Abstract
It is not yet known, if critically ill COVID-19 patients are prone to fungal infections. We report a 69-year-old patient without typical risk factors for invasive pulmonary aspergillosis (IPA), who developed IPA two weeks after onset of symptoms. Our report shows that IPA may occur in critically ill COVID-19 patients.
Keywords: Aspergillosis, COVID-19, ICU
Highlights
-
•
Invasive pulmonary aspergillosis may complicate severe Covid-19.
-
•
Physicians should be aware for aspergillosis in critically ill Covid-19 patients.
-
•
Screening for aspergillosis may be indicated in these patients.
1. Introduction
Aspergillus is an opportunistic fungal pathogen that may cause devastating disease in immunocompromised hosts, including those with hematological malignancies. Aspergillus is an air-borne pathogen, bringing the lungs in the frontline for defense. Recently, severe viral pulmonary infections have shown to be associated with an increased risk for invasive pulmonary aspergillosis (IPA). Severe influenza in critically ill patients for example, is complicated by IPA in 7–23% of cases and associated with a case fatality rate of more than 50% [1]. Similar to influenza, the newly emerged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may cause severe lower respiratory tract infections leading to acute respiratory distress syndrome (ARDS). The proportion of patients with coronavirus disease 2019 (COVID-19) who develop severe disease with ARDS is considerably high [2]. Consequently, there is a large number of patients in need for intensive care unit (ICU) admission. It is not yet known, if patients with severe COVID-19 are at similar risk for IPA development as patients with severe influenza.
2. Case
Here we report a 70-year-old male who was diagnosed with COVID-19 in March 2020. Underlying diseases included chronic obstructive pulmonary disease (COPD) GOLD grade 2, obstructive sleep apnea syndrome, insulin-dependent type 2 diabetes with end-organ damage [retinopathy, nephropathy (CKD 3b according to the KDIGO classification at first presentation), polyneuropathy], arterial hypertension, coronary heart disease and obesity (body mass index: 38 kg/m2). Past medical history included proximal deep vein thrombosis four months prior to presentation. His medication consisted of a long-acting beta agonist/long-acting muscarinic antagonist combination, inhaled glucocorticoid (400 μg budesonide per day), valsartan, spironolactone, ivabradine, atorvastatin, metformin, liraglutide, insulin glargine and enoxaparin.
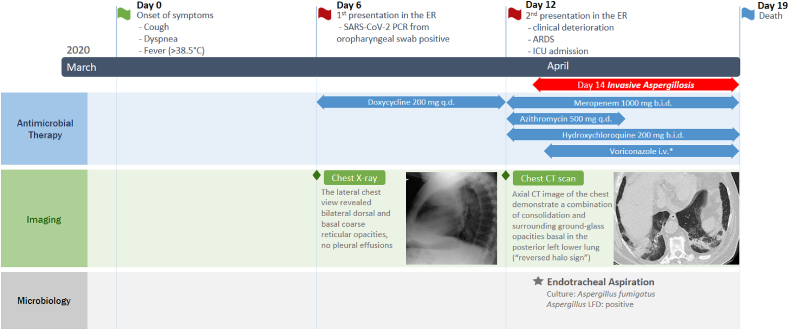
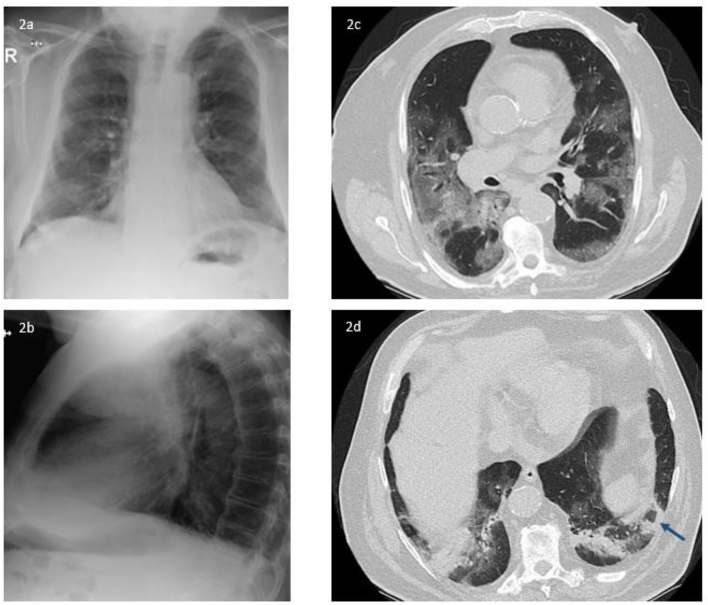
The patient presented with productive cough, dyspnea and intermittent fever (>38.5 °C) for one week (Fig. 1). Arterial pO2 was 67 mmHg while breathing ambient air. White blood cell count was within the normal limits and C-reactive protein (CRP) was slightly elevated (33 mg/dl; upper limit of norm 5 mg/dl). D-dimer was within the normal range. Chest X-ray revealed bilateral basal coarse reticular opacities (Fig. 2a and b). Real-time polymerase chain reaction (PCR) from oropharyngeal swab was positive for SARS-CoV-2. However, the patient refused to be hospitalized due to personal reasons and was discharged home with oral doxycycline 200 mg q.d. One week later, the patient returned to the emergency department because of clinical deterioration and hypoxemia (arterial pO2 46 mmHg while breathing ambient air). Chest CT-scan was performed and showed multiple bilateral ground-glass opacities with a crazy paving appearance (Fig. 2c). In addition, a reversed halo sign was described (Fig. 2d). White blood-cell count was slightly elevated (11.65 × 10^9 per L), neutrophils were elevated (9.7 × 10^9 per L), CRP level was 144 mg/dl, interleukin-6 was 396 pg/ml and ferritin 465 ng/ml. Creatinine levels increased from 1.85 mg/dl (first presentation) to 3.26 mg/dl. Lymphocytes were within the normal range. The patient was admitted to ICU with moderate ARDS (oxygenation index 154) and received meropenem 1 g b.i.d., azithromycin 500mg once q.d., and hydroxychloroquine 200mg b.i.d. He was intubated and mechanically ventilated. Chest X-ray revealed a progression of the bilateral infiltrates on day 2 after ICU admission concordant with significant pulmonary deterioration (oxygenation index <100). On day 3 of ICU stay (day 14 after onset of symptoms) endotracheal aspiration was obtained. Culture grew Aspergillus fumigatus (voriconazole minimal inhibitory concentration 0.125 mg/L), without bacterial growth. Aspergillus lateral-flow device (LFD) [3], detecting an Aspergillus specific antigen secreted during active growth of Aspergillus and not during colonization, was performed from the endotracheal aspirate and revealed a positive result. Due to low sample volume, galactomannan could not be performed from this respiratory specimen. Serum fungal biomarkers (galactomannan and 1,3-ß-D-glucan) remained negative. Based on progression of pulmonary infiltrates, recovery of Aspergillus fumigatus in endotracheal aspirate, positive LFD in endotracheal aspirate and the clinical deterioration the patient was diagnosed with putative invasive pulmonary aspergillosis according to Blot et al. [4] and intravenous voriconazole (6 mg/kg b.i.d followed by 4 mg/kg b.i.d.) was initiated on day 4 of ICU stay. Despite treatment of ICU and addition of voriconazole, the patient deceases 3 days after initiation of antifungal treatment due to multiorgan failure. Autopsy was not performed.
Fig. 1.
Timeline representing the course of COVID-19 in the reported patient
Abbreviations: q.d. = once daily; b.i.d. = twice daily, i.v. = intravenous; LFD = lateral-flow device; ER = emergency room; ARDS = acute respiratory distress syndrome; ICU = intensive care unit; SARS-CoV-2 = Severe acute respiratory syndrome coronavirus 2, CT = computed tomography
* Voriconazole dosage: 6 mg/kg twice daily on day 1, followed by 4 mg/kg twice daily. Chest imagings displayed here are also displayed in more detail in Fig. 2.
Fig. 2.
Fig. 2a and b representing findings on chest X-ray performed on day 6 of symptom onset. The lateral chest view revealed bilateral dorsal and basal coarse reticular opacities, no pleural effusions. Fig. 2c and d representing chest CT scans on day 12 of symptom onset. Axial CT image of the chest demonstrate extensive-ground glass opacities with bilateral and multi-lobar distribution (2c) and a combination of consolidation and surrounding ground-glass opacities basal in the posterior left lower lung (“reversed halo sign” - shown by the arrow) (2d).
3. Discussion
From early reports from Wuhan, we know that patients with COVID-19 may develop complicating fungal infections [5]. However, galactomannan testing and other fungal diagnostics for further differentiating these infections are rarely available in the Wuhan region and China in general [6].
Here we report a case of putative invasive aspergillosis in a patient with COVID-19 associated ARDS, with growth of Aspergillus fumigatus and a positive Aspergillus-Ag test in endotracheal aspirate. The patient presented in this report had a two-week history of symptoms before he was admitted to the ICU. On ICU admission, a CT scan revealed a reversed halo sign that is usually suspicious for the presence of mucormycosis [7] but may also be found in other mold infections, particularly IPA [8]. Chest CT scans are a valuable tool for COVID-19 diagnosis and management. Ground-glass opacities with or without consolidations are observed in the majority of COVID-19 cases [9,10] but a reversed halo may be observed in some patients [9]. Similar to patients with severe influenza a halo or reversed halo sign may be indicative for presence of a pulmonary mold infection [11] and should therefore trigger further diagnostic steps to confirm or rule out IPA.
For further diagnostic workup, endotracheal aspiration was performed and yielded growth of Aspergillus fumigatus. To further discriminate between colonization and infection we tested the Aspergillus specific LFD, a CE-certified point of care assay, specific for Aspergillus. This test detects actively growing hyphae during invasive infection and remains negative in case of Aspergillus colonization of the airways. The LFD was recently given an BII recommendation for diagnosis of invasive aspergillosis in the ESCMID/ECMM/ERS guidelines [12].
While serum galactomannan generally lacks sensitivity in non-neutropenic patients [13], and also turned out negative in this patient, further studies are needed to evaluate the benefit of serum GM screening in COVID-19 patients where bronchoalveolar lavage fluid sampling for culture and biomarker testing is often avoided due to the risk of aerosol spreading and health care worker infections. This seems to be of special interest as based on the data from a recently published trial, showing that GM in serum is indeed elevated in the majority of patients with severe influenza [14] on ICU and the fact that a significant amount of IPA cases on ICU are still diagnosed post-mortem only, serum GM screening may be an important tool for early recognition of IPA in critically ill COVID-19 patients.
Diagnosis of IPA in ICU patients without classical risk factors for IPA remains challenging. The well-established criteria of the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) [14] are not applicable for many of the ICU patients due to a missing host factor. Thus, new diagnostic criteria for ICU patients were established to overcome these limitations [4]. This criteria base on clinical signs and symptoms compatible with IPA, abnormal chest imaging of the lungs and microbiological evidence of presence of Aspergillus. This criteria turned out to be superior to the EORCT/MSG criteria in the ICU setting [4]. Also the patient reported in this case report would not have been classified as IPA according to the EORTC/MSG criteria from 2008 due to a missing host factor. Recently, in the influenza and aspergillosis trial, GM in serum and bronchoalveolar lavage fluid was added to the ICU criteria as an additional mycological criterium to overcome the limitation of imperfect culture sensitivity for aspergillosis [15].
The patient reported here had no classical risk factors for IPA, but it is well known, that viral infections like influenza or cytomegalovirus infections increase the risk for IPA. The pathogenetic mechanisms rendering patients susceptible for IPA during such viral infections are not completely understood at the moment but probable include viral induced overexpression of anti-inflammatory cytokines, dysregulation of T-helper cell differentiation and impaired cell-mediated immune response [16,17]. It is likely, that during SARS-CoV-2 infections similar impairment are present putting the patients at risk for complicating mold infections as seen in our patient.
Our case report highlights that IPA may occur in in critically ill COVID-19 patients. Screening studies are needed to evaluate the prevalence of IPA in COVID-19 patients admitted to ICU.
Funding
There was no funding for this manuscript.
Declaration of competing interest
All authors declare that they have no conflicts of interest regarding this manuscript.
References
- 1.Schwartz I.S., Friedman D.Z.P., Zapernick L., Dingle T.C., Lee N., Sligl W. High rates of influenza-associated invasive pulmonary aspergillosis may not be universal: a retrospective cohort study from Alberta, Canada. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2020 doi: 10.1093/cid/ciaa007. [DOI] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. J. Am. Med. Assoc. 2020;(13) doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Prattes J., Flick H., Pruller F., Koidl C., Raggam R.B., Palfner M. Novel tests for diagnosis of invasive aspergillosis in patients with underlying respiratory diseases. Am. J. Respir. Crit. Care Med. 2014;190(8):922–929. doi: 10.1164/rccm.201407-1275OC. [DOI] [PubMed] [Google Scholar]
- 4.Blot S.I., Taccone F.S., Van den Abeele A.M., Bulpa P., Meersseman W., Brusselaers N. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am. J. Respir. Crit. Care Med. 2012;186(1):56–64. doi: 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]
- 5.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chindamporn A., Chakrabarti A., Li R., Sun P.-L., Tan B.-H., Chua M. Survey of laboratory practices for diagnosis of fungal infection in seven Asian countries: an Asia Fungal Working Group (AFWG) initiative. Med. Mycol. 2018;56(4):416–425. doi: 10.1093/mmy/myx066. [DOI] [PubMed] [Google Scholar]
- 7.Cornely O.A., Alastruey-Izquierdo A., Arenz D., Chen S.C.A., Dannaoui E., Hochhegger B. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical mycology in cooperation with the mycoses study Group education and Research consortium. Lancet Infect. Dis. 2019;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godoy M.C., Viswanathan C., Marchiori E., Truong M.T., Benveniste M.F., Rossi S. The reversed halo sign: update and differential diagnosis. Br. J. Radiol. 2012;85(1017):1226–1235. doi: 10.1259/bjr/54532316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y., Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am. J. Roentgenol. 2020:1–7. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- 10.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229) doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang L., Zhang N., Huang X., Xiong S., Feng Y., Zhang Y. Invasive pulmonary aspergillosis in patients with influenza infection: a retrospective study and review of the literature. Clin. Res. J. 2019;13(4):202–211. doi: 10.1111/crj.12995. [DOI] [PubMed] [Google Scholar]
- 12.Ullmann A.J., Aguado J.M., Arikan-Akdagli S., Denning D.W., Groll A.H., Lagrou K. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clinical microbiology and infection. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2018;24(Suppl 1):e1–e38. doi: 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Jenks J.D., Mehta S.R., Taplitz R., Aslam S., Reed S.L., Hoenigl M. Point-of-care diagnosis of invasive aspergillosis in non-neutropenic patients: Aspergillus Galactomannan Lateral Flow Assay versus Aspergillus-specific Lateral Flow Device test in bronchoalveolar lavage. Mycoses. 2019;62(3):230–236. doi: 10.1111/myc.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Pauw B., Walsh T.J., Donnelly J.P., Stevens D.A., Edwards J.E., Calandra T. Revised definitions of invasive fungal disease from the European organization for Research and treatment of cancer/invasive fungal infections cooperative Group and the national institute of allergy and infectious diseases mycoses study Group (EORTC/MSG) consensus Group. Clin. Infect. Dis. : Off. Publ. Infect. Dis. Soc. Am. 2008;46(12):1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schauwvlieghe A., Rijnders B.J.A., Philips N., Verwijs R., Vanderbeke L., Van Tienen C. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir. Med. 2018;6(10):782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 16.McCullers J.A. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat. Rev. Microbiol. 2014;12(4):252–262. doi: 10.1038/nrmicro3231. [DOI] [PubMed] [Google Scholar]
- 17.Cunha C., Goncalves S.M., Duarte-Oliveira C., Leite L., Lagrou K., Marques A. IL-10 overexpression predisposes to invasive aspergillosis by suppressing antifungal immunity. J. Allergy Clin. Immunol. 2017;140(3):867–870 e9. doi: 10.1016/j.jaci.2017.02.034. [DOI] [PubMed] [Google Scholar]