Abstract
BACKGROUND
Cholesteryl ester storage disease (CESD) is a rare genetic disease. Its symptoms and severity are highly variable. CESD is a systemic disease that can lead to the accumulation of fat and inflammation in the liver, as well as gastrointestinal and cardiovascular disease. The majority of patients require liver transplantation due to decompensated cirrhosis. Enzyme replacement therapy has been approved based on a randomized trial. Our study aims to clinically and genetically evaluate two siblings with CESD who underwent liver transplantation, as well as their first-degree family members.
CASE SUMMARY
The siblings were compound heterozygous for the missense variant in LIPA exon 8, c.894G>A, (p.Gln298Gln) and a single base pair deletion, c.482del (p.Asn161Ilefs*19). Analyses of single nucleotide polymorphisms showed variants with an increased risk of fatty liver disease and fibrosis for both patients. Clinically, both patients show signs of recurrence of CESD in the liver after transplantation and additional gastrointestinal and cardiovascular signs of CESD. Three family members who were LIPA heterozygous had a lysosomal acid lipase activity below the reference value. One of these carriers, a seven-year-old boy, was found to have severe dyslipidemia and was subsequently treated with statins.
CONCLUSION
Our study underlines that CESD is a multi-organ disease, the progression of which may occur post-liver transplantation. Our findings underline the need for monitoring of complications and assessment of possible further treatment.
Keywords: Lysosomal acid lipase deficiency, Lysosomal storage disease, Non-alcoholic fatty liver disease, Liver transplantation, Sebelipase alfa, Case report
Core tip: Cholesteryl ester storage disease is a multisystemic disease affecting several organs. Accordingly, the disease will progress after liver transplantation and may recur in the transplanted liver. Abnormal lipid accumulation in the vascular endothelium causes cardiovascular diseases. The occurrence of single nucleotide polymorphisms associated with fatty liver disease may increase the risk of cirrhosis and of recurrence of fatty liver disease after transplantation. Our case presents findings that underline the importance of monitoring and treatment.
INTRODUCTION
Cholesteryl ester storage disease (CESD) is a type of lysosomal acid lipase (LAL) deficiency. LAL deficiency (LAL-D) is caused by pathogenic variants in the LIPA, which encodes the enzyme LAL[1-3]. LAL-D is inherited in an autosomal recessive manner. Affected individuals are homozygous or compound heterozygous for pathogenic variants in LIPA. The LAL enzyme is required for intracellular hydrolysis of cholesteryl esters and triglycerides within low-density lipoprotein (LDL) particles into free cholesterol and free fatty acids[4]. Deficiency of the enzyme leads to accumulation of mucolipids and mucopolysaccharides. The most common pathogenic variant associated with CESD is variant c.894G>A, p.(Gln298Gln), also named E8SJM, in exon 8 in LIPA[5-8]. In Western countries, the allele frequency in the general population is about 0.0025, corresponding to a carrier frequency of about one in 200. The variant results in a transcript with an in-frame deletion of exon 8 and can result in some residual LAL activity when only small amounts of mRNA are spliced correctly[9]. About 50%-70% of patients with CESD have the c.894G>A, p.(Gln298Gln) variant, which corresponds to a prevalence of the disease ranging from 1 in 40000 to 1 in 300000[10,11].
The clinical spectrum and rate of progression of LAL-D is wide[3,12]. Wolman disease, the severe infantile and rapidly progressive disorder, is associated with complete loss of enzyme activity[13,14]; survival beyond one year is rare[15]. CESD, the attenuated form of the disease, may manifest in childhood or adulthood as a progressive multi-organ disease and is associated with a variation in LAL activity from 1% to 12%[14,16,17]. Its clinical manifestations are less well defined than in Wolman disease and the diagnosis of CESD is often incidental[12]. Abnormal lipid accumulation occurs in the liver, spleen, adrenal glands, lymph nodes, intestinal mucosa, vascular endothelium, and skeletal muscle. Lipid abnormalities are common, and patients may present early signs of atherosclerosis, including presence of macrophage foam cells when cholesteryl ester accumulates in foam cell lysosomes[18]. CESD leads to progressive liver damage with cirrhosis and liver failure, as well as atherosclerosis with a risk of stroke, heart disease and aneurysms[12,14-16]. The disease is often misdiagnosed as non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, or cryptogenic cirrhosis[15]. In addition, patients with CESD may experience gastrointestinal symptoms including diarrhoea, abdominal pain, and malabsorption[8]. Individuals with CESD often have elevated transaminases and hypercholesterolemia, with elevated serum low-density and very LDL-cholesterol and normal or elevated triglycerides[19]. High-density lipoprotein-cholesterol is typically low. Hepatomegaly and hyperlipidaemia may be the only abnormalities prompting the diagnosis, which could explain why LAL-D is underdiagnosed.
The initial test recommended to establish a diagnosis of LAL-D is measurement of the enzymatic activity of LAL in leukocytes in a blood sample or on a dried blood spot[20,21], followed by targeted DNA sequencing of LIPA to identify the pathogenic variant(s).
Dietary interventions may help alleviate gastrointestinal symptoms associated with LAL-D[22]. In addition, treatment with statins is needed to reduce the risk of cardiovascular disease[23]. Unfortunately, the therapeutic response is often insufficient despite high-dose treatment, and upregulation of LDL receptors in hepatocytes may occur. Liver transplantation may be necessary, although longitudinal data about subsequent transplant rejection are scarce[24]. Sebelipase alfa is a recombinant human LAL that is produced in egg white from transgenic hens[25]. The attached mannose-6-phosphate residue facilitates the lysosomal uptake of the recombinant enzyme, leading to hydrolysis of cholesteryl esters and triglycerides. The drug was approved for the treatment of LAL-D in 2015.
We undertook a cross-sectional study evaluating two siblings with CESD and their first-line family members. The protocol was approved by the Danish Regional Ethics Committee (H-18020170). All participants gave their informed consent. All participants underwent abdominal ultrasound and fibroscan with controlled attenuation parameter (CAP) and routine blood test. LAL activity was determined in dried blood test (DBS) samples. Further, genetic tests were carried out with targeted DNA sequencing of LIPA to identify the variant(s) causing LAL-D in the siblings, and potential variants in the family members, was undertaken with whole genome sequencing[12]. In addition, genetic variants affecting the interaction between pathways that could increase the risk of developing fibrosis and cirrhosis in CESD were assessed based on analyses for single nucleotide polymorphisms (SNPs)[26-29].
CESD patients underwent extended diagnostic imaging with myocardial perfusion scintigraphy, combined with a cardiac single photon emission computed tomography (SPECT), with stress test as indicated for the diagnosis of coronary artery diseases and to evaluate myocardial blood flow, as well as cardiac magnetic resonance imaging (MRI) and gastroduodenoscopy.
CASE PRESENTATION
Chief complaints
We assessed two siblings with confirmed CESD, a 59-year-old female and a 61-year-old male, of Scandinavian heritage, in order to systematically evaluate CESD in these patients, and evaluate possible LIPA carriers.
History of present illness
The patients have experienced persisting gastrointestinal symptoms ever since the first diagnosis.
History of past illness
The female patient with CESD was diagnosed as a child after developing symptoms at the age of eight. Symptoms included hepatosplenomegaly, malabsorption and diarrhoea, combined with elevated transaminases and dyslipidemia. As an adult, the female patient underwent surgery for an arterial aneurism in her leg and adrenal insufficiency. At the age of 45, the patient developed progressive liver failure. A liver biopsy revealed microvesicular and micronodular cirrhosis, lobular inflammation and hepatocyte ballooning, and numerous macrophages with Periodic Acid-Schiff positive and diastase-resistant material. The patient underwent liver transplantation. Afterwards, hepatosplenomegaly recurred and a post-transplant biopsy in 2018 showed 60% macrovesicular and microvesicular steatosis, a mild degree of ballooning and lobular inflammation, as well as perisinusoidal fibrosis. The findings were consistent with LAL-D liver pathology.
The male patient with CESD was diagnosed as an adolescent, following his sister’s diagnosis. The patient had elevated transaminases and dyslipidaemia at the time of the diagnosis, but no clinical symptoms. As an adult, the patient developed progressive liver failure and a liver biopsy found severe microvesicular and micronodular cirrhosis and inflammation consistent with CESD. The patient underwent liver transplantation at the age of 53. A post-transplant liver biopsy showed mild-to-moderate inflammation and F1 fibrosis. After the liver transplant, the patient also developed cardiovascular disease with an abdominal aortic aneurysm and underwent an endovascular abdominal aortic aneurysm repair.
Physical examination upon admission
Both patients presented with steatorrhea and upper abdominal discomfort. The female patient suffered from abdominal pain.
Laboratory examinations
The LAL activity was < 0.01 nmol/(punch∙h) for both siblings [normal range: 0.37-2.30 nmol/(punch∙h)]. Routine laboratory tests showed that the female patient had mildly elevated ALT, VLDL, and triglycerides, as well as low platelets (Table 1). The male patient had normal blood tests.
Table 1.
Routine blood tests of patients with cholesteryl ester storage disease and their adult family members
| Blood tests |
Patients |
||||||
| F | M | M, 5 yr | F, 9 yr | M, 31 yr | M, 34 yr | M, 34 yr | |
| Alanine amino transferase (U/L) | 86 | 38 | 19 | 19 | 66 | 28 | 18 |
| Alkaline phosphatase (U/L) | 49 | 96 | 269 | 234 | 61 | 79 | 52 |
| Total bilirubin (mg/dL) | 0.94 | 0.35 | 0.58 | 0.29 | 0.41 | 0.94 | 0.41 |
| International normalised ratio (INR) | 0.9 | 1.0 | 1.1 | 1.1 | 0.9 | 1.0 | 0.9 |
| Haemoglobin (g/L) | 132.1 | 137 | 120.8 | 127.3 | 133.7 | 148.2 | 161.1 |
| Platelets (109/L) | 121 | 168 | 230 | 329 | 234 | 192 | 229 |
F: Female; M: Male. Blood tests for 7 years child unavailable.
LIPA gene sequencing revealed two previously reported variants, including the well-known missense variant in exon 8, c.894G>A, (p.Gln298Gln), and a single base pair deletion, c.482del (p.Asn161Ilefs*19). Accordingly, the siblings were compound heterozygote for these variants. Additional analyses showed that the female patient had two SNP polymorphisms in the TM6SF2 [ Rs58542926(C/T) type CT] and in MBOAT7 [ Rs641738(C/T) type CT], suggesting an increased risk of fatty liver disease. The male patient had Rs738409 (C/G) type CG, suggesting an increased risk of fibrosis[28,30].
Imaging examinations
The abdominal ultrasound and Fibroscan confirmed that the patients had steatosis with Cap values of 281 and 342 dB/m for the female and male patient, respectively. The male patient also had evidence of mild fibrosis, with a median score of 9.2 kPa in the Fibroscan.
Both patients underwent additional tests to assess evidence of cardiovascular disease. The female patient had a moderate stenosis of up to 70% on ultrasound imaging of the carotid arteries. Transthoracic echocardiography identified a sclerotic aorta valve, mitral annular calcification, and mild left ventricular hypertrophy. Myocardial perfusion scintigraphy combined with SPECT showed a small, reversible perfusion defect near the septal basis and a moderately elevated coronary artery calcium score. The findings were confirmed with cardiac MRI, which found the same reversible perfusion defect near the septal basis and mild left ventricular hypertrophy (Space between the CESD patients).
The male patient had no evidence of stenosis in a carotid artery ultrasound. Transthoracic echocardiography found severe aortic valve stenosis with calcified cusps and mild left ventricular hypertrophy. Myocardial scintigraphy with SPECT showed a small, reversible perfusion defect in the inferior lateral left ventricle and a moderately elevated calcium score. Cardiac MRI showed aortic valves with restrictive movement due to calcified cups, resulting in severe aorta stenosis and left ventricular hypertrophy. Gastroduodenoscopy was normal. Duodenal biopsies showed no inflammation of foamy cells.
Family members
None of the six family members showed evidence of LAL-D in dried blood test (Table 2). Two adult family members and one child were heterozygotes and had LAL activity below the reference value of 0.37 (Table 2, Table 3). The child, a seven-year-old boy, also had elevated LDL cholesterol (166.3 mg/dL; reference value < 116). Both 34-year-old males had increased controlled attenuation parameter values of 330 and 400, respectively they had LAL activity of 0.37 and 0.51 nmol/(punch∙h) (Table 2). The latter was also morbidly obese with a body mass index of 58 kg/m2 and had an increased median value of 24.2 Kpa on the Fibroscan. A percutaneous liver biopsy showed steatosis and inflammation, but no fibrosis. Lifestyle interventions were initiated, and the patient was referred for assessment for possible bariatric surgery.
Table 2.
Lysosomal acid lipase activity and LIPA variants in family members of two patients with cholesteryl ester storage disease
| Age | Gender | DBS | LIPA variant | Genotype |
| 5 | Male | 1.03 | c.482del, p.Asn161Ilefs*19 | Heterozygote |
| 7 | Male | 0.34 | c.894G>A, p.Gln298Gln | Heterozygote |
| 9 | Female | 1.33 | WT | Normal |
| 31 | Male | 0.22 | c.894G>A, p.Gln298Gln | Heterozygote |
| 34 34 | Male Male | 0.37 0.51 | c.482del, p.Asn161Ilefs*19 c.482del, p.Asn161Ilefs*19 | Heterozygote Heterozygote |
DBS: Dried blood spot nmol/punch per hour; WT: Wildtype.
Table 3.
Analysis of single nucleotide polymorphisms in family members of patients with cholesteryl ester storage disease
| Age | Gender | MBOAT7 | PNPLA3 |
| 5 | Male | Rs641738 [C/T]; CT | |
| 7 | Male | Rs641738 [C/T]; CT | |
| 9 | Female | Rs641738 [C/T]; TT | |
| 31 34 | Male Male | Rs641738 [C/T]; CT Rs641738 [C/T]; CT | Rs738409 [C/G]; CG |
| 341 | Male | Rs738409 [C/G]; GG |
The patient also had TM6SF2 Rs58542926 [C/T]; CT.
FINAL DIAGNOSIS
The final diagnosis of this presented case is LAL-D and its attenuated phenotype CESD disease. SNPs variants associated with development of fatty liver disease were also found in all the participants.
TREATMENT
Treatment has been limited to statins. Both patients receive ongoing treatment with statin. However, the female patient has dyslipidemia and does not respond to statin medication. The male patient shows effect of the latter and has normal blood concentration of lipids. Previously, the female patient had received treatment with enzyme replacement therapy, Sebelipase alfa. Unfortunately, the treatment was assessed as having no clear effect and treatment was stopped. The price and availability of the medicine have blocked new attempts to retry the enzyme replacement therapy.
OUTCOME AND FOLLOW-UP
Our clinical work-up showed evidence of recurrence of LAL-D pathology in liver and abnormal lipid accumulation in the vascular endothelium leading to cardiovascular diseases in both patients. Consequently, these findings have led to multidisciplinary follow up control of the patients by the department of hepatology and cardiology. They will be assessed once a year to monitor the disease progression. None of the family members showed any symptoms and they all had normal LAL activity. One of the family members, the seven-year-old boy was referred for treatment with statins.
DISCUSSION
This is a clinical and genetic study of two siblings with CESD and their first-degree family members. The siblings were compound heterozygotes for the missense variant in LIPA, c.894G>A, (p.Gln298Gln) and a single base pair deletion, c.482del (p.Asn161Ilefs*19). Analyses of SNPs showed an increased risk of fatty liver disease and fibrosis. One patient was treated with Sebelipase alfa and was classified as non-responsive. It is possible that genetic factors influence the response to therapy. Both patients underwent liver transplantation, and both had symptoms in the gastrointestinal tract, as well as cardiovascular disease, underlining that CESD is a systemic disease. None of the family members had CESD, but one child had dyslipidemia and required treatment.
Our clinical examinations showed evidence of abnormal lipid accumulation in the transplanted liver and vascular endothelium in both patients. Our findings included reversible perfusion defects in coronary arteries and severe aorta valve stenosis, with calcified cusps and mild left ventricular hypertrophy in the male patient. Both patients underwent surgery for arterial aneurysms. Additionally, our findings confirm previous evidence that liver transplantation does not prevent the multi-systemic progression of CESD[16]. Patients have increased mortality and morbidity post-transplantation, which underlines the importance of adequate monitoring and treatment of complications.
Recurrence of CESD in a transplanted liver may occur when deficient bone marrow-derived monocyte macrophages migrate to the liver and mature to Kupffer cells, thereby repeating the pre-liver transplantation pathophysiology[31,32]. In our patients, the histological assessment found mild-to-moderate inflammation, fibrosis, and microvesicular steatosis, which is likely an outcome of the combination of their medication and, for one patient, her obesity, but is also consistent with recurrent CESD in the transplanted liver[16]. A previous study assessing 18 patients with LAL-D who underwent liver transplantation found severe disease progression post-transplant[32]; six of these patients died.
The siblings described in our paper were carriers of the most commonly reported variant in the literature, c.894G>A, (p.Gln298Gln) in a compound heterozygous state[17,33]. The other variant was a single base pair deletion, c.482del (p.Asn161Ilefs*19), which has an allele frequency rate of 0.0032%[7,34], compared with 0.0090% for c.894G>A, (p.Gln298Gln). This corresponds with the critically low LAL activity of < 0.01 nmol/(punch∙h). In addition, our female patient had two SNP polymorphisms in TM6SF2 [Rs58542926 (C/T) type CT] and MBOAT7 [Rs641738 (C/T) type CT], suggesting a significant predisposition to fatty liver disease development, in addition to the impact of the pathogenic LIPA variants[30,35]. The male patient had CG genotype in Rs738409 (C/G), suggesting an increased risk of fibrosis in alcoholic liver disease[27,30]. It is possible that the additional genetic assessment of patients with LAL-D, including SNPs associated with liver disease, may provide important prognostic information and be an indicator of treatment requirements.
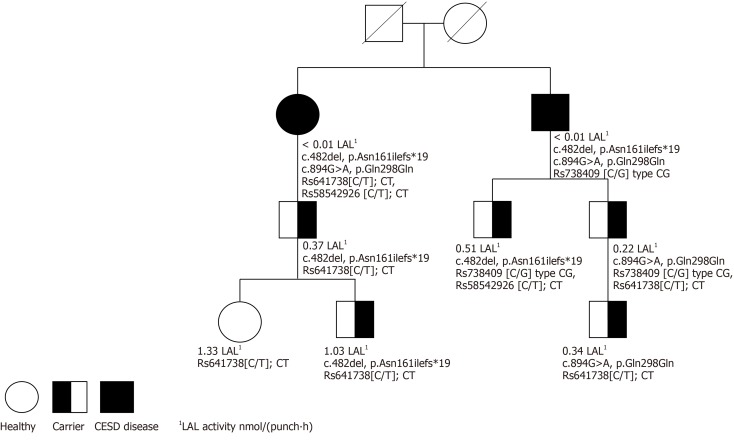
The role of LAL-D heterozygosity in presenting LAL-D phenomena has not yet been defined, and there are few reports assessing LAL-D carriers. However, we found a correlation between heterozygosity and LAL activity. Three heterozygous family members presented a LAL activity value below the reference value of 0.37 nmol/(punch∙h) (Figure 1). Two of the adult heterozygous family members had moderate steatosis, one of them verified with a liver biopsy showing severe steatosis and inflammation. It is possible that LAL activity can be used as a biomarker in heterozygotes[16,36-38]. In addition, one of the family members in the study was a seven-year-old boy with LAL activity of 0.34 nmol/(punch∙h). The child also had severe dyslipidemia, indicating that (as previously suggested[19]) reduced LAL activity may contribute to dyslipidemia, as well as to non-specific symptoms[8,12,16,39]. The choice was therefore made to monitor the child closely.
Figure 1.
Family pedigree with lysosomal acid lipase activity and single nucleotide polymorphisms. CESD: Cholesteryl ester storage disease; LAL: Lysosomal acid lipase.
Treatment for LAL-D has been limited to lipid-lowering medications, statins, or liver transplantation. The patients in our study have been treated with statins for more than 15 years. Our results indicate that this treatment does not prevent the progression of CESD. Even with statins, the female patient continued to have elevated VLDL and triglycerides. These findings accord with the conclusions of previous studies that treatment with statins is not beneficial to the progression of liver diseases associated with LAL-D due to enhanced acceleration of intracellular accumulation of cholesterol ester[8,16,19,37-39]. The current treatment of choice is Sebelipase alfa, based on a randomized controlled trial showing beneficial effects on several clinical outcomes[40]. Our assessment of the male patient suggests that Sebelipase alfa should be considered for use based on a high risk of recurrence of cirrhosis in the transplanted liver. Furthermore, Sebelipase alfa might have beneficial effects on cardiovascular outcomes. However, based on its price and availability in Denmark, treatment is not readily available without prior approval.
CONCLUSION
Our study underlines that CESD is a multi-organ disease, the progression of which may occur post-transplantation. The findings reiterate the need for monitoring of complications and assessment of possible additional treatment. The findings also suggest that despite the high carrier frequency for CESD, one must consider testing family members for possible dyslipidemia, as well as LAL-D, as even heterozygote patients might present with dyslipidemia.
Footnotes
Informed consent statement: Informed written consent was obtained from the patients for their inclusion in this report, including any accompanying images.
Conflict-of-interest statement: This study has received financial support for research from Alexion in the form of an unrestricted grant.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: January 15, 2020
First decision: February 29, 2020
Article in press: April 17, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: Denmark
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Angelico F, Morini S S-Editor: Dou Y L-Editor: A E-Editor: Xing YX
Contributor Information
Elias Badal Rashu, Gastrounit, Copenhagen University Hospital Hvidovre, Hvidovre 2650, Denmark.
Anders Ellekær Junker, Gastrounit, Copenhagen University Hospital Hvidovre, Hvidovre 2650, Denmark.
Karen Vagner Danielsen, Gastrounit, Copenhagen University Hospital Hvidovre, Hvidovre 2650, Denmark.
Emilie Dahl, Department of Hepatology, Rigshospitalet, Copenhagen University, Copenhagen 2100, Denmark.
Ole Hamberg, Department of Hepatology, Rigshospitalet, Copenhagen University, Copenhagen 2100, Denmark.
Line Borgwardt, Centre of Genomic Medicine, Rigshospitalet, Copenhagen University, Copenhagen 2100, Denmark.
Vibeke Brix Christensen, Department of Paediatrics and Adolescent Medicine, Rigshospitalet, Copenhagen University, Copenhagen 2100, Denmark.
Nicolai J Wewer Albrechtsen, Novo Nordisk Foundation Center for Protein Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen 2200, Denmark; Department for Clinical Biochemistry, Rigshospitalet, University of Copenhagen, Copenhagen 2200, Denmark.
Lise L Gluud, Gastrounit, Copenhagen University Hospital Hvidovre, Hvidovre 2650, Denmark. lise.lotte.gluud.01@regionh.dk.
References
- 1.Bay L, Canero Velasco C, Ciocca M, Cotti A, Cuarterolo M, Fainboim A, Fassio E, Galoppo M, Pinero F, Rozenfeld P. Liver disease and dyslipidemia as a manifestation of lysosomal acid lipase deficiency (LAL-D). Clinical and diagnostic aspects, and a new treatment. An update. Arch Argent Pediatr. 2017;115:287–293. doi: 10.5546/aap.2017.eng.287. [DOI] [PubMed] [Google Scholar]
- 2.Maciejko JJ, Anne P, Raza S, Lyons HJ. Lysosomal acid lipase deficiency in all siblings of the same parents. J Clin Lipidol. 2017;11:567–574. doi: 10.1016/j.jacl.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Burton BK, Silliman N, Marulkar S. Progression of liver disease in children and adults with lysosomal acid lipase deficiency. Curr Med Res Opin. 2017;33:1211–1214. doi: 10.1080/03007995.2017.1309371. [DOI] [PubMed] [Google Scholar]
- 4.Du H, Duanmu M, Rosa LR. Mouse lysosomal acid lipase: characterization of the gene and analysis of promoter activity. Gene. 1998;208:285–295. doi: 10.1016/s0378-1119(98)00019-5. [DOI] [PubMed] [Google Scholar]
- 5.Stitziel NO, Fouchier SW, Sjouke B, Peloso GM, Moscoso AM, Auer PL, Goel A, Gigante B, Barnes TA, Melander O, Orho-Melander M, Duga S, Sivapalaratnam S, Nikpay M, Martinelli N, Girelli D, Jackson RD, Kooperberg C, Lange LA, Ardissino D, McPherson R, Farrall M, Watkins H, Reilly MP, Rader DJ, de Faire U, Schunkert H, Erdmann J, Samani NJ, Charnas L, Altshuler D, Gabriel S, Kastelein JJ, Defesche JC, Nederveen AJ, Kathiresan S, Hovingh GK National Heart, Lung, and Blood Institute GO Exome Sequencing Project. Exome sequencing and directed clinical phenotyping diagnose cholesterol ester storage disease presenting as autosomal recessive hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2013;33:2909–2914. doi: 10.1161/ATVBAHA.113.302426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott SA, Liu B, Nazarenko I, Martis S, Kozlitina J, Yang Y, Ramirez C, Kasai Y, Hyatt T, Peter I, Desnick RJ. Frequency of the cholesteryl ester storage disease common LIPA E8SJM mutation (c.894G>A) in various racial and ethnic groups. Hepatology. 2013;58:958–965. doi: 10.1002/hep.26327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muntoni S, Wiebusch H, Jansen-Rust M, Rust S, Schulte H, Berger K, Pisciotta L, Bertolini S, Funke H, Seedorf U, Assmann G. Heterozygosity for lysosomal acid lipase E8SJM mutation and serum lipid concentrations. Nutr Metab Cardiovasc Dis. 2013;23:732–736. doi: 10.1016/j.numecd.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Reiner Ž, Guardamagna O, Nair D, Soran H, Hovingh K, Bertolini S, Jones S, Ćorić M, Calandra S, Hamilton J, Eagleton T, Ros E. Lysosomal acid lipase deficiency--an under-recognized cause of dyslipidaemia and liver dysfunction. Atherosclerosis. 2014;235:21–30. doi: 10.1016/j.atherosclerosis.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Pagani F, Pariyarath R, Garcia R, Stuani C, Burlina AB, Ruotolo G, Rabusin M, Baralle FE. New lysosomal acid lipase gene mutants explain the phenotype of Wolman disease and cholesteryl ester storage disease. J Lipid Res. 1998;39:1382–1388. [PubMed] [Google Scholar]
- 10.Muntoni S, Wiebusch H, Jansen-Rust M, Rust S, Seedorf U, Schulte H, Berger K, Funke H, Assmann G. Prevalence of cholesteryl ester storage disease. Arterioscler Thromb Vasc Biol. 2007;27:1866–1868. doi: 10.1161/ATVBAHA.107.146639. [DOI] [PubMed] [Google Scholar]
- 11.Vinje T, Wierød L, Leren TP, Strøm TB. Prevalence of cholesteryl ester storage disease among hypercholesterolemic subjects and functional characterization of mutations in the lysosomal acid lipase gene. Mol Genet Metab. 2018;123:169–176. doi: 10.1016/j.ymgme.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Pisciotta L, Fresa R, Bellocchio A, Pino E, Guido V, Cantafora A, Di Rocco M, Calandra S, Bertolini S. Cholesteryl Ester Storage Disease (CESD) due to novel mutations in the LIPA gene. Mol Genet Metab. 2009;97:143–148. doi: 10.1016/j.ymgme.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 13.ABRAMOV A, SCHORR S, WOLMAN M. Generalized xanthomatosis with calcified adrenals. AMA J Dis Child. 1956;91:282–286. doi: 10.1001/archpedi.1956.02060020284010. [DOI] [PubMed] [Google Scholar]
- 14.Porto AF. Lysosomal acid lipase deficiency: diagnosis and treatment of Wolman and Cholesteryl Ester Storage Diseases. Pediatr Endocrinol Rev. 2014;12 Suppl 1:125–132. [PubMed] [Google Scholar]
- 15.Pericleous M, Kelly C, Wang T, Livingstone C, Ala A. Wolman's disease and cholesteryl ester storage disorder: the phenotypic spectrum of lysosomal acid lipase deficiency. Lancet Gastroenterol Hepatol. 2017;2:670–679. doi: 10.1016/S2468-1253(17)30052-3. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein DL, Hülkova H, Bialer MG, Desnick RJ. Cholesteryl ester storage disease: review of the findings in 135 reported patients with an underdiagnosed disease. J Hepatol. 2013;58:1230–1243. doi: 10.1016/j.jhep.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Aguisanda F, Thorne N, Zheng W. Targeting Wolman Disease and Cholesteryl Ester Storage Disease: Disease Pathogenesis and Therapeutic Development. Curr Chem Genom Transl Med. 2017;11:1–18. doi: 10.2174/2213988501711010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sargolzaei J, Chamani E, Kazemi T, Fallah S, Soori H. The role of adiponectin and adipolin as anti-inflammatory adipokines in the formation of macrophage foam cells and their association with cardiovascular diseases. Clin Biochem. 2018;54:1–10. doi: 10.1016/j.clinbiochem.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Chora JR, Alves AC, Medeiros AM, Mariano C, Lobarinhas G, Guerra A, Mansilha H, Cortez-Pinto H, Bourbon M. Lysosomal acid lipase deficiency: A hidden disease among cohorts of familial hypercholesterolemia? J Clin Lipidol. 2017;11:477–484.e2. doi: 10.1016/j.jacl.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Dairaku T, Iwamoto T, Nishimura M, Endo M, Ohashi T, Eto Y. A practical fluorometric assay method to measure lysosomal acid lipase activity in dried blood spots for the screening of cholesteryl ester storage disease and Wolman disease. Mol Genet Metab. 2014;111:193–196. doi: 10.1016/j.ymgme.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Lukacs Z, Barr M, Hamilton J. Best practice in the measurement and interpretation of lysosomal acid lipase in dried blood spots using the inhibitor Lalistat 2. Clin Chim Acta. 2017;471:201–205. doi: 10.1016/j.cca.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 22.Erwin AL. The role of sebelipase alfa in the treatment of lysosomal acid lipase deficiency. Therap Adv Gastroenterol. 2017;10:553–562. doi: 10.1177/1756283X17705775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gasche C, Aslanidis C, Kain R, Exner M, Helbich T, Dejaco C, Schmitz G, Ferenci P. A novel variant of lysosomal acid lipase in cholesteryl ester storage disease associated with mild phenotype and improvement on lovastatin. J Hepatol. 1997;27:744–750. doi: 10.1016/s0168-8278(97)80092-x. [DOI] [PubMed] [Google Scholar]
- 24.Sreekantam S, Nicklaus-Wollenteit I, Orr J, Sharif K, Vijay S, McKiernan PJ, Santra S. Successful long-term outcome of liver transplantation in late-onset lysosomal acid lipase deficiency. Pediatr Transplant. 2016;20:851–854. doi: 10.1111/petr.12748. [DOI] [PubMed] [Google Scholar]
- 25.Balwani M, Breen C, Enns GM, Deegan PB, Honzík T, Jones S, Kane JP, Malinova V, Sharma R, Stock EO, Valayannopoulos V, Wraith JE, Burg J, Eckert S, Schneider E, Quinn AG. Clinical effect and safety profile of recombinant human lysosomal acid lipase in patients with cholesteryl ester storage disease. Hepatology. 2013;58:950–957. doi: 10.1002/hep.26289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falleti E, Fabris C, Cmet S, Cussigh A, Bitetto D, Fontanini E, Fornasiere E, Bignulin S, Fumolo E, Bignulin E, Pirisi M, Toniutto P. PNPLA3 rs738409C/G polymorphism in cirrhosis: relationship with the aetiology of liver disease and hepatocellular carcinoma occurrence. Liver Int. 2011;31:1137–1143. doi: 10.1111/j.1478-3231.2011.02534.x. [DOI] [PubMed] [Google Scholar]
- 27.Salameh H, Raff E, Erwin A, Seth D, Nischalke HD, Falleti E, Burza MA, Leathert J, Romeo S, Molinaro A, Corradini SG, Toniutto P, Spengler U, Daly A, Day CP, Kuo YF, Singal AK. PNPLA3 Gene Polymorphism Is Associated With Predisposition to and Severity of Alcoholic Liver Disease. Am J Gastroenterol. 2015;110:846–856. doi: 10.1038/ajg.2015.137. [DOI] [PubMed] [Google Scholar]
- 28.Buch S, Stickel F, Trépo E, Way M, Herrmann A, Nischalke HD, Brosch M, Rosendahl J, Berg T, Ridinger M, Rietschel M, McQuillin A, Frank J, Kiefer F, Schreiber S, Lieb W, Soyka M, Semmo N, Aigner E, Datz C, Schmelz R, Brückner S, Zeissig S, Stephan AM, Wodarz N, Devière J, Clumeck N, Sarrazin C, Lammert F, Gustot T, Deltenre P, Völzke H, Lerch MM, Mayerle J, Eyer F, Schafmayer C, Cichon S, Nöthen MM, Nothnagel M, Ellinghaus D, Huse K, Franke A, Zopf S, Hellerbrand C, Moreno C, Franchimont D, Morgan MY, Hampe J. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet. 2015;47:1443–1448. doi: 10.1038/ng.3417. [DOI] [PubMed] [Google Scholar]
- 29.Mancina RM, Dongiovanni P, Petta S, Pingitore P, Meroni M, Rametta R, Borén J, Montalcini T, Pujia A, Wiklund O, Hindy G, Spagnuolo R, Motta BM, Pipitone RM, Craxì A, Fargion S, Nobili V, Käkelä P, Kärjä V, Männistö V, Pihlajamäki J, Reilly DF, Castro-Perez J, Kozlitina J, Valenti L, Romeo S. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology. 2016;150:1219–1230.e6. doi: 10.1053/j.gastro.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Trépo E, Nahon P, Cao Q, Moreno C, Letouzé E, Imbeaud S, Gustot T, Deviere J, Debette S, Amouyel P, Bioulac-Sage P, Calderaro J, Ganne-Carrié N, Laurent A, Blanc JF, Guyot E, Sutton A, Ziol M, Zucman-Rossi J, Nault JC. PNPLA3 and TM6SF2 variants as risk factors of hepatocellular carcinoma across various etiologies and severity of underlying liver diseases. Int J Cancer. 2019;144:533–544. doi: 10.1002/ijc.31910. [DOI] [PubMed] [Google Scholar]
- 31.Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE. Kupffer cells in the liver. Compr Physiol. 2013;3:785–797. doi: 10.1002/cphy.c120026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernstein DL, Lobritto S, Iuga A, Remotti H, Schiano T, Fiel MI, Balwani M. Lysosomal acid lipase deficiency allograft recurrence and liver failure- clinical outcomes of 18 liver transplantation patients. Mol Genet Metab. 2018;124:11–19. doi: 10.1016/j.ymgme.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Zhang B, Porto AF. Cholesteryl ester storage disease: protean presentations of lysosomal acid lipase deficiency. J Pediatr Gastroenterol Nutr. 2013;56:682–685. doi: 10.1097/MPG.0b013e31828b36ac. [DOI] [PubMed] [Google Scholar]
- 34.Carter A, Brackley SM, Gao J, Mann JP. The global prevalence and genetic spectrum of lysosomal acid lipase deficiency: A rare condition that mimics NAFLD. J Hepatol. 2019;70:142–150. doi: 10.1016/j.jhep.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 35.Thabet K, Asimakopoulos A, Shojaei M, Romero-Gomez M, Mangia A, Irving WL, Berg T, Dore GJ, Grønbæk H, Sheridan D, Abate ML, Bugianesi E, Weltman M, Mollison L, Cheng W, Riordan S, Fischer J, Spengler U, Nattermann J, Wahid A, Rojas A, White R, Douglas MW, McLeod D, Powell E, Liddle C, van der Poorten D, George J, Eslam M International Liver Disease Genetics Consortium. MBOAT7 rs641738 increases risk of liver inflammation and transition to fibrosis in chronic hepatitis C. Nat Commun. 2016;7:12757. doi: 10.1038/ncomms12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baratta F, Pastori D, Ferro D, Carluccio G, Tozzi G, Angelico F, Violi F, Del Ben M. Reduced lysosomal acid lipase activity: A new marker of liver disease severity across the clinical continuum of non-alcoholic fatty liver disease? World J Gastroenterol. 2019;25:4172–4180. doi: 10.3748/wjg.v25.i30.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baratta F, Pastori D, Tozzi G, D'Erasmo L, Di Costanzo A, Arca M, Ettorre E, Ginanni Corradini S, Violi F, Angelico F, Del Ben M. Lysosomal acid lipase activity and liver fibrosis in the clinical continuum of non-alcoholic fatty liver disease. Liver Int. 2019;39:2301–2308. doi: 10.1111/liv.14206. [DOI] [PubMed] [Google Scholar]
- 38.Angelico F, Corradini SG, Pastori D, Fargion S, Fracanzani AL, Angelico M, Bolondi L, Tozzi G, Pujatti PL, Labbadia G, Corazza GR, Averna M, Perticone F, Croce G, Persico M, Bucci T, Baratta F, Polimeni L, Del Ben M, Violi F LAL-Cirrhosis Collaborative Research Group. Severe reduction of blood lysosomal acid lipase activity in cryptogenic cirrhosis: A nationwide multicentre cohort study. Atherosclerosis. 2017;262:179–184. doi: 10.1016/j.atherosclerosis.2017.03.038. [DOI] [PubMed] [Google Scholar]
- 39.Vespasiani-Gentilucci U, Gallo P, Piemonte F, Riva E, Porcari A, Vorini F, Tozzi G, Piccioni L, Galati G, De Vincentis A, Carotti S, Morini S, D'Amico J, Angeletti S, Pedone C, Picardi A. Lysosomal Acid Lipase Activity Is Reduced Both in Cryptogenic Cirrhosis and in Cirrhosis of Known Etiology. PLoS One. 2016;11:e0156113. doi: 10.1371/journal.pone.0156113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burton BK, Balwani M, Feillet F, Barić I, Burrow TA, Camarena Grande C, Coker M, Consuelo-Sánchez A, Deegan P, Di Rocco M, Enns GM, Erbe R, Ezgu F, Ficicioglu C, Furuya KN, Kane J, Laukaitis C, Mengel E, Neilan EG, Nightingale S, Peters H, Scarpa M, Schwab KO, Smolka V, Valayannopoulos V, Wood M, Goodman Z, Yang Y, Eckert S, Rojas-Caro S, Quinn AG. A Phase 3 Trial of Sebelipase Alfa in Lysosomal Acid Lipase Deficiency. N Engl J Med. 2015;373:1010–1020. doi: 10.1056/NEJMoa1501365. [DOI] [PubMed] [Google Scholar]