Abstract
Objectives/Hypothesis:
Human papillomavirus (HPV) is a DNA virus that causes cancer in multiple sites. Although sexual activity is the primary means of oropharyngeal HPV acquisition, studies suggest HPV transmission through occupational exposure from medical instruments and surgical fumes. We assess if aerosolization of HPV16 DNA via electrocautery places otolaryngologists at risk for exposure.
Study Design:
Animal and human laboratory model.
Methods:
Plasmid (pLXSN16E6E7) expressing HPV p16 E6/E7 genes was transformed into DH5α Escherichia coli cells using the heat shock method. Miniprep and maxiprep purification of transformed DNA with subsequent restriction enzyme double digestion confirmed presence of E6E7 fragment. We injected 2 μg plasmid DNA in 20 μL TE (Tris and ethylenediaminetetraacetic acid) buffer intradermally into freshly severed mouse tail then cauterized for 5 to 10 seconds. Generated fumes were collected through a suction tube fitted with Whatman filter paper. Filter paper was placed in 100 μL TE buffer. Additionally, six patients undergoing transoral robotic surgery for resection of oropharyngeal cancer were identified, three with p16-negative tumors and three with p16-positive tumors. Intraoperatively, Whatman filter paper was exposed to electrocautery fumes, then placed in 100 uL TE buffer. Additional samples were collected from the suction tubing and filter, the surgical mask of the surgeon at head of the bed, and the robot arm.
Results:
Samples were analyzed via polymerase chain reaction with an assay sensitivity of 1.5 ng E6E7 DNA. None of the patient or mouse tail samples yielded detectable HPV16 DNA in the electrocautery fumes. We did not detect HPV16 DNA on the surgical masks, suction apparatus, or robot arm intraoperatively.
Conclusions:
There is likely minimal risk of occupational exposure to HPV16 via electrocautery fumes.
Keywords: Human papillomavirus, human papillomavirus occupational exposure, human papillomavirus risk factor, human papillomavirus transmission
INTRODUCTION
The human papillomavirus (HPV) is a DNA virus from the papillomavirus family and one of the most common sexually transmitted infections in the world.1 HPV is known to cause cancer in multiple sites including the anus, penis, vagina, cervix, vulva, and oropharynx. Investigators continue to seek ways to identify groups at high risk for HPV exposure, because HPV-related cancers are arguably prevented with the multivalent HPV vaccine.2,3
HPV is causally associated with a subset of squamous cell cancers of the head and neck, specifically cancers of the tonsils and base of tongue.4,5 As of 2018, the number of HPV-associated head and neck cancers has surpassed HPV-associated cervical cancers. There has been a rapid rise in the incidence of oropharyngeal squamous cell carcinoma. In the United States, between 2008 and 2012, 38,793 HPV-related cancers were diagnosed annually.6 Interestingly and pertinent to otolaryngologists, the most common cancer among these was oropharyngeal squamous cell carcinoma, with approximately 3,100 cases in women and 12,638 cases in men.6,7 HPV is now associated with 40% to 80% of all head and neck cancers, with HPV16 accounting for the majority of these cancers.8 The incidence of HPV-related oropharyngeal cancers has risen since 1984 and is projected to comprise the majority of head and neck cancers in the next 20 years.9 It is interesting to note that the percentage of oropharyngeal cancer attributed to HPV varies greatly depending on geo-graphic location, with the incidence consistently increasing in some areas such as North America and Northern Europe while staying low and stable in others.10
Although no studies have definitively identified an increasing prevalence of oral HPV16 over time, a rise in oral HPV16 is generally assumed to be a major causal factor in the rapid rise in squamous cell carcinoma of the oropharynx (SCCOP) amongst patients.7,11 Although sexual transmission has been established as the primary role of HPV acquisition in the oropharynx, some preliminary data suggest that HPV may be transmitted through occupational exposure from medical instruments and surgical fumes.12 With the increase in oropharyngeal cancer, a subsequent rise is expected in transoral robotic surgery for T1 and T2 HPV SCCOP and neck dissections for more advanced disease. Surgeons routinely use high-powered Bovie cautery in these resections, which generates a significant amount of smoke that may contain HPV. Therefore, the potential risk of contracting HPV via occupational exposure may be on the rise for head and neck surgeons.
Nononcogenic HPV 6 and 11 can be detected on medical devices, nasolabial folds of treating physicians,13 gloves, and within laser-generated plumes from HPV lesions.14,15 This suggests a potential risk for otolaryngologists who treat HPV-related SCCOP. Understanding the potential risk is extremely important, as epidemiological evidence suggests HPV is the etiological agent of oral cancers. Although HPV is a necessary, though not sufficient, cause of several cancers, targeted interventions that protect against HPV infections have clearly demonstrated the ability to prevent progression to cancer.16,17 Although it is difficult to prove direct infectivity, there are multiple case reports of HPV infection and disease in treating physicians. A 44-year-old laser gynecologist was found to have laryngeal biopsy-positive HPV subtypes 6 and 11 with a negative evaluation for genital or sexual transmission.15 Rioux et al. reported two gynecologists, with minimal risk factors other than busy laser practices for various dysplastic cervical and vulvar lesions, who developed HPV16-positive oral cavity squamous cell carcinoma.18 Although these case reports are limited by their lack of commentary on general sexual history, the presence of frequent HPV-positive patient–provider interactions is a common and concerning theme.
Further data are needed to better understand and identify risk factors for HPV among head and neck surgeons. Some case series have demonstrated that HPV is detectable in laser generated plumes using polymerase chain reaction (PCR),14,15,19 whereas others have not been able to detect it.20,21 However, no studies have examined fumes generated from Bovie cautery. If exposure in the operating room increases the risk of HPV infections, targeted interventions, such as the use of the HPV vaccine or specialized masks, would be justified to reduce the likelihood of cancer among surgeons. The aim of this study was to examine the potential occupational risk of acquiring HPV16 within the operating room as an otolaryngologist. In this study, we collected a series of cautery-generated fumes within the operating room and laboratory to test whether HPV16 could be objectively detected and whether this could be a potential risk for patient-to-provider transmission.
MATERIALS AND METHODS
Not for Human Subjects Institutional Review Board approval was obtained. This study did not involve direct patient contact. The authors were informed of tumor status of operating room patients by the attending physician preoperatively and postoperatively. No chart review or patient identification was performed by the authors.
Operating Room Sample Collection
Six patients with biopsy-proven oropharyngeal squamous cell carcinoma who sought oncologic care at the Department of Otolaryngology–Head and Neck Surgery of the University of Kansas Medical Center (KUMC) were identified. Included patients had opted to pursue surgical management of their tumors, which entailed transoral robotic resection. Patients were identified by the collaborating attending surgeon as p16 positive or negative based on their preoperative biopsy results. All pathology reports were reviewed by the KUMC Department of Pathology, and results were provided by the collaborating attending surgeon. Per guidelines established by the College of American Pathologists,22 positivity was determined by p16 immunohistochemistry performed using a 70% nuclear and cytoplasmic staining cutoff. Three patients with p16-positive tumors and three patients with p16-negative tumors were selected.
Samples were obtained during the surgical procedure. Under sterile conditions, a 1-cm2 piece of Whatman filter paper was individually cut. During the operation, filter paper was held within the plume generated by the robotic Bovie electrocautery using sterile forceps in the operative field for between 5 and 10 seconds. Additional swabs using separate pieces of filter paper were obtained from the areas listed in Table I. Swabs were then placed in 100 μL of TE (Tris and ethylenediaminetetraacetic acid) buffer, then held at −20°C for 0 to 30 days following transport to the laboratory.
TABLE I.
Collection Sites of Samples From the Operating Room.
| Sample Collection |
|---|
| Bovie electrocautery fumes |
| Surgical mask of assistant surgeon at head of bed |
| Suction tubing at attachment point to Yankauer |
| Suction filter |
| Robot arms |
Samples were collected under sterile conditions using a 1-cm2 piece of Whatman filter paper.
Laboratory Sample Creation and Collection
Using a sterile technique, Luria-Bertani (LB) agar plates with ampicillin (75 μg/μL) were poured and stored at 4°C. Bacterial transformation and growth experiment was then performed. HPV16E6E7 Plasmid DNA expressing E6 and E7 genes (Addgene pLXSN16E6E7) and DH5α competent bacterial cells (#18258–012; Invitrogen, Carlsbad, CA) were thawed on ice. Plasmid DNA (10 ng) was transferred to an Eppendorf tube and allowed to incubate on ice for 1 minute. We then added a 10-μL aliquot of DH5α cells to plasmid DNA and mixed by pipetting. The mixture was incubated on ice for 30 minutes. Next, the tube was heat-shocked in a water bath at 42°C for 30 seconds, then placed back on ice for 2 minutes. Under a sterile technique, 500 μL of room temperature LB broth was added to the tube, and the tube was sealed with parafilm. The tube was placed in shaker at 37°C at 225 rpm for 1 hour. Using a sterile technique, 150 μL of transformed cells were plated onto an LB agar plate with ampicillin (75 μg/μL). Plates were then incubated overnight at 37°C. Plates were organized into 10 separate divisions, which were labeled. Using a sterile technique, plaques from each division were transferred into separate tubes containing a 5-mL aliquot of LB broth with an antibiotic. Tubes were sealed with parafilm, then placed in shaker at 37°C at 225 rpm for 4 hours. Tubes were removed, and 1.3 mL from each tube was transferred to a new 1.5-mL tube under sterile conditions. Tubes were centrifuged for 30 seconds at 11,000 relative centrifugal force.
DNA was extracted using the NucleoSpin Plasmid miniprep kit (Macherey-Nagel, Düren, Germany) according to the instructions of the manufacturer. Concentration of each purified plasmid sample was then quantified using a Beckman Coulter (Brea, CA) DU 640 Spectrophotomoter. We then performed a restriction enzyme double digestion experiment utilizing BamHIHF (NEB #R3136; New England Biolabs, Ipswich, MA) and EcoRI-HF (NEB #R3101; New England Biolabs). Double digestion was carried out in 20-μL volumes consisting of 800 ng of purified DNA, 2 μL of CutSmart 10x buffer (NEB #B7204; New England Biolabs), 1 μL of BamHI-HF, 1 μL of EcoRI-HF, and the requisite volume of nuclease-free water. Mixtures were then covered with parafilm and incubated in a 37°C water bath for 15 minutes followed by heat inactivation at 65°C for 20 minutes. Double digestion products were then visualized by staining with GelRed Nucleic Acid Stain (#41003; Biotium, Fremont, CA) on 1% agarose gels. Transformation and digestion was considered successful if bands of the expected size (approximately 5858 bp and 856 bp) were visible.
Successfully transformed cells where then grown in the same fashion as above and purified using Qiagen (Hilden, Germany) Plasmid Maxi Kit according to the instructions of the manufacturer. Double digestion restriction enzyme was again performed to confirm that the necessary cells were created. Stock plasmid DNA was stored at −20°C.
We cut a 1-inch piece of the tail from a euthanized mouse, placed it in an Eppendorf tube, and kept it on ice. Mouse tail and equipment where then brought to an operating room. Under sterile conditions, 5 μL of stock plasmid DNA was mixed with 15 μL of TE buffer to generate a mixture containing 2 μg of DNA. Three separate 20-μL injections were prepared then injected intradermally into separate tail specimens. A negative control consisting of 20 μL of TE buffer was also injected intradermally into a rat tail specimen. Next, Bovie electrocautery was set at 20 W, and the injected area of each sample were cauterized for 5 seconds. Electrocautery fumes were evacuated utilizing a sterile Yankauer suction that was fitted with Whatman filter paper at the end of the suction. Separate Yankauer suction and tubing were used for each specimen. Filter paper was then placed in 100 μL of TE buffer, then held at −20°C for 0 to 30 days following transport to the laboratory.
HPV Detection
Tubes containing filter paper were allowed to thaw on ice, then were centrifuged at 8,000g for 15 minutes. Laboratory and operating room samples were tested separately. Amplification of HPV16 DNA was performed with custom E6 and E7 primers (Invitrogen). This primer system is well suited for detection of HPV16 E6 and E7. Positive controls were generated by serial dilution of plasmid DNA with TE buffer until final concentration of 1.5 ng per 2.5 μL was achieved. PCR was carried out in a 25-μL volume. For positive control, the preparation consisted of a 2.5-μL solution containing 1.5 ng of DNA, 12.5 μL of Quiagen HotStart buffer (Qiagen), 0.5 μL of forward primer, 0.5 μL of reverse primer, and 9 uL of ddH20. Laboratory and operating room sample preparation consisted of 5 μL of sample, 12.5 μL of Quiagen HotStart buffer, 0.5 μL of forward primer, 0.5 μL of reverse primer, and 6.5 μL of ddH20. E6 and E7 primers were separately tested for each sample. The cycling conditions were 50 cycles at 94°C for 1 minute, 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds.
After amplification, PCR products were visualized by staining with GelRed Nucleic Acid Stain (#41003; Biotium) on 1% agarose gels. PCR was considered tentatively positive if a band of the expected size (approximately 320 bp for E6 and 196 bp for E7) was visible. General laboratory procedures to prevent PCR contamination were strictly adhered to. One negative control was also included for both sets of experiments. All negative controls were found to be negative for HPV16 DNA.
Characterization of Plasmid DNA
To detect HPV DNA, we first used plasmid DNA expressing the E6/E7 proteins to develop an assay. Plasmid DNA was digested with restriction enzymes that linearized the plasmid. Plasmid DNA was also digested simultaneously with two single cutters. Restriction digestion products were then visualized by staining with GelRed Nucleic Acid Stain (#41003; Biotium) on 1% agarose gels. Transformation and digestion was considered successful if bands of the expected size (approximately 5,858 bp and 856 bp) were visible in the lane with plasmid DNA subjected to double digestion.
Optimization of Assay
To ascertain the sensitivity of our assay, we generated solutions of varying concentrations then assessed via PCR. We took 1 μL of our stock plasmid solution (399.9 ng/μL) and combined it with 999 μL of TE buffer to create a solution with a concentration of 399.9 pg/μL (picogram stock). We then combined 5 uL of our stock plasmid solution (399.9 ng/μL) with 15 uL of TE buffer to create a solution with DNA concentration of 100 ng/μL. Similarity, we combined 5 μL of the picogram stock with 15 μL of TE buffer to create a solution with DNA concentration of 100 pg/μL. PCR was carried out in a 25-μL reaction. A master mix consisting of 27.5 Qiagen HotStar buffer, 1.1 μL of E6 forward primer, and 1.1 μL of E6 reverse primer was created on ice. This was then mixed with 11.5 μL of DNA from either the 100 ng/μL solution or 100 pg/uL solutions. The cycling conditions were 50 cycles of 94°C for 1 minute, 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. A PCR was considered tentatively positive if a band of the expected size (approximately 320 bp for E6 and 196 bp for E7) was visible.
RESULTS
Characterization of Plasmid DNA
Transformation and digestion was considered successful if bands of the expected size (approximately 5,858 bp and 856 bp) were visible in the lane with plasmid DNA subjected to double digestion. Figure 1 represents the gel film depicting successful double digestion with the expected molecular weights. Subsequent growth and purification yielded a stock solution of DNA at a concentration of 399.99 ng/μL.
Fig. 1.
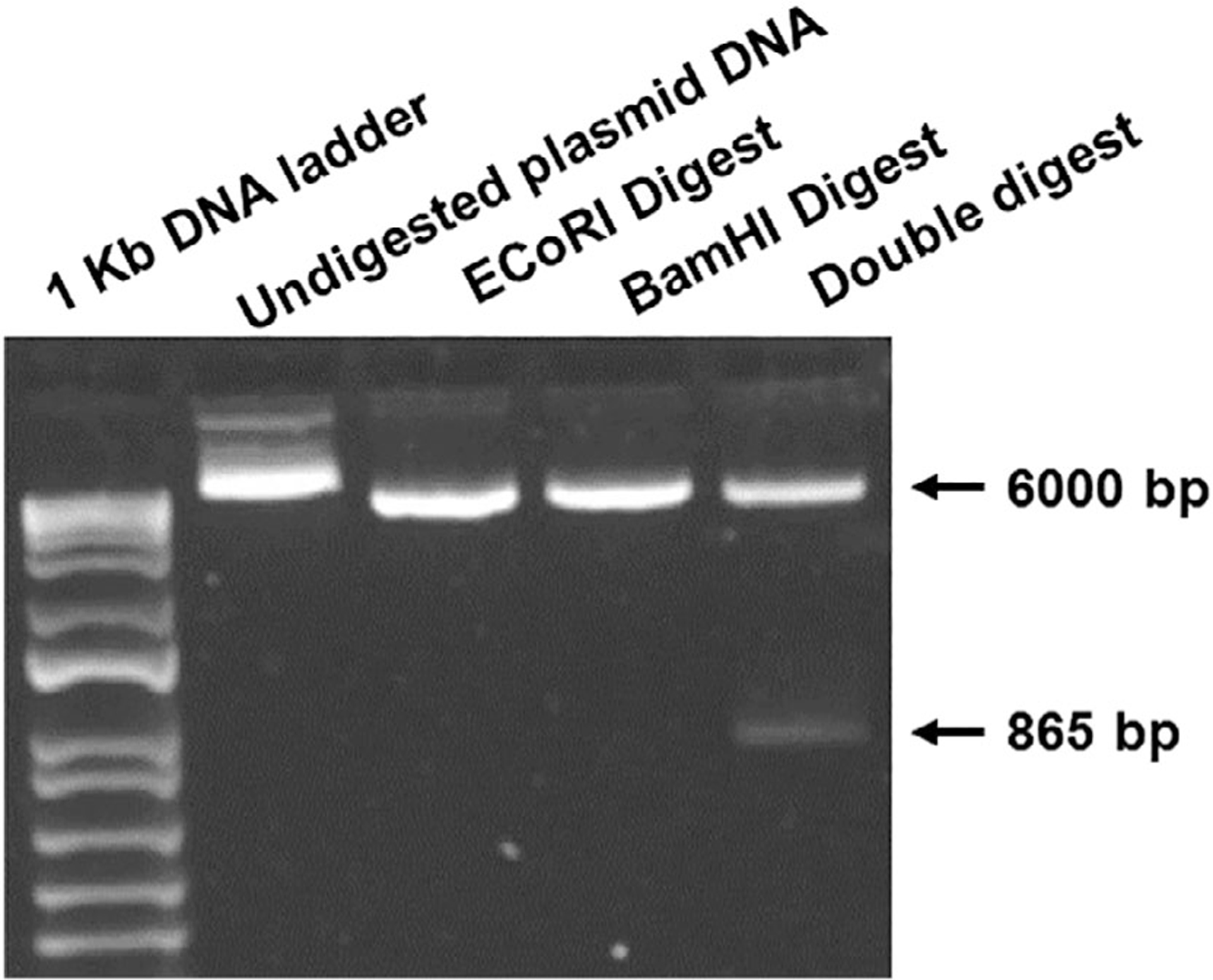
Agarose gel electrophoresis (1% agarose) utilizing GelRed Nucleic Acid Stain (#41003; Biotium, Fremont, CA) to visualize double digestion products. Transformation and digestion was considered successful if bands of the expected size (approximately 5,858 bp and 856 bp) were visible.
Optimization of Assay
PCR was considered tentatively positive if a band of the expected size (approximately 320 bp for E6 and 196 bp for E7) was visible. Our assay was sensitive down to ~1 ng of DNA. We then performed a serial dilution starting at 25 ng down to 1.5 ng plasmid DNA. Figure 2 depicts these results.
Fig. 2.
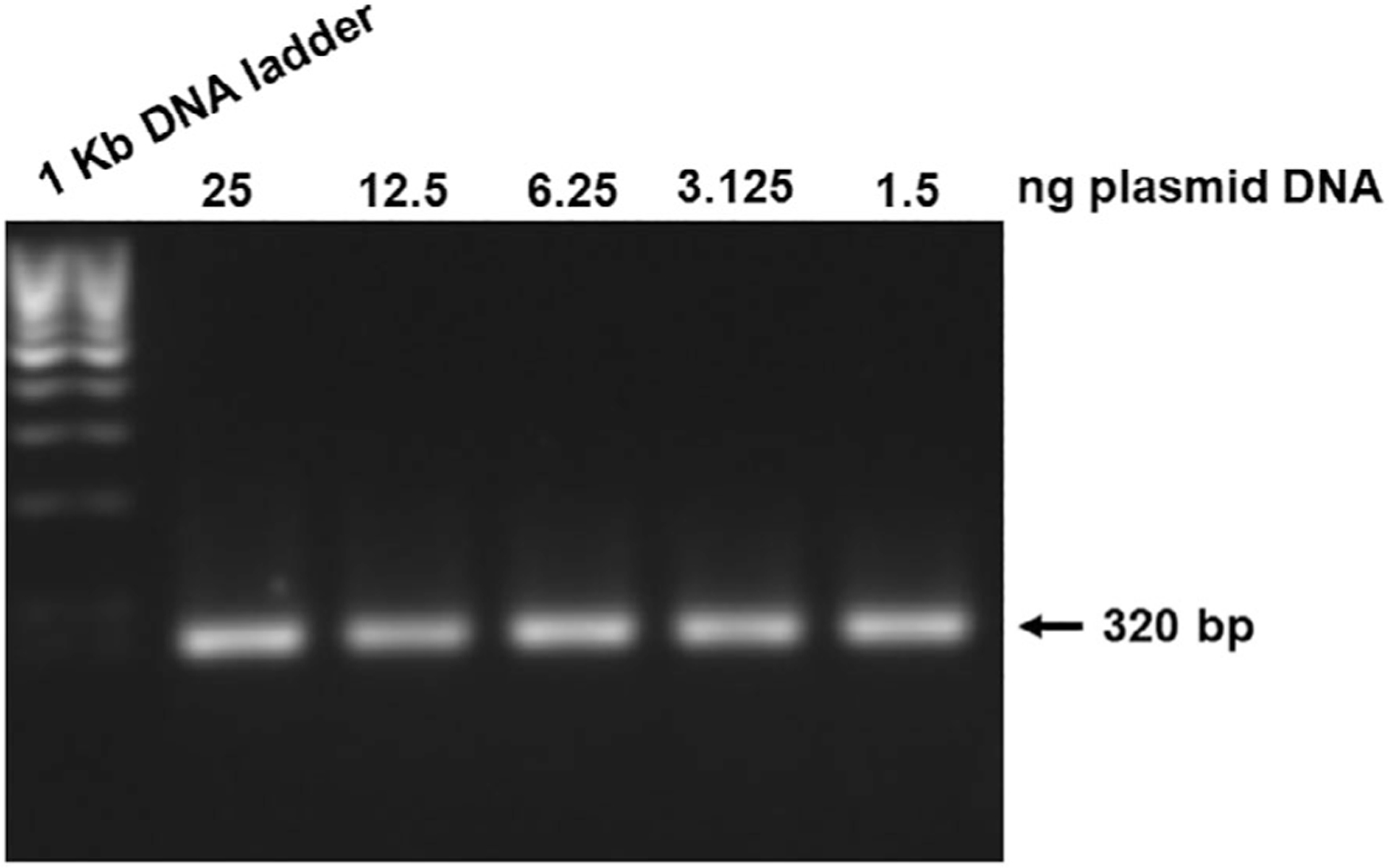
Agarose gel electrophoresis (1% agarose) of polymerase chain reaction (PCR) amplified products using HPV16 E6-specific primer sets. Gel film depicts assay sensitivity. PCR was considered tentatively positive if a band of the expected size (approximately 320 bp) was visible. Serial dilution went from 25 ng down to 1.5 ng.
Detection of DNA in the Operating Room and Laboratory Samples
In the operating room, 30 total samples were collected from six patients. Collected samples included Bovie fumes, surgical mask, suction tubing, suction filter, and robot arm from all six patients. Fifteen samples were obtained from patients with biopsy-proven p16-negative SCCOP, and 15 samples were obtained from patients with biopsy-proven p16-positive SCCOP. p16 status was confirmed on final pathology reports for all patients. All samples were tested for both E6 and E7 plasmid DNA. Samples were compared to a positive control containing 1.5 ng of plasmid DNA.
We did not detect any E6 or E7 plasmid DNA in the electrocautery fumes. We were also unable to detect DNA on any surgical equipment including surgical masks, suction apparatus, or robot arms. Samples were compared to both negative and positive controls. Figure 3 is a representative gel film of an HPV-positive operating room sample. This film is representative of all samples collected.
Fig. 3.

Agarose gel electrophoresis (1% agarose) of polymerase chain reaction (PCR) amplified products using HPV16 E6-specific primer sets. Gel film depicts results of samples obtained from the operating room of a p16-positive tumor. The positive control contained 1.5 μg of DNA. The negative control consisted of a Tris and ethylenediaminetetraacetic acid buffer. PCR was considered tentatively positive if a band of the expected size (approximately 320 bp for E6) was visible.
In the laboratory, one negative control as well as three positive control samples were collected. Samples consisted of aerosolized fumes from 2 μg of plasmid DNA contained in the TE buffer injected intradermally into the mouse tail. We did not detect any E6 or E7 plasmid DNA in any of the electrocautery fumes. Samples were compared to a positive control.
DISCUSSION
The aim of this study was to examine the potential occupational risk of acquiring HPV within the operating room as an otolaryngologist by conducting a series of experiments in the operating room and laboratory. To our knowledge, this is the first study to examine fumes generated from Bovie cautery as a proxy for the potential risk for patients to provider transmission. We were unable to detect HPV16 DNA in any laboratory or operating room samples that we examined. This suggests that the risk of occupational exposure among head and neck surgeons is low.
Although the primary mode of HPV16 transmission is sexual activity, there are data that support horizontal transmission and dissemination of HPV. In a systematic review, over 15 studies were identified that support objective evidence of HPV in the medical or public environment.12 HPV DNA has been found on many different medical devices out-side of the otolaryngology field both before and after chemical disinfection; HPV DNA after disinfection has been identified on transrectal and transvaginal probes,23–25 speculums,26 and surgical gloves.21,27 Of further concern, HPV DNA has been detected within the plume produced during CO2 laser vaporization of genital warts13,28 and papillomas due to respiratory papillomatosis.14,15,19 Bergbrant et al. demonstrated HPV DNA within the nasolabial fold and nostrils of a treating physician during both CO2 laser and electrocoagulation of genital warts.13 On the other hand, other studies have failed to detect HPV on various surgical equipment and materials.20,21
With the advent of transoral robotic surgery, early-stage T1 and T2 oral cavity tonsil and base of tongue HPV+ squamous cell carcinoma are amenable to surgical resection with or without neck dissection. If patients are good candidates, this is often the preferred treatment modality, as surgery avoids some of the long-term morbidities associated with radiation with or without chemotherapy. Although favorable to patients, resection of the tonsil and base of tongue malignancies with high-powered Bovie cautery generates significant amounts of smoke. Surgeons and surgical assistants are exposed to this smoke, and to date, no studies have evaluated the risk of Bovie electrocautery-related smoke fumes specifically as it relates to their contamination with HPV DNA particles.
In our study, we were unable to detect HPV16 DNA on surgical equipment and protective masks of surgeons. Furthermore, we were unable to identify HPV16 DNA within electrocautery fumes in both the operating room and laboratory. This suggests that there is a lower risk of exposure and subsequent transmission of HPV16 during operative interventions targeted at p16-positive tumors. Studies have shown that the use of a surgical mask prevented transmission of bovine papilloma virus DNA. Our finding that Bovie-related smoke fumes did not contain high-risk HPV16 DNA suggests that continued routine use of basic surgical masks is adequate to protect surgeons and their assistants. Furthermore, HPV vaccination should be routine for patients and providers, especially now that the Food and Drug Administration has approved it for up to the age of 49 years.29
Limitations of this study include that a sound oncologic surgery is to obtain adequate negative margins. Thus, direct cauterization of the tumor containing the virus may not occur frequently, thus preventing aerosolization of viral particles. In addition, the degree in which Whatman filter paper captures aerosolized DNA is unclear. However, Whatman filter paper is commonly used to store and transport DNA, and ultimately should capture any relevant aerosolized viral DNA if present. Also, we did not perform HPV in situ hybridization on patients with p16+ tumors. Although p16 is considered a surrogate for HPV16 infection, studies have shown that 15% of SCCOP can be p16 positive/HPV negative.30 Given the smaller sample size of our study, there is a risk of p16 positive/HPV negative patients being included, so further studies with larger sample sizes should be performed to confirm our findings and overcome this limitation. Finally, the sensitivity of our PCR may not have been adequate to detect the viral load within Bovie electrocautery fumes. Further studies focused on optimizing capture of fumes coupled with improved PCR assay sensitivity are needed.
CONCLUSION
The aim of this study was to objectively assess the presence of HPV16 in smoke generated from Bovie cautery as a proxy for the potential risk for patient to provider transmission. We were unable to detect any HPV16 DNA in operating room samples or laboratory samples, suggesting a low risk of occupational exposure. Continued use of routine safety measures should be adequate to safely reduce theoretical risk of exposure.
ACKNOWLEDGMENTS
The authors would like to acknowledge the following individuals: Dr. Nicholas A. Wallace for providing HPV16 plasmid DNA, Dr. Dharmalingam Subramaniam for providing DH5α cells, Dr. Shrikant Anant and his lab members for providing various materials critical to the study, and the University of Kansas Medical Center head and neck oncology team for allowing us access to their operating room.
This work was supported by a National Institutes of Health grant (CA227838) to S.M.T. and the National Cancer Institute Cancer Center Support Grant to the University of Kansas Cancer Center (P30CA168524).
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Footnotes
Presented at the Triological Society Annual Meeting at COSM, Austin, Texas, U.S.A, May 1–5, 2019.
BIBLIOGRAPHY
- 1.Harden ME, Munger K. Human papillomavirus molecular biology. Mutat Res Rev Mutat Res 2017;772:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer 2008;113:3036–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowy DR, Solomon D, Hildesheim A, Schiller JT, Schiffman M. Human papillomavirus infection and the primary and secondary prevention of cervical cancer. Cancer 2008;113:1980–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anantharaman D, Muller DC, Lagiou P, et al. Combined effects of smoking and HPV16 in oropharyngeal cancer. Int J Epidemiol 2016;45:752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Human Papillomaviruses. Vol. 90. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon, France: International Agency for Research on Cancer; 2007. [Google Scholar]
- 6.Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep 2016; 65:661–666. [DOI] [PubMed] [Google Scholar]
- 7.Sonawane K, Suk R, Chiao EY, et al. Oral human papillomavirus infection: differences in prevalence between sexes and concordance with genital human papillomavirus infection, NHANES 2011 to 2014. Ann Intern Med 2017;167:714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus–positive head and neck squamous cell carcinoma. J Clin Oncol 2015;33:3235–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29:4294–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castellsague X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst 2016;108:djv403. [DOI] [PubMed] [Google Scholar]
- 11.Woods KV, Shillitoe EJ, Spitz MR, Schantz SP, Adler-Storthz K. Analysis of human papillomavirus DNA in oral squamous cell carcinomas. J Oral Pathol Med 1993;22:101–108. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Rashid T, Nyitray AG. Penises not required: a systematic review of the potential for human papillomavirus horizontal transmission that is non-sexual or does not include penile penetration. Sex Health 2016;13: 10–21. [DOI] [PubMed] [Google Scholar]
- 13.Bergbrant IM, Samuelsson L, Olofsson S, Jonassen F, Ricksten A. Polymerase chain reaction for monitoring human papillomavirus contamination of medical personnel during treatment of genital warts with CO2 laser and electrocoagulation. Acta Derm Venereol 1994;74:393–395. [DOI] [PubMed] [Google Scholar]
- 14.Kashima HK, Kessis T, Mounts P, Shah K. Polymerase chain reaction identification of human papillomavirus DNA in CO2 laser plume from recurrent respiratory papillomatosis. Otolaryngol Head Neck Surg 1991;104: 191–195. [DOI] [PubMed] [Google Scholar]
- 15.Hallmo P, Naess O. Laryngeal papillomatosis with human papillomavirus DNA contracted by a laser surgeon. Eur Arch Otorhinolaryngol 1991;248: 425–427. [DOI] [PubMed] [Google Scholar]
- 16.Huh WK, Joura EA, Giuliano AR, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomised, double-blind trial. Lancet 2017;390: 2143–2159. [DOI] [PubMed] [Google Scholar]
- 17.Garland SM, Kjaer SK, Muñoz N, et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin Infect Dis 2016;63:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rioux M, Garland A, Webster D, Reardon E. HPV positive tonsillar cancer in two laser surgeons: case reports. J Otolaryngol Head Neck Surg 2013; 42:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrett WL, Garber SM. Surgical smoke: a review of the literature. Is this just a lot of hot air? Surg Endosc 2003;17:979–987. [DOI] [PubMed] [Google Scholar]
- 20.Hughes PS, Hughes AP. Absence of human papillomavirus DNA in the plume of erbium:YAG laser-treated warts. J Am Acad Dermatol 1998;38: 426–428. [DOI] [PubMed] [Google Scholar]
- 21.Ilmarinen T, Auvinen E, Hiltunen-Back E, Ranki A, Aaltonen LM, Pitkaranta A. Transmission of human papillomavirus DNA from patient to surgical masks, gloves and oral mucosa of medical personnel during treatment of laryngeal papillomas and genital warts. Eur Arch Otorhinolaryngol 2012;269:2367–2371. [DOI] [PubMed] [Google Scholar]
- 22.Lewis JS Jr, Beadle B, Bishop JA, et al. Human papillomavirus testing in head and neck carcinomas: guideline from the College of American Pathologists. Arch Pathol Lab Med 2018;142:559–597. [DOI] [PubMed] [Google Scholar]
- 23.Kac G, Podglajen I, Si-Mohamed A, Rodi A, Grataloup C, Meyer G. Evaluation of ultraviolet C for disinfection of endocavitary ultrasound transducers persistently contaminated despite probe covers. Infect Control Hosp Epidemiol 2010;31:165–170. [DOI] [PubMed] [Google Scholar]
- 24.Casalegno JS, Le Bail Carval K, Eibach D, et al. High risk HPV contamination of endocavity vaginal ultrasound probes: an underestimated route of nosocomial infection? PLoS One 2012;7:e48137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma ST, Yeung AC, Chan PK, Graham CA. Transvaginal ultrasound probe contamination by the human papillomavirus in the emergency department. Emerg Med J 2013;30:472–475. [DOI] [PubMed] [Google Scholar]
- 26.McCance DJ, Campion MJ, Baram A, Singer A. Risk of transmission of human papillomavirus by vaginal specula. Lancet 1986;2:816–817. [DOI] [PubMed] [Google Scholar]
- 27.Ferenczy A, Bergeron C, Richart RM. Human papillomavirus DNA in fomites on objects used for the management of patients with genital human papillomavirus infections. Obstet Gynecol 1989;74:950–954. [PubMed] [Google Scholar]
- 28.Andre P, Orth G, Evenou P, Guillaume JC, Avril MF. Risk of papillomavirus infection in carbon dioxide laser treatment of genital lesions. J Am Acad Dermatol 1990;22:131–132. [DOI] [PubMed] [Google Scholar]
- 29.Head American and Society Neck. Position statement on human papillomavirus (HPV) vaccination for prevention of HPV-related oropharyngeal cancer. Available at: https://www.ahns.info/wp-content/uploads/2019/03/Signed-Joint-position-statement-HPV.pdf. Published December 18, 2018 Accessed October 4, 2019.
- 30.Smeets SJ, Hesselink AT, Speel EJ, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer 2007;121:2465–2472. [DOI] [PubMed] [Google Scholar]


