Abstract
Background:
Controversy exists regarding optimal treatment of occult breast cancer (OBC). Treatment options include mastectomy alone (MAST), radiation alone (XRT), or mastectomy with radiation (MXRT).
Methods:
We queried the National Cancer Database from 2004-2014 for patients with OBC who underwent MXRT, XRT, or MAST. We utilized propensity score matching to perform three head-to-head comparisons. Kaplan-Meier analysis was performed to compare overall survival (OS).
Results:
A total of 190 patients received XRT, 237 received MAST, and 244 received MXRT. In the MXRT vs. XRT comparison, 5-year OS was 78.2% and 82.8%, respectively. In the MXRT vs. MAST comparison, 5-year OS was 81.5% and 86.7%, respectively. In the MAST vs XRT comparison, 5-year OS was 83.2% and 82.5%, respectively. There was no difference in OS for all paired comparisons.
Conclusions:
There were no OS differences in patients undergoing MAST, XRT, or MXRT, suggesting breast conservation can be considered in patients with OBC.
Keywords: Occult breast cancer, NCDB, propensity score matching, radiation therapy, mastectomy
PRECISE
Occult breast cancer presents a clinical challenge for clinicians. Using propensity score matching, we found no difference in overall survival among mastectomy alone, radiation alone, or mastectomy and radiation.
INTRODUCTION
Occult breast cancer most often presents as axillary lymph node metastases without evidence of a primary breast tumor.1,2 Because of its rarity,3 prospective randomized control trials have not been conducted and treatment strategies are based on few small retrospective and case studies.4–6 For patients with ≤3 positive lymph nodes, the National Comprehensive Cancer Network (NCCN) guidelines recommend either 1) mastectomy with axillary lymph node dissection (ALND) +/− postmastectomy radiation or 2) ALND with whole-breast irradiation +/− lymph node irradiation. For patients with >3 positive lymph nodes, the NCCN recommends neoadjuvant therapy followed by mastectomy with ALND.7 However, controversy still exists regarding the optimal surgical treatment as practice patterns are highly varied. For example, a 2005 survey of American Society of Breast Surgeons revealed that 43% of surgeons preferred mastectomy while 37% preferred whole breast radiation, highlighting the lack of standardization in treatment of occult breast cancer.8
Several recent studies turned to large cancer databases to gather insights into the appropriate treatment of occult breast cancer.3,9–11 Analyses of the Surveillance, Epidemiology, and End Results Program (SEER) database and National Cancer Database (NCDB) provide invaluable information on outcomes of various treatment combinations of chemotherapy, mastectomy, ALND, and radiation therapy. However, none of the prior studies specifically address whether a mastectomy should still be performed in patients already receiving radiation therapy and ALND.
The most common treatment options in addressing the breast for these challenging patients are mastectomy alone, radiation alone, or mastectomy with radiation. The decision to provide single or combined therapy is dependent on a multitude of patient and external factors. Thus, previous studies may be subject to selection bias, especially given their retrospective nature. Advanced statistical techniques like propensity score matching can minimize these biases. Therefore, the aim of this study is to determine the optimal treatment of patients with occult breast cancer by analyzing data from the NCDB using propensity score matching.
MATERIALS AND METHODS
Patients and Variables
The NCDB is a national oncology outcomes database that is jointly sponsored by the American College of Surgeons and the American Cancer Society. It contains clinical oncology data sourced from over 1,500 Commission on Cancer (CoC) accredited centers, accounting for approximately 70% of newly diagnosed cancer cases nationwide. Due to the de-identified nature of the data, this study was exempt from institutional review board approval.
We identified patients diagnosed with occult breast cancer in the NCDB from 2004 to 2014 using a combination of factors: 1) no primary tumor found, 2) positive pathologic lymph nodes, and 3) International Classification of Disease for Oncology, 3rd edition histology codes for ductal and lobular carcinoma (8500 and 8520). This combination of factors was used because occult breast cancer is typically diagnosed from a positive lymph node biopsy showing invasive breast cancer but no primary cancer in the breast is identified.
Patients were categorized into three treatment groups: 1) mastectomy alone (MAST), 2) radiotherapy alone (XRT), and 3) mastectomy with radiotherapy (MXRT). As per the NCDB, radiotherapy is limited to treatment of the primary site (e.g. chest wall). We excluded patients with distant metastatic disease at diagnosis, missing survival or treatment data, and those who received no radiation or mastectomy (i.e. chemotherapy only). A CONSORT diagram is shown in Figure 1.
Figure 1.
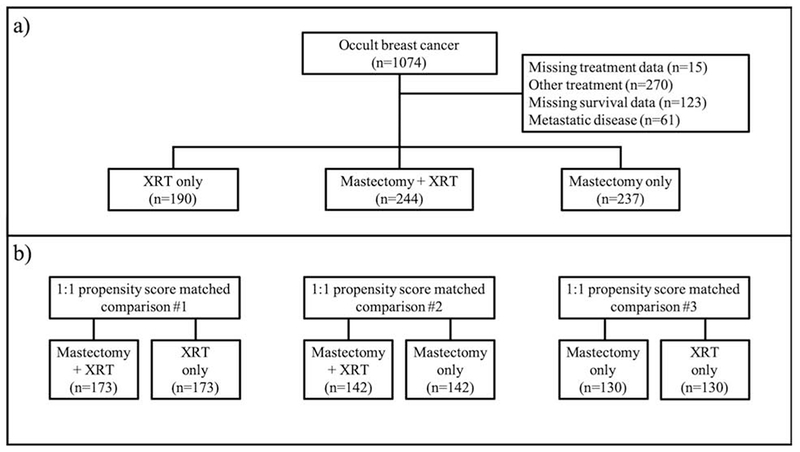
CONSORT Diagram
We included the following variables in our analysis based on availability and clinical significance. Patient age was categorized into discrete groups (<50, 50-59, 60-69, >69). Patient race categorized into white, black, and other (American Indian, Asian, Pacific Islander, and unknown race). Treatment facility type categorized into academic, non-academic (community, comprehensive community, and integrated network cancer program), and unknown. We kept clinical M stage as a variable because a number of patients had missing clinical M stage data. Because the NCDB does not capture whether an ALND was performed, we considered patients with ≥10 lymph nodes examined as undergoing an ALND. Charlson-Deyo Comorbidity Score (CDCS), year of diagnosis (categorized into before or after 2010), pathologic N stage, estrogen receptor (ER) status, progesterone receptor (PR) status, HER2/neu receptor status, and whether hormonal therapy and chemotherapy (both adjuvant and/or neoadjuvant) were performed were also included in our analysis.
Propensity Score Matching and Statistical Analysis
Propensity score matching is a statistical method used to minimize selection bias in non-randomized studies. Cohorts are split into “control” and “case” matched on variables that would otherwise confound comparisons between them. After matching, treatment effect can be estimated by directly comparing outcomes between control and case patients. We generated propensity scores by using a multivariable logistic regression model. Missing values were included in the model as missing data have the potential to systematically differ between the two treatment groups.12 After including all variables above, a 1:1 match (without replacement) by propensity score was performed using nearest neighbor method with caliper width set to 0.2 standard deviations.13 We examined balance in the baseline and matched variables by using standardized mean differences (SMD). A SMD <0.25, ideally <0.10, indicates an acceptable balanced match.14 To determine optimal treatment for patients with occult breast cancer, three paired comparisons were performed: 1) MXRT versus XRT, 2) MXRT versus MAST, and 3) MAST versus XRT.
After successfully matching patients in each comparison, we estimated the overall survival from the time of diagnosis for each group by using Kaplan-Meier analysis and compared the overall survival using the log-rank test. We performed a subset analysis of each matched comparison of patients with pN2 or pN3 disease. Because matching properties may be lost after sub-setting, we performed a multivariable Cox proportional hazard analysis to investigate whether the treatment is associated with overall survival. All predictors used in the matching process were also used in the multivariable model. Two-sided p-values <0.05 were considered significant. All statistical analyses were performed using R Studio (version 3.4.1, The R Foundation, Vienna, Austria). The matching procedure was performed using the Matching Package in R.15
RESULTS
Mastectomy + XRT versus XRT Alone
A total of 434 patients were included prior to matching. A comparison of the two groups before and after propensity score matching is shown in Table 1. After matching, the SMD of the matched variables were all <0.10, indicating negligible difference between the two groups.14
Table 1.
Comparison of Variables Between Mastectomy + Radiotherapy (MXRT) and Radiotherapy Only (XRT) Groups in the Original Dataset and Matched Dataset
| Original Dataset | Matched Dataset | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | MXRT | XRT | SMD* | p-value | MXRT | XRT | SMD* | p-value |
| Number of Patients | 244 (56.2) | 190 (43.8) | - | - | 173 (50.0) | 173 (50.0) | - | - |
| Age | 0.266 | 0.010 | 0.041 | 0.974 | ||||
| <50 | 67 (27.4) | 27 (14.2) | 29 (16.8) | 26 (15.0) | ||||
| 50-59 | 78 (32.0) | 68 (35.8) | 64 (37.0) | 64 (37.0) | ||||
| 60-69 | 60 (24.6) | 57 (30.0) | 47 (27.2) | 49 (28.3) | ||||
| >69 | 39 (16.0) | 38 (20.0) | 33 (19.1) | 34 (19.7) | ||||
| Race | 0.057 | 0.445 | 0.059 | 0.858 | ||||
| White | 195 (79.9) | 153 (80.5) | 137 (79.2) | 141 (81.5) | ||||
| Black | 37 (15.2) | 32 (16.8) | 30 (17.3) | 27 (15.6) | ||||
| Other | 12 (4.9) | 5 (2.6) | 6 (3.5) | 5 (2.9) | ||||
| Charlson-Deyo Score | 0.083 | 0.627 | 0.038 | 0.808 | ||||
| 0 | 201 (82.4) | 163 (85.8) | 147 (85.0) | 146 (84.4) | ||||
| 1 | 34 (13.9) | 21 (11.1) | 22 (12.7) | 21 (12.1) | ||||
| ≥2 | 9 (3.7) | 6 (3.2) | 4 (2.3) | 6 (3.5) | ||||
| Facility Type | 0.035 | 0.007 | 0.091 | 0.645 | ||||
| Non-Academic | 165 (67.6) | 122 (64.2) | 121 (69.9) | 113 (65.3) | ||||
| Academic | 62 (25.4) | 65 (34.2) | 49 (28.3) | 57 (32.9) | ||||
| Unknown | 17 (7.0) | 3 (1.6) | 3 (1.7) | 3 (1.7) | ||||
| Year of Diagnosis | 0.045 | 0.642 | 0.049 | 0.646 | ||||
| <2010 | 72 (29.5) | 60 (31.6) | 58 (33.5) | 54 (31.2) | ||||
| ≥2010 | 172 (70.5) | 130 (68.4) | 115 (66.5) | 119 (68.8) | ||||
| Pathologic N Stage | 0.030 | 0.917 | 0.021 | 0.711 | ||||
| 1 | 116 (47.5) | 94 (49.5) | 76 (43.9) | 81 (46.8) | ||||
| 2 | 69 (28.3) | 51 (26.8) | 55 (31.8) | 48 (27.7) | ||||
| 3 | 59 (24.2) | 45 (23.7) | 42 (24.3) | 44 (25.4) | ||||
| Clinical M Stage | 0.094 | 0.337 | <0.001 | 1.0 | ||||
| cM0 | 235 (96.3) | 186 (97.9) | 169 (97.7) | 169 (97.7) | ||||
| cMx | 9 (3.7) | 4 (2.1) | 4 (2.3) | 4 (2.3) | ||||
| ER† Status | 0.007 | 0.154 | 0.055 | 0.578 | ||||
| Negative | 71 (29.1) | 49 (25.8) | 53 (30.6) | 46 (26.6) | ||||
| Positive | 155 (63.5) | 134 (70.5) | 111 (64.2) | 120 (69.4) | ||||
| Unknown | 18 (7.4) | 7 (3.7) | 9 (5.2) | 7 (4.0)) | ||||
| PR‡ Status | 0.100 | 0.033 | <0.001 | 0.690 | ||||
| Negative | 125 (51.2) | 80 (42.1) | 80 (46.2) | 77 (44.5) | ||||
| Positive | 100 (41.0) | 101 (53.2) | 82 (47.4) | 88 (50.9) | ||||
| Unknown | 19 (7.8) | 9 (4.7) | 11 (6.4) | 8 (4.6) | ||||
| HER2/neu Status | 0.061 | 0.476 | 0.068 | 0.811 | ||||
| Negative | 116 (47.5) | 99 (52.1) | 84 (48.6) | 90 (52.0) | ||||
| Positive | 34 (13.9) | 20 (10.5) | 19 (11.0) | 18 (10.4) | ||||
| Unknown | 94 (38.5) | 71 (37.4) | 70 (40.4) | 65 (37.6) | ||||
| Hormonal Therapy | 0.055 | 0.821 | 0.042 | 0.904 | ||||
| No | 91 (37.3) | 67 (35.2) | 67 (38.7) | 63 (36.4) | ||||
| Yes | 145 (59.4) | 115 (60.5) | 99 (57.2) | 103 (59.5) | ||||
| Unknown | 8 (3.3) | 8 (4.2) | 7 (4.0) | 7 (4.0) | ||||
| Chemotherapy | 0.230 | 0.049 | 0.069 | 0.519 | ||||
| No | 22 (9.0) | 31 (16.3) | 20 (11.6) | 24 (13.9) | ||||
| Yes | 221 (90.6) | 159 (83.7) | 153 (88.4) | 149 (86.1) | ||||
| Unknown | 1 (0.4) | 0 (0) | - | - | ||||
| ALND§ | 0.068 | 0.676 | 0.055 | 0.852 | ||||
| No | 83 (34.0) | 72 (37.9) | 59 (34.1) | 64 (37.0) | ||||
| Yes | 154 (63.1) | 112 (58.9) | 109 (63.0) | 104 (60.1) | ||||
| Unknown | 7 (2.9) | 6 (3.2) | 5 (2.9) | 5 (2.9) | ||||
Standardized mean difference
Estrogen receptor
Progesterone receptor
Axillary lymph node dissection
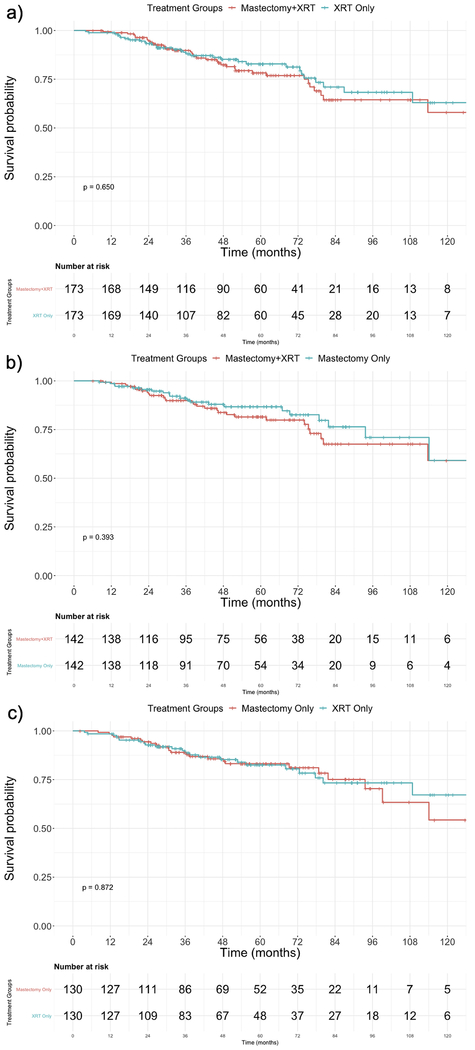
The Kaplan-Meier curve comparing the two treatment groups is shown in Figure 2a. The 5- and 10-year overall survival of the MXRT group was 78.2% (95% CI 71.3 – 85.8) and 58.0% (95% CI 44.3 – 75.9), respectively. The 5- and 10-year overall survival of the XRT group was 82.8% (95% CI 76.5 – 89.8) and 63.0% (95% CI 50.1 – 79.3), respectively (Table 2). There was no difference in overall survival by log-rank test (p=0.650). Table 3 shows the results of the subset analysis of pN2 and pN3 patients. After controlling for multiple variables, MXRT and XRT had no effect on overall survival (p=0.952).
Figure 2.
Kaplan-Meier Survival Curves for the Three Matched Comparisons. a) MXRT vs. XRT, b) MXRT vs. MAST, c) MAST vs. XRT
Table 2.
5- and 10-Year Overall Survival for All Matched Comparisons
| Comparison Groups | 5-Year Overall Survival | 10-Year Overall Survival | Median Follow-Up (Months) | p-value |
|---|---|---|---|---|
| MXRT* | 0.782 (0.713 – 0.858) | 0.580 (0.443 – 0.759) | 47.87 | 0.650 |
| XRT† | 0.828 (0.765 – 0.898) | 0.630 (0.501 – 0.793) | ||
| MXRT | 0.815 (0.745 – 0.891) | 0.591 (0.430 – 0.811) | 48.53 | 0.393 |
| MAST‡ | 0.867 (0.804 – 0.934) | 0.591 (0.391 – 0.894) | ||
| MAST | 0.832 (0.762 – 0.907) | 0.543 (0.359 – 0.822) | 50.33 | 0.872 |
| XRT | 0.825 (0.753 – 0.905) | 0.672 (0.536 – 0.843) |
Mastectomy and radiotherapy
Radiotherapy alone
Mastectomy alone
Table 3.
Cox Proportional Hazard Analysis of Matched Patients with pN2 or pN3 Disease
| Comparison Groups | p-value | 95% Confidence Interval | Variables Included in Model |
|---|---|---|---|
| MXRT* | 0.952 | Reference | Age, race, Charlson-Deyo comorbidity score, treatment facility type, diagnosis year, clinical M stage, ER status, PR status, HER2/neu status, hormonal therapy, chemotherapy, and axillary lymph node dissection status |
| XRT† | 0.501 – 1.916 | ||
| MXRT | 0.415 | Reference | |
| MAST‡ | 0.558 – 4.112 | ||
| MAST | 0.582 | Reference | |
| XRT | 0.222 – 2.327 |
Mastectomy and radiotherapy
Radiotherapy alone
Mastectomy alone
Mastectomy + XRT versus Mastectomy Alone
A total of 481 patients were included prior to matching. A comparison of the two groups before and after propensity score matching is shown in Table 4. After matching, the SMD of the matched variables were all <0.10, except for pathologic N stage, which was 0.109. An SMD of <0.25 still indicates an acceptable match balance, especially given the small sample size.
Table 4.
Comparison of Variables Between Mastectomy + Radiotherapy (MXRT) and Mastectomy Only (MAST) Groups in the Original Dataset and Matched Dataset
| Original Dataset | Matched Dataset | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | MXRT | MAST | SMD* | p-value | MXRT | MAST | SMD* | p-value |
| Number of Patients | 244 (50.7) | 237 (49.3) | - | - | 142 (50.0) | 142 (50.0) | - | - |
| Age | 0.004 | 0.564 | 0.054 | 0.953 | ||||
| <50 | 67 (27.4) | 73 (30.8) | 43 (30.3) | 40 (28.2) | ||||
| 50-59 | 78 (32.0) | 62 (26.2) | 43 (30.3) | 44 (31.0) | ||||
| 60-69 | 60 (24.6) | 61 (25.7) | 35 (24.6) | 34 (23.9) | ||||
| >69 | 39 (16.0) | 41 (17.3) | 21 (14.8) | 24 (16.9) | ||||
| Race | 0.021 | 0.961 | 0.064 | 0.698 | ||||
| White | 195 (79.9) | 187 (78.9) | 111 (78.2) | 116 (81.7) | ||||
| Black | 37 (15.2) | 38 (16.0) | 23 (16.2) | 18 (12.7) | ||||
| Other | 12 (4.9) | 12 (5.1) | 8 (5.6) | 8 (5.6) | ||||
| Charlson-Deyo Comorbidity Score | 0.021 | 0.675 | 0.079 | 0.734 | ||||
| 0 | 201 (82.4) | 196 (82.7) | 119 (83.8) | 114 (80.3) | ||||
| 1 | 34 (13.9) | 29 (12.2) | 16 (11.3) | 20 (14.1) | ||||
| ≥2 | 9 (3.7) | 12 (5.1) | 7 (4.9) | 8 (5.6) | ||||
| Facility Type | 0.084 | 0.546 | 0.065 | 0.815 | ||||
| Non-Academic | 165 (67.6) | 154 (65.0) | 90 (63.4) | 93 (65.5) | ||||
| Academic | 62 (25.4) | 60 (25.3) | 38 (26.7) | 38 (26.7) | ||||
| Unknown | 17 (7.0) | 23 (9.7) | 14 (9.9) | 11 (7.7) | ||||
| Year of Diagnosis | 0.344 | <0.001 | 0.044 | 0.711 | ||||
| <2010 | 72 (29.5) | 109 (46.0) | 50 (35.2) | 53 (37.3) | ||||
| ≥2010 | 172 (70.5) | 128 (54.0) | 92 (64.8) | 89 (62.7) | ||||
| Pathologic N Stage | 0.704 | <0.001 | 0.109 | 0.646 | ||||
| 1 | 116 (47.5) | 195 (82.3) | 100 (70.4) | 106 (74.6) | ||||
| 2 | 69 (28.3) | 22 (9.3) | 21 (14.8) | 20 (14.1) | ||||
| 3 | 59 (24.2) | 20 (8.4) | 21 (14.8) | 16 (11.3) | ||||
| Clinical M Stage | 0.067 | 0.466 | 0.098 | 0.409 | ||||
| cM0 | 235 (96.3) | 231 (97.5) | 138 (97.2) | 140 (98.6) | ||||
| cMx | 9 (3.7) | 6 (2.5) | 4 (2.8) | 2 (1.4) | ||||
| ER† Status | 0.129 | 0.340 | 0.048 | 0.821 | ||||
| Negative | 71 (29.1) | 59 (24.9) | 42 (29.6) | 41 (28.9) | ||||
| Positive | 155 (63.5) | 153 (64.6) | 89 (62.7) | 87 (61.3) | ||||
| Unknown | 18 (7.4) | 25 (10.5) | 11 (7.7) | 14 (9.9) | ||||
| PR‡ Status | 0.253 | 0.021 | 0.022 | 0.978 | ||||
| Negative | 125 (51.2) | 93 (39.2) | 66 (46.5) | 65 (45.8) | ||||
| Positive | 100 (41.0) | 115 (48.5) | 63 (44.4) | 63 (44.4) | ||||
| Unknown | 19 (7.8) | 29 (12.2) | 13 (9.1) | 14 (9.8) | ||||
| HER2/neu Status | 0.384 | <0.001 | 0.053 | 0.789 | ||||
| Negative | 116 (47.5) | 70 (29.5) | 58 (40.8) | 53 (37.3) | ||||
| Positive | 34 (13.9) | 36 (15.2) | 19 (13.4) | 22 (15.5) | ||||
| Unknown | 94 (38.5) | 131 (55.3) | 65 (45.8) | 67 (47.2) | ||||
| Hormonal Therapy | 0.104 | 0.492 | 0.013 | 0.993 | ||||
| No | 91 (37.3) | 101 (42.6) | 59 (41.5) | 60 (42.3) | ||||
| Yes | 145 (59.4) | 129 (54.4) | 79 (55.6) | 78 (54.9) | ||||
| Unknown | 8 (3.3) | 7 (3.0) | 4 (2.8) | 4 (2.8) | ||||
| Chemotherapy | 0.513 | <0.001 | 0.019 | 0.986 | ||||
| No | 22 (9.0) | 72 (30.4) | 20 (14.1) | 21 (14.8) | ||||
| Yes | 221 (90.6) | 162 (68.4) | 121 (85.2) | 120 (84.5) | ||||
| Unknown | 1 (0.4) | 3 (1.2) | 1 (0.7) | 1 (0.7) | ||||
| ALND§ | 0.285 | 0.004 | 0.039 | 0.937 | ||||
| No | 83 (34.0) | 116 (48.9) | 61 (43.0) | 64 (45.1) | ||||
| Yes | 154 (63.1) | 115 (48.5) | 78 (54.9) | 75 (52.8) | ||||
| Unknown | 7 (2.9) | 6 (2.5) | 3 (2.1) | 3 (2.1) | ||||
Standardized mean difference
Estrogen receptor
Progesterone receptor
Axillary lymph node dissection
The Kaplan-Meier curve comparing the two treatment groups is shown in Figure 2b. The 5- and 10-year overall survival of the MXRT group was 81.5% (95% CI 74.5 – 89.1) and 59.1% (95% CI 43.0 – 81.1), respectively. The 5- and 10-year overall survival of the MAST group was 86.7% (95% CI 80.4 – 93.4) and 59.1% (95% CI 39.1 – 89.4), respectively (Table 2). There was no difference in overall survival by log-rank test (p=0.393). Table 3 shows the results of the subset analysis of pN2 and pN3 patients. After controlling for multiple variables, MXRT and MAST had no effect on overall survival (p=0.415).
Mastectomy Alone versus XRT Alone
A total of 427 patients were included prior to matching. A comparison of the two groups before and after propensity score matching is shown in Table 5. After matching, the SMD of the matched variables were all <0.10 except for pathologic N stage, which was 0.161.
Table 5.
Comparison of Variables Between Mastectomy Only (MAST) and Radiotherapy Only (XRT) Groups in the Original Dataset and Matched Dataset
| Original Dataset | Matched Dataset | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | MAST | XRT | SMD* | p-value | MAST | XRT | SMD* | p-value |
| Number of Patients | 237 (55.5) | 190 (44.5) | - | - | 130 (50.0) | 130 (50.0) | - | - |
| Age | 0.256 | <0.001 | 0.055 | 0.762 | ||||
| <50 | 73 (30.8) | 27 (14.2) | 23 (17.7) | 23 (17.7) | ||||
| 50-59 | 62 (26.2) | 68 (35.8) | 43 (33.1) | 50 (38.5) | ||||
| 60-69 | 61 (25.7) | 57 (30.0) | 42 (32.3) | 35 (26.9) | ||||
| >69 | 41 (17.3) | 38 (20.0) | 22 (16.9) | 22 (16.9) | ||||
| Race | 0.079 | 0.440 | 0.016 | 0.985 | ||||
| White | 187 (78.9) | 153 (80.5) | 107 (82.3) | 106 (81.5) | ||||
| Black | 38 (16.0) | 32 (16.8) | 19 (14.6) | 20 (15.4) | ||||
| Other | 12 (5.1) | 5 (2.6) | 4 (3.1) | 4 (3.1) | ||||
| Charlson-Deyo Comorbidity Score | 0.102 | 0.562 | 0.088 | 0.693 | ||||
| 0 | 196 (82.7) | 163 (85.8) | 106 (81.5) | 109 (83.9) | ||||
| 1 | 29 (12.2) | 21 (11.1) | 16 (12.3) | 16 (12.3) | ||||
| ≥2 | 12 (5.1) | 6 (3.2) | 8 (6.2) | 5 (3.8) | ||||
| Facility Type | 0.123 | <0.001 | 0.060 | 0.860 | ||||
| Non-Academic | 154 (65.0) | 122 (64.2) | 91 (70.0) | 88 (67.7) | ||||
| Academic | 60 (25.3) | 65 (34.2) | 37 (28.5) | 39 (30.0) | ||||
| Unknown | 23 (9.7) | 3 (1.6) | 2 (1.5) | 3 (2.3) | ||||
| Year of Diagnosis | 0.298 | 0.002 | <0.001 | 1.0 | ||||
| <2010 | 109 (46.0) | 60 (31.6) | 50 (38.5) | 50 (38.5) | ||||
| ≥2010 | 128 (54.0) | 130 (68.4) | 80 (61.5) | 80 (61.5) | ||||
| Pathologic N Stage | 0.669 | <0.001 | 0.161 | 0.428 | ||||
| 1 | 195 (82.3) | 94 (49.5) | 93 (71.5) | 84 (64.6) | ||||
| 2 | 22 (9.3) | 51 (26.8) | 19 (14.6) | 21 (16.2) | ||||
| 3 | 20 (8.4) | 45 (23.7) | 18 (13.8) | 25 (19.2) | ||||
| Clinical M Stage | 0.028 | 0.772 | 0.056 | 0.652 | ||||
| cM0 | 231 (97.5) | 186 (97.9) | 128 (98.5) | 127 (97.7) | ||||
| cMx | 6 (2.5) | 4 (2.1) | 2 (1.5) | 2 (1.5) | ||||
| ER† Status | 0.144 | 0.027 | 0.059 | 0.652 | ||||
| Negative | 59 (24.9) | 49 (25.8) | 38 (29.2) | 37 (28.5) | ||||
| Positive | 153 (64.6) | 134 (70.5) | 88 (67.7) | 86 (66.1) | ||||
| Unknown | 25 (10.5) | 7 (3.7) | 4 (3.1) | 7 (5.4) | ||||
| PR‡ Status | 0.167 | 0.026 | 0.079 | 0.680 | ||||
| Negative | 93 (39.2) | 80 (42.1) | 58 (44.6) | 55 (42.3) | ||||
| Positive | 115 (48.5) | 101 (53.2) | 67 (51.5) | 67 (51.5) | ||||
| Unknown | 29 (12.2) | 9 (4.7) | 5 (3.8) | 8 (6.2) | ||||
| HER2/neu Status | 0.444 | <0.001 | 0.033 | 0.966 | ||||
| Negative | 70 (29.5) | 99 (52.1) | 54 (41.5) | 56 (43.1) | ||||
| Positive | 36 (15.2) | 20 (10.5) | 16 (12.3) | 16 (12.3) | ||||
| Unknown | 131 (55.3) | 71 (37.4) | 60 (46.2) | 58 (44.6) | ||||
| Hormonal Therapy | 0.157 | 0.271 | 0.027 | 0.951 | ||||
| No | 101 (42.6) | 67 (35.2) | 55 (42.3) | 54 (41.5) | ||||
| Yes | 129 (54.4) | 115 (60.5) | 70 (53.8) | 70 (53.9) | ||||
| Unknown | 7 (3.0) | 8 (4.2) | 5 (4.9) | 6 (4.6) | ||||
| Chemotherapy | 0.298 | <0.001 | 0.038 | 0.757 | ||||
| No | 72 (30.4) | 31 (16.3) | 27 (20.8) | 25 (19.2) | ||||
| Yes | 162 (68.4) | 159 (83.7) | 103 (79.2) | 105 (80.8) | ||||
| Unknown | 3 (1.2) | 0 (0) | - | - | ||||
| ALND§ | 0.215 | 0.073 | 0.042 | 0.926 | ||||
| No | 83 (34.0) | 72 (37.9) | 56 (43.1) | 54 (41.5) | ||||
| Yes | 154 (63.1) | 112 (58.9) | 70 (53.8) | 71 (54.6) | ||||
| Unknown | 7 (2.9) | 6 (3.2) | 4 (3.1) | 5 (3.9) | ||||
Standardized mean difference
Estrogen receptor
Progesterone receptor
Axillary lymph node dissection
The Kaplan-Meier curve comparing the two treatment groups is shown in Figure 2c. The 5- and 10-year overall survival of the MAST group was 83.2% (95% CI 76.2 – 90.7) and 54.3% (95% CI 35.9 – 82.2), respectively. The 5- and 10-year overall survival of the XRT group was 82.5% (95% CI 75.3 – 90.5) and 67.2% (95% CI 53.6 – 84.3), respectively (Table 2). There was no difference in overall survival by log-rank test (p=0.872). Table 3 shows the results of the subset analysis of pN2 and pN3 patients. After controlling for multiple variables, MAST and XRT had no effect on overall survival (p=0.582).
DISCUSSION
Occult breast cancer is a rare clinical entity and represents a therapeutic challenge for physicians. In our analysis of the NCDB, we found that of the 671 patients analyzed, 35.3% underwent mastectomy alone, 28.3% underwent radiation alone, and 36.4% underwent both. This relatively equal distribution suggests that optimal treatment for this disease is not well-defined. To identify the best treatment for these patients, we performed three propensity score matched analyses comparing the various treatment options and found no differences in overall survival among the groups.
Mastectomy+XRT versus XRT Alone
In the pre-matched cohort, there was a slight majority of patients who underwent MXRT compared to XRT alone. Key differences in patients prior to matching were evident and may reflect when each treatment is being utilized in clinical practice. For example, patients who received XRT alone tended to be older, perhaps indicating the reluctance of physicians to subject older patients to MXRT.16 There was no difference in CDCS, suggesting comorbidities in the CDCS alone were not a deciding factor. Conversely, patients receiving XRT were also more likely to be treated at an academic center, a phenomenon seen in previous studies.17 Lastly, patients receiving XRT were more likely to be PR positive and not receive chemotherapy. Patient preference may play a role, as these patients are not receiving more aggressive surgical and medical therapy such as mastectomy or chemotherapy. Unfortunately, patient choice and some comorbidities are not captured in NCDB. In order to minimize the bias that these differences may produce, we performed a propensity score-matched analysis, which showed no difference in overall survival between MXRT and XRT. The Kaplan-Meier survival curves for these patients were similar up to 10 years after diagnosis, suggesting that the addition of mastectomy to XRT-treated patients may not be necessary.
Mastectomy+XRT versus Mastectomy Alone
Prior to matching, there were several key differences between these two evenly-split groups. MXRT was utilized to a greater extent after 2010 (70.5% of patients) compared to MAST (54.0% of patients), which may reflect a change in the utilization of post-mastectomy radiation to include N1 as well as N2 disease.18 In addition, patients in the MXRT group tended to have higher pathologic N stage. Though all patients in this study had at least pN1 disease, the MXRT group had higher proportions of pN2 or pN3 disease. MAST patients were more likely to have PR positivity and higher unknown HER2 status. The latter may be due to the fact that HER2/neu status was only available after 2010 in the NCDB. Surprisingly, 30.4% of the MAST group had no chemotherapy. In other words, these patients with lymph node positive disease underwent mastectomy plus ALND but no XRT or chemotherapy.
In the matched comparison, the 5-year overall survival of MAST was 86.7%, which was higher than the 81.5% of MXRT, although not statistically significant. In addition, at 10 years, the overall survival of MAST was identical to MXRT. This may be explained by findings in multiple prior studies that the addition of XRT reduces local recurrence in the long-term, which provides a more sustained disease control.18,19
Mastectomy Alone versus XRT Alone
Compared to MAST, XRT patients tended to be older, a phenomenon also seen in the XRT versus MXRT comparison. XRT patients also tended to have more advanced pN stages, suggesting that radiation in both XRT and MXRT groups is utilized when patients have more advanced lymph node disease. Lastly, patients in the XRT group were more likely to have received chemotherapy compared to the MAST group. This may reflect the desire to maximize adjunctive therapies in patients who do not undergo surgical resection, although other reasons could be treatment at an academic center or patient preference.17 In the matched comparison, MAST patients had lower 10-year overall survival rates compared to XRT patients. Though this difference was not significant, it again suggests that radiotherapy in these patients may be beneficial for long-term overall survival.10,18,19
Our main finding that all three treatment options yielded no significant differences in overall survival after propensity score matching supports the oncologic safety of omitting mastectomy after radiotherapy in patients with occult breast cancer. This is true even for pN2 and pN3 patients, although the NCCN recommends mastectomy for all these patients.7 Our findings are consistent with several prior studies suggesting breast preservation is feasible in the context of occult breast cancer and does not negatively impact local control or survival.6,20,21 Furthermore, mastectomy is a more invasive procedure with associated risks and negative impacts on quality of life.22,23 In addition, previous studies showed that a primary tumor is found in only 31% of mastectomy specimen, suggesting that patients with occult cancers in the breast have very low volume disease that can be treated with radiation as a primary local treatment to the breast.4,24 Therefore, we believe that patients undergoing radiation alone may be preferable.
There are limitations to our study. This is a retrospective study which limits the variables that can be used in the propensity score matching process and leads to the possibility of uncontrolled confounders.25 For example, the extent of lymphadenectomy is not included in the NCDB. Also, while the role of MRI has increased in the identification of occult breast lesions, this data is not available in the NCDB. If an occult lesion is identified using MRI, then it would not be labelled as “occult” and would not be included in this study. There is currently no variable for complete pathologic response in the NCDB, which may occur in patients undergoing neoadjuvant systemic therapy. The presence of complete pathologic response may have affected clinical decision-making. Unfortunately, it is not possible to analyze the complex decision-making process that is involved in the care of these patients, which may have contributed to why certain patients received specific treatment regimens. Disease-specific survival and recurrence data is not currently available in the NCDB, so we are limited to overall survival as our outcome of interest. Patients included in the NCDB are treated at CoC-accredited centers, which are often referral centers for more complex or aggressive disease, leading to potential referral bias. In addition, these findings may not be applicable to non-CoC-accredited centers, limiting generalizability.
CONCLUSIONS
Patients with occult breast cancer present a unique clinical challenge to physicians. In our propensity score matched analysis, we found that there was no difference in overall survival in patients undergoing MAST, XRT, or MXRT. The invasive nature of mastectomy and the morbidities associated with it should not be overlooked. Given the equipoise in overall survival among the treatment options, we conclude that after axillary clearance, breast preservation and radiation therapy alone may be sufficient in the treatment of patients with occult breast cancer.
HIGHLIGHTS.
Occult breast cancer presents a clinical challenge for clinicians
Treatment options include mastectomy alone, radiotherapy alone, or both
Using propensity score matching, we compared these treatment options
We found no difference in overall survival among the treatment groups
These results suggest that radiotherapy alone may be sufficient
FUNDING SUPPORT AND ACKNOWLEDGEMENTS
Dr. Beiqun Zhao is supported by the National Library of Medicine Training Grant [NIH Grant: T15LM011271].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST AND DISCLOSURE STATEMENT
All co-authors have no conflicts of interest to disclose.
REFERENCES
- 1.Wang X Presentation of axillary metastases from occult breast carcinoma. Chin J Clin Oncol. 2007;4(1):1–5. doi: 10.1007/s11805-007-0001-3 [DOI] [Google Scholar]
- 2.Rosen PP, Kimmel M. Occult breast carcinoma presenting with axillary lymph node metastases: a follow-up study of 48 patients. Hum Pathol. 1990;21(5):518–523. [DOI] [PubMed] [Google Scholar]
- 3.Walker GV, Smith GL, Perkins GH, et al. Population-based analysis of occult primary breast cancer with axillary lymph node metastasis. Cancer. 2010;116(17):4000–4006. doi: 10.1002/cncr.25197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He M, Tang L-C, Yu K-D, et al. Treatment outcomes and unfavorable prognostic factors in patients with occult breast cancer. Eur J Surg Oncol EJSO. 2012;38(11):1022–1028. doi: 10.1016/j.ejso.2012.08.022 [DOI] [PubMed] [Google Scholar]
- 5.Sohn G, Son BH, Lee SJ, et al. Treatment and survival of patients with occult breast cancer with axillary lymph node metastasis: A nationwide retrospective study: Survival of Occult Breast Cancer. J Surg Oncol. 2014;110(3):270–274. doi: 10.1002/jso.23644 [DOI] [PubMed] [Google Scholar]
- 6.Rueth NM, Black DM, Limmer AR, et al. Breast Conservation in the Setting of Contemporary Multimodality Treatment Provides Excellent Outcomes for Patients with Occult Primary Breast Cancer. Ann Surg Oncol. 2015;22(1):90–95. doi: 10.1245/s10434-014-3991-0 [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network Guidelines, Breast Cancer 2019. https://www.nccn.org/professionals/physician_gls/default.aspx. [Google Scholar]
- 8.Khandelwal AK, Garguilo GA. Therapeutic options for occult breast cancer: a survey of the American Society of Breast Surgeons and review of the literature. Am J Surg. 2005;190(4):609–613. doi: 10.1016/j.amjsurg.2005.06.026 [DOI] [PubMed] [Google Scholar]
- 9.Hessler LK, Molitoris JK, Rosenblatt PY, et al. Factors Influencing Management and Outcome in Patients with Occult Breast Cancer with Axillary Lymph Node Involvement: Analysis of the National Cancer Database. Ann Surg Oncol. 2017;24(10):2907–2914. doi: 10.1245/s10434-017-5928-x [DOI] [PubMed] [Google Scholar]
- 10.Kim BH, Kwon J, Kim K. Evaluation of the Benefit of Radiotherapy in Patients with Occult Breast Cancer: A Population-Based Analysis of the SEER Database. Cancer Res Treat. 2018;50(2):551–561. doi: 10.4143/crt.2017.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu S-G, Zhang W-W, Sun J-Y, et al. Comparable Survival between Additional Radiotherapy and Local Surgery in Occult Breast Cancer after Axillary Lymph Node Dissection: A Population-based Analysis. J Cancer. 2017;8(18):3849–3855. doi: 10.7150/jca.21217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattei A. Estimating and using propensity score in presence of missing background data: an application to assess the impact of childbearing on wellbeing. Stat Methods Appl. 2009;18(2) :257–273. doi: 10.1007/s10260-007-0086-0 [DOI] [Google Scholar]
- 13.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments: Propensity scores and survival analysis. Stat Med. 2014;33(7):1242–1258. doi: 10.1002/sim.5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sekhon JS. Multivariate and Propensity Score Matching Software with Automated Balance Optimization: The Matching Package for R. J Stat Softw. 2011;42(7). doi: 10.18637/jss.v042.i07 [DOI] [Google Scholar]
- 16.Malik MK, Tartter PI, Belfer R. Undertreated breast cancer in the elderly. J Cancer Epidemiol. 2013;2013:893104. doi: 10.1155/2013/893104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasan Y, Waller J, Yao K, et al. Utilization trend and regimens of hypofractionated whole breast radiation therapy in the United States. Breast Cancer Res Treat. 2017;162(2):317–328. doi: 10.1007/s10549-017-4120-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet Lond Engl. 2014;383(9935):2127–2135. doi: 10.1016/S0140-6736(14)60488-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masinghe SP, Faluyi OO, Kerr GR, Kunkler IH. Breast Radiotherapy for Occult Breast Cancer with Axillary Nodal Metastases — Does it Reduce the Local Recurrence Rate and Increase Overall Survival? Clin Oncol. 2011;23(2):95–100. doi: 10.1016/j.clon.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 20.Vlastos G, Jean ME, Mirza AN, et al. Feasibility of breast preservation in the treatment of occult primary carcinoma presenting with axillary metastases. Ann Surg Oncol. 2001;8(5):425–431. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Zhang Y, Wang X, et al. Treatment outcomes of occult breast carcinoma and prognostic analyses. Chin Med J (Engl). 2013;126(16):3026–3029. [PubMed] [Google Scholar]
- 22.Kaviani A, Sodagari N, Sheikhbahaei S, et al. From radical mastectomy to breast-conserving therapy and oncoplastic breast surgery: a narrative review comparing oncological result, cosmetic outcome, quality of life, and health economy. ISRN Oncol. 2013;2013:742462. doi: 10.1155/2013/742462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Tamer MB, Ward BM, Schifftner T, Neumayer L, Khuri S, Henderson W. Morbidity and Mortality Following Breast Cancer Surgery in Women: National Benchmarks for Standards of Care. Ann Surg. 2007;245(5):665–671. doi: 10.1097/01.sla.0000245833.48399.9a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanchard DK, Farley DR. Retrospective study of women presenting with axillary metastases from occult breast carcinoma. World J Surg. 2004;28(6):535–539. [DOI] [PubMed] [Google Scholar]
- 25.Andrade C Propensity Score Matching in Nonrandomized Studies: A Concept Simply Explained Using Antidepressant Treatment During Pregnancy as an Example: (Clincial and Practical Psychopharmacology). J Clin Psychiatry. 2017;78(02):e162–e165. doi: 10.4088/JCP.17f11446 [DOI] [PubMed] [Google Scholar]