Abstract
Purpose
Both body mass index (BMI) and breast density impact breast cancer risk in the general population. Whether obesity and density represent additive risk factors in women with lobular carcinoma in situ (LCIS) is unknown.
Methods
Patients diagnosed with LCIS from 1988-2017 were identified from a prospectively maintained database. BMI was categorized by World Health Organization classification. Density was captured as the mammographic BIRADS value. Other covariates included age at LCIS diagnosis, menopausal status, family history, chemoprevention, and prophylactic mastectomy. Cancer-free probability was estimated using the Kaplan-Meier method, and Cox regression models were used for univariable and multivariable analyses.
Results
1222 women with LCIS were identified. At a median follow-up of 7 years, 179 women developed breast cancer (121 invasive, 58 ductal carcinoma in situ); 5- and 10-year cumulative incidences of breast cancer were 10% and 17%, respectively. In multivariable analysis, increased breast density (BIRADS C/D vs A/B) was significantly associated with increased hazard of breast cancer (hazard ratio [HR] 2.42, 95% confidence interval [CI] 1.52-3.88) whereas BMI was not. On multivariable analysis, chemoprevention use was associated with a significantly decreased hazard of breast cancer (HR 0.49, 95% CI 0.29-0.84). Exploratory analyses did not demonstrate significant interaction between BMI and menopausal status, BMI and breast density, BMI and chemoprevention use, or breast density and chemoprevention.
Conclusions
Breast cancer risk among women with LCIS is impacted by breast density. These results aid in personalizing risk assessment among women with LCIS and highlight the importance of chemoprevention counseling for risk reduction.
Keywords: body mass index, lobular carcinoma in situ, breast cancer, breast density
INTRODUCTION
The presence of lobular carcinoma in situ (LCIS) portends a significantly increased risk of developing either ductal carcinoma in situ (DCIS) or invasive breast cancer (BC). Studies with long-term follow-up report a 7-to-10-fold increased risk of developing cancer in either breast in women with LCIS,1–4 amounting to an approximately 2% annual incidence of BC in this population.5,6 Although LCIS is a relatively rare finding (found in approximately 0.5-5.3% of benign breast biopsies2,7–9), its incidence has been increasing in recent years, from approximately 0.9/100,000 women in the late 1970s to 2.75/100,000 in the early 2000s.10,11 As LCIS is being diagnosed more frequently, understanding the relationship between LCIS and other risk factors for developing BC is becoming increasingly important.
In a previous analysis of our institution’s LCIS cohort,6 we found that LCIS volume was associated with significantly increased cancer risk, but that family history, menopausal status, bilateral synchronous LCIS, concurrent atypical ductal hyperplasia (ADH) or atypical lobular hyperplasia (ALH), and number of breast biopsies after initial LCIS diagnosis were not. Breast density, a well-established BC risk factor in general populations,12–14 also had no significant association with increased risk in this analysis. Other studies exploring the possible additive risk of breast density in women with high-risk lesions have had mixed results, with 2 large studies demonstrating a non-significant trend toward increased risk associated with increased breast density15,16 among women with atypical hyperplasia (AH), and 2 others with null results.17,18 The relationship is thus left unclear in populations with high-risk breast lesions.
Despite the known inverse association between breast density and body mass index (BMI),19–22 this data point was previously unavailable and not controlled for in our prior analysis. In the general population, the relationship between increased BMI and BC risk is complex, appearing to differ by menopausal status, with obesity representing an established risk factor for postmenopausal BC.23,24 Moreover, the interplay between increased BMI and cancer risk—like that of increased breast density—remains poorly characterized in populations with high-risk breast lesions. We thus sought to further examine the relationship between BMI and breast density, and the development of DCIS and invasive BC, among women with LCIS.
METHODS
Data Source
Upon MSK institutional review board approval, women diagnosed with LCIS from 1988-2017 were identified from a prospectively maintained database of women with a diagnosis of LCIS participating in high-risk surveillance at MSK. Women were excluded if they had a known BRCA mutation, a prior or concurrent cancer (defined as cancer diagnosed within 6 months of LCIS diagnosis), pleomorphic LCIS, missing BMI or breast density values, or if they did not return for at least 1 follow-up appointment after their initial visit.
Variables
BMI was measured as continuous and was then categorized according to World Health Organization classification (normal: <25; overweight: 25 to <30; obese: 30 to <35; very obese: ≥35). Analyses of BMI were restricted to patients with a BMI measurement within 1 year (before or after) of their LCIS diagnosis date.
Density was captured by mammographic Breast Imaging Reporting and Data System (BIRADS) value at imaging performed nearest to the LCIS diagnosis (BIRADS A: fatty; BIRADS B: scattered fibroglandular density; BIRADS C: heterogeneous/moderately dense; BIRADS D: extremely dense). All mammograms were read by dedicated MSK breast imagers, and the BIRADS density classification was assigned from the MSK review. Analyses were restricted to patients with an available BIRADS measurement.
Screening by MRI in this population was carried out at the discretion of the physician and patient at our institution. For the purposes of this analysis, a woman was coded as having undergone MRI screening if she underwent 1 or more MRIs for screening purposes following LCIS diagnosis. Our experience with MRI screening in the LCIS population has previously been published.25
Risk-reduction strategies used by our population included chemoprevention use and prophylactic mastectomy. The use of chemoprevention with a selective estrogen receptor modulator (SERM) or an aromatase inhibitor (AI) for LCIS was approved in 1998. Chemoprevention was treated as a time-dependent covariate in our analysis. Women undergoing bilateral prophylactic mastectomy were included in this analysis but censored at the date of prophylactic surgery.
Our primary outcome of interest was the development of DCIS or invasive BC. All women were censored at date of bilateral prophylactic mastectomy, cancer diagnosis, or last follow-up.
Statistical Analysis
To examine factors associated with breast density at baseline, the Wilcoxon rank sum test and Fisher’s exact test were used for comparisons across BIRADS categories for continuous and categorical variables, respectively. Cancer-free probability was estimated using the Kaplan-Meier method. Cancer-free survival (CFS) was defined as time from LCIS diagnosis to development of first BC (either DCIS or invasive cancer). Patients who did not develop cancer were censored at their date of prophylactic mastectomy or last follow-up. Cox regression models were used for univariable and multivariable analyses, with predictors of interest determined a priori. A p-value <0.05 was considered statistically significant. Interaction testing was carried out using a likelihood ratio test, considering any p-value < 0.1 significant to test for interactions between continuous BMI and both 4-category breast density and 2-category breast density, as well as BMI and chemoprevention use, and breast density and chemoprevention use. All statistical analyses were conducted in R software version 3.5.0 (R Core Development Team, Vienna, Austria).
RESULTS
In total, 1222 women met inclusion criteria. Median BMI was 24 (interquartile range [IQR] 21.6-28.0), and 56% of the population fell within the normal weight category. Nearly 80% of the cohort had a BIRADS C or D breast density. Patients with dense breasts were more frequently <50 years of age, more frequently premenopausal, had lower BMIs, and more frequently underwent MRI screening (Table 1). Sixty-three women with dense breasts (BIRADS C/D) had a prophylactic mastectomy during follow-up as compared with only 5 women with BIRADS A/B breast density. Chemoprevention was used in 185 patients, 50 with BIRADS A/B breast density and 135 with BIRADS C/D breast density. The majority (88%) of the cohort is non-Hispanic White, with < 5% of the cohort represented by each of the following self-reported ethnic/racial groups: Black, Hispanic, Asian, and Other.
TABLE 1.
Baseline Patient Characteristics by Breast Density Category
| Patient Characteristics | BIRADS A/B n = 260 (21%) | BIRADS C/D n = 962 (79%) | p-value |
|---|---|---|---|
| Age at LCIS diagnosis, years | |||
| Median | 55 | 49 | |
| Interquartile range | 50-61 | 44-54 | |
| < 50 | 65 (25%) | 524 (54%) | < 0.001 |
| ≥ 50 | 195 (75%) | 438 (46%) | |
| Menopausal status | < 0.001 | ||
| Pre/perimenopausal | 76 (30%) | 627 (67%) | |
| Postmenopausal | 177 (70%) | 310 (33%) | |
| Unknown | 7 | 25 | |
| ≥ 1 FDR with breast cancer | 0.6 | ||
| No | 181 (71%) | 653 (69%) | |
| Yes | 74 (29%) | 290 (31%) | |
| Unknown | 5 | 19 | |
| ≥ 2 SDRs with breast cancer | 0.8 | ||
| No | 226 (89%) | 843 (89%) | |
| Yes | 29 (11%) | 100 (11%) | |
| Unknown | 5 | 19 | |
| Any (≥ 1 FDR with breast cancer or ≥ 2 SDRs with breast cancer) | > 0.9 | ||
| No | 161 (63%) | 592 (63%) | |
| Yes | 94 (37%) | 351 (37%) | |
| Unknown | 5 | 19 | |
| BMI in kg/m2 | |||
| Median | 28.4 | 23.4 | |
| Interquartile Range | 24.7-32.1 | 21.2-26.5 | |
| Normal weight (BMI < 25) | 71 (27%) | 614 (64%) | < 0.001 |
| Overweight (BMI ≥ 25, < 30) | 86 (33%) | 247 (26%) | |
| Obese (BMI ≥ 30, < 35) | 61 (23%) | 69 (7%) | |
| Very obese (BMI ≥ 35) | 42 (16%) | 32 (3%) | |
| MRI screening | 0.007 | ||
| No | 147 (57%) | 451 (47%) | |
| Yes | 113 (43%) | 511 (53%) |
LCIS lobular carcinoma in situ, BIRADS Breast Imaging Reporting and Data System, FDR first-degree relative, SDR second-degree relative, BMI body mass index
At a median follow-up of 7 years (IQR 3.1-10.4), 179 women developed BC (122 invasive, 58 DCIS). Fig. 1a shows the Kaplan-Meier curve for overall cancer-free probability; 5- and 10-year cancer-free survival (CFS) were 0.90 (95% confidence interval [CI] 0.88-0.91) and 0.83 (95% CI 0.80-0.86), respectively. There was no significant difference in cancer-free probability by BMI category (p=0.9)(Fig. 1b). Cancer-free probability, however, was significantly lower in patients with BIRADS C/D versus A/B breast density (p<0.001), with a 10-year CFS of 0.81 (95% CI 0.78-0.84) in patients with BIRADS C/D breast density as compared to a 10-year CFS of 0.89 (95% CI 0.85-0.94) in patients with BIRADS A/B breast density (Fig. 1c).
Fig. 1.
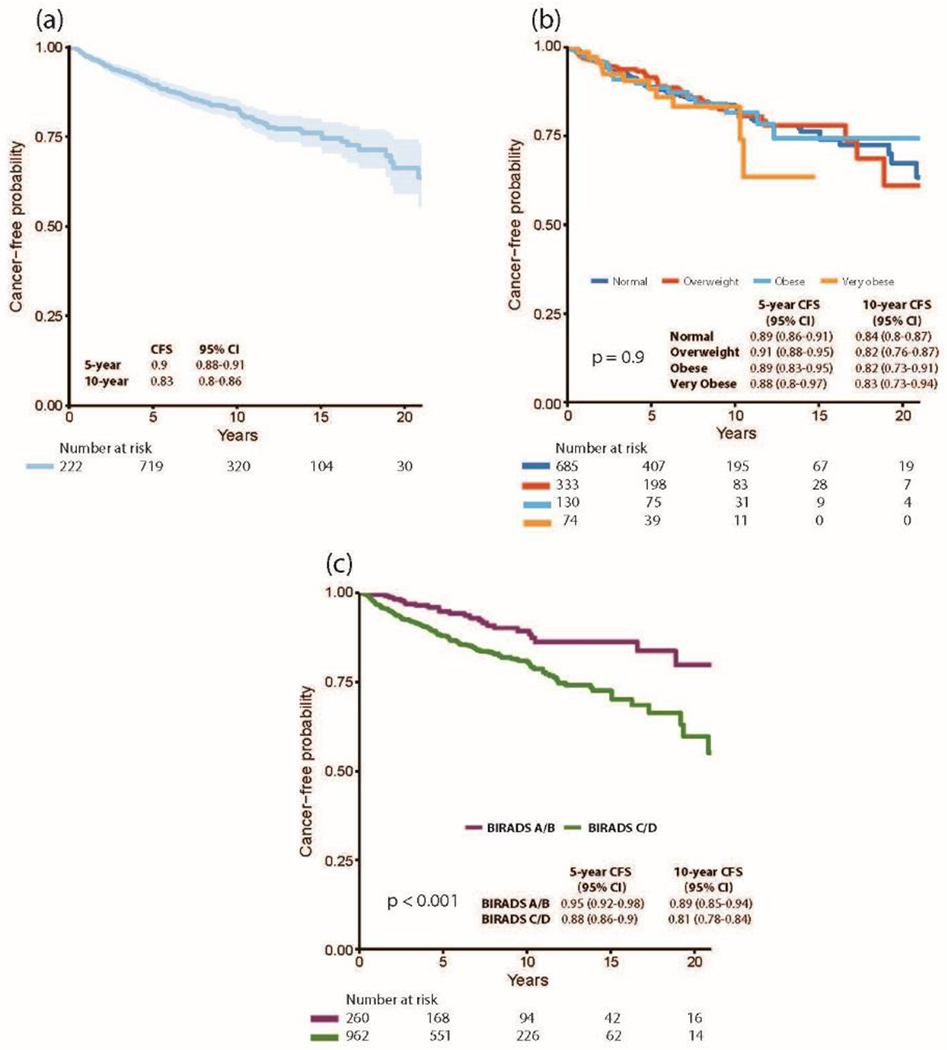
(a) Kaplan-Meier curve for time to cancer events (DCIS or invasive cancer) (b) Kaplan-Meier curve for time to cancer events (DCIS or invasive cancer) by BMI category (Normal weight [BMI < 25]; Overweight [BMI ≥ 25, < 30]; Obese [BMI ≥ 30, < 35]; Very obese [BMI ≥ 35] (c) Kaplan-Meier curve for time to cancer events (DCIS or invasive cancer) by breast density (BIRADS A/B vs C/D).
CFS cancer-free survival, CI confidence interval, DCIS ductal carcinoma in situ, BMI body mass index, BIRADS Breast Imaging Reporting and Data System
In univariable analysis, increased breast density (BIRADS C/D vs A/B) was significantly associated with increased hazard of BC (hazard ratio [HR] 2.13, 95% CI 1.39-3.26). The use of chemoprevention was associated with a significantly lower hazard for developing cancer (HR 0.49, 95% CI 0.29-0.83). BMI category, age, menopausal status, family history, and MRI screening were not significantly associated with BC on univariable analysis (Table 2).
TABLE 2.
Univariable Analysis of Factors Associated With the Development of Breast Cancer Among Women with LCIS
| Characteristic | n | HR | 95% CI | p-value |
|---|---|---|---|---|
| Age at LCIS diagnosis | 1222 | 0.6 | ||
| < 50 | Ref. | |||
| ≥ 50 | 1.08 | 0.81-1.45 | ||
| Menopausal status | 1190 | 0.9 | ||
| Pre/perimenopausal | Ref. | |||
| Postmenopausal | 0.98 | 0.72-1.33 | ||
| Family History | ||||
| ≥ 1 FDR with breast cancer | 1198 | 1.33 | 0.97-1.81 | 0.077 |
| ≥ 2 SDRs with breast cancer | 1198 | 0.83 | 0.50-1.39 | 0.5 |
| Any (≥ 1 FDR with breast cancer or ≥ 2 SDRs with breast cancer) | 1198 | 1.24 | 0.92-1.68 | 0.2 |
| BIRADS | 1222 | < 0.001 | ||
| A/B | Ref. | |||
| C/D | 2.13 | 1.39-3.26 | ||
| BMI categories | 1222 | > 0.9 | ||
| Normal weight (BMI < 25) | Ref. | |||
| Overweight (BMI ≥ 25, < 30) | 1.00 | 0.71-1.41 | ||
| Obese (BMI ≥ 30, < 35) | 1.00 | 0.61-1.66 | ||
| Very obese (BMI ≥ 35) | 1.25 | 0.67-2.34 | ||
| MRI screening | 1222 | 0.88 | 0.66-1.19 | 0.4 |
| Chemoprevention | 1222 | 0.49 | 0.29-0.83 | 0.007 |
HR hazard ratio, CI confidence interval, LCIS lobular carcinoma in situ, BIRADS Breast Imaging Reporting and Data System, FDR first-degree relative, SDR second-degree relative, BMI body mass index
Findings on multivariable analysis were similar to those from univariable analysis. Breast density (BIRADS C/D vs A/B) was associated with significantly increased hazard of BC (HR 2.42, 95% CI 1.52-3.88), and use of chemoprevention was associated with significantly decreased hazard of BC (HR 0.49, 95% CI 0.29-0.84). BMI category, menopausal status, family history, and MRI screening were not significantly associated with BC on multivariable analysis (Table 3). Interaction testing did not demonstrate any significant interactions between BMI and menopausal status, BMI and breast density, BMI and chemoprevention use, or breast density and chemoprevention (all p>0.1).
TABLE 3.
Multivariable Analysis of Factors Associated with the Development of Breast Cancer Among Women with LCIS*
| Characteristic | HR | 95% CI | p-value |
|---|---|---|---|
|
Menopausal status (postmenopausal vs pre/perimenopausal) |
1.15 | 0.83-1.60 | 0.4 |
|
Family History (≥ 1 FDR with breast cancer or 2 SDRs with breast cancer) |
1.29 | 0.95-1.77 | 0.11 |
| Breast density (BIRADS C/D vs A/B) | 2.42 | 1.52-3.88 | < 0.001 |
| BMI categories | 0.5 | ||
| Normal weight (BMI < 25) | Ref. | ||
| Overweight (BMI ≥ 25, < 30) | 1.10 | 0.77-1.58 | |
| Obese (BMI ≥ 30, < 35) | 1.38 | 0.82-2.32 | |
| Very obese (BMI ≥ 35) | 1.48 | 0.75-2.91 | |
| MRI screening (yes vs no) | 0.87 | 0.64-1.20 | 0.4 |
| Chemoprevention (yes vs no) | 0.49 | 0.29-0.84 | 0.01 |
Analysis includes 1170 women with complete data
HR hazard ratio, CI confidence interval, LCIS lobular carcinoma in situ, BIRADS Breast Imaging Reporting and Data System, FDR first-degree relative, SDR second-degree relative, BMI body mass index
DISCUSSION
Given increasing emphasis on personalized risk prediction, we sought to evaluate factors that may impact overall BC risk among a large population of women with LCIS with longitudinal follow-up. Here we report that increased breast density is associated with BC risk among this high-risk population, and that chemoprevention use remains a durable risk-reducing strategy. We see that family history of BC, menopausal status, as well as BMI, are variables not significantly associated with increased risk in this cohort.
Breast density is well documented as an independent risk factor in the development of BC in the general population, as women with dense breasts have been shown to carry a 2-to-6-fold increased risk of developing BC compared to those with less-dense breasts.14,26 This phenomenon has not, however, been consistently shown to carry over to women with high-risk lesions. In populations with AH, null findings were reported in 2 series.17,18 Vierkant et al. examined a cohort of 6271 women with non-proliferative breast disease, proliferative disease without atypia, and AH in a cohort from the Mayo clinic. There was a strong association between increased breast density and increased cancer risk amongst women with non-proliferative disease, but none in the subgroup of 470 patients with AH. A case-control study by Byrne et al.,18 using the Breast Cancer Detection Demonstration Project, echoed these findings, showing a strong dose-related association with density in women with non-proliferative disease, but no apparent association between increased breast density and cancer risk among those with AH.
Conversely, other analyses have reported an association between breast density and cancer risk among those with high-risk breast lesions. Reimers et al.16 used the Women at Risk Registry to examine this association amongst 815 women with biopsy-proven AH, and found that those classified as BIRADS 3 or 4 (equivalent to BIRADS C/D27) had a relative risk of 4.4 for developing BC when compared to those classified as BIRADS 1 or 2 (equivalent to BIRADS A/B). Tice et al.15 compared BC risk among differing combinations of benign breast lesions and BIRADS density, and reported increasing relative risk among those with AH and increasing breast density. They found that when compared to women with non-proliferative disease and BIRADS 2 (equivalent to BIRADS B) density, women with AH/BIRADS 4 (equivalent to BIRADS D) density had a relative risk of 5.3, those with AH/BIRADS 3 (equivalent to BIRADS C) had a relative risk of 3.4, and those with AH/BIRADS 2 (equivalent to BIRADS B) had a relative risk of 2.6. The results of our updated analysis are consistent with these studies, showing that increased breast density was in fact significantly associated with increased BC risk among a group of women with LCIS. In our previous analysis using a slightly smaller LCIS cohort, we did not find any significant association between breast density and cancer risk; however, our findings from this analysis, with more patients and longer follow-up, suggest that our previous analysis may have been underpowered to detect this particular association.
Although often cited as a well-known BC risk factor, increased BMI has a more complicated relationship with cancer risk than breast density. Obesity’s effect on BC development in the general population appears to differ by menopausal status, with obese postmenopausal patients carrying modestly increased relative risks of 1.6 in large cohorts,23 while obesity in premenopausal patients may in fact be protective.24 How these associations specifically play out in populations with high-risk lesions has not been well defined. Our finding that increased BMI is not associated with increased cancer risk in a high-risk population of women with LCIS differs from the findings among a more heterogeneous population of high-risk women in the Women At Risk Registry. In their population of high-risk women (defined as having ≥1 first-degree relatives with premenopausal BC, ≥2 first-degree relatives with postmenopausal BC, or a biopsy-proven history of LCIS, ADH, or ALH), Chun et al.28 found that women with a BMI >30 had a 2-fold increased risk of BC compared with women of normal weight. However, only 307 (19.8%) women in their population of 1553 had LCIS, and thus the incremental effect of obesity among women with LCIS cannot be determined from their study.
Analysis of high-risk women enrolled in the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 and STAR (Study of Tamoxifen and Raloxifene) P-2 BC prevention trials29 further illustrates the complicated relationship between BMI and cancer risk in high-risk populations. Among 12,243 participants in the P-1 trial, which examined the effect of tamoxifen versus placebo on the development of BC in high-risk premenopausal and postmenopausal women, and 19,488 participants from the STAR P-2 trial, which compared raloxifene versus tamoxifen in high-risk postmenopausal women, Cecchini et al. did not find a significantly increased risk of BC among overweight and obese postmenopausal trial participants, but did find an increased risk of BC in overweight and obese premenopausal participants. Compared to those with a BMI <25, premenopausal women with a BMI of 25-29 had an adjusted hazard ratio of 1.6 (95% CI 1.05-2.42), and those with a BMI >30 had a hazard ratio of 1.7 (95% CI 1.10-2.63). In our own results, the lack of interaction between BMI and menopausal status on exploratory analysis indicates that the association between BMI and cancer risk did not differ by menopausal status in our cohort.
The protective nature of a SERM or AI against the development of BC in high-risk populations has been well established by randomized controlled trial data and large observational studies.5,30,31 We again demonstrate that chemoprevention use is protective against BC development irrespective of breast density. Chemoprevention was used in 185 patients (15%), which is similar to reported rates of observed uptake of BC chemoprevention in a recent meta-analysis.32 In our prior analysis, the reported use of chemoprevention differed by year, with notably lower rates of chemoprevention use prior to 1998 than subsequent years, but with a protective effect demonstrated throughout the study period.6
While starting chemoprevention is a preference-sensitive decision, in which a good decision is one that is both informed and consistent with one’s values, these uptake rates seem objectively low.32 Completion of the 5-year recommended course, once begun, is seen in approximately 55-60% of women.33–35 To address both uptake and adherence, a better understanding of barriers and facilitators to chemoprevention use is necessary. A meta-analysis by Ropka et al. found that actual risk, as calculated by the Gail model, did not significantly correlate with chemoprevention uptake; rather, a patient’s perceived risk appeared to be a stronger motivator. In addition, a recent study from our own institution’s high-risk population demonstrated that one of the most common reasons for chemoprevention refusal was fear of side effects.35
Ongoing efforts to identify more appealing chemoprevention options for high-risk women with improved side-effect profiles include low-dose (5mg) daily tamoxifen, as reported in the TAM-01 study,36 and topical chemoprevention,37 an area under current investigation. Results from these trials will hopefully shift the risk/benefit ratio of chemoprevention use in a greater proportion of the LCIS population. Given our current findings, when counseling women on their BC risk, history of LCIS as well as mammographic breast density should be included in the discussion and decision making regarding the appropriateness of chemoprevention use.
Limitations
To our knowledge, this study is reported from one of largest longitudinal experiences with LCIS. Its single-institutional nature, however, may limit its applicability to other populations, and despite the relative size of our database, we acknowledge that different patterns of association may reveal themselves over time. In addition, this is a very homogeneous population in respect to race. Given the differences in breast density by ethnicity, this may impact the generalizability of these findings. Furthermore, nearly all women included in this analysis underwent surgical excision following diagnosis of LCIS. Recently, however, following publication of a prospective study showing very low rates of upgrade for concordant lobular neoplasia,38 our practice in recent years has changed, and patients with asymptomatic concordant classic-type LCIS identified by core needle biopsy are now managed with core biopsy alone. This is the topic of ongoing research.
Conclusions
BMI was not significantly associated with the development of DCIS or invasive BC in women with LCIS, but increased breast density does appear to be an additive risk factor in this already high-risk population. Use of chemoprevention is a durable protective strategy against the development of cancer in this cohort. This understanding of the interplay of breast density and LCIS can aid in personalizing risk assessment and in counseling women on the appropriateness of chemoprevention.
Synopsis:
Here we examine the relationship between BMI and breast density, and the development of DCIS and invasive breast cancer among women with LCIS. We find that breast cancer risk among women with LCIS is affected by breast density.
ACKNOWLEDGEMENTS
Disclosures: The preparation of this study was funded in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center. This study was presented in podium format at the Society of Surgical Oncology 72nd Annual Cancer Symposium, March 27-30, 2019, San Diego, CA. Dr. Tari A. King has received honoraria as a speaker for Genomic Health. All other authors have no conflict of interest disclosures to report.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Rosen PP, Kosloff C, Lieberman PH, Adair F, Braun DW Jr. Lobular carcinoma in situ of the breast. Detailed analysis of 99 patients with average follow-up of 24 years. Am J Surg Pathol. 1978;2(3):225–251. [DOI] [PubMed] [Google Scholar]
- 2.Page DL, Kidd TE Jr., Dupont WD, Simpson JF, Rogers LW. Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol. 1991;22(12):1232–1239. [DOI] [PubMed] [Google Scholar]
- 3.Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312(3):146–151. [DOI] [PubMed] [Google Scholar]
- 4.Page DL, Dupont WD, Rogers LW, Rados MS. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer. 1985;55(11):2698–2708. [DOI] [PubMed] [Google Scholar]
- 5.Coopey SB, Mazzola E, Buckley JM, et al. The role of chemoprevention in modifying the risk of breast cancer in women with atypical breast lesions. Breast Cancer Res Treat. 2012;136(3):627–633. [DOI] [PubMed] [Google Scholar]
- 6.King TA, Pilewskie M, Muhsen S, et al. Lobular Carcinoma in Situ: A 29-Year Longitudinal Experience Evaluating Clinicopathologic Features and Breast Cancer Risk. J Clin Oncol. 2015;33(33):3945–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hussain M, Cunnick GH. Management of lobular carcinoma in-situ and atypical lobular hyperplasia of the breast--a review. Eur J Surg Oncol. 2011;37(4):279–289. [DOI] [PubMed] [Google Scholar]
- 8.Frykberg ER. Lobular Carcinoma In Situ of the Breast. Breast J 1999;5(5):296–303. [DOI] [PubMed] [Google Scholar]
- 9.Cheng P, Huang Q, Shou J, Hu G, Han M, Huang J. Treatment and survival outcomes of lobular carcinoma in situ of the breast: a SEER population based study. Oncotarget. 2017;8(61):103047–103054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li CI, Anderson BO, Daling JR, Moe RE. Changing incidence of lobular carcinoma in situ of the breast. Breast Cancer Res Treat. 2002;75(3):259–268. [DOI] [PubMed] [Google Scholar]
- 11.Portschy PR, Marmor S, Nzara R, Virnig BA, Tuttle TM. Trends in incidence and management of lobular carcinoma in situ: a population-based analysis. Ann Surg Oncol. 2013;20(10):3240–3246. [DOI] [PubMed] [Google Scholar]
- 12.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. [DOI] [PubMed] [Google Scholar]
- 13.Vachon CM, van Gils CH, Sellers TA, et al. Mammographic density, breast cancer risk and risk prediction. Breast Cancer Res. 2007;9(6):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159–1169. [DOI] [PubMed] [Google Scholar]
- 15.Tice JA, O’Meara ES, Weaver DL, Vachon C, Ballard-Barbash R, Kerlikowske K. Benign breast disease, mammographic breast density, and the risk of breast cancer. J Natl Cancer Inst. 2013;105(14):1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reimers LL, Crew KD, Terry MB. Atypical hyperplasia of the breast. N Engl J Med. 2015;372(13):1270–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vierkant RA, Degnim AC, Radisky DC, et al. Mammographic breast density and risk of breast cancer in women with atypical hyperplasia: an observational cohort study from the Mayo Clinic Benign Breast Disease (BBD) cohort. BMC Cancer. 2017;17(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrne C, Schairer C, Brinton LA, et al. Effects of mammographic density and benign breast disease on breast cancer risk (United States). Cancer Causes Control. 2001;12(2):103–110. [DOI] [PubMed] [Google Scholar]
- 19.Hudson S, Vik Hjerkind K, Vinnicombe S, et al. Adjusting for BMI in analyses of volumetric mammographic density and breast cancer risk. Breast Cancer Res. 2018;20(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soguel L, Durocher F, Tchernof A, Diorio C. Adiposity, breast density, and breast cancer risk: epidemiological and biological considerations. Eur J Cancer Prev. 2017;26(6):511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baglietto L, Krishnan K, Stone J, et al. Associations of mammographic dense and nondense areas and body mass index with risk of breast cancer. Am J Epidemiol. 2014;179(4):475–483. [DOI] [PubMed] [Google Scholar]
- 22.Hart V, Reeves KW, Sturgeon SR, et al. The effect of change in body mass index on volumetric measures of mammographic density. Cancer Epidemiol Biomarkers Prev. 2015;24(11):1724–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Z, Hankinson SE, Colditz GA, et al. Dual effects of weight and weight gain on breast cancer risk. Jama. 1997;278(17):1407–1411. [PubMed] [Google Scholar]
- 24.Schoemaker MJ, Nichols HB, Wright LB, et al. Association of Body Mass Index and Age With Subsequent Breast Cancer Risk in Premenopausal Women. JAMA Oncol. 2018;4(11):e181771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King TA, Muhsen S, Patil S, et al. Is there a role for routine screening MRI in women with LCIS? Breast Cancer Res Treat. 2013;142(2):445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vachon CM, Pankratz VS, Scott CG, et al. The contributions of breast density and common genetic variation to breast cancer risk. J Natl Cancer Inst. 2015;107(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American College of Radiology. Breast Imaging and Reporting and Data System (ACR BI-RADS® Atlas) teR, VA: American College of Radiology, 2013. [Google Scholar]
- 28.Chun J, El-Tamer M, Joseph KA, Ditkoff BA, Schnabel F. Predictors of breast cancer development in a high-risk population. Am J Surg. 2006;192(4):474–477. [DOI] [PubMed] [Google Scholar]
- 29.Cecchini RS, Costantino JP, Cauley JA, et al. Body mass index and the risk for developing invasive breast cancer among high-risk women in NSABP P-1 and STAR breast cancer prevention trials. Cancer Prev Res (Phila). 2012;5(4):583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–1388. [DOI] [PubMed] [Google Scholar]
- 31.Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila). 2010;3(6):696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28(18):3090–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roetzheim RG, Lee JH, Fulp W, et al. Acceptance and adherence to chemoprevention among women at increased risk of breast cancer. Breast. 2015;24(1):51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roche CA, Tang R, Coopey SB, Hughes KS. Chemoprevention acceptance and adherence in women with high-risk breast lesions. Breast J. 2019;25(2):190–195. [DOI] [PubMed] [Google Scholar]
- 35.Flanagan MR, Zabor EC, Stempel M, Mangino DA, Morrow M, Pilewskie ML. Chemoprevention Uptake for Breast Cancer Risk Reduction Varies by Risk Factor. Ann Surg Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeCensi APM, Guerrieri Gonzaga A, Avino F, Cortesi L, Donadio M, Pacquola M, Falcini F, Gulisano M, Digennaro M, Tienghi A, Cagossi K, Pinotti G, Varicchio C, Caviglia S, Boni L, Bonanni BEO A randomized placebo controlled phase III trial of low dose tamoxifen for the prevention of recurrence in women with operated hormone sensitive breast ductal or lobular carcinoma in situ. San Antonio Breast Cancer Symposium. 2018;abstract GS3-01. [Google Scholar]
- 37.Cheraghi Z, Poorolajal J, Hashem T, Esmailnasab N, Doosti Irani A. Effect of body mass index on breast cancer during premenopausal and postmenopausal periods: a meta-analysis. PLoS One. 2012;7(12):e51446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakhlis F, Gilmore L, Gelman R, et al. Incidence of Adjacent Synchronous Invasive Carcinoma and/or Ductal Carcinoma In-situ in Patients with Lobular Neoplasia on Core Biopsy: Results from a Prospective Multi-Institutional Registry (TBCRC 020). Ann Surg Oncol. 2016;23(3):722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]


