Abstract
Coronavirus pneumonia is accompanied by rapid virus replication, where a large number of inflammatory cell infiltration and cytokine storm may lead to acute lung injury, acute respiratory distress syndrome (ARDS) and death. The uncontrolled release of pro-inflammatory cytokines, including interleukin (IL)-1β and IL-6, is associated with ARDS. This constituted the first study to report on the variability in physicochemical properties of β-glucans extracts from the same edible mushroom Lentinus edodes on the reduction of these pro-inflammatory cytokines and oxidative stress. Specifically, the impact on the immunomodulatory and cytoprotective properties of our novel in ‘house’ (IH-Lentinan, IHL) and a commercial (Carbosynth-Lentinan, CL) Lentinan extract were investigated using in vitro models of lung injury and macrophage phagocytosis. CL comprised higher amounts of α-glucans and correspondingly less β-glucans. The two lentinan extracts demonstrated varying immunomodulatory activities. Both Lentinan extracts reduced cytokine-induced NF-κB activation in human alveolar epithelial A549 cells, with the IHL extract proving more effective at lower doses. In contrast, in activated THP-1 derived macrophages, the CL extract more effectively attenuated pro-inflammatory cytokine production (TNF-α, IL-8, IL-2, IL-6, IL-22) as well as TGF-β and IL-10. The CL extract attenuated oxidative stress-induced early apoptosis, while the IHL extract attenuated late apoptosis. Our findings demonstrate significant physicochemical differences between Lentinan extracts, which produce differential in vitro immunomodulatory and pulmonary cytoprotective effects that may also have positive relevance to candidate COVID-19 therapeutics targeting cytokine storm.
Keywords: β-Glucans, Medicinal mushroom, COVID-19, Immunomodulation, Cytokine storm, Disease mitigation
Graphical abstract
Highlights
-
•
β-Glucans from shiitake mushroom reduces IL-1β, IL-6 in in vitro lung injury model.
-
•
β-Glucans from same source can differ in immunomodulatory and pulmonary cytoprotective effects.
-
•
β-Glucans can reduce oxidative stress and activate macrophages.
-
•
β-Glucans may ameliorate cytokine storm that causes ARDS as seen with COVID-19.
1. Introduction
The pandemic outbreak of coronavirus disease 2019 (COVID-19) is rapidly spreading globally (Zhang et al., 2020; Rowan and Laffey, 2020). Reports from China showed that about 20% of COVID-19 patients developed severe disease, resulting in a fatality of 4% (Zhang et al., 2020). A large part of COVID-19 patients in China experienced severe complications including acute respiratory distress syndrome (ARDS) requiring admission to intensive care unit (ICU) (Zhang et al., 2020). ARDS is a devastating condition of severe respiratory failure with 40% mortality and for which novel therapies are urgently needed (Rezoagli et al., 2017). It is characterized by widespread inflammation of the lungs, where extrapulmonary infections are a key aetiology of ARDS onset (Laffey and Matthay, 2017). The inflammatory insult results in lung parenchyma injury and activation of the immune system with an up-regulation of pro-inflammatory cytokines (Laffey and Matthay, 2017). The current international standard of intervention includes ventilatory management and organ support (Bellani et al., 2016). The innate immune system plays a pivotal role in the pathophysiology of ARDS (Chousterman et al., 2017).
COVID-19 causes an inflammatory or cytokine storm (CS) in the lungs with the excessive and uncontrolled release of pro-inflammatory cytokines, including interleukin (IL)-1β and IL-6 (Conti et al., 2020). The binding of COVID-19 to the Toll-Like Receptor (TLR) causes the release of pro-IL-1β which is cleaved by caspase-1, followed by the production of IL-1β that is a mediator of lung inflammation, fever and fibrosis. Researchers have confirmed level of inflammatory factors in patients with COVID-19 including elevation of IL-6 in non-survival groups (Huang et al., 2020), as compared with that of the survivals. Therefore, how to block the CS and when to initiate anti-inflammation therapy is critical for reducing the death rate of COVID-19 (Channappanavar and Perlman, 2017; Chousterman et al., 2017). Suppression of pro-inflammatory IL-1 family members and IL-6 has been shown to have a therapeutic effect in many inflammatory diseases, including viral infections (Zhang et al., 2020).
There is an increasing interest in the medicinal use of mushrooms nutraceuticals that have been previously reported to exhibit wide-ranging activities including anti-inflammatory, anti-tumor and immune-modulating capabilities (Pelizon et al., 2005; Zheng et al., 2005; Akramiene et al., 2007; Kumar, 2015). β-Glucans are one of the main active components derived from mushrooms (Smith et al., 2002; Zhu et al., 2015). These are glucose polymers that are linked together through 1,3 linear β-glycosidic chains. Complexity and variation in the compound derive from side branching structures (Stone, 2009). β-glucans isolated from fungi commonly possess side branching at the 1,4 or 1,6 position (Kaur et al., 2020). Variance will also occur with chain length and many these variances are species-specific and dictate biological activity (Sullivan et al., 2006; Chaichian et al., 2020). Lentinans are a specific class of β-glucans extracted from the edible mushroom Lentinus edodes, and are composed of a β-(1–3)-glucose backbone with two (1–6)-β-glucose branches of each five glucose units (Sullivan et al., 2006; Kaur et al., 2020). There has been an increasing interest in their use for treating disease in animals and humans (Carballo et al., 2019; McCarty and DiNicolantonio, 2020). McCarty and DiNicolantonio (2020) recently described the potential role of β-glucan as a natural nutraceutical for boosting type 1 interferon response to RNA viruses such as influenza and coronavirus.
Putative use of β-glucans in mitigating lung infections correlates with findings from our recent in vivo studies to address ARDS (Masterson et al., 2019). However, there were significant challenges in identifying a reliable and repeatable source of β-glucans suitable for lung delivery as findings from screening of over 20 natural and commercial products screened revealed that they were unsuitable for lung delivery due to microbial contamination or exhibiting very low levels of β-glucan. We recently reported that purified β-glucans (Lentinan) from the Shiitake mushroom Lentinus edodes, obtained using our in-house novel extraction method can be used to reduce populations of clinical isolate Klebsiella pneumoniae harbouring multiple antibiotic resistances in an in vivo lung infection model (Masterson et al., 2019). We reported that administration of Lentinan shows potential for treating sepsis-induced lung injury as it effectively reduces bacterial load in arterial blood and BAL, reduces white cell count protein inflammation to the lungs, and improves lung physiological parameters. Evidence also showed that in-house Lentinan extracted supported vital pO2 along with promoting lung cellular repair.
This constitutes the first study to compare a commercially sourced Lentinan extract from the edible mushroom Lentinus edodes (referred to as Carbosynth-Lentinan) to that of an in-house hot-water extract (IHL) of the same mushroom in order to evaluate immunomodulatory properties. These were characterized using an in vitro lung injury model with a focus on profiling components associated with cytokine storm. It is hypothesized that β-glucans derived from exotic mushrooms have the potential to alleviate the immune cascade in pathological conditions, such as ARDS that is experienced by COVID-19 patients.
2. Materials and methods
2.1. Materials
Commercial Lentinan (CL) was sourced from Carbosynth (FL33321, Compton, Berkshire, UK). Fruiting bodies of Lentinus edodes were purchased from Fancy Fungi (Ringaheen, Co. Wexford Ireland). IHL was extracted from the fruiting bodies using a novel process. A549 cells (used at passage 90) and THP-1 cells (used at passage 10) were obtained from the American Type Culture Collection, (ATCC, Rockville, MD, USA). Cells were cultured in RPMI-1640 (Sigma-Aldrich, St. Louis, MO, USA), supplemented with 10% fetal calf serum (Sigma-Aldrich), 1% penicillin G (100 U/mL) and streptomycin (100 μg/mL) solution (Sigma-Aldrich) at 37 °C in 95% air/5% CO2 environment. For differentiation into macrophages, THP-1 cells were treated with phorbol 12-myristate 13-acetate (PMA) (Peprotech EC, London, UK) at a concentration of 100 ng/mL for 48 h.
2.2. Physicochemical characterization of β-glucan samples
2.2.1. Megazyme analysis
Extracts were analyzed for (1,3)-(1,6)-β-glucan content using the Megazyme yeast and mushroom kit (K-YBGL) (Megazyme Ltd., Bray, Co. Wicklow, Ireland). Assays were carried out according to manufacturer's instructions. Briefly, samples were milled, and placed in 12 M H2SO4 at −4 °C for 2 h to solubilize the glucans. The samples were then hydrolyzed in 2 M H2SO4 at 100 °C for 2 h. After incubation, any remaining glucan fragments were quantitatively hydrolyzed to glucose using a mixture of exo-1,3-β-glucanase and β-glucosidase which gives a measurement of total glucan. The alpha glucan and sucrose content of the sample is determined by hydrolyzing specifically to d-glucose and d-fructose. Glucose was measured with amyloglucosidase and invertase using a glucose oxidase peroxidase GOPOD reagent. β-Glucan was determined by the difference in both measurements.
Attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR).
ATR-FTIR was carried out on a Perkin Elmer Spectrum fitted with a universal ATR sampling accessory. All data were recorded at ambient temperature, in the spectral range of 4000–650 cm−1, utilizing a 16 scan per sample cycle and a fixed universal compression force of 80 N. Subsequent analysis was carried out using Spectrum software.
2.2.2. Scanning electron microscopy (SEM)
SEM was performed on a Mira SEM (Tescan Oxford Instruments, UK) using a range of magnifications to evaluate the surface morphology of the extracts using the function of secondary electrons. Samples were placed on an aluminum stub and were gold-coated using Baltec SCD 005 sputter coater (BAL-TEC GmbH, Chemnitz Germany) for 110 s at 0.1 mbar vacuum before observation.
2.2.3. Nuclear magnetic resonance (NMR)
1H NMR spectra were obtained using an Agilent Technologies Ultra High Field (UHF) 800 MHz NMR system. Spectra were analyzed using ACD NMR software. Samples were prepared in deuterated water (D2O).
2.3. Immunomodulatory properties of β-glucan samples from Lentinus edodes
2.3.1. Cell viability assays
Cells were seeded at a density of 4 × 105 cells/well in a 96 well plate. After 24 h, cells were treated with varying concentrations of both CL and IHL. Cells were incubated with samples for a further 24 h. For MTT assay, media was aspirated and cells were treated with 10% 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) in RPMI for 3.5 h at 37 °C in a humidified incubator with 5% CO2. MTT and media were aspirated and formazan product was solubilized through the addition of 100 μL of DMSO per well. Solubilized product was quantitatively measured at 540 nm using a Synergy™ HT Multi- Mode Microplate Reader (BioTek, Winooski, USA). Results were expressed as percentage viability with respect to vehicle control.
2.3.2. Enzyme linked immunosorbent assay (ELISA)
Human Duoset sandwich ELISA kit (RnD Systems MN, USA) was used to measure cytokine levels in the medium after β-glucan exposure. All ELISAs were performed according to manufacturer's instructions. Results were expressed either in pg/mL or in ng/mL.
2.3.3. Cell injury
Pulmonary alveolar type II A549 cells were seeded at a density of 4 × 105 cells/well in 96 well plates. After 24 h cells were injured with 1 ng/mL of IL-1β (Peprotech, Rocky Hill, NJ) in RPMI supplemented with 1% penicillin/streptomycin. THP-1 cells were seeded at a density of 4 × 105 cells/well in 96 well plates and 24 h later injured with 100 ng/mL LPS (Sigma) in RPMI supplemented with 1% penicillin/streptomycin. Cells were then treated with β-glucan samples for a further 24 h.
2.3.4. Luciferase assays
A549 cells stably transfected with NF-κB-luc reporter gene were obtained from the National University of Ireland Galway. These A549 cells were seeded at a density of 4 × 105 cells/well in 96 well plates. After 24 h, IL-1β and β-glucan samples were added to each well for 3.5 h at 37 °C in a humidified incubator with 5% CO2. 100 μL of luciferase substrate (SolarGlow Molecutools, Dublin, Ireland) which includes lysis buffer, was added to each well. After brief agitation on an orbital shaker, luminescence was assessed in a Microplate Reader (BioTek, USA). Results were expressed as fold induction with respect to vehicle treated controls.
2.3.5. Phagocytosis assay
The Vybrant™ phagocytosis assay kit (Invitrogen, Carlsbad, CA) was used to measure the phagocytosis capacity of THP-1 cells after exposure to β-glucan samples. Samples were analyzed according to manufacturer's instructions. Phagocytic index was calculated and results were graphed as percentage phagocytic index with respect to vehicle treated controls.
2.3.6. Hydrogen peroxide (H2O2) injury assay
To determine the effect of H2O2 on cell viability, cell apoptosis and necrosis were assessed using propidium iodide (PI) and Annexin-VFITC conjugated antibodies (Miltenyl Biotech). Cells were initially treated with a dose range of H2O2 from 0.001 mM to 20 mM. As 10 mM of H2O2, reduced cell viability by 50%, it was selected as the injuring concentration for further experiments. Cells were simultaneously treated with β-glucan samples. After 24 h of incubation, cells were complexed with Annexin-VFITC conjugated antibody at 1:1000 dilution for a further 10 min in the dark. Cells were washed in PBS three times and resuspended in flow buffer (Miltenyl Biotech). Cells were then automatically incubated with PI using the MACS Quant analyzer. Cells from each sample were then analyzed by MACSQUANT Analyzer 10 (Miltenyl Biotech). Data was analyzed using Flowlogic software(Miltenyl Biotech) with a guide to interpretation of findings represented in Table 1 .
Table 1.
Interpretation of H2O2 assay derived from using flow-cytometry data.
| Quadrant location: | Location representation: | Annexin V: | Propidium iodide: | Cell condition: |
|---|---|---|---|---|
| Lower left |  |
Negative | Negative | Living cells |
| Lower right |  |
Positive | Negative | Early apoptotic |
| Upper right |  |
Negative | Positive | Late apoptotic cells |
| Upper left |  |
Positive | Positive | Necrotic cells |
2.4. Statistical data analysis
Continuous data were described as mean ± standard deviation (SD). Differences in glucan content between CL and IHL were tested using unpaired Student's t-test. Differences of κb induction in A459 cells over increasing doses of CL and IHL were explored using a two-way analysis of variance. The viability of A549 cells was tested using increasing doses of β-glucans (i.e. IHL or CL) using a one-way analysis of variance. Post-hoc multiple comparison of increasing doses of CL and IHL versus 0 mg/mL was performed by controlling the false discovery rate using the two-stage step-up method of Benjamini, Krieger and Yekuteli. Differences in cytokine levels and phagocytosis were tested between different groups (i.e. LPS, CL and IHL) or between different doses (i.e. 1, 5 and 10 mg/mL) of β-glucans using a one-way analysis of variance. Post-hoc multiple comparison versus PBS was performed by controlling the false discovery rate using the two-stage step-up methods of Benjamini, Krieger and Yekuteli. Statistical significance was reached with a p-value < .05 (2-tailed). Statistical analyses were performed using GraphPad Prism 7a (GraphPad Software, San Diego, CA, USA) and Microsoft Excel for Mac 2017, Version 15.32 (Microsoft Corporation, Redmond, WA).
3. Results & discussion
3.1. Physicochemical characterization of β-glucan samples
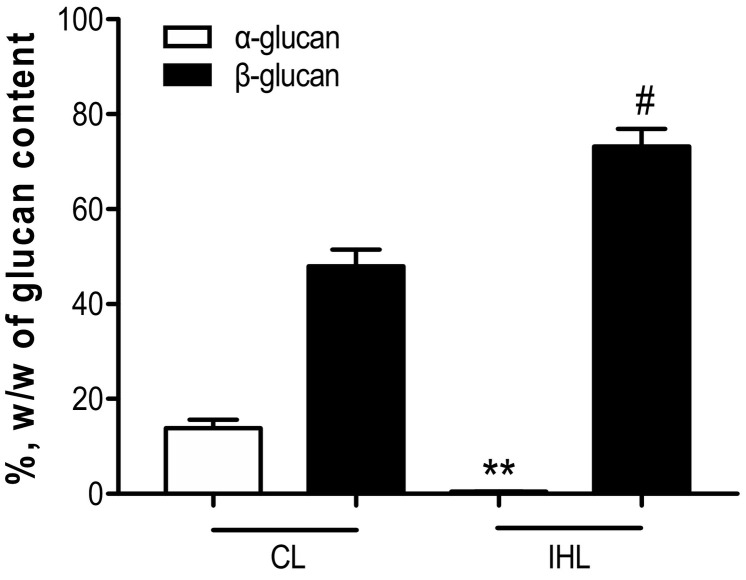
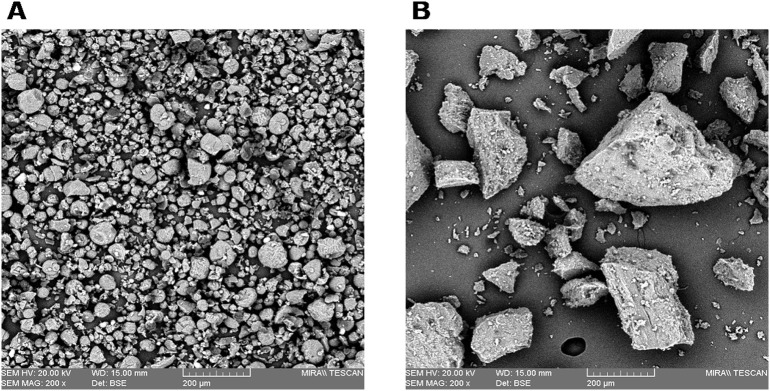
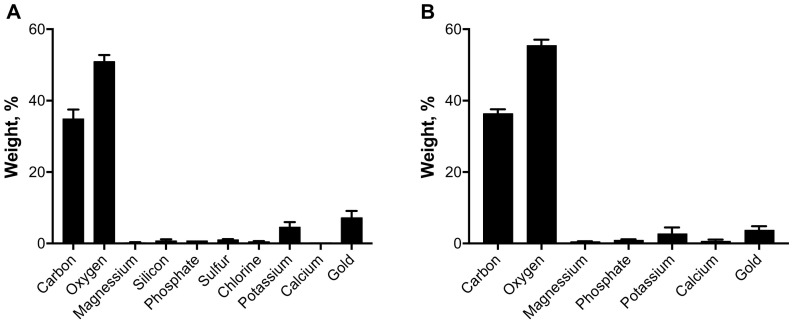
Megazyme analysis of Carbosynth-lentinan (CL) and In-house Lentinan (IHL) samples are displayed in Fig. 1 . Data shows both β and alpha glucan % w/w content. CL was shown to have a significantly higher alpha glucan content. IHL was shown to have a significantly higher β-glucan content and were purer and cleaner in composition. Scanning electron microscopy paired with energy dispersive X-ray analysis was carried out to determine particle size and elemental content, with results displayed in Fig. 2, Fig. 3 respectively. Fig. 2 , a dimensional representation of particle size, shows that CL has a uniform particle dispersity. Conversely, IHL shows a more heterogeneous particle mix. Fig. 3 shows that IHL contains fewer elements compared to CL, as sulfur, silicon and chlorine were not present in IHL. These results, paired with Megazyme results, reaffirm that IHL is a cleaner preparation with fewer constituents.
Fig. 1.
Comparison of glucan content in commercial and in-house samples using Megazyme analysis. **p < .01 versus alpha glucan C; #p < .05 versus β-glucan CL.
Fig. 2.
Dimensional representation of commercial (A) and in-house (B) samples analyzed by scanning electron microscopy.
Fig. 3.
Quantitative analysis of elemental contents in commercial (A) and in-house (B) samples determined by energy dispersive X-ray analyzer.
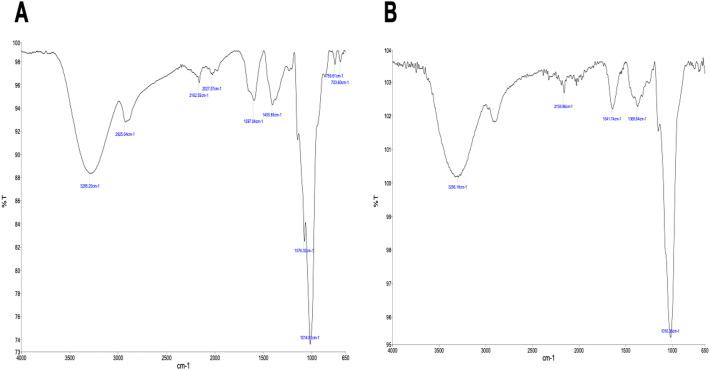
FT-IR spectra of the CL and IHL are shown in Fig. 4 . The IR analysis reveals a strong absorption peak in the fingerprint region at approximately 1015 cm−1 in both the commercial and in-house lentinan samples. The presence of equivalent absorption peaks, in the absorption range characteristic of polysaccharides, strongly suggests that both samples contain the same β-glucan compound. Preliminary NMR analysis of the commercial and in-house Lentinan suggests significant differences between the products. The 1 H spectra of the compounds are shown in Fig. 5 .
Fig. 4.
FTIR spectra of the commercial (A) and in-house lentinan (B).
Fig. 5.
A549 cells transfected with NF-ΚB reporter gene were stimulated with IL-1β for 1 h before administration of β-glucan samples. *p < .05; **p < .01; ***p < .001 versus 0 mg/mL.
Previous researchers have reported that variability in β-glucan chain length, branching and composition can vary based principally on on source ( Kaur et al., 2020 ). Extraction procedures will have an effect on the structure and purity of the β-glucan product, which may have an effect of its bioactivity. Thus, there has been an obstacle in understanding biological activities and this has hindered potential therapeutic development (Zhang et al., 2011). Scanning electron microscopy paired with energy dispersive X-ray analysis showed that the IHL had a diverse heterogenous particle composition in comparison to CL, which had a more uniform composition. IHL was also found to have less chemical elements (sulfur, silicon and chlorine) compared to CL. These results suggest that IHL is a purer β-glucan product, compared to the commercially available sample. FTIR characterization of the samples shows that both samples contain the same β-glucan compound ( Fig. 4 ). NMR analysis contradicted this and showed that there is a significant difference between both samples (refer to supplementary information). Bak and colleagues carried out a study to measure the glucan contents in the fruiting bodies of L. edodes mushroom from various cultivars which was found to be variable based on cultivar ( Bak et al., 2014). Gil-Ramirez and co-authors found a variance between mushroom samples based on growth conditions, degree of fruiting body maturing body as well as a difference between fresh and fruiting bodies (Gil-Ramirez et al., 2011). Studies have also shown variance in β-glucan content dependent on the country of origin (Bak et al., 2014). L. edodes from Japan (49.5% β-glucan w/w) having a higher content than that of mushrooms purchased in Iran (38% β-glucan w/w).
Immunomodulatory properties of β-glucan samples from Shiitake mushroom L. edodes.
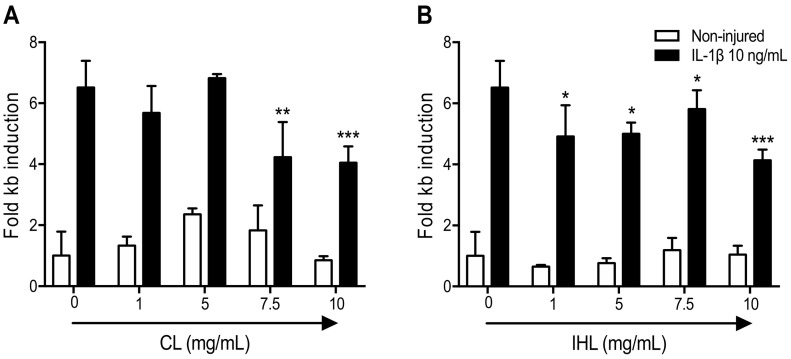
To investigate the anti-inflammatory effects of the β-glucan samples, transformed human airway epithelial cells (A549) were used. Initial experiments showed that a dose of 10 ng/mL of IL-1β gave an IL-8 release response, indicative of NF-κB activation. To further examine the effects of the β-glucan samples on the NF-κB pathway, A549 cells stably transfected with the NF-κB luciferase reporter gene were used. IL-1β stimulation caused a 6-fold increase in luciferase expression. Both β-glucan samples reduced this expression, with IHL inhibiting this increase at a dose of 1 mg/mL (Fig. 5B). CL was able to reduce IL-1β induced NF-κB pathway activation at the higher concentrations (Fig. 5A). This IL-1β pulmonary model finding is directly relevant to COVID-19 as IL-1β is a prominent part of the ‘cytokine storm’ response (Zhang et al., 2020). Hypercytokinaemia is considered a prominent mechanism of injury in COVID-19 patients. Furthermore, there are reports of the IL-1 Ra blocker Anakinra being effective for COVID-19, where Anakinra is being tested as a potential therapy for clinical trials. There are currently no treatments directed at halting the cytokine storm and acute lung injury to stop the progression from manageable hypoxia to frank respiratory failure and ARDS in patients with COVID-19 infection. Preventing progression from early acute hypoxia and cytokine release syndrome to frank hypoxic respiratory failure and ARDS could have a huge impact on the foreseeable overflow of the ICU units (https://clinicaltrials.gov/ct2/show/NCT04330638). The aforementioned reported that in ventilated patients, preventing the onset of ARDS, or shortening ICU stay could also be crucial in this regard. Furthermore, the clinical status after 15 days treatment was evaluated to measure the effectiveness of tocilizumab, tocilizumab and anakinra, siltuximab, siltuximab and anakinra and anakinra on restoring lung homeostasis, using single IV injection (siltuximab or tocilizumab) combined or not with daily subcutaneous injections of anakinra until 28 days or hospital discharge, whichever is first.
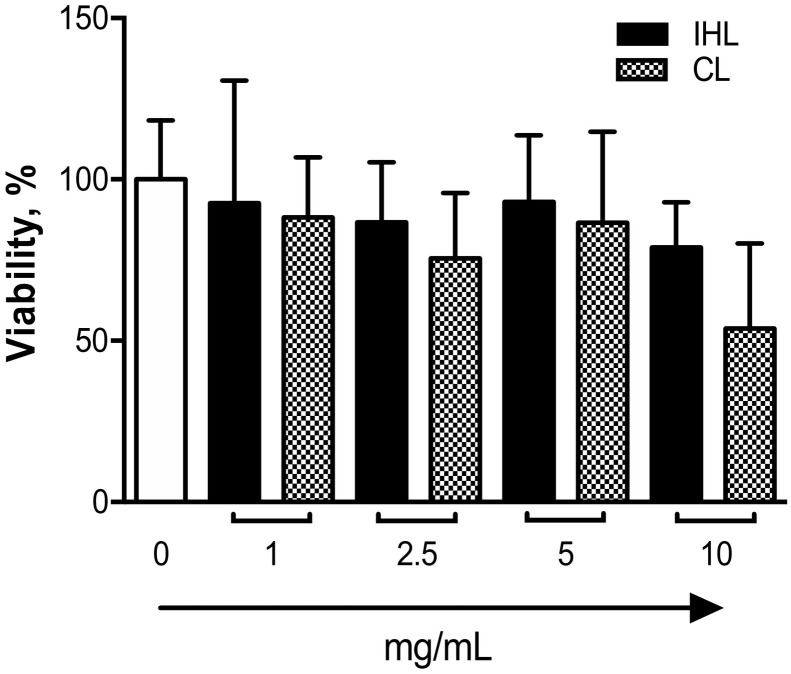
IHL was able to achieve these effects at the lower concentrations analyzed. MTT assays were preforming to ensure that cell viability was not contributing to the anti-inflammatory effects observed. Results show that both extracts did not elicit any cytotoxic effects at all concentrations, despite a trend toward a decrease in viability that was seen at the highest dose tested (10 mg/mL), but was only evident for the CL group (p = .096) (Fig. 6 ). No statistical differences were observed for multiple comparisons of singe p-values for MTT findings, which is attributed to the broad data variability represented by large standard deviation observed (Fig. 6). Post-hoc multiple comparisons of increasing doses of CL and IHL versus test control (0 mg/mL) were used to ensure that MTT findings were not over-stated in the assessment of preclinical data to reduce risk of false discovery. Statistical analysis data of multiple comparisons for single p-values for MTT findings are also provided in supporting information.
Fig. 6.
MTT analysis of A549 cells treated with β-glucan samples to ensure anti-inflammatory properties' are not related to a reduction in toxicity.
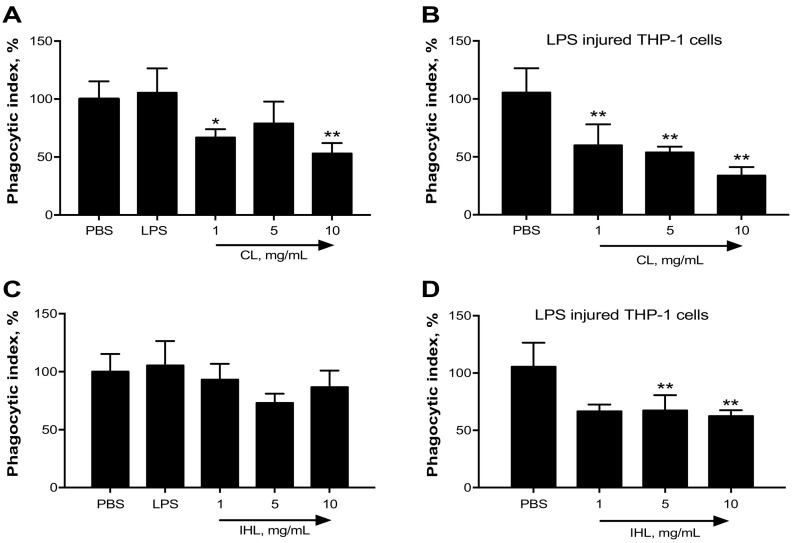
The capability of the β-glucan samples to induce or inhibit phagocytosis was also investigated. In the absence of injury, both CL (Fig. 7A+C) and IHL (Fig. 7B+C) appeared to reduce phagocytic index. After injury, both samples reduced phagocytic index (Fig. 7).
Fig. 7.
The potential of β-glucan samples to alleviate phagocytic index of THP-1 cells was determined using Vybrant Phagocytosis assay kit *p < .05; **p < .01; ***p < .001 versus PBS.
Macrophages and monocytes recognize β-glucans by various receptors present on their membrane (Vaclav et al., 2013). This recognition will result in the secretion of cytokines (Netea et al., 2008). Therefore, the immunomodulatory activity of the β-glucan samples was analyzed, in relation to their ability to influence the secretion of cytokines from macrophages. Results demonstrated that the samples induced/suppressed cytokine release at different concentrations. For example, IHL induced the secretion of IL-6 — conversely CL suppressed it. Lentinan analyzed by previous researchers was found to increase the release of TNF-α and IL-6 (Morales et al., 2019). This immunomodulatory effect was also analyzed by their ability to influence the phagocytic activity of macrophages. Both samples suppressed phagocytosis after LPS insult, but CL exclusively suppressed phagocytosis in the absence of injury. Dectin-1 receptor is assumed to be one of the main receptors responsible for the recognition of β-glucans. Activation of dectin-1 with 1,3–1,6 β-glucans can trigger cytokine release and phagocytosis (Brown and Gordon, 2001, Brown and Gordon, 2005; Willment et al., 2003; Herre et al., 2004).
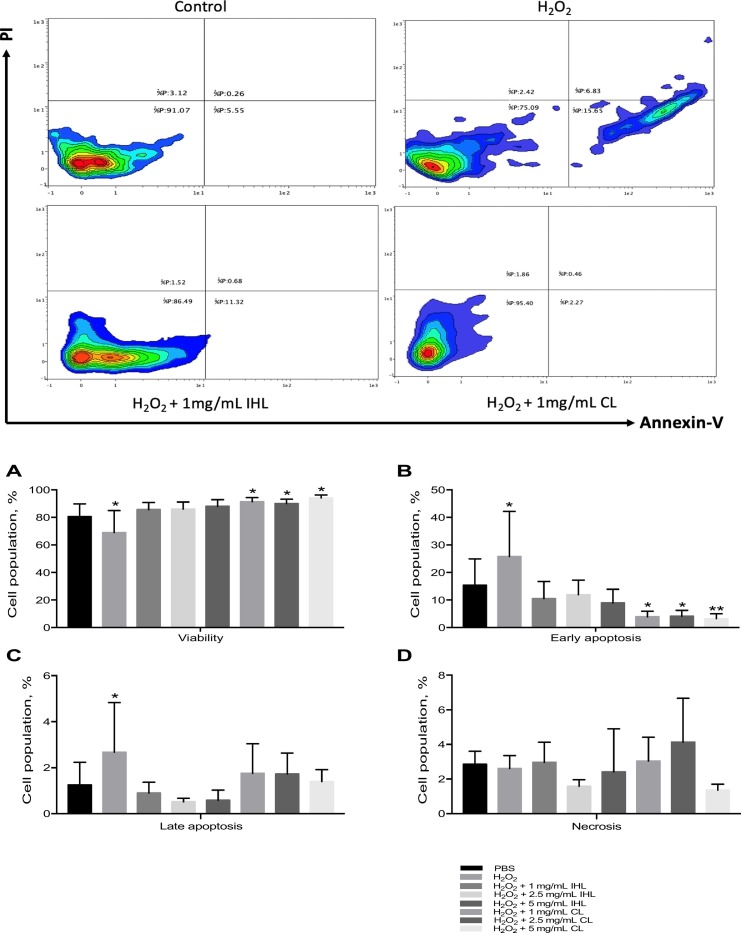
To determine the potential alleviating effects of the β-glucans on oxidative stress injury, THP-1 cells were treated with 10 mM of H2O2 to induce oxidative stress subsequent to β-glucan treatment. In terms of viability (Panel a), all doses of β-glucans increased viability after injury, CL however significantly increased viability at all doses administered (1, 2.5 and 5 mg/mL). CL significantly reduced early apoptosis after injury at all doses tested (Fig. 8B). IHL appeared to reduce both early and late apoptosis (Fig. 8A+B), however this was not significant. IHL at 1 mg/mL and 5 mg/mL, or CL at 1 mg/mL and 2.5 mg/mL appear to increase necrosis although not significantly (Fig. 8C).
Fig. 8.
Flow cytometric analysis of apoptosis (early and late) and necrosis of THP-1 cells after oxidative stress induction and subsequent β-glucan treatment *p < .05; **p < .01; ***p < .001 versus 0 mg/mL.
Reactive oxygen species (ROS), which are generated as intermediates in metabolic pathways, are prevalent in pathological conditions. Oxidative stress can occur at a pulmonary level (Rahman and MacNee, 2000). Cellular-derived ROS are produced enzymatically by inflammatory and epithelial cells (Marwick et al., 2007). It has been suggested that oxidants play a contributing role to cell injury as well as leakage of fluid into the lung interstitial space (Liu, 2008). The potential of β-glucan to alleviate oxidative stress is relatively unknown. β-Glucans were used to investigate the ability to alleviate oxidative stress in H2O2-treated THP-1 cells where apoptosis and necrosis were measured using flow cytometry. Propidium iodide (PI) in conjunction with Annexin V was used to determine if THP-1 cells were viable, apoptotic or necrotic. Cell status was assessed based on differences in plasma membrane integrity and permeability (Vermes et al., 2000; Farrell et al., 2011). The results from this experiment were variable and effects were source dependent. Both CL and IHL sources at both conditions tested had the ability to increase viability after injury. CL significantly reduced early apoptosis, and IHL showed a trend to reduce early apoptosis although not significantly. Our results are in line with the findings reported by Zi and colleagues who observed that Lentinan had the ability to alleviate oxidative stress (Zi et al., 2019). Reduction in oxidative stress by use of Lentinan is relevant to COVID-19 intervention. The renin-angiotensin (RAS) signaling pathway, oxidative stress and cell death, cytokines storm and endothelial dysfunction are four major pathways involved in the pathogenesis of COVID-19 (Kouhpayeh et al., 2020). Therapeutic candidates that inhibit RAS and quench oxidative stress would be relevant for COVID-19.
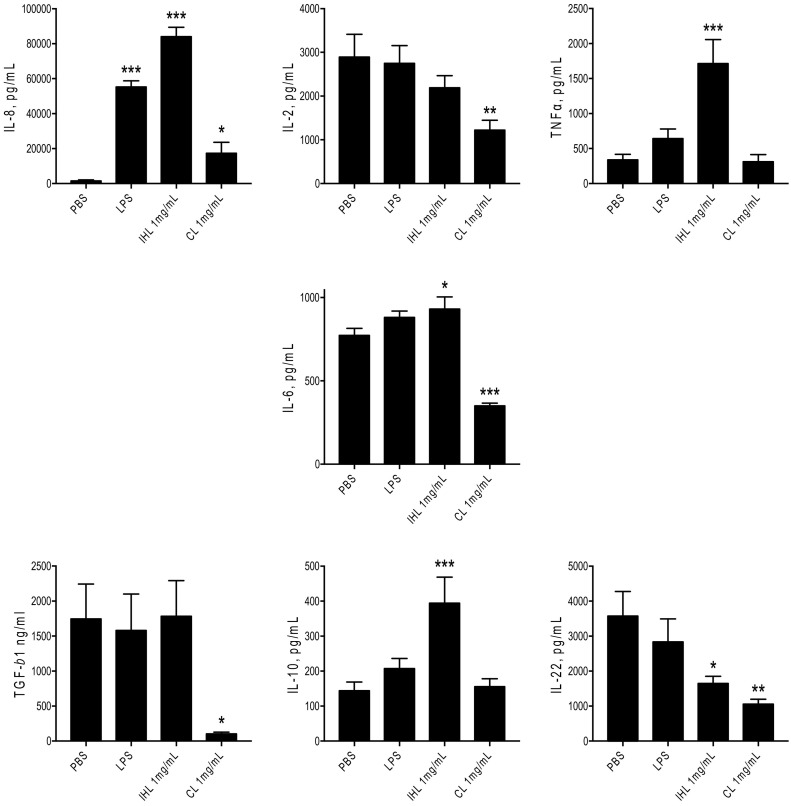
An important assay to determine glucan effect is measurement of cytokine production. In this study, we investigated the effect β-glucan samples had on pro and anti-inflammatory cytokine expression. Inflammatory chemokines and cytokines measured included IL-8, IL-2 and TNF-α. Pleiotropic cytokines included TGF-β1 and IL-6. Anti-inflammatory cytokines included IL-10 and IL-22 (Fig. 9 ). LPS (100 ng/mL) was also tested as it is a known inducer of inflammatory cytokines in immune cells. IHL (1 mg/mL) significantly induced the secretion of inflammatory mediators IL-8 and TNF-α. CL significantly induced the secretion of IL-8. IHL significantly increased the secretion of IL-10 and significantly decreased the secretion IL-22, as did CL. Other cytokines measured included IL-6 and TGF-β1. CL reduced the secretion of IL-6 and TGF-β1. Conversely, IHL significantly increased the secretion of IL-6 and CL decreased secretion. No differences in TGF-β1 concentration were observed when IHL was administered, however CL significantly reduced secretion.
Fig. 9.
The effects of β-glucan samples on cytokine expression of THP-1 cells preactivated with PMA. *p < .05; **p < .01; ***p < .001 versus PBS.
In conclusion, findings of this in-vitro investigation showed that β-glucan from Lentinus edodes demonstrated potential for the treatment of lung injury. When compared to a commercial source of the same mushroom, the in-house Lentinan extract contained higher levels of β-glucan and lower levels of α-glucan. Both Lentinan products reduced inflammation in a lung epithelial model, and IHL achieved this effect at lower doses. Physicochemical characterization studies found important differences in the composition of the Lentinan extracts, as determined by SEM, ATR-FTIR, and NMR, with the CL exhibiting higher amounts of alpha-glucans and correspondingly less β-glucans. The two Lentinan extracts demonstrated varying immunomodulatory activities. Both Lentinan extracts reduced cytokine induced NF-κB activation in human alveolar epithelial A549 cells, with the IHL extract proving more effective at lower doses. In contrast, in activated THP-1 derived macrophages, the CL extract more effectively attenuated pro-inflammatory cytokine production (TNF-α, IL-8, IL-2, IL-6, IL-22). The CL extract attenuated oxidative stress-induced early apoptosis, while the IHL extract attenuated late apoptosis. The implications of these findings infers that defining a reliable and repeatable source of β-glucan, where processes can be tailored to control chain lengths may potentially reduce key cytokines involved in cytokine storm experienced in severe cases of COVID-19. In the future, β-glucans may be delivered as a tailored cocktail matched against critical time-points in the form of future nutraceutical-based intervention for tackling COVID-19. Such β-glucans immunomodulatory cocktails may also have adjacent applications for addressing ARDS that is an important pathophysiological event seen with sepsis. These purified β-glucan combinational cocktails may be produced on a large commercial scale using bioreactors for global deployment (Tafuek et al., 2020).
To maintain functional bioactivity and to increase β-glucan yield, less harmful extraction processes are required that includes cessation of enzyme and harsh chemical usage as adopted using this IHL approach. Findings from this timely study highlight the putative potential for use β-glucan extractions from the edible mushroom Future research is also required to study putative relationship (if any) of variation in the extraction methods producing different β-glucan preparations and cytokine storm associated with COVID-19. L. edodes may also have future potential by way of influencing immunotherapies for addressing COVID-19 that rely on reducing cytokine storm. Further clinical studies are merited to refine β-glucan as a countermeasure for tackling cytokine storm that causes ARDS, as evident with COVID-19.
Funding
The authors acknowledge funding support from the Health Research Board Project [grant number HRB-HRA-POR-2014-664], Republic of Ireland, and Athlone Institute of Technology Doctoral Scholarship [grant number AIT0614], Republic of Ireland.
Contributions to publication
All author contributed equally to this research paper.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
The authors are grateful to all the researchers whom we cited in this study for their significant and valuable research.
Editor: Damia Barcelo
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2020.139330.
Appendix A. Supplementary data
Beta-glucan characterization supporting data from IHL and CL samples.
References
- Akramiene D. Effects of beta-glucans on the immune system. Medicina (Kaunas, Lithuania) 2007 doi: 10.3390/medicina43080076. [DOI] [PubMed] [Google Scholar]
- Bak W.C. Determination of glucan contents in the fruiting bodies and mycelia of Lentinula edodes cultivars. Mycobiology. 2014 doi: 10.5941/MYCO.2014.42.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellani G. The LUNG SAFE study: a presentation of the prevalence of ARDS according to the Berlin definition! Crit. Care. 2016 doi: 10.1186/s13054-016-1443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G.D., Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001 doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- Brown G.D., Gordon S. Immune recognition of fungal β-glucans. Cell. Microbiol. 2005 doi: 10.1111/j.1462-5822.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- Carballo C., Pinto P.I.S., Mateus A.P., Berbei C., Guerreiro C.C., Martinez-Blanch J.F., Condoner F.M., Mantecon L., Power D.M., Manchado M. Yeast beta-glucans and microalgal extracts modulate the immune response and gut microbiome in Senegalese sole (Solea senegalensis) Fish Shellfish Immunol. 2019;92:31–39. doi: 10.1016/j.fsi.2019.05.044. [DOI] [PubMed] [Google Scholar]
- Channappanavar R., Perlman S. Pathogenic human coronovirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichian S., Moazzami B., Sadoughi F., Haddad H., Marsa Zaroudi K., Azemi Z. Functional activities of beta-glucans in the prevention or treatment of cervical cancer. J. Ovarian Res. 2020;13:24. doi: 10.1186/s13048-020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017;39:517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- Conti P., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Frydas I., Kritas S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34(2) doi: 10.23812/CONTI-E. Mar 14. pii: 1. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Farrell H., Hayes J., Laffey J.G., Rowan N.J. Studies on the relationship between pulsed UV light irradiation and the simultaneous occurrence of molecular and cellular damage in clinically-relevant Candida albicans. J. Microbiol. Methods. 2011;84(2):317–326. doi: 10.1016/j.mimet.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Gil-Ramirez A. Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products. 2011. Edible mushrooms as potential sources of new hypocholesterolemic compounds. [Google Scholar]
- Herre J. Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood. 2004 doi: 10.1182/blood-2004-03-1140. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur, R., Sharma, M., Ji D, Xu M, Agyei, D. 2020. Structural features, modification, and functionalities of beta-glucan. Fibers. 8(1):1. https://doi.org/ 10.3390/fib8010001. [DOI]
- Kouhpayeh S., Shariati L., Boshtam M., Rahimmanesh I., Mirian M., Zeinalian M., Salari-jazi A., Khanahmad N., Damavandi M.S., Sadeghi P., Khanahmad H. 2020. The Molecular Story of COVID-19; NAD+ Depletion Addresses all Questions in this Infection. Preprints. 2020030346. [DOI] [Google Scholar]
- Kumar P. Role of edible mushrooms as functional foods — a review. South Asian J. Food Technol. Environ. 2015;1(3):211–218. [Google Scholar]
- Laffey J.G., Matthay M.A. Fifty years of research in ARDS. Cell based therapy for ARDS: biology and potential therapeutic value. Am. J. Respir. Crit. Care Med. 2017 doi: 10.1164/rccm.201701-0107CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.M. Oxidative stress, plasminogen activator inhibitor 1, and lung fibrosis. Antioxid. Redox Signal. 2008 doi: 10.1089/ars.2007.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwick J.A. Oxidative stress and steroid resistance in asthma and COPD: pharmacological manipulation of HDAC-2 as a therapeutic strategy. Expert Opin. Ther. Targets. 2007 doi: 10.1517/14728222.11.6.745. [DOI] [PubMed] [Google Scholar]
- Masterson C.H., Murphy E., Major I., Gonzalez H., O’Toole D., McCarthy S., Laffey J.G., Rowan N. Purified beta-glucan from the Lentinus edodes mushroom attenuates antibiotic resistant Klebsiella pneumonia-induced pulmonary sepsis. Am. J. Respir. Crit. Care Med. 2019;199:A1222. doi: 10.1111/lam.13358. https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A1222 2019. [DOI] [PubMed] [Google Scholar]
- McCarty M.F., DiNicolantonio J.J. Nutraceuticals have potential for boosting the type 1 interferon response to RNA viruses including influenza and coronavirus. Prog. Cardiovasc. Dis. 2020 doi: 10.1016/j.pcad.202.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales D. Carbohydrate Polymers. Elsevier Ltd; 2019. Isolation and comparison of α- and β-d-glucans from shiitake mushrooms (Lentinula edodes) with different biological activities; p. 115521. [DOI] [PubMed] [Google Scholar]
- Netea M.G. An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 2008 doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- Pelizon A.C. Immunomodulatory activities associated with β-glucan derived from Saccharomyces cerevisiae. Physiol. Res. 2005;54:557–564. [PubMed] [Google Scholar]
- Rahman I., MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur. Respir. J. 2000 doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]
- Rezoagli E., Fumagalli R., Bellani G. Definition and epidemiology of acute respiratory distress syndrome. Ann. Transl. Med. 2017 doi: 10.21037/atm.2017.06.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan N.J., Laffey J.G. Challenges and solutions for addressing critical shortage of supply chain for personal and protective equipment (PPE) arising from coronavirus disease (COVID-19) pandemic — case study from the Republic of Ireland. Sci. Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138532. https://www.sciencedirect.com/science/article/pii/S0048969720320453?via%3Dihub (Online pre-print 5th April, 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.E., Rowan N.J., Sullivan R. Medicinal mushrooms: a rapidly developing area of biotechnology for cancer therapy and other bioactives. Biotechnol. Lett. 2002;24(22):1839–1845. [Google Scholar]
- Stone B.A. Chemistry, Biochemistry, and Biology of 1–3 Beta Glucans and Related Polysaccharides. 2009. Chemistry of β-glucans. [DOI] [Google Scholar]
- Sullivan R., Smith J.E., Rowan N.J. Medicinal mushrooms and cancer therapy: translating a traditional practice into Western medicine. Perspect. Biol. Med. 2006;49(2):159–170. doi: 10.1353/pbm.2006.0034. [DOI] [PubMed] [Google Scholar]
- Taufek, N.M., Harith, H.H., Hafiz, M., ZulIlhamd, A. R., Rowan, N., Wan-Mohtar W.A.A.Q.I., 2020. Performance of Mycelial Biomass and Exopolysaccharide from Malaysian Ganoderma lucidum for the Fungivore Red Hybrid Tilapia (Oreochromis Sp.) in Zebrafish Embryo. vol 17 10.1016/j.aqrep.2020.100322. [DOI]
- Vaclav V. Placebo-driven clinical trials of transfer point glucan #300 in children with chronic respiratory problems: III. Clinical findings. Am. J. Immunol. 2013 doi: 10.3844/ajisp.2013.88.93. [DOI] [Google Scholar]
- Vermes I., Haanen C., Reutelingsperger C. Flow cytometry of apoptotic cell death. J. Immunol. Methods. 2000 doi: 10.1016/S0022-1759(00)00233-7. [DOI] [PubMed] [Google Scholar]
- Willment J.A. Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J. Immunol. 2003 doi: 10.4049/jimmunol.171.9.4569. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Advances in lentinan: isolation, structure, chain conformation and bioactivities. Food Hydrocoll. 2011 doi: 10.1016/j.foodhyd.2010.02.001. [DOI] [Google Scholar]
- Zhang, W., Zhao, Y., Zhang, F., Wang, Q., Li, T., Liu, Z., Wang, J., Qin, Y., Zhang, X., Yan, X., Zeng, Z., Zhang, S. 2020. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China, Clin. Immunol. (published online 25 March, 2025) https://doi.org/ 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed]
- Zheng R. Characterization and immunomodulating activities of polysaccharide from Lentinus edodes. Int. Immunopharmacol. 2005 doi: 10.1016/j.intimp.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Zhu F. β-Glucans from edible and medicinal mushrooms: characteristics, physicochemical and biological activities. J. Food Compos. Anal. 2015 doi: 10.1016/j.jfca.2015.01.019. [DOI] [Google Scholar]
- Zi Y. Lentinan inhibits oxidative stress and inflammatory cytokine production induced by benzo(a)pyrene in human keratinocytes. J. Cosmet. Dermatol. 2019 doi: 10.1111/jocd.13005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Beta-glucan characterization supporting data from IHL and CL samples.