Abstract
Purpose:
Few studies examine the genomics of axillary lymph node (ALN) metastasis in triple negative breast cancer (TNBC). The aim was to characterize and compare gene expression patterns of primary breast cancers and paired ALN metastases.
Methods:
Patients with stage 2–3 ER/PR negative, HER2 negative TNBC with ALN macrometastasis without neoadjuvant therapy were selected. Tumor-specific area was isolated from breast and ALN tissue sections. Gene expression of 2,567 cancer-associated genes was analyzed with the HTG EdgeSeq system coupled with Illumina Next Generation Sequencing (NGS).
Results:
17 pairs of TNBC and autologous ALN metastasis were analyzed. Compared with the primary, ALN metastasis had 257 statistically significant differentially expressed genes, including 123 upregulated genes and 134 downregulated genes. Notably, there was an upregulation of anti-apoptosis and survival signaling genes (BIRC3, TCL1A, FLT3, VCAM1) in the ALN metastasis. There was also an upregulation of chemotaxis genes (CCL19, CCL21, CXCL13, TNFSF11). The most striking feature is the downregulation of genes known to regulate cell microenvironment interaction (MMP2, MMP 3, MMP 7, MMP 11, MMP14, COL1A1, COL1A2, COL3A1, COL5A1, COL5A2, COL6A6, COL11A1, COL17A1).
Conclusion:
In TNBC, ALN metastases have a distinct gene expression profile. Genes associated with anti-apoptosis, survival responses, and chemotaxis are upregulated, and genes associated with regulation of extracellular matrix are downregulated when compared to autologous primary cancer. TNBC cells metastatic to lymph nodes undergo a change in order to metastasize and survive in the new microenvironment, which may lead to insights into the metastatic process
Keywords: Gene expression, triple negative breast cancer, lymph node metastasis
Introduction
Axillary lymph node (ALN) metastases not only often guide breast cancer treatment and prognosis, but as early manifestations of cancer spread, they may harbor clues to understanding the metastatic process. Triple negative breast cancer (TNBC) occurs in about 10–15% of women with breast cancer, and more than 30% TNBC of patients present with positive nodesi. These patients have a poor prognosis compared to those with hormone receptor positive cancerii, iii, iv. Understanding lymph node metastasis could aid not only in understanding breast cancer biology, but also for aid in developing new therapiesv, vi.
To date, there have been few studies examining the genomics of axillary lymph node metastasis in triple negative breast cancer. Previous research comparing gene expression profiles of primary breast cancer (PBC) with paired lymph node metastasis had variable results, rarely controlled for breast cancer histologic type and biomarker profile, and used techniques for tissue isolation, which may have lead to contamination of samples with lymphoid cells.
The purpose of this study was to characterize and compare gene expression patterns of primary TNBC to paired axillary lymph node (ALN) metastases, including sentinel nodes.
Methods
Patient Selection -
After Institutional Review Board (IRB) approval, patients with stage 2–3 invasive ductal carcinoma (IDC) with lymph node macrometastasis were identified from a prospectively maintained database from January 2007 to December 2016. According to the American Society of Clinical Oncology (ASCO) and College of American Pathologists guidelines for HER2 testing in breast cancer, patients who had tumors that were estrogen receptor negative, progesterone receptor negative, and HER2-neu negative on Fluorescence in Situ Hybridization (FISH) (triple negative) were further selectedvii. The sentinel lymph node was selected when a sentinel node biopsy was performed and it harbored metastasis. When an axillary lymph node dissection was performed, the lymph node with the largest macrometastasis was selected.
Patients were further selected with primary breast cancer tumor size <5cm and lymph node metastasis tumor size >5mm. Patients were excluded if they received neoadjuvant chemotherapy or hormonal therapy, had distant metastatic disease at diagnosis, or a personal history of a previous primary breast cancer.
Tissue Samples -
Formalin fixed paraffin embedded (FFPE) tissue blocks of the primary breast cancer (PBC) and paired lymph node metastasis were obtained. Unstained FFPE tissue sections of the PBC and lymph node metastasis were cut into glass slides at 4μm thickness. A corresponding Hematoxylin & Eosin (H&E) stained section of the same block for each sample was obtained to identify and mark tumor area. Each slide was reviewed by a pathologist who circled the tumor area in both the PBC and lymph node metastasis by using a microscope. The tissue was submitted to HTG for further processing, where the tissue was removed from the marked section and placed into the HTG lysis buffer (see methods below).
HTG EdgeSeq combined with Next Generation Sequencing (NGS)
HTG EdgeSeq system combines HTG’s proprietary quantitative nuclease protection assay (qNPA) chemistry with Illumina Next Generation Sequencing (NGS) platform to enable a semi-quantitative analysis of a panel of targeted genes in a single assay. This allows for mRNA quantization without nucleic acid extraction. Samples are added to the HTG lysis buffer. Functional DNA nuclease protection probes (NPPs) flanked by universal sequences were hybridized to target RNAs. Universal DNA wingmen probes were hybridized to the wings to prevent S1 nuclease digestion. Next, S1 nuclease was added to digest excess non-hybridized DNA probes and non-hybridized RNA. Heat denaturation was used to release the protection probes from the DNA:RNA duplexes. Target capture was performed with polymerase chain reaction (PCR) amplification and barcoding. Sequencing was performed using Illumina NextSeq platform. All samples passed HTG post-sequencing metricsviii.
HTG EdgeSeq Oncology Biomarker Panel
An HTG EdgeSeq oncology biomarker panel which is an assay containing probes for 2,567 genes, including 15 housekeeper genes, four negative process controls, and four positive process controls was selected for use on each PBC and lymph node metastasis tumor.
Data Analysis
Data was returned from the sequencer as a demultiplexed FASTQ file, with four files per original assay well. The relative expression abundances of genes were calculated as Count-Per-Million (CPM) within each sample. This result allows for evaluation of gene expression as a proportion of total counts on a sample level. Fifteen genes with average abundance less than 5 CPM were filtered out. Read counts of remaining 2,544 genes were used to calculate scaling factors with trimmed mean of M-value (TMM) method for normalization. The differential expression (DE) analysis were performed by fitting a negative binomial generalized log-linear model for each gene using the edgeR package (v 3.24.3)ix. For the comparison between ALN and PBC, the DE analysis was adjusted by patients. The p-values of multiple tests were adjusted using Benjamini-Hochberg’s methodx and the significant level is designed as false discovery rate (FDR) <0.05.
Statistical Analysis
The upregulation and downregulation of genes were reviewed and the fold change (FC) was defined as the ratio of the normalized intensity of axillary lymph node metastasis to normalized intensity in the PBC. A FC >1 meant genes were upregulated in the ALN compared to the PBC. A FC between 0 and 1 meant genes were downregulated in the ALN compared to the PBC. Genes were considered significantly differentially expressed if they had an adjusted p-value, FDR <0.05. The functions of the differentially expressed genes were retrieved using National Center for Biotechnology Information (NCBI) genomic data source.
Ingenuity Pathway Analysis (IPA)
Ingenuity Pathway Analysis software (IPA, QIAGEN Redwood City, CA, USA) analytics tools of “Bio Function” and “Upstream Transcriptional Regulators” were used. The “Bio Functions” analysis predicts the downstream biological processes, which are increased or decreased based on input data. The “Upstream Transcriptional Regulator” analysis is based on prior knowledge of expected effects between transcriptional regulators and their target genes stored in the Ingenuity Knowledge Base, to compare the expression in axillary lymph node metastases relative to expression in primary breast cancer and predict likely relevant transcriptional regulators based on expected literature. The activation z-score is used to infer likely activation states of upstream regulators based on comparison with a model that assigns random regulation directions. All analyses were carried out with differentially expressed genes with an FDR <0.05.
Results
Clinicopathologic Characteristics
Women with TNBC were a median age of 62 years (range 30–88). Median PBC tumor size was 2.5cm (range 1.2–4.0cm). Thirteen (76.5%) patients had stage 2 disease and 4 (23.5%) patients had stage 3 disease. Other clinical and pathologic characteristics are shown in Table 1. The median lymph node tumor size was 1.0cm (range 0.6–2.5cm). Eight (47.1%) patients had a SLN with metastasis examined, and 9 (52.9%) patients had a positive non-sentinel axillary node examined [Table 1].
Table 1:
Clinical, Tumor, and Lymph Node Characteristics
| All patients (N = 17) | |
|---|---|
| Age (years), median, (range) | 62 (30–88) |
| Type of operation | |
| Partial mastectomy | 15 (88.2) |
| Mastectomy | 2 (11.8) |
| IDC Histology | 17 (100.0) |
| TNBC Subtype | 17 (100.0) |
| Grade | |
| Well differentiated | 0 (0.0) |
| Moderately differentiated | 4 (23.5) |
| Poorly differentiated | 13 (76.5) |
| Tumor size (cm), median (range) | 2.5 (1.2–4.0) |
| Focality | |
| Single focus | 16 (94.1) |
| Multifocal | 1 (5.9) |
| LVI | 13 (76.5) |
| Ki-67 (%), average (range) | 50.7 (1–86) |
| Low (<11%) | 2 (11.8) |
| Intermediate (11–20%) | 3 (17.7) |
| High (>20%) | 12 (70.6) |
| Genetic Mutation | |
| BRCA1 | 3 (17.6) |
| BARD1 | 1 (5.9) |
| Pathologic T Stage | |
| 1 | 3 (17.6) |
| 2 | 14 (82.4) |
| Pathologic N Stage | |
| 1 | 13 (76.5) |
| 2 | 3 (17.7) |
| 3 | 1 (5.9) |
| Stage | |
| 2 | 13 (76.5) |
| 3 | 4 (23.5) |
| Axillary operation | |
| SNB | 6 (35.3) |
| ALND | 8 (47.1) |
| SNB + ALND | 3 (17.6) |
| Number LN sampled, median (range) | 13 (1–20) |
| Number positive LN, median (range) | 2 (1–4) |
| LN tumor size (cm), median (range) | 1.0 (0.6–2.5) |
| Extranodal extension | 8 (47.1) |
| Size extranodal extension (cm), median (range) | 0.4 (0.25–0.6) |
| SLN analyzed | 8 (47.1) |
Abbreviations: IDC, Intraductal carcinoma; TNBC, Triple negative breast cancer; LVI, Lymphovascular invasion; SNB, Sentinel Lymph Node Biopsy; ALND, Axillary Lymph Node Dissection; LN, Lymph node; SLN, Sentinel lymph node;
ALN compared to PBC
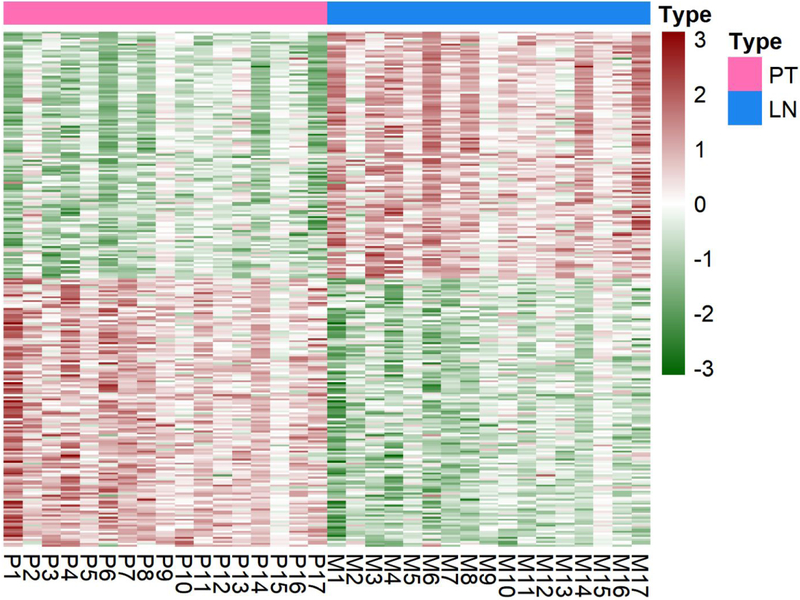
Thirty-four samples comprised of 17 pairs of PBC and autologous ALN metastases were analyzed for 2,567 cancer-associated genes [Figure 1]. Compared with the PBC, ALN metastases had 257 statistically significant differentially expressed genes, including 123 upregulated genes and 134 downregulated genes. There were 5 upregulated genes that had log2FC ≥2.00 FC gene expression and 9 downregulated genes that had log2FC ≤−2.00 FC in gene expression, which is shown in Table 2. Figure 2 shows the heatmap for the 257 genes and 17 patients.
Figure 1.
Methods for Paired Gene Expression Analysis
Abbreviations: PBC, Primary breast cancer; ALN, axillary lymph node; PCR, Polymerase chain reaction
Table 2:
Upregulated and Downregulated Genes with at least a two log2 fold-change in gene expression in Lymph Node Metastasis Compared with TNBC
| Gene | Log2 Fold-Change | Fold-Change | FDR |
|---|---|---|---|
| CCL21 | 3.38 | 10.38 | <0.001 |
| CACNA2D3 | 3.03 | 8.16 | 0.003 |
| CD19 | 2.34 | 5.07 | <0.001 |
| FCER2 | 2.26 | 4.78 | <0.001 |
| PGC | 2.24 | 4.72 | <0.001 |
| COL11A1 | −2.04 | 0.24 | <0.001 |
| MMP3 | −2.06 | 0.24 | <0.001 |
| MMP2 | −2.12 | 0.23 | <0.001 |
| WNT2 | −2.30 | 0.20 | <0.001 |
| COL6A6 | −2.60 | 0.17 | <0.001 |
| SFRP2 | −2.70 | 0.16 | <0.001 |
| COL17A1 | −3.29 | 0.10 | 0.004 |
| CHRNA1 | −3.94 | 0.07 | 0.023 |
| FGF5 | −5.36 | 0.02 | <0.001 |
Figure 2.
Heatmap of the 257 statistically significant differentially expressed genes in the Primary Tumor (PT) compared to the Lymph Node (LN). Each column represents the profile of the 257 marker genes for one tumor, and each row represents the relative level of expression of each gene. On the x-axis, P1–17 represent the 17 primary tumors, and M1–17 represent the 17 metastatic lymph node samples. A red color indicates a high level of gene expression, and a green color indicates a low level of expression.
Upregulated Genes: ALN compared to PBC
Compared with the PBC, ALN metastases had 123 statistically significant differentially expressed upregulated genes. There were 5 upregulated genes that had log2FC ≥2.00 in gene expression which include CCL21 (FC 10.38, FDR<0.001), CACNA2D3 (FC 8.16, FDR=0.003), CD19 (FC 5.07, FDR<0.001), FCER2 (FC 4.78, FDR<0.001), and PGC (FC 4.72, FDR<0.001) [Table 2]. There was an upregulation of anti-apoptosis and survival signaling genes in the SLN metastasis, notably of BIRC3 (FC 1.91, FDR<0.001). BIRC3 is an inhibitor of apoptosis protein that serves as an endogenous inhibitor of programmed cell death. There was an upregulation of chemotaxis genes including CCL19 (FC 3.19, FDR<0.001), CCL21 (FC 10.38, FDR<0.001), CXCL13 (FC 2.97, FDR<0.001), and TNFSF11 (FC 3.00, FDR=0.008).
Downregulated Genes: ALN compared to PBC
Compared with the PBC, ALN metastasis had 134 statistically significant differentially expressed downregulated genes. There were 9 downregulated genes that had log2FC <−2.00 in gene expression which include FGF5 (FC 0.02, FDR<0.001), CHRNA1 (FC 0.07, FDR=0.023), COL17A1 (FC 0.10, FDR=0.005), SFRP2 (FC 0.16, FDR<0.001), COL6A6 (FC 0.17, FDR<0.001), WNT2 (FC 0.20, FDR<0.001), MMP2 (FC 0.23, FDR<0.001), MMP3 (FC 0.24, FDR<0.001), and COL11A1 (FC 0.24, FDR<0.001) [Table 2]. The most striking feature is the downregulation of genes known to regulate cell microenvironment interaction including Matrix Metallopeptidases (MMP2, MMP 3, MMP 7, MMP 11, MMP14) and Collagens (COL1A1, COL1A2, COL3A1, COL5A1, COL5A2, COL6A6, COL11A1, COL17A1).
Comparison of SLN versus ALN
Eight patients had tumor isolated from a sentinel lymph node, and 9 patients had tumor isolated from a non-sentinel axillary lymph node. Compared with the ALN (n=9), SLN (n=8) metastasis had no statistically significant difference in gene expression (FDR=NS) for any of the 2,567 genes. The two genes that approached statistically significant differences in expression included AHNAK2 (FC 0.25, FDR=0.0058) and FOS (FC 0.289, FDR=0.0058).
Ingenuity Pathway Analysis (IPA)
Ingenuity Pathway Analysis (IPA, QIAGEN Redwood City, CA, USA) of breast cancer cells in the axillary lymph node (ALN) compared to the primary breast cancer (PBC) showed distinct cellular functions [Figure 3a]. Positive activation z-scores that represent the activation of cellular functions notably include cell survival, cell viability, and cell invasion. Negative activation z-scores that represent the inhibition of cellular functions include apoptosis and organismal death. Breast cancer cells in the axillary lymph nodes show distinct regulation of predicted upstream transcriptional factors compared to the primary tumors [Figure 3b].
Figure 3.
Ingenuity Pathway Analysis (IPA) of breast cancer cells in the axillary lymph node (ALN) compared to the primary breast cancer (PBC). Activation z-scores represent the activation (positive values in red) or inhibition (negative values in green) of cellular functions. 3a. Breast cancer cells in the axillary lymph nodes show distinct cellular functions compared to the primary tumors. 3b. Breast cancer cells in the axillary lymph nodes show distinct regulation of predicted upstream transcriptional factors compared to the primary tumors.
Discussion
Gene expression studies for breast cancer have been performed to better understand breast cancer biology and metastasis, aid in drug development, understand drug resistance mechanisms, and develop prognostic gene expression signaturesxi. Gene expression studies of primary breast cancer have been compared with paired distant organ metastasis to better understand the mechanism of distant metastasisxii. In 2003, Weigelt et al. evaluated gene expression in 8 patients with breast cancer compared to paired distant metastasis to the ovary, lung, arm, and lymph node and showed similar gene expressionxiii. Bell et al. performed a meta-analysis of 12 studies comparing gene expression of PBC to breast cancer metastases and concluded that COX2 and RRM2 are likely prominent markers for breast cancer metastasisxiv. While our study did not include COX2 in our panel of cancer-associated genes, COX5A and COX7B, along with RRM2 were not differentially expressed in our study.
More recently, several studies have analyzed gene expression of primary breast cancer compared with paired lymph node metastasis. In 2004, Hao et al. analyzed gene expression in 9 patients with primary breast cancer compared to matched lymph node metastasis using a cDNA microarray for 3800 genes, and showed 280 statistically significant differentially expressed genesxv. Similarly, they showed a downregulation of FN and MMP2, which are important for tissue modeling in the lymph node compared to the primary breast cancer. Furthermore, they showed an upregulation of IGFBP5 and cyclinD1, important in cell cycle regulators, which were not differentially expressed in our study. A limitation to their study is they did not evaluate the breast cancer subtype and receptor profiles used. Weigelt et al. analyzed 15 patients with breast cancer and concluded that no classifier gene in primary breast tumors could be identified to predict lymph node statusxvi.
Suzuki et al. evaluated 10 patients with primary breast cancer and paired LN metastasis for 22,000 genes, and showed that the gene expression profile of the metastases resembled the primary tumorxvii. Similar to our study, they showed downregulation of MMP and Collagen genes in the LN compared to the PBC. Their study evaluates a combination of ductal, lobular, and mixed invasive cancer and does not differentiate receptor profile. Feng et al, analyzed 26 patients with primary IDC and paired lymph node metastasis and showed 79 differentially expressed genesxviii. They similarly showed downregulation of MMP, fibronectin, and COL11A1 genes in the lymph node compared to the PBC. In our study, this downregulation of several collagen and matrix metallopeptidase genes, which are known to regulate cell microenvironment interactions, may provide a unique environment for TNBC. This study differs from ours because they did not control for breast cancer subtype by including both ER positive and negative tumors, and did not evaluate for HER2 receptor. Differences in breast cancer subtype may have meaningful differences in gene expression.
Smeets et al. evaluated 96 paired PBC compared to LN metastasis in patients with Estrogen Receptor (ER)-positive IDC for 241 genes, and demonstrated that multiple kinases, apoptosis-related, and zinc ion-binding genes were differentially expressed compared to node-negative patientsxix. While they showed an overexpression of apoptosis related genes in the lymph node positive group (BCL6, CDKN1A, CEBPB, KRT18P19, MAPK9, TNFRSF12A, XRCC4), these genes were not differentially expressed in our study. Conversely, our study demonstrated a downregulation of genes related to apoptosis and an upregulation of anti-apoptosis and survival signaling genes in the ALN. Differences in findings between our studies may be due to differences in the PBC receptor subtype being studied or the inclusion of a larger gene-panel by our study. This overall ability for triple negative breast cancer metastases to survive in the sentinel and axillary lymph node may portend a worse prognosis for tumors that are able to survive in the new microenvironment in the lymph node.
The majority of studies that have evaluated TNBC compared to metastasis have been of distant organ sites including the brain, lung, liver, and adrenal gland, and few studies that have evaluated lymph nodes alonexx, xxi, xxii, xxiii, xxiv. Our study evaluates gene expression in triple negative IDC that has metastasized to both sentinel and axillary lymph nodes, which showed similar gene expression. This similar gene expression and microenvironment between sentinel and non-sentinel lymph nodes may allow for TNBC to survive and spread through the axillary lymphatic system.
Our study is limited by its small sample size, as it is difficult to find contemporary patients at our institution with TNBC who did not have neoadjuvant chemotherapy. Next, an appropriate tissue sample with a sufficient percentage of tumor content is critical. Our study importantly uses a microdissection technique to include a predicted >90% tumor cells. Lastly, variation in results between NGS studies may be due to tumor heterogeneity and a difference in the number of genes evaluated.
Conclusion
In TNBC, ALN metastases have a distinct gene expression profile. Genes associated with anti-apoptosis, survival responses, and chemotaxis are upregulated, and genes associated with regulation of extracellular matrix are downregulated when compared to autologous primary cancer. TNBC cells metastatic to lymph nodes undergo a change in order to metastasize and survive in the new microenvironment, which may lead to insights into the metastatic process.
Acknowledgements:
Armando E. Giuliano is supported by the Fashion Footwear Charitable Foundation of New York, Inc., the Margie and Robert E. Petersen Foundation, and Linda and Jim Lippman. Xiaojiang Cui is supported by National Institutes of Health (2R01CA151610) and Department of Defense (W81XWH-18-1-0067). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Meeting Presentation: Presented at the annual meeting of the American Society of Clinical Oncology (ASCO), Merit Award poster presentation, Chicago, IL, June 2019.
Conflict of Interest Statement: All authors have no conflicts of interest or other disclosures.
References:
- i.Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LAG, Cronin KA. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. Journal of the National Cancer Institute. 2014. 106(5): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ii.Dings PJ, Elferink MA, Stobbe LJ, de Wilt JH. The prognostic value of lymph node ratio in node-positive breast cancer: a Dutch nationwide population-based study. Annals of Surgical Oncology. 2013. 20(8): 2607–14. [DOI] [PubMed] [Google Scholar]
- iii.Liao GS, Chou YC, Golshan M, Hsu HM, Hong ZJ, Yu JC, Zhu JH. Prognostic value of the lymph node ratio in breast cancer subtypes. American Journal of Surgery. 2015. 210(4):749–54. [DOI] [PubMed] [Google Scholar]
- iv.O’Brien KM, Cole SR, Tse CK, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina breast cancer study. Clin Cancer Res. 2010. 16(24):6100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- v.Jang N, Choi JE, Kang SH, Bae YK. Validation of the pathological prognostic staging sytem proposed in the revised eighth edition of the AJCC staging manual in different molecular subtypes of breast cancer. Virchows Arch. 2019. 474(2):193–200. [DOI] [PubMed] [Google Scholar]
- vi.Yates LR, Desmedt C. Translational genomics: practical applications of the genomic revolution in breast cancer. Clin Cancer Res. 23(11):2630–2639. [DOI] [PubMed] [Google Scholar]
- vii.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Onocology/College of American Pahtologists clinical practice guideline update. J Clin Oncol. 2013. 31(31):3997–4013. [DOI] [PubMed] [Google Scholar]
- viii.Schwartz M, Kremer J. Verio Laboratory Services: HTG EdgeSeq oncology biomarker panel. 2018. [Google Scholar]
- ix.Robinson MD, McCarthy DJ and Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010; 26(1): 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- x. Benjamini Y and Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995; 57: 289–300. [Google Scholar]
- xi.Perou CM, Sorlie T, Eisen MB et al. Molecular portraits of human breast tumours. Nature. 2000. 406(6797):747–52. [DOI] [PubMed] [Google Scholar]
- xii.Lee JY, Park K, Lee E, et al. Gene expression profiling of breast cancer brain metastasis. Sci Rep. 2016. 6:28623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xiii.Weigelt B, Glas AM, Wessels LFA, Witteveen AT, Peterse JL, van’t Veer LJ. Gene expression profiles of primary breast tumors maintained in distant metastases. PNAS. 2003. 100(26): 15901–15905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xiv.Bell R, Barraclough R, Vasieva O. Gene expression meta-analysis of potential metastatic breast cancer markers. Current Molecular Medicine. 2017. 17: 200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xv.Hao X, Sun B, Hu L, et al. Differential gene and protein expression in primary breast malignancies and their lymph node metastases as revealed by combined cDNA microarray and tissue microarray analysis. Cancer. 2004. 100(6):1110–22. [DOI] [PubMed] [Google Scholar]
- xvi.Weigelt B, Wessels LF, Bosma AJ, et al. No common denominator for breast cancer lymph node metastasis. Br J Cancer. 2005. 93(8):924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xvii.Suzuki M, Tarin D. Gene expression profiling of human lymph node metastases and matched primary breast carcinomas: clinical implications. Molecular Oncology. 2007. 1(2):172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xviii.Feng Y, Sun B, Li X, et al. Differentially expressed genes between primary cancer and paired lymph node metastases predict clinical outcome of node-positive breast cancer patients. Breast Cancer Res Treat. 2007. 103(3):319–29. [DOI] [PubMed] [Google Scholar]
- xix.Smeets A, Daemen A, Bempt IV, et al. Prediction of lymph node involvement in breast cancer from primary tumor tissue using gene expression profiling and miRNAs. Breast Cancer Res Treat. 2011. 129(3):767–776. [DOI] [PubMed] [Google Scholar]
- xx.Ding L, Ellis M, Li S, et al. Genome remodeling in a basal-like breast cancer metastasis and xenograft. Nature. 2010. 464(7291):999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxi.Hoadley KA, Siegel MB, Kanchi KL, et al. Tumor evoluation in two patients with basal-like breast cancer: a retrospective genomics study of multiple metastases. PLOS Medicine. 2016. 13(2):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxii.Zhou L, Gao HF, Liu DS, Feng JY, Gao DD, Xia W. Gene expression profiling of brain metastatic cell from triple negative breast cancer: Understanding the molecular events. Gene. 2018. 640:21–27. [DOI] [PubMed] [Google Scholar]
- xxiii.Lin FM, Yost SE, Wen W, et al. Differential gene expression and AKT targeting in triple negative breast cancer. Oncotarget. 2019. 10(43):4356–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxiv.Zhao ZM, Yost SE, Hutchinson KE, et al. CCNE1 amplification is associated with poor prognosis in patients with triple negative breast cancer. BMC Cancer. 2019. 19(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]