual and cognitive tasks, including arithmetic processing [58,61], executive function tasks [17,58,69–70], and action observation [71], and are modulated by difficulty, with stronger responses to conditions that require greater effort [58,69–70] (Figure 2). These domain-general regions share this response profile with several other frontal, parietal, cingular, and insular regions spread across both hemispheres, which jointly form the bilateral “multiple- demand (MD)” network [17–18]. This network has been linked to several theoretical constructs, including attention, working memory, inhibitory control, planning/goal-directed behaviors, fluid intelligence, and consciousness [17,72–73].
It is important to note that some linguistic manipulations may elicit a response in both language- selective and domain-general MD regions. For example, regions in both networks—at least under some task conditions—have been shown to be sensitive to violations of linguistic structure [74–75], and to manipulations of linguistic complexity in well-formed sentences [76–78]. Therefore, sensitivity within Broca’s area to manipulations of linguistic materials that also affect processing difficulty cannot be used as evidence for the recruitment of a language-specific region (because they could instead, or in addition, reflect the recruitment of the domain-general region). Conversely, sensitivity to manipulations of processing difficulty that focus on the linguistic domain cannot be used as evidence for the recruitment of a domain-general, effort-modulated region (although lack of sensitivity to difficulty modulations may provide evidence against the recruitment of this region, assuming sufficient power).
Critically, the sensitivity of both regions to manipulations that confound linguistic processing and general effort is not an argument against the need to segregate these regions and the associated networks in analyses and theorizing, for two reasons. First, unlike language regions, the engagement of MD regions during task-based linguistic processing does not necessarily generalize to naturalistic comprehension scenarios [79–81], and the functional relevance of the MD network to linguistic behavior remains unclear. And second, and more importantly, the overall response patterns of the language-selective and domain-general regions to linguistic materials are strikingly opposite: the language-selective region responds most strongly to meaningful and structured linguistic stimuli (sentences), with the response falling off as the meaning/structure of the sentence become degraded [41,82] (Figure 2). In contrast, the domain-general region responds more strongly to meaningless and unstructured nonword sequences [58], or to acoustically degraded indecipherable speech [83], compared to well-formed and perceptually clear sentences (Figure 2). This evidence for functional dissociation overrides evidence of association: the fact that for many stimuli and tasks, the two networks respond in distinct ways suggests that we should treat them as functionally separate entities. Unfortunately, many standard language tasks, especially ones popular in clinical practice, like verb generation [84], confound linguistic and general cognitive demands, making the resulting activations difficult to interpret.
Distinct Patterns of Functional Correlations During Naturalistic Cognition
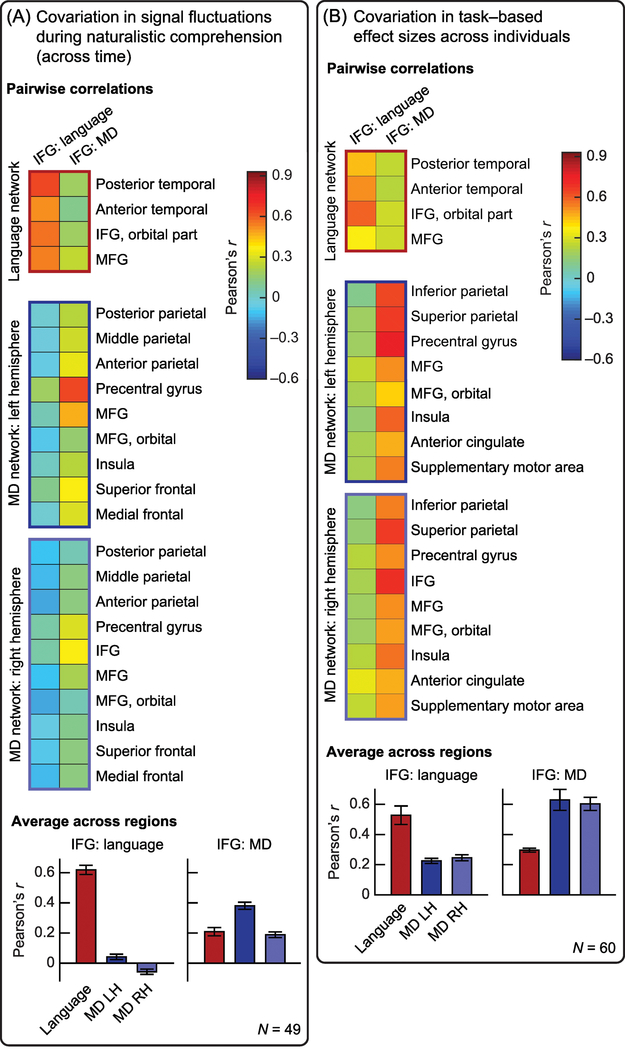
One possible criticism of the functional dissociations in task-based paradigms is that such paradigms underestimate the complexity of real-world cognition [85]. Thus, perhaps in scenarios that more strongly resemble everyday experiences where we simultaneously engage a host of mental processes, we would observe less functional separation and more integration and similarity among different brain areas. Contra this intuition, dozens of studies have now shown that the functional networks that emerge in naturalistic-cognition paradigms closely resemble the ones discovered previously with task- based paradigms [56,86–87]. Similarly, the language-selective and domain-general regions within Broca’s area also dissociate sharply in their pattern of signal fluctuations during rich naturalistic cognition, including both “resting state” and story comprehension paradigms [50–51] (Figure 3a). In particular, the language-selective region exhibits synchronized activity with all core regions of the language network, whereas the domain-general region synchronizes with some regions of the fronto- parietal MD network (mostly in the left hemisphere), and the correlations across these two regions are substantially weaker (the correlations between the two larger networks are non-existent). Moreover, data-driven clustering of signal time-courses in language and MD regions, including the functionally distinct regions within Broca’s area, recovers a clear and replicable parcellation into two networks [50–51].
Figure 3. Functional co-variation between the regions within Broca’s area and their networks.
(a) Co-variation between the two regions within Broca’s area and each of the remaining functional regions in the language and MD networks, based on regional fluctuations in the BOLD signal while listening to naturalistic stories [50–51]. Signal fluctuations (over time) in the language-selective IFG region correlate with fluctuations elsewhere in the language (but not MD) network; and, to a lesser extent, fluctuations in the domain-general IFG region correlate with those in other left-hemispheric MD regions. (b) Co-variation between the two regions within Broca’s area and each of the remaining functional regions in the language and MD networks based on regional effect sizes across individuals [49]. Individuals with stronger (weaker) responses in the language-selective region are likely to also exhibit strong (weak) responses in other language (but not MD) regions, and vice-versa for the domain-general region.
Distinct Patterns of Inter-Region Correlations in Effect Size
A novel approach was recently introduced to probe the relationships among brain regions within and between functional networks, by examining co-variation in the strength of their functional responses across individuals. First, our group [88] found that different language regions robustly exhibit such co-variation in their effect sizes for the contrast between reading sentences and nonword lists. In other words, individuals who show a relatively strong response to the sentences>nonwords contrast in one language region (e.g., the language-selective component of Broca’s area) are also likely to show a strong response to that contrast in other language regions. And importantly, these effect sizes appear to be stable within participants across runs and scanning sessions, suggesting that they tap some time-invariant idiosyncratic properties of individual brains. Additional studies [49; also 89] further showed that MD regions similarly co-vary in their effect sizes across individuals. Critically, they also demonstrated that these effect-size correlations respect functional dissociations between brain networks: across individuals, language and MD regions show relatively weak co-variation with one another in their degree of responsiveness (Figure 3b). Therefore, effect-size correlations across individuals do not simply reflect brain-wide properties like the degree of vascularization, arousal, or generalized processing abilities. From a theoretical standpoint, this finding provides further evidence that the language and MD networks, including their respective components within Broca’s area, are functionally distinct. From an experimental standpoint, it suggests that the response strength in each network can be used as a specific neural marker, to be related to behavior [88,90–91].
Distinct Cognitive Deficits Following Brain Damage
A strong advantage of the functional localization approach—in addition to establishing a cumulative research enterprise and facilitating replications and comparisons across studies [cf. 92]—is that it allows for the accumulation of large datasets where the same functional paradigm is used across hundreds of participants. These datasets can then be used to construct probabilistic activation atlases [42], which capture not only the areas of most consistent responses across individuals, but also the variability in the locations of functional areas. Such atlases can aid the interpretation of group-level activation peaks or lesion data from prior studies.
This approach was recently used [52] to demonstrate a dissociation between the MD and language networks in neuropsychological data, whereby damage to coordinates that likely house the MD network leads to decreases in fluid intelligence, but damage to coordinates that likely house the language network leads to linguistic deficits. Of most relevance to the current piece, this dissociation obtained for lesions limited to the left frontal cortex. This dissociation aligns with other studies that have reported that individuals with even extensive damage to the language network retain their ability to perform diverse non-linguistic tasks, from solving arithmetic problems [93], to causal reasoning [94–95], to representing others’ mental states [96–97], to producing and processing music [98].
Re-evaluating Inferences about Broca’s Area from Prior Studies
Many incorrect inferences in past investigations of Broca’s area may be attributed to reliance on analysis methods that fail to take inter-individual variability in the precise locations of functional regions into account. Specifically, in traditional group analyses, two kinds of problems arise [36]. First, some activations may not be detectable at the group level despite being robustly present in each individual brain, due to little/no overlap across participants (low sensitivity); and second, two regions that are adjacent but functionally distinct in each individual brain may appear as one region at the group level (or when group-level activation peaks are pooled across studies) due to the same stereotactic coordinate showing one functional profile in some participants and a different, sometimes opposite, profile in other participants (Figure 1c) (low functional resolution). Both problems are attested in investigations of Broca’s area, as illustrated with three examples below.
A number of studies have failed to observe activation within Broca’s area for contrasts between sentences and linguistically/perceptually degraded control conditions [99–101]. This has led some to argue against the existence of sentence-responsive areas in Broca’s area/LIFG and for functional differences between the inferior frontal and posterior temporal components of the language network [101–102]. However, this claim is misleading because—given sufficient data per participant—almost every individual shows robust responses within Broca’s area for such contrasts [88,103], suggesting that the occasional lack of group-level effects is due to insufficient spatial overlap among individual participants.
Second, task activations across diverse cognitive domains have long been reported within Broca’s area, including executive tasks like those requiring working memory and cognitive control [69]. Consequently, some have argued that, to the extent that frontal activations are observed for language tasks, they arise from the general executive demands that language processing may impose, like the need to keep information active in working memory or inhibit irrelevant meanings or parses [104–106]. For example, in an early meta-analysis of PET and fMRI activation peaks, Kaan & Swaab [105] concluded, “Broca’s area is only systematically activated when processing demands increase due to working memory demands or task requirements”. Similarly, following activations around Broca’s area for simple articulatory tasks [107], some have argued that activations during language comprehension tasks reflect the need to engage articulatory rehearsal mechanisms [108]. Thus, in a review article, Rogalsky & Hickok [102] suggested that “posterior portions of Broca’s area [...] and surrounding regions support sentence comprehension via articulatory rehearsal” [cf. 109]. In our view, these proposals are misguided because the region within Broca’s area that responds selectively during language processing (including under passive listening/reading conditions [41,53]) is distinct from both the domain-general executive region (Figure 1b) and the articulatory region (Box 2), although the latter two may also be active during language processing under certain conditions.
Finally, according to a prominent hypothesis in the literature, Broca’s area houses an abstract hierarchical-structure processor, which supports syntactic processing in language but also other domains, like arithmetic processing, music perception, and action observation/planning [7–10]. This hypothesis does not fit well with the available data. Most studies that have observed activation for non-linguistic tasks with a hierarchical component have assumed that these activations arise within the same region that processes linguistic syntax, presumably due to the long-standing association between Broca’s area and “language/syntax” [2]. However, as discussed above, the region of Broca’s area that responds more to structured linguistic stimuli (sentences) than to lists of unconnected words is (i) highly selective for language relative to non-linguistic tasks, including ones that involve hierarchical structure and/or recursion, like arithmetic and music [16,60] (Figure 2), which puts into question the domain-general hierarchical processing idea; and (ii) is sensitive not only to syntactic manipulations, but also to those to do with word-level meanings [41,67,75,110], suggesting that any syntax-focused account would need to accommodate these non-syntactic effects. Furthermore, most studies that have reported activation for structural complexity effects (in language and other domains) within Broca’s area have relied on difficulty manipulations, with the more structurally complex condition being more cognitively demanding. Given that the domain-general region of Broca’s area is robustly sensitive to effort [58,69–70], those effects could well have arisen in this region. It is important to keep in mind, however, that this domain-general region appears to also be sensitive to effort in manipulations that seemingly have nothing to do with processing more vs. less complex structures [111], arguing against complex syntactic operations as the core computation of this region [cf. 112].
Contributions of Language-Selective versus Domain-General MD Networks to Language Processing
A core goal of cognitive neuroscience is to develop computationally precise accounts of how different brain areas accomplish perceptual, motor, and cognitive tasks. This is a challenging pursuit in domains like language, where we lack animal models. Nevertheless, recent work has begun illuminating key differences in whether and how the language-selective and domain-general MD regions contribute to language processing.
First, as we have emphasized throughout this piece, the two components of Broca’s area discussed here each belong to a different large-scale brain network. Evidence is accumulating that many functional properties are shared among the regions within each network (Figures 1–3), contra proposals that the language-selective component of Broca’s area differs functionally from the rest of the language areas [2–6]. So, going forward, these regions’ contributions should be considered in light of the networks they belong to [56,66,68,75].
Second, a key constraint on what computations a brain region carries out comes from understanding how its activity is modulated by the properties of different stimuli. We recently showed that the language-selective but not the domain-general MD regions closely “track” linguistic input during naturalistic language comprehension [80] (as evidenced by high inter-subject correlations [113]). Relatedly, the language regions, but not the MD regions, show robust sensitivity to predictability as measured both via lexicalized (5-gram) and hierarchical structure-based surprisal [114]. These results suggest that the MD regions’ contribution to language processing is unlikely to be tied to particular features of the linguistic input like the ambiguity or frequency of a word or structure [cf. 11,13]. Finally, absent a secondary task, MD regions show little or no response to naturalistic language processing [81], suggesting that the MD regions’ contribution likely reflects secondary task demands.
With respect to the language network, its selective responses to linguistic input dramatically narrow the range of hypotheses to be considered by ruling out the domain-general ones. Further, as noted above, its regions respond robustly to both lexico-semantic and syntactic manipulations across diverse paradigms [75], in line with how linguistic knowledge is construed in usage-based and construction- grammar approaches, where the lexicon and grammar are strongly interlinked [115–117]. Further, using a word-order-scrambling manipulation, a recent study [118] found that composition may be the core computation implemented in the language-selective network: even in scrambled sentences, as long as words within local contexts are semantically and syntactically combinable (to a similar degree as in natural linguistic input), the stimulus elicits the maximal response—a response as high as that elicited by well-formed meaningful sentences. Critically, this effect is observed even if the morphosyntactic frame does not support composition. This result makes sense in light of the cross-linguistic universality of composition, and the robustness of language processing mechanisms to morphosyntactic noise [119].
Concluding Remarks
Broca’s area contains a language-selective region that belongs to the fronto-temporal core language network, and a domain-general region that belongs to the fronto-parietal MD network. These two regions clearly dissociate across numerous brain imaging measures—in many cases, exhibiting diametrically opposing response profiles—and likely contribute to linguistic and cognitive processing in computationally distinct ways. These areas do not correspond to visible macroanatomic landmarks, so referring to Broca’s area, LIFG, or LIFG’s anatomical sub-divisions without specifying the relevant functional component(s) is counter-productive, and analogous to attributing a cognitive function to the entire fusiform gyrus, or the cerebellum. Any empirical claim about Broca’s area construed at this level of specificity is therefore difficult, or impossible, to evaluate and test against competing hypotheses. These scientific processes are at the heart of robust and replicable research [120] and are critical for approaching a mechanistic-level understanding of different frontal areas and the cognitive architecture they support.
To make progress in deciphering the contributions of different components of Broca’s area to human cognition, we can identify the relevant regions of Broca’s area using task-based functional markers at the single-participant level [30,35,41], much like vision researchers have long identified face-selective portions of the fusiform gyrus by searching for a stronger response to faces than non-face objects [121–122]. In the future, identification of these regions can perhaps rely on functional correlations [55–56] or anatomical connectivity data [57]. In cases when these approaches are not feasible, we can use probabilistic activation atlases to interpret group-level data [42,52]. But regardless of the approach one adopts, any future hypothesis or proposal about Broca’s area needs to specify whether it refers to the language-selective or the domain-general region therein, or to yet another candidate functional region (e.g., the articulation-responsive region; Box 2). This approach has long been the gold standard in other areas of cognitive neuroscience, including those that, in our view, have made more significant progress towards understanding the targeted mental processes.
A lot of exciting progress remains to be made in deciphering the precise computations carried out by the different regions within Broca’s area and their associated networks (see Outstanding Questions). We hope that the evidence reviewed here—of two functionally distinct regions within Broca’s area— and the approach we advocate bring us closer to realizing this goal.
Outstanding questions.
What functions do different regions of Broca’s area implement? Through a systematic characterization of the functional profiles of the language-selective and domain- general regions in Broca’s area, we are narrowing down the hypotheses for possible representations that these regions store/build and the computations they perform. For example, by establishing that the language-selective region does not respond to music or arithmetic, we have ruled out the hypothesis whereby this region operates over abstract hierarchically structured representations (e.g., [7–9]). However, more work is needed to develop an algorithmic and computationally precise understanding of what these regions do, and whether they differ functionally from the rest of their associated functional networks.
How do micro-structural (cyto-/myelo-/recepto-architectonic) sub-divisions of the lateral frontal cortex correspond to the functional dissociations discussed here? Linking micro-anatomical properties of different patches of cortex with their functional response profiles would give us unprecedented insights into the neural circuitry underlying different cognitive operations, and—through cross-species comparisons—into potential evolutionary trajectories of speech and language. Such investigations could also tell us whether the underlying architecture is comprised of true “regions” with sharp boundaries vs. continuous, gradient “maps” whose extremes we sample. Addressing these questions would only be possible through post-mortem micro-structural analyses of brains of individuals for whom we have functional data. With the spread of brain donation programs, this research may become possible in the decades to come.
How do the language-selective and domain-general MD regions of Broca’s area interact during language comprehension and production? The fact that two or more brain areas are functionally dissociated does not mean that they cannot, or do not, interact. Even though, during naturalistic cognition, functional correlations between the language and MD networks are on average ~0 when measured with fMRI [50–51], it remains possible that they interact on faster time-scales, and/or in ways that have not been measured yet using current techniques. Characterizing the mechanisms via which the language and the MD networks may influence each other’s activity and transfer information remains an important goal for future research.
Highlights.
“Broca’s area” is structurally and functionally heterogeneous, containing a language- selective region and a domain-general Multiple Demand (MD) region.
These two regions exhibit drastically different functional response profiles and co-vary with two distinct brain networks: the core language-selective fronto-temporal network and the domain-general fronto-parietal MD network.
High inter-individual variability in the locations of different functional regions has muddled prior theorizing and empirical work, much of which has relied on traditional group analyses.
Functional localization in individual participants in future investigations of Broca’s area can help establish a robust and cumulative research enterprise.
Any future theoretical proposal or empirical claim needs to specify the relevant functional component of Broca’s area.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Broca P (1861) Remarques Sur le Siége de la Faculté Du Langage Articulé, Suivies D’une Observation D’aphémie (Perte de la Parole). Bulletin Society Anatomique 6, 330–357. [Google Scholar]
- 2.Caramazza A & Zurif EB (1976) Dissociation of algorithmic and heuristic processes in language comprehension: Evidence from aphasia. Brain Lang. 3(4), 572–582. [DOI] [PubMed] [Google Scholar]
- 3.Hagoort P (2005) On Broca, brain, and binding: a new framework. Trends Cogn. Sci. 9(9), 416–423. [DOI] [PubMed] [Google Scholar]
- 4.Grodzinsky Y & Santi A (2008) The battle for Broca’s region. Trends Cogn. Sci. 12(12), 474–480. [DOI] [PubMed] [Google Scholar]
- 5.Friederici AD (2011) The Brain Basis of Language Processing: From Structure to Function. Physiol. Rev. 91, 1357–1392. [DOI] [PubMed] [Google Scholar]
- 6.Friederici AD (2019). Hierarchy processing in human neurobiology: How specific is it? Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences 375(1789), 20180391 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tettamanti M, & Weniger D (2006) Broca’s area: a supramodal hierarchical processor? Cortex, 42(4), 491–494. [DOI] [PubMed] [Google Scholar]
- 8.Fadiga L et al. (2009) Broca’s Area in Language, Action, and Music. Broca’s Area in Language, Action, and Music. Ann. N. Y. Acad. Sci. 1169, 448–458. [DOI] [PubMed] [Google Scholar]
- 9.Fitch WT & Martins MD (2014) Hierarchical processing in music, language, and action: Lashley revisited. Ann. N. Y. Acad. Sci. 1316(1), 87–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L et al. (2015) Representation of numerical and sequential patterns in macaque and human brains. Curr.Biol. 25(15), 1966–1974. [DOI] [PubMed] [Google Scholar]
- 11.Thompson-Schill SL et al. (1997) Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proc. Natl. Acad. Sci. U. S. A. 94(26): 14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabeza R & Nyberg L (2000) Imaging Cognition II: An Empirical review of 275 PET and fMRI Studies. J. Cogn. Neurosci. 12(1), 1–47. [DOI] [PubMed] [Google Scholar]
- 13.Novick JM et al. (2005) Cognitive control and parsing: Reexamining the role of Broca’s area in sentence comprehension. Cogn. Affect. Behav. Neurosci. 5(3), 263–281. [DOI] [PubMed] [Google Scholar]
- 14.Tremblay P & Dick AS (2016) Broca and Wernicke are dead, or moving past the classic model of language neurobiology. Brain Lang. 162:60–71. [DOI] [PubMed] [Google Scholar]
- 15.Brodmann K (1909) Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues, Leipzig: Barth JA. [Google Scholar]
- 16.Fedorenko E et al. (2011) Functional specificity for high-level linguistic processing in the human brain. Proc. Natl. Acad. Sci. U. S. A. 108(39), 16428–16433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duncan J (2010) The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn. Sci. 14(4), 172–179. [DOI] [PubMed] [Google Scholar]
- 18.Duncan J (2013) The Structure of Cognition: Attentional Episodes in Mind and Brain. Neuron 80(1), 35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ono M et al. (1990) Atlas of the cerebral sulci. Stuttgart: Thieme Verlag. [Google Scholar]
- 20.Duvernoy HM et al. (1991) The Human brain: surface, three-dimensional sectional anatomy and MRI. Wien: Springer-Verlag. [Google Scholar]
- 21.Zilles K et al. (1997) Quantitative analysis of sulci in the human cerebral cortex: Development, regional heterogeneity, gender difference, asymmetry, intersubject variability and cortical architecture. Hum. Brain Mapp. 5(4), 218–221. [DOI] [PubMed] [Google Scholar]
- 22.Amunts K et al. (1999) Broca’s region revisited: cytoarchitecture and intersubject variability. J. Comp. Neurol. 412, 319–341. [DOI] [PubMed] [Google Scholar]
- 23.Tomaiuolo F et al. (1999) Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: An in vivo MRI analysis. Eur. J. Neurosci. 11(9), 3033–3046. [DOI] [PubMed] [Google Scholar]
- 24.Juch H et al. (2005) Anatomical variability of the lateral front lobe surface: implication for intersubject variability in language neuroimaging. Neuroimage 24, 504–514. [DOI] [PubMed] [Google Scholar]
- 25.Von Economo CF & Koskinas GN (1925) Die cytoarchitektonik der hirnrinde des erwachsenen menschen. Berlin: Springer. [Google Scholar]
- 26.Sarkisov SA et al. (eds) (1949) Cytoarchitecture of the Human Cortex Cerebri, Moscow: Medgiz. [Google Scholar]
- 27.Amunts K et al. (2010) Broca’s region: novel organizational principles and multiple receptor mapping. PLoS Biol. 8 (9), e1000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amunts K & Zilles K (2012) Architecture and organizational principles of Broca’s region. Trends Cogn. Sci. 16(8), 418–426. [DOI] [PubMed] [Google Scholar]
- 29.Zilles K, & Amunts K (2018). Cytoarchitectonic and receptorarchitectonic organization in Broca’s region and surrounding cortex. Current opinion in behavioral sciences, 21, 93–105. [Google Scholar]
- 30.Brett M et al. (2002) The problem of functional localization in human brain. Nat. Rev. Neurosci. 3, 243–249. [DOI] [PubMed] [Google Scholar]
- 31.Holmes AP & Friston KJ (1998) Generalisability, random effects and population inference. Neuroimage 7, S754. [Google Scholar]
- 32.Stanley ML et al. (2013). Defining nodes in complex brain networks. Frontiers in computational neuroscience, 7, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashburner J, & Friston KJ (2000). Voxel-based morphometry—the methods. Neuroimage, 11(6), 805–821 [DOI] [PubMed] [Google Scholar]
- 34.Yarkoni T et al. (2011) Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 8, 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saxe R et al. (2006) Divide and conquer: A defense of functional localizers. Neuroimage 30(4), 1088–1096. [DOI] [PubMed] [Google Scholar]
- 36.Nieto-Castañon A & Fedorenko E (2012) Subject-specific functional localizers increase sensitivity and functional resolution of multi-subject analyses. Neuroimage 63(3), 1646–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frost MA & Goebel R (2011) Measuring structural-functional correspondence: spatial variability of specialised brain regions after macro-anatomical alignment. Neuroimage 59(2), 1369–1381. [DOI] [PubMed] [Google Scholar]
- 38.Tahmasebi AM et al. (2012) Is the Link between Anatomical Structure and Function Equally Strong at All Cognitive Levels of Processing? Cereb. Cortex 22(7), 1593–1603. [DOI] [PubMed] [Google Scholar]
- 39.Vázquez-Rodríguez B, Suárez LE, Markello RD, Shafiei G, Paquola C, Hagmann P, … & Misic, B. (2019) Gradients of structure–function tethering across neocortex. Proceedings of the National Academy of Sciences, 116(42), 21219–21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nieto-Castanon A, Ghosh SS, Tourville JA, & Guenther FH (2003). Region of interest based analysis of functional imaging data. Neuroimage, 19(4), 1303–1316. [DOI] [PubMed] [Google Scholar]
- 41.Fedorenko E et al. (2010) A new method for fMRI investigations of language: Defining ROIs functionally in individual subjects. J. Neurophysiol. 104(2), 1177–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Mruczek RE, Arcaro MJ, & Kastner S (2014) Probabilistic maps of visual topography in human cortex. Cerebral cortex, 25(10), 3911–3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bookheimer S (2002) Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu. Rev. Neurosci. 25, 151–188. [DOI] [PubMed] [Google Scholar]
- 44.Chein JM et al. (2002) Functional heterogeneity within Broca’s area during verbal working memory. Physiol Behav. 77(4–5): 635–639. [DOI] [PubMed] [Google Scholar]
- 45.Costafreda SG et al. (2006) A systematic review and quantitative appraisal of fMRI studies of verbal fluency: Role of the left inferior frontal gyrus. Hum. Brain Mapp. 27: 799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fedorenko E et al. (2012) Language-selective and domain-general regions lie side by side within Broca’s area. Curr. Biol. 22(21), 2059–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clos M et al. (2013). Tackling the multifunctional nature of Broca’s region meta-analytically: co-activation-based parcellation of area 44. Neuroimage, 83, 174–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goucha T & Friederici AD (2015) The language skeleton after dissecting meaning: a functional segregation within Broca’s Area. Neuroimage 114, 294–302. [DOI] [PubMed] [Google Scholar]
- 49.Mineroff Z et al. (2018) A robust dissociation among the language, multiple demand, and default mode networks: Evidence from inter-region correlations in effect size. Neuropsychologia 119, 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blank I et al. (2014) A functional dissociation between language and multiple-demand systems revealed in patterns of BOLD signal fluctuations. J. Neurophysiol. 112(5), 1105–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paunov. A. M. et al. (2019) Functionally distinct language and Theory of Mind networks are synchronized at rest and during language comprehension. J. Neurophysiol. 121(4), 1244–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woolgar A et al. (2018) Fluid intelligence is supported by the multiple-demand system not the language system. Nat. Hum. Behav. 2(3), 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scott TL et al. (2016) A new fun and robust version of an fMRI localizer for the frontotemporal language system. Cogn. Neurosci. 8(3), 167–176. [DOI] [PubMed] [Google Scholar]
- 54.Friston KJ et al. (2006) A critique of functional localisers. Neuroimage 30(4), 1077–1087. [DOI] [PubMed] [Google Scholar]
- 55.Tavor I et al. (2016) Task-free MRI predicts individual differences in brain activity during task performance. Science 352, 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Braga R et al. (2019) Situating the Left-Lateralized Language Network in the Broader Organization of Multiple Specialized Large-Scale Distributed Networks. BioRxiv, doi: https://doi.org/10.110½019.12.11.873174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saygin ZM et al. (2012) Anatomical connectivity patterns predict face-selectivity in the fusiform gyrus. Nat. Neurosci.15(2), 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fedorenko E et al. (2013) Broad domain-generality in focal regions of frontal and parietal cortex. Proc. Natl. Acad. Sci. U. S. A. 110(41), 16616–16621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vagharchakian L et al. (2012) A Temporal Bottleneck in the Language Comprehension Network. J. Neurosci, 32(26), 9089–9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monti MM et al. (2012) Thought Beyond Language: Neural Dissociation of Algebra and Natural Language. Psychol. Sci. 23(8), 914–922. [DOI] [PubMed] [Google Scholar]
- 61.Amalric M, & Dehaene S (2016). Origins of the brain networks for advanced mathematics in expert mathematicians. Proceedings of the National Academy of Sciences, 113(18), 4909–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paunov AM (2019) FMRI studies of the relationship between language and theory of mind in adult cognition (unpublished doctoral dissertation). MIT, Cambridge, MA. [Google Scholar]
- 63.Pritchett BL et al. (2018) High-level language processing regions are not engaged in action observation or imitation. J. Neurophysiol. 120(5), 2555–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jouravlev O, Zheng D, Balewski Z, Pongos ALA, Levan Z, Goldin-Meadow S, & Fedorenko E (2019). Speech-accompanying gestures are not processed by the language- processing mechanisms. Neuropsychologia, 132, 107132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Binder JR et al. (1997) Human brain language areas identified by functional magnetic resonance imaging. J. Neurosci. 17(1), 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fedorenko E & Thompson-Schill SL (2014) Reworking the language network. Trends Cogn. Sci. 18(3), 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fedorenko E et al. (2012) Lexical and syntactic representations in the brain: An fMRI investigation with multi-voxel pattern analyses. Neuropsychologia 50(4), 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fedorenko E (in press). The brain network that supports high-level language processing. In Gazzaniga Ivry, Mangun (Ed.), Cognitive Neuroscience: The Biology of the Mind (5th edition). [Google Scholar]
- 69.Duncan J & Owen AM (2000) Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci, 23(10), 475–483. [DOI] [PubMed] [Google Scholar]
- 70.Hugdahl K et al. (2015) On the existence of a generalized non-specific task-dependent network. Front. Hum. Neurosci. 9, 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caspers S et al. (2010) ALE meta-analysis of action observation and imitation in the human brain. Neuroimage, 50(3) 1148–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cole MW, & Schneider W (2007). The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage, 37(1), 343–360. [DOI] [PubMed] [Google Scholar]
- 73.Dehaene S, & Changeux JP (2011). Experimental and theoretical approaches to conscious processing. Neuron, 70(2), 200–227. [DOI] [PubMed] [Google Scholar]
- 74.Kuperberg GR et al. (2008) Neuroanatomical Distinctions within the Semantic System during Sentence Comprehension: Evidence from functional Magnetic Resonance Imaging. Neuroimage 40(1), 367–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fedorenko E et al. (2018). Word meanings and sentence structure recruit the same set of fronto-temporal regions during comprehension. bioRxiv 477851; doi: https://doi.org/10.110¼77851 [Google Scholar]
- 76.Peelle JE et al. (2010) Neural Processing during Older Adults’ Comprehension of Spoken Sentences: Age Differences in Resource Allocation and Connectivity. Cereb. Cortex 20(4), 773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blank I et al. (2016) Syntactic processing is distributed across the language system. Neuroimage 127, 307–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodd JM, Davis MH, & Johnsrude IS (2005). The neural mechanisms of speech comprehension: fMRI studies of semantic ambiguity. Cerebral Cortex, 15(8), 1261–1269. [DOI] [PubMed] [Google Scholar]
- 79.Wright P et al. (2011) Dissociating linguistic and task-related activity in the left inferior frontal gyrus. J. Cogn. Neurosci. 23(2), 404–413. [DOI] [PubMed] [Google Scholar]
- 80.Blank I & Fedorenko E (2017) Domain-General Brain Regions Do Not Track Linguistic Input as Closely as Language-Selective Regions. J. Neurosci. 37(41), 9999–10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Diachek E et al. (2019). The domain-general multiple demand network cannot support core aspects of sentence interpretation: a large-scale fMRI investigation. BioRxiv, https://www.biorxiv.org/content/10.1101/744094v1.full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pallier C et al. (2011) Cortical representation of the constituent structure of sentences. PNAS 108(6), 2522–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wild CJ et al. (2012) Effortful Listening: The Processing of Degraded Speech Depends Critically on Attention. J. Neurosci. 32(40), 14010–14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thompson-Schill SL, Swick D, Farah MJ, D’Esposito M, Kan IP, & Knight RT (1998). Verb generation in patients with focal frontal lesions: A neuropsychological test of neuroimaging findings. Proceedings of the National Academy of Sciences, 95(26), 15855–15860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hasson U et al. (2018) Grounding the neurobiology of language in first principles: The necessity of non-language-centric explanations for language comprehension. Cognition 180, 135–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Power JD et al. (2011) Functional network organization of the human brain. Neuron 72(4), 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yeo BT et al. (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106(3), 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mahowald K & Fedorenko E (2016) Reliable individual-level neural markers of high-level language processing: A necessary precursor for relating neural variability to behavioral and genetic variability. Neuroimage 139, 74–93. [DOI] [PubMed] [Google Scholar]
- 89.Assem M et al. (2017) Activity in the Fronto-Parietal Multiple-demand Network is Robustly Associated with Individual Differences in Working Memory and Fluid Intelligence. bioRxiv 110270; doi: 10.1101/110270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dubois J & Adolphs R (2016) Building a Science of Individual Differences from fMRI. Trends Cogn. Sci. 20(6), 425–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seghier ML & Price CJ (2018) Interpreting and Utilising Intersubject Variability in Brain Function. Trends Cogn. Sci. 22(6), 517–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hong Y et al. (2019) False-positive neuroimaging: Undisclosed flexibility in testing spatial hypotheses allows presenting anything as a replicated finding. Neuroimage 195, 384–395. [DOI] [PubMed] [Google Scholar]
- 93.Varley RA et al. (2005) Agrammatic but numerate. Proc. Natl. Acad. Sci. U. S. A. 102(9), 3519–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sirigu A et al. (1998) Distinct Frontal Regions for Processing Sentence Syntax and Story Grammar. Cortex 34(5), 771–778. [DOI] [PubMed] [Google Scholar]
- 95.Varley R & Siegal M (2000) Evidence for cognition without grammar from causal reasoning and “theory of mind” in an agrammatic aphasic patient. Curr. Biol. 10(12), 723–726. [DOI] [PubMed] [Google Scholar]
- 96.Varley R et al. (2001) Severe impairment in grammar does not preclude theory of mind. NeuroCase 7, 489–493. [DOI] [PubMed] [Google Scholar]
- 97.Apperly IA et al. (2006) Intact first-and second-order false belief reasoning in a patient with severely impaired grammar. Soc. Neurosci. 1(3-4), 334–348. [DOI] [PubMed] [Google Scholar]
- 98.Polk M & Kertesz A (1993) Music and Language in Degenerative Disease of the Brain. Brain Cogn. 22(1), 98–117. [DOI] [PubMed] [Google Scholar]
- 99.Mazoyer BM et al. (1993) The cortical representation of speech. J. Cogn. Neurosci. 5(4), 467–479. [DOI] [PubMed] [Google Scholar]
- 100.Scott SK et al. (2000) Identification of a pathway for intelligible speech in the left temporal lobe. Brain 123, 2400–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Friederici AD et al. (2000). Auditory language comprehension: An event-related fMRI study on the processing of syntactic and lexical information. Brain Lang. 74, 289–300. [DOI] [PubMed] [Google Scholar]
- 102.Rogalsky C & Hickok G (2011) The role of Broca’s area in sentence comprehension. J. Cogn. Neurosci. 23(7), 1664–1680. [DOI] [PubMed] [Google Scholar]
- 103.Fedorenko E & Kanwisher N (2011). Some regions within Broca’s area do respond more strongly to sentences than to linguistically degraded stimuli: A comment on Rogalsky & Hickok (2010). J. Cogn. Neurosci. 23(10), 2632–2635. [Google Scholar]
- 104.Just MA et al. (1996) Brain activation modulated by sentence comprehension. Science 274(5284), 114–116. [DOI] [PubMed] [Google Scholar]
- 105.Kaan E & Swaab TY (2002) The brain circuitry of syntactic comprehension. Trends Cogn. Sci. 6(8), 350–356. [DOI] [PubMed] [Google Scholar]
- 106.Novick JM et al. (2010) Broca’s area and language processing: evidence for the cognitive control connection. Lang. Linguist. Compass 4, 906–924. [Google Scholar]
- 107.Basilakos A, Smith K, Fillmore P, Fridriksson J & Fedorenko E (2018). Functional characterization of the human speech articulation network. Cerebral Cortex, 28(5), 1816–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rogalsky C et al. (2008) Broca’s area, sentence comprehension, and working memory: an fMRI study. Front. Hum. Neurosci. 2(14), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rogalsky C et al. (2015) Sentence processing selectivity in Broca’s area: evident for structure but not syntactic movement. Lang. Cogn. Neurosci. 30, 10, 1326–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bautista A & Wilson SM (2016) Neural responses to grammatically and lexically degraded speech. Lang. Cogn. Neurosci. 31(4), 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Crittenden BM & Duncan J (2012) Task difficulty manipulation reveals multiple demand activity but no frontal lobe hierarchy. Cereb. Cortex 24(2), 532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Berwick RC et al. (2013) Evolution, brain, and the nature of language. Trends Cogn. Sci. 17, 89–98. [DOI] [PubMed] [Google Scholar]
- 113.Hasson U, Malach R, & Heeger DJ (2010). Reliability of cortical activity during natural stimulation. Trends in cognitive sciences, 14(1), 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shain C*, Blank I*, Van Shijndel MW & Fedorenko E (in press). fMRI reveals language-specific predictive coding during naturalistic sentence comprehension. Neuropsychologia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Goldberg A (2002). Construction Grammar. In Encyclopedia of Cognitive Science: Macmillan Reference Limited Nature Publishing Group. [Google Scholar]
- 116.Bybee J (2006). From usage to grammar: The mind’s response to repetition. Language, 82, 711–733. [Google Scholar]
- 117.Jackendoff R (2007). A Parallel Architecture perspective on language processing. Brain Research, 1146, 2–22. [DOI] [PubMed] [Google Scholar]
- 118.Mollica F*, Siegelman M*, Diachek E, Piantadosi S, Mineroff Z, Futrell R, Kean H, Qian P & Fedorenko E (in press). Composition is the core driver of the language-selective network. Neurobiology of Language. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gibson E, Futrell R, Piantadosi ST, Dautriche I, Mahowald K, Bergen L & Levy R (2019). How efficiency shapes human language. Trends in Cognitive Sciences. [DOI] [PubMed] [Google Scholar]
- 120.Poldrack RA et al. (2017) Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci. 18, 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kanwisher N, McDermott J, & Chun M (1997) The Fusiform Face Area: A Module in Human Extrastriate Cortex Specialized for the Perception of Faces. Journal of Neuroscience, 17, 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chang L, & Tsao DY (2017). The code for facial identity in the primate brain. Cell, 169(6), 1013–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gagneux P & Varki A (2001) Genetic Differences between Humans and Great Apes. Mol. Phylogenet. and Evol. 18(1), 2–13. [DOI] [PubMed] [Google Scholar]
- 124.Donahue CJ et al. (2018) Quantitative assessment of prefrontal cortex in humans relative to nonhuman primates. PNAS 115(22), E5183–E5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Petrides M & Pandya DN (1994) Comparative architectonic analysis of the human and the macaque frontal cortex. In Handbook of neuropsychology (Boller F, Grafman J, eds), pp. 17–58. [Google Scholar]
- 126.Palomero-Gallagher N & Zilles K (2018) Differences in cytoarchitecture of Broca’s region between human, ape and macaque brains. Cortex. 10.1016/j.cortex.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 127.Sallet J et al. (2013) The Organization of Dorsal Frontal Cortex in Humans and Macaques. J. Neurosci. 33(30), 12255–12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mitchell DJ et al. (2016). A putative multiple-demand system in the macaque brain. J. Neurosci. 36(33), 8574–8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shepherd SV & Freiwald WA (2018) Functional Networks for Social Communication in the Macaque Monkey. Neuron 99, 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Freedman DJ et al. (2001) Categorical representation of visual stimuli in the primate prefrontal cortex. Science 291, 312–316. [DOI] [PubMed] [Google Scholar]
- 131.Freedman DJ & Assad J (2006) Experience-dependent representation of visual categories in parietal cortex. Nature 443(7107), 85–88. [DOI] [PubMed] [Google Scholar]
- 132.Roy JE et al. (2010). Prefrontal cortex activity during flexible categorization. Journal of Neuroscience, 30(25), 8519–8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fitch WT (2018) The Biology and Evolution of Speech: A Comparative Analysis. Annu. Rev. Liguist. 4, 255–279. [Google Scholar]
- 134.Guenther FH (2016). Neural control of speech. MIT Press. [Google Scholar]
- 135.Townsend SW et al. (2018) Compositionality in animals and humans. PLOS Biol. 16(8), e2006425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Heesen R et al. (2018) Linguistic laws in chimpanzee gestural communication, Linguistic laws in chimpanzee gestural communication. Proc. R. Soc. B, 286, 20182900. doi: 10.1098/rspb.2018.2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mantini D et al. (2013) Evolutionarily novel functional networks in the human brain. J. Neurosci. 33(8), 3259–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bornkessel-Schlesewsky I et al. (2015) Neurobiological roots of language in primate audition: common computational properties. Trends Cogn. Sci. 19, 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wise R et al. (1999) Brain regions involved in articulation. Lancet 353, 1057–1061. [DOI] [PubMed] [Google Scholar]
- 140.Eickhoff SB, Heim S, Zilles K, Amunts K. 2009. A systems per- spective on the effective connectivity of overt speech pro- duction. Phil Trans R Soc A. 367:2399–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dronkers N 1996. A new brain region for coordinating speech articulation. Nature. 384:159–161. [DOI] [PubMed] [Google Scholar]
- 142.Hillis AE et al. (2004) Re-examining the brain regions crucial for orchestrating speech articulation. Brain 127, 1479–1487. [DOI] [PubMed] [Google Scholar]
- 143.Richardson JD et al. (2012) Re-establishing Broca’s initial findings. Brain Lang. 123, 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hickok G (2014) The architecture of speech production and the role of the phoneme in speech processing. Lang. Cogn. Process 29(1), 2–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Flinker A et al. (2015) Redefining the role of Broca’s area in speech. PNAS 112(9), 2871–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hickok G (2009) The functional neuroanatomy of language. Phys Life Rev. 6:121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Long MA et al. (2016) Functional Segregation of Cortical Regions Underlying Speech Timing and Articulation. Neuron 89, 1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Desikan RS et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–980. [DOI] [PubMed] [Google Scholar]
- 149.Fischl B et al. (2008) Cortical folding patterns and predicting cytoarchitecture. Cereb. Cortex 18, 1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tzourio-Mazoyer N et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15(1), 273–289. [DOI] [PubMed] [Google Scholar]