Since the emergence of Coronavirus Disease 2019 (COVID-19) in December 2019, this latest pandemic has represented a major global threat. The disease is caused by a novel coronavirus (SARS-CoV-2) which structurally resembles other coronaviruses (severe acute respiratory syndrome [SARS-CoV] and the Middle East Respiratory Syndrome [MERS-CoV]). Although we are in the process of learning about this disease and trying to identify interventions that could slow this pandemic, several experiences from outside the United States, namely from China, Italy, and Iceland, and from New York have generated some interesting observations. Even though the virus affects both women and men, men appear to have disproportionately higher rates of mortality [1,2,8].
SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) for entry into cells. Limited human data suggest that SARS-CoV-2 binding to ACE2 may attenuate residual ACE2 activity, skewing ACE/ACE2 balance toward a state of heightened angiotensin II production, leading to pulmonary vasoconstriction, inflammation, and oxidative-related organ damage resulting in an increased risk for acute lung injury. However, as ACE2 is an “X” linked enzyme and some reports indicate an increase in plasma ACE2 in older women, they would appear to be more vulnerable to SARS-CoV-2. Yet, reports from China, Italy, and New York clearly document that women have about half of the COVID-19 incidence with much less disease severity and mortality compared with men. Perhaps higher ACE2 produces more Ang-1-7, which provides more protection? It may also be possible that higher ACE2 activity provides a more efficient “sink” in eliminating the virus or preventing its attachment for target cell entry. Differential regulation of ACE2 with sex hormones may be working, as well. Investigation of this disparity could provide important clues to better understanding complexities of this enzyme and improving COVID-19 outcomes.
Based on the pathophysiology of SARS-CoV-2 infection and pleiotropic effects of ACE inhibitors (ACE—Is)/angiotensin receptor blockers (ARBs), these agents may have a potential role in the management of select patients with severe COVID-19. Studies to fill important knowledge gaps in human participants regarding the basic science of ACE2, COVID-19 serology and outcomes are clearly needed.
An ongoing clinical trial of convenience, WARRIOR, with projected enrollment of 4422 women with symptoms and signs of ischemia but no obstructive coronary artery disease is randomly assigning participants to either usual care or intensive medical treatment (IMT) consisting of high dose ACE-I (or ARB), high potency statin, and low-dose aspirin. Currently ~1000 women are enrolled and available for COVID-19 investigation, at 50 sites across the US.
Epidemiological data from the epidemics of SARS-CoV in 2003–2004 and MERS-CoV in 2014 showed a similar sex-related pattern independent of age [3]. Although the US currently has the largest number of confirmed cases of COVID-19 worldwide, the latest update from the Centers for Disease Control does not provide any sex-specific data regarding prevalence or mortality related to COVID-19. This is an important observation which deserves a pause to reflect upon and identify some potential reasons.
In China and Italy, advancing age of the hospitalized COVID-19 patients, as well as cardiovascular co-morbidities and pre-existing cardiovascular disease, were predictors of worse outcomes [2,3]. As epidemiological studies have shown that the prevalence of cardiovascular disease and certain risk factors such as smoking tend to be higher among men, this finding might not be surprising. But this alone is not likely to explain the remarkable difference in mortality between sexes (~1.7 fold in Italy).
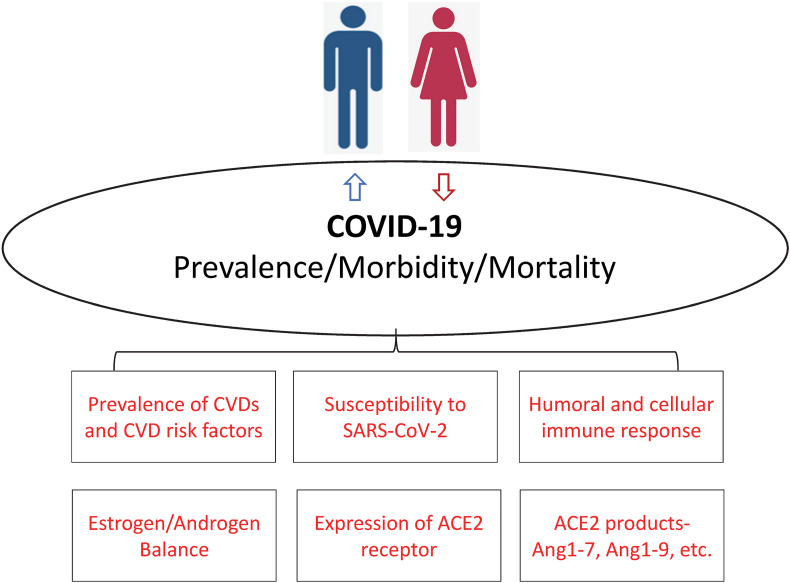
Animal models suggest that male mice were more susceptible to SARS-CoV compared with female mice with matched age. This enhanced susceptibility was associated with accumulation of inflammatory monocyte macrophages and neutrophils resulting in vascular leakage and alveolar edema. Moreover, blocking estrogen receptors among female mice was associated with increased mortality suggesting that estrogen receptor signaling might offer some protective effect for female mice [4]. Human studies had long suggested that women have a stronger humoral and cellular immune response to viral infections in general [5]. This might be the case for coronaviruses as well. In a study, which has not undergone peer review, of 331 Chinese patients with COVID-19, there was no difference in plasma IgG levels among mild and recovering cases between men and women. But among severely diseased patients, plasma levels of IgG were higher among women compared with men [6]. The viral surface spike protein of SARS-CoV-2 enters the host cell by binding to the human ACE2 receptor, which is prevalent in the lung but also present in the endothelium of blood vessels of other organs including myocardium and brain. Animal models have also suggested that male sex is associated with increased expression of ACE2 receptors [7]. In addition, it is well known that the prevalence of autoimmune disorders such as rheumatoid arthritis and systemic lupus erythematosus is much higher among women. Perhaps the therapeutic agents used to manage these disorders such as hydroxychloroquine, which in-vitro blocks the entry of SARS-CoV-2 into the endothelial cells, and interleukin-6 receptor antagonists (e.g., tocilizumab and sarilumab), which work to modify the cytokine storm in severe cases, might play a role in treating COVID-19 patients (Fig. 1 ).
Fig. 1.
Postulated mechanisms for the worse outcomes observed among men with Coronavirus Disease 2019 (Covid-19).
By observing this pattern of worse outcomes among men again with COVID-19, how could this help us moving forward? First, future studies, including the anticipated studies from the US, should address the sex-differences in the prevalence and outcomes of this disease, and try to identify the predictors of worse outcomes among both sexes, as it appears that the virus behaves differently in men versus women. Better understanding of these prognostic differences could help us better risk-stratify patients. Second, therapies for certain auto-immune and viral diseases such as interleukin-6 receptor antagonists and remdesiver might be potential therapies. There are several ongoing trials evaluating the merits of these agents in improving clinical outcomes. Finally, there are two recently launched randomized phase 1 trials to evaluate for a newly developed vaccine. It is imperative that randomized trials of the vaccines as well as therapies consider recruiting an equivalent proportion of women and men, as there have been many examples in the past of therapies later shown to be ineffective in women due to under-representation of women in the landmark trials.
Sources of funding
None.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., Cereda D., Coluccello A., Foti G., Fumagalli R., Iotti G., Latronico N., Lorini L., Merler S., Natalini G., Piatti A., Ranieri M.V., Scandroglio A.M., Storti E., Cecconi M., Pesenti A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Br. Med. J. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karlberg J., Chong D.S., Lai W.Y. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am. J. Epidemiol. 2004;159:229–231. doi: 10.1093/aje/kwh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Channappanavar R., Fett C., Mack M., Ten Eyck P.P., Meyerholz D.K., Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J. Immunol. 2017;198:4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 6.Zeng F, Dai C, Cai P, Wang J, Xu L, Li J, Hu G, Wang L. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: a possible reason underlying different outcome between gender. medRxiv 2020.03.26.20040709 [pre-print] doi: 10.1101/2020.03.26.20040709 [DOI] [PMC free article] [PubMed]
- 7.Fernández-Atucha A., Izagirre A., Fraile-Bermúdez A.B., Kortajarena M., Larrinaga G., Martinez-Lage P., Echevarría E., Gil J. Sex differences in the aging pattern of renin-angiotensin system serum peptidases. Biol. Sex Differ. 2017;8:5. doi: 10.1186/s13293-017-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gudbjartsson D.F., Helgason A., Jonsson H., Magnusson O.T., Melsted P, Norddahl G.L., Saemundsdottir J. Spread of SARS-CoV-2 in the Icelandic population. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]