Abstract
Background
Immunologic dysfunction due to coronavirus disease 2019 (COVID-19) is closely related to clinical prognosis, and the inflammatory response of pregnant women may affect the directional differentiation and function of fetal immune cells.
Objective
We sought to analyze the immune status of newborns from mothers with COVID-19 in the third trimester.
Methods
Along with collecting the clinical data from 51 newborns and their respective mothers, we recorded the immunophenotypes and cytokine and immunoglobulin levels of the newborns.
Results
None of the 51 newborns showed fever or respiratory distress during hospitalization. Detection of severe acute respiratory syndrome coronavirus 2 nucleic acid in pharyngeal swabs was negative. Except for the low level of CD16-CD56 cells, the count and proportion of lymphocytes, CD3, CD4, CD8, and CD19 were all in the normal range. Moreover, the serum IgG and IgM levels were within the normal range, whereas IL-6 showed increased levels. There was no correlation between maternal COVID-19 duration and the lymphocyte subsets or cytokine levels (IFN-γ, IL-2, IL-4, IL-6, IL-10, and TNF-α). There was a positive correlation between IL-6 and IL-10 levels and CD16-CD56 cells. One (1.96%) infant with an extremely elevated IL-6 concentration developed necrotizing enterocolitis in the third week after birth, and the remaining 50 infants did not show abnormal symptoms through the end of the follow-up period.
Conclusions
COVID-19 in the third trimester did not significantly affect the cellular and humoral immunity of the fetus, and there was no evidence that the differentiation of lymphocyte subsets was seriously unbalanced.
Key words: Newborn, pregnant women, COVID-19, third-trimester, lymphocyte subsets and cytokines
Abbreviations used: ALC, Absolute lymphocyte count; BW, Birth weight; COVID-19, Coronavirus disease 2019; GA, Gestational age; NEC, Necrotizing enterocolitis; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
Humans are generally susceptible to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),1 , 2 and pregnant women and newborns are potential at-risk groups for coronavirus disease 2019 (COVID-19).3, 4, 5 As of April 13, there were 1,773,084 people globally who were confirmed infected with SARS-CoV-2 in the COVID-19 pandemic, resulting in 111,652 deaths (World Health Organization, COVID-19 daily update).
Coronavirus infection in pregnant women can cause adverse consequences, such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, and stillbirth.6, 7, 8 Although there is no evidence of mother-to-child vertical transmission of SARS-CoV-2,3 , 9 it is urgent to clarify whether the maternal inflammatory response in the third trimester affects the development and physiological state of newborns.
The incidence of critical illness caused by COVID-19 is near 19%,2 , 5 , 10 of which most cases progress to acute respiratory distress syndrome and respiratory failure, accompanied by acute immune dysfunction.10 SARS-CoV-2 infection causes a sharp decline in lymphocyte counts, especially a reduction in CD4 T cells, accompanied by an uncontrolled release of inflammatory cytokines, leading to the second strike and aggravating pathological changes in the respiratory system.10 The clinical symptoms vary among the infected population,11 , 12 suggesting that individual immune status is related to COVID-19 susceptibility and that immune dysfunction may play an essential role in developing critical illness.
Because of the special immunologic status of pregnant women, the maternal inflammatory response to coronavirus infection may affect the structural and functional development of the fetus and neonate.6 , 13 In children, COVID-19 is mild or asymptomatic12; however, the virus can remain in the body for a long time, and viral nucleic acids can persist in feces, which implies that there is a possibility of nonrespiratory transmission in children.14 The immaturity of immunologic function in children and newborns leads to their increased susceptibility to viral infections, while the immaturity of adaptive immunologic development may make their clinical symptoms different from those in adults.13 Together, these aspects raise serious questions as to why clinical manifestations of infected children and newborns are different from those of adults with immunosuppression and what impact the inflammatory reaction caused by maternal infection has on the immunologic function of the fetus.
We speculate that the answers may be related to the immaturity of fetal immunity, especially dominant immune tolerance. Given the critical role of immunologic activity in COVID-19 pathogenesis and the possible influence of infected mothers on the differentiation of immunologic cells in newborns, we analyzed the immunologic status of newborns born to mothers with COVID-19 in the third trimester.15
Methods
Subjects
From January 20 to March 3, 2020, 71 newborns born to mothers with COVID-19 were admitted to the neonatal intensive care unit isolation ward of Zhongnan Hospital of Wuhan University. The included criterion was newborns whose mother had clinically confirmed or confirmed COVID-19.16 The exclusion criteria were premature infants of gestational age (GA) less than 35 weeks (12 cases), newborns with suspected congenital malformations (1 case), and children with incomplete laboratory data (7 cases). Thus, a total of 20 cases were excluded from the total cases enrolled in the study. Ultimately, 51 cases were eligible for further analysis.
Patient management during hospitalization
In Wuhan, as the epidemic center at the beginning of the COVID-19 pandemic, all patients including the pregnant woman and children who were admitted for hospitalization would take chest imaging scans during the epidemic as a supplementary tool for the detection of COVID-19,17 given the reality that the SARS-CoV-2 nucleic acid test showed a certain false-negative rate in the diagnosis of COVID-19.18 Hence, 51 pregnant women underwent chest imaging before delivery, with symptoms such as fever, cough, vomiting, and running nose, or were asymptomatic at a routine prenatal examination in this particular period. All of them showed imaging changes corresponding to viral pneumonia. Among them, 48 women had a cesarean section, and 3 women delivered naturally. All the women were wearing the medical surgical masks during surgery or delivery. The newborns were quickly isolated from the mothers within 10 minutes after delivery, transferred to the neonatal intensive care unit negative pressure isolation ward (−10 Pa), and placed in an incubator for observation. Apgar scores at 1 and 5 minutes were recorded, and the first pharyngeal swab was collected within 30 minutes after birth.
Specimen sample collection and detection
Pharyngeal swabs collected on days 0, 1, and 5 after birth were sealed in preservation solution and sent to the P3 laboratory of Zhongnan Hospital. Peripheral blood was collected on the day of birth and analyzed for routine blood tests, blood biochemistry, coagulation assays, and blood culture. Blood samples were collected within 3 days after birth for the detection of immunoglobulin levels, cytokine concentrations, and lymphocyte subsets.
Viral nucleic acid detection
RNA extraction and real-time quantitative RT-PCR were carried out to detect the SARS-CoV-2 nucleic acid according to a 2019 novel coronavirus (ORF1ab/N gene) nucleic acid detection kit (Bio-Germ Medical Technology Co., Ltd, Shanghai, China). When the RT-PCR results of pharyngeal swabs were “suspected to be positive,” the 3 consecutive follow-up pharyngeal swabs (1 day apart each time) were taken. The final result was judged as “false positive” if the 3 consecutive follow-up pharyngeal swab test results were negative. Once a positive result appeared, it would be defined as “positive.”
Detection of lymphocyte subsets, cytokines, and immunoglobulins
The expression of CD3, CD4, CD8, CD19, and CD16-CD56 on cells in peripheral blood cells was detected by a FACS CantoII Flow Cytometer (BD Biosciences, San Jose, Calif) according to the BD Multitest 6-Color TBNK Reagent instructions. Absolute cell counts of lymphocytes were calculated using BD Trucount tubes and analyzed by BD FACSCanto.
Serum levels of IFN-γ, IL-2, IL-4, IL-6, IL-10, and TNF-α were assayed by a FACS Calibur Flow Cytometer (BD Biosciences) according to the instructions of a flow fluorometer from Jiangxi Nuo De Medical Instrument Co., Ltd. Serum immunoglobulin levels of IgA, IgG, IgM, and IgE were determined by nephelometry (Siemens Healthcare Diag Prod GmbH, Marburg, Germany).
Treatment and outcomes
All newborns did not require mechanical ventilation during hospitalization, and few of them received low-flow nasal catheter oxygen for less than 3 days. Those with premature rupture of membranes or increased inflammation indicators received empirical antibiotics (piperacillin-tazobactam). Upon the negative blood culture and the rebound in inflammation indicators back to normal, the antibiotics were ceased. All infants were fed formula milk during hospitalization, and none of them received antiviral treatment. The newborns were discharged home for isolation until 3 consecutive nucleic acid test results were negative. Breast-feeding was recommended when the mother had been isolated for more than 14 days and the SARS-CoV-2 nucleic acid test result was negative.
Data collection
During hospitalization, the general vital signs, feeding, neurological reactions, and laboratory results of the newborns were continuously recorded. The families of the newborns received telephone follow-up of the baby’s general condition and abnormal clinical signs after discharge.
Statistical analysis
All the data are presented as the mean ± SD. Statistical analyses were performed using a 2-tailed unpaired t test, 1-way ANOVA, or linear regression analyses. A P value of less than .05 was considered significant. All statistical calculations were performed with Prism 8 (GraphPad Software, Inc, La Jolla, Calif).
Ethics approval
The present study was registered as a clinical study with the Chinese Clinical Trial Registry (ChiCTR-ORC-16008872), and the Institutional Review Board of Zhongnan Hospital of Wuhan University approved the study (approval no. 2015019). All guardians signed informed consent forms.
Results
Clinical features of pregnant women and newborns
Maternal status
Among the 51 pregnant women, the average age was 31.94 ± 4.02 years. Seven cases were positive for SARS-CoV-2 nucleic acid, of which 4 cases presented clinical symptoms (fever, cough) and the remaining 3 cases were asymptomatic. All mothers underwent chest computed tomography examination before admission, and all showed specific signs of COVID-19–induced pneumonia changes (ground-glass opacity, patchy or consolidation). Among them, 37 cases took chest computed tomography scans on the day of or 1 day before delivery; 14 cases took chest computed tomography scans 2 to 19 days before delivery. Clinical symptoms were presented in 24 (47.06%) of 51 cases before delivery. For the disease course, 10 cases (19.6%) had begun within 1 week, 14 cases (27.5%) had begun over 1 week before, and the longest onset was 28 days before delivery. The postpartum conditions of the women were stable, and no critical illness or death occurred (Table I ).
Table I.
Demographic and clinical features of 51 recruited newborns
| Categories | No. of infants | Percentage within group |
|---|---|---|
| Total | 51 | 100.00 |
| Sex | ||
| Female | 27 | 52.94 |
| Male | 24 | 47.06 |
| GA (wk) | ||
| ≥35-37 | 6 | 11.76 |
| >37-40 | 34 | 66.67 |
| >40 | 11 | 21.57 |
| BW (g) | ||
| <1500 | 0 | 0.00 |
| 1500-2500 | 4 | 7.84 |
| >2500 | 47 | 92.16 |
| Delivery mode | ||
| Eutocia | 3 | 5.88 |
| Cesarean | 48 | 94.12 |
| Clinical symptom | ||
| None | 41 | 80.39 |
| Fever, cough | 0 | 0.00 |
| Abdominal distension, vomiting | 10 | 19.61 |
| Ventilation support | ||
| Yes | 0 | 0.00 |
| No | 51 | 100.00 |
| Chest imaging result | ||
| Normal | 51 | 100.00 |
| Pneumonia changes | 0 | 0.00 |
| SARS-CoV-2 nucleic acid results | ||
| First | ||
| Positive, false positive, negative | 0, 3, 48 | 0.00, 5.89, 94.12 |
| Second | ||
| Positive, false positive, negative | 0, 3, 48 | 0.00, 5.89, 94.12 |
| Third | ||
| Positive, false positive, negative | 0, 0, 51 | 0.00, 0.00, 100.00 |
| Maternal basic information | ||
| Clinical symptom | ||
| None | 27 | 52.94 |
| Fever, cough, vomiting, running nose | ||
| Within 1 wk of delivery | 10 | 19.61 |
| Over than 1 wk of delivery | 14 | 27.45 |
| Chest CT result | ||
| Viral pneumonia changes | 51 | 100.00 |
| SARS-CoV-2 nucleic acid results | ||
| Positive, false positive, negative | 7, 0, 44 | 13.73, 0.00, 86.27 |
CT, Computed tomography.
Neonatal status
A total of 51 neonates (27 females [52.94%] and 24 males [47.06%]) were enrolled, and the median GA was 38 weeks (35+1 to 41+2 week), of which 6 (11.76%) were late preterm (≥35 week and <37 week) and 45 (88.24%) were full-term. The median birth weight (BW) was 3080 g (1990-3950 g), 47 (92.16%) with a BW above 2500 g. Apgar scores at 1 and 5 minutes were 8.90 and 9.90 points, respectively (Table I).
Pharyngeal swab virus nucleic acid monitoring
All newborns were monitored by throat swabs on days 0, 1, and 5 of hospitalization. SARS-CoV-2 nucleic acid results were judged to be “false positive” in 3 of 51 neonates at the first sampling and 3 at the second sampling. One case showed 2 consecutive false-positive results of pharyngeal swab nucleic acid tests after birth (while chest computed tomography examination result was normal); however, the 3 consecutive follow-up examination results of the 5 cases were negative. The remaining 46 cases had negative results for 3 consecutive nucleic acid tests (Table I).
Treatment of newborns
The 51 neonates were monitored by electrocardiograph after birth. No fever, cough, or nasal congestion occurred during hospitalization. Ten patients showed a transient sign of abdominal distension or vomiting. A chest imaging test was performed on the day of or the next day after admission, which showed no specific viral pneumonia changes (see Fig E1, A and B, in this article’s Online Repository at www.jacionline.org). Thirty-one neonates (60.8%) received antibiotics, 20 (39.2%) did not receive antibacterial treatment, and none received antiviral therapy. Except for 1 case of a GA 35+5-week neonate who had abdominal distension and bloody stools in the third week after birth and was diagnosed with necrotizing enterocolitis (NEC), the other neonates were in stable condition. The average hospital stay was 8.96 ± 4.96 days.
Fig E1.
Chest radiographs were performed on the day or the next day of admission, which showed no pneumonia changes. A, The chest computed tomography examination of 1 case with 2 consecutive suspected positive results of pharyngeal swab nucleic acid after birth. B, The chest x-ray picture of neonate born to SARS-CoV-2–infected mother.
Assessment of immune status in newborns
Newborn immune cell distribution
The routine blood and immune cell characterization within 3 days after birth is presented in Table II . The white blood cell count, absolute lymphocyte count (ALC), CD3 cells as percent of ALC (CD3%), CD3 cell absolute value, CD4 cells as percent of ALC (CD4%), CD4 cell absolute value, CD8 cells as percent of ALC (CD8%), CD8 cell absolute value, CD19 cells as percent of ALC (CD19%), and CD19 cell absolute value were all in the normal range.19 CD16-CD56 cells as percent of ALC (CD16-CD56%) and the CD16-CD56 cell absolute value were lower than those of reported references.19
Table II.
Laboratory results of white blood cell count, lymphocyte subsets, and cytokines in 51 newborns
| Test items | Median (95% CI) | Median (95% CI) | |
|---|---|---|---|
| WBC count (109/L) | 13.86 (12.40-15.33) | Lymphocyte count (109/L) | 3.20 (2.85-3.54) |
| CD3 T cells | 2442 (2196-2689) | CD3 as % of ALC | 77.66 (75.30-80.03) |
| CD4 T cells | 1852 (1656-2048) | CD4 as % of ALC | 58.68 (56.35-61.00) |
| CD8 T cells | 573.10 (497.80-648.40) | CD8 as % of ALC | 18.42 (16.69-20.15) |
| CD4/CD8 | 3.73 (3.16-4.30) | ||
| CD19 B cells | 443.70 (369.90-517.50) | CD19 as % of ALC | 13.56 (11.86-15.26) |
| CD16-CD56 natural killer cells | 238.70 (168.00-309.40) | CD16-CD56 as % of ALC | 6.62 (5.29-7.95) |
| IFN-γ (pg/mL) | 2.14 (0.86-1.91) | IL-6/IFN-γ | 21.48 (2.25-7.20) |
| IL-2 (pg/mL) | 0.99 (0.42-1.02) | IL-6/IL-2 | 42.64 (3.80-19.07) |
| IL-4 (pg/mL) | 0.71 (0.11-0.63) | IL-6/IL-4 | 69.53 (6.31-33.00) |
| IL-6 (pg/mL) | 10.82 (3.37-8.01) | ||
| IL-10 (pg/mL) | 3.59 (2.17-3.61) | IL-6/IL-10 | 3.67 (1.34-2.39) |
| TNF-α (pg/mL) | 0.41 (0.10-0.16) | IL-6/TNF-α | 65.17 (13.15-34.90) |
WBC, White blood cell.
Effect of maternal prepartum infection on the distribution of newborn immune cells
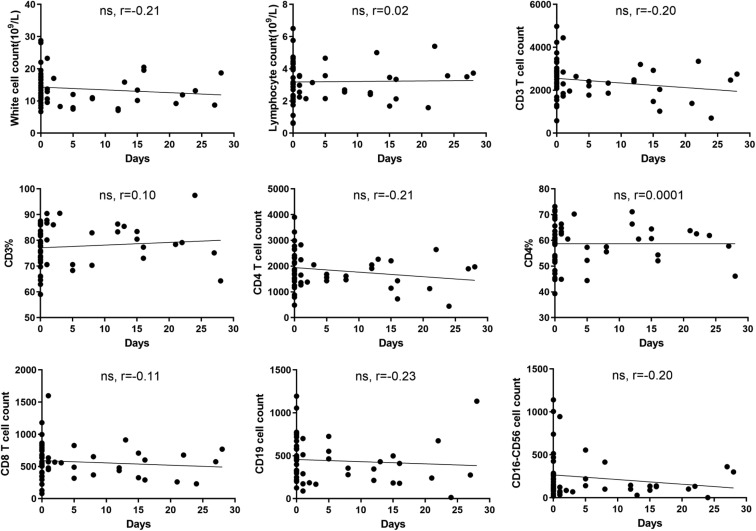
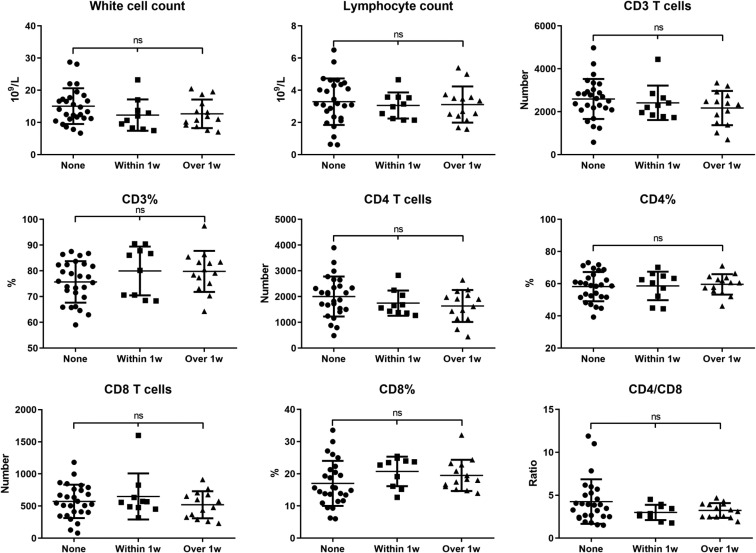
Because maternal infection in different stages may affect the immune status of the fetus, we performed correlation analysis between different immune cell subtypes and maternal infection duration. The results showed that as the time from onset to delivery extended, the white blood cell and CD3, CD4, CD8, CD19, and CD16-CD56 cell counts gradually decreased, but there was no statistical correlation among them (P > .05) (Fig 1 ). We therefore divided the 51 cases into 3 groups according to maternal clinical symptoms: the asymptomatic group (None, n = 27), onset to delivery within 1 week group (Within 1 week, n = 10), and onset to delivery over 1 week group (Over 1 week, n = 14). The results showed that there was no statistically significant difference in white blood cell, ALC, or CD3, CD4, and CD8 numbers among the 3 groups (P > .05) (Fig 2 ), which indicated that the span of maternal prepartum infection did not affect fetal lymphocyte differentiation. Among the 3 groups, the CD19% and CD19 cell counts of the Within and Over 1 week groups were lower than those of the None group (Fig 3 , A); the CD16-CD56% and CD16-CD56 cell counts in the Within and Over 1 week groups were slightly lower than those in the None group, but there was no statistical significance (P > .05) (Fig 4 , A).
Fig 1.
Maternal disease duration may not affect the number or percentage of immune cells in newborns. The data showed the correlations between maternal disease duration and white cells, lymphocytes, CD3 counts, CD3 counts as a percent of lymphocytes (CD3%), CD4 counts, CD4 counts as a percent of lymphocytes (CD4%), CD8 counts, CD19 counts, and CD16-CD56 counts. ns, Not significant.
Fig 2.
Preexisting maternal COVID-19–specific clinical symptoms may not affect lymphocyte subsets of newborns. We compared white cells, lymphocytes, CD3 counts, CD3 counts as a percent of lymphocytes (CD3%), CD4 counts, CD4 counts as a percent of lymphocytes (CD4%), CD8 counts, CD8 counts as a percent of lymphocytes (CD8%), and the CD4/CD8 ratio in the None, Within 1 w, and Over 1 w groups. Data are presented as the mean ± SD. ns, Not significant.
Fig 3.
Neonatal CD19 cell and IgG levels were not affected by maternal COVID-19; the IgG concentration was independent of the CD19 cell count. Number and percentages of CD19 cells (A) and IgG concentration (B) in the None, Within 1 w, and Over 1 w groups. Data are presented as the mean ± SD. ns, Not significant.
Fig 4.
Effect of maternal SARS-CoV-2 infection on CD16-CD56 cells and cytokines in newborns. A, Numbers and percentages of CD16-CD56 cells and CD16-CD56 cells as a percent of lymphocytes (CD16-CD56 cells%) in None, Within 1 w, and Over 1 w groups. B, Concentration of IFN-γ, IL-2, IL-4, IL-6, IL-10, and TNF-α in the None, Within 1 w, and Over 1 w groups. Data are presented as the mean ± SD. ns, Not significant.
The newborn serum immunoglobulin levels were analyzed and showed that IgG levels were similar in the None, Within 1 week, and Over 1 week groups and that there was no significant difference among them (Fig 3, B). IgM content was slightly higher (at an average level of 0.22 ± 0.04 g/L) than the detection threshold (<0.175 g/L) in 10 cases, whereas in the other 41 cases, the level of IgM was under the detection threshold. The CD19 lymphocyte cell counts in these 10 cases were within the normal range, which suggested that fetal CD19 cells were not activated in the uterus.
Effects on cytokines
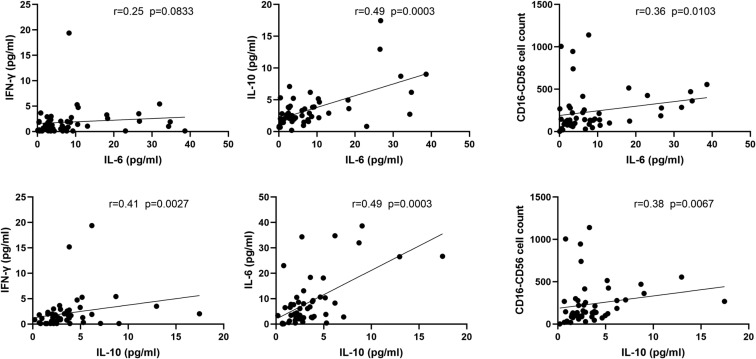
The cytokine levels of IFN-γ, IL-2, IL-4, IL-6, IL-10, and TNF-α among the 3 groups were analyzed (Fig 4, B). The levels of TNF-α and IL-6 were decreased uniquely in the Within and Over 1 week groups, but there was no significant difference; the levels of IFN-γ, IL-4, and IL-10 were decreased only in the Within 1 week group but showed no significant difference as well. However, IL-6 content correlated positively with IL-10 content and CD16-CD56 cell counts (P < .05) (Fig 5 ). In this data set, IL-6 content was not normally distributed, and the mean level (10.82 pg/mL) was higher than the reference range (0.1-2.9 pg/mL). Because IL-6 and IL-10 have promoting/tolerance effects in the inflammation process to control the usual inflammatory response, the imbalance between the 2 may lead to variable inflammation or immunosuppression,13 and we speculated that the imbalance between the 2 cytokines may lead to excessive inflammation or immunosuppression.
Fig 5.
A significant relationship was found between IL-6 and IL-10. The data present the concentrations of IL-6, IL-10, and IFN-γ and the number of CD16-CD56 cells. There were significant positive correlations between IL-6 and IL-10, IL 455 -6 and CD16-CD56 cell counts, IL-10 and IFN-γ, and IL-10 and CD16-CD56 cell counts.
For further study, we divided the patients into 4 groups: the IL-6 normal group (0.1-2.9 pg/mL, n = 17), the IL-6 elevated group (>2.9 pg/mL, n = 34), the IL-10 normal group (0.1-5.0 pg/mL, n = 41), and the IL-10 elevated group (>5.0 pg/mL, n = 10), according to the reference ranges of IL-6 and IL-10. The results showed that there were no significant differences in the newborns’ sex, GA, BW, positive rate of viral nucleic acid test results, chest imaging results, incidence of abnormal symptoms, or maternal COVID-19 progression clinical features (Table III ). However, 1 newborn (GA of 35+5 weeks, BW of 2.26 kg) was generally in good condition at birth and had no difficulty feeding, but her IL-6 level was the highest among the 51 neonates, with a value as high as 109.42 pg/mL, and her procalcitonin level was slightly augmented, reaching a maximum level of 6.74 ng/mL. Twenty-two days after birth, severe NEC occurred.
Table III.
Clinical features of IL-6, IL-10 normal and elevated groups
| Categories | IL-6 |
IL-10 |
||||
|---|---|---|---|---|---|---|
| Normal | Elevated | P value | Normal | Elevated | P value | |
| No. of cases | 17 | 34 | 41 | 10 | ||
| Sex | ||||||
| F | 9 | 18 | 22 | 5 | ||
| M | 8 | 16 | >.9999 | 19 | 5 | >.9999 |
| GA (wk) | ||||||
| <37 | 3 | 3 | 5 | 1 | ||
| ≥37 | 14 | 31 | .3871 | 36 | 9 | >.9999 |
| BW (kg) | ||||||
| <2.5 | 1 | 3 | 3 | 1 | ||
| ≥2.5 | 16 | 31 | >.9999 | 38 | 9 | >.9999 |
| SARS-CoV-2 nucleic acid results | ||||||
| First | ||||||
| Positive | 0 | 0 | 0 | 0 | ||
| False positive | 0 | 3 | 2 | 1 | ||
| Negative | 17 | 31 | .542 | 39 | 9 | .4881 |
| Second | ||||||
| Positive | 0 | 0 | 0 | 0 | ||
| False positive | 2 | 1 | 3 | 0 | ||
| Negative | 15 | 33 | .2547 | 38 | 10 | >.9999 |
| Third | ||||||
| Positive | 0 | 0 | 0 | 0 | ||
| False positive | 0 | 0 | 0 | 0 | ||
| Negative | 17 | 34 | 41 | 10 | ||
| Chest imaging results | ||||||
| Normal | 17 | 34 | 41 | 10 | ||
| Viral pneumonia changes | 0 | 0 | 0 | 0 | ||
| Maternal basic information | ||||||
| Clinical symptom | ||||||
| None | 10 | 17 | 22 | 5 | ||
| Fever, cough, vomiting | 7 | 17 | .7666 | 19 | 5 | .355 |
| Chest CT results | ||||||
| Normal | 0 | 0 | 0 | 0 | ||
| Viral pneumonia changes | 17 | 34 | 41 | 10 | ||
| SARS-CoV-2 nucleic acid results | ||||||
| Positive | 4 | 3 | 4 | 3 | ||
| False positive | 0 | 0 | 0 | 0 | ||
| Negative | 13 | 31 | .2033 | 37 | 7 | .1262 |
CT, Computed tomography.
Discussion
In this article, we have discussed the immunologic characteristics of 51 neonates with a risk of intrauterine exposure to SARS-CoV-2 in the COVID-19 pandemic from Wuhan, China. With the strictly protective measures, none of the 51 newborns suffered from COVID-19.
Our study is unique in demonstrating that intrauterine exposure poses no serious threat to the development of cellular and humoral immune function in near-term and full-term infants. However, the biased expression of inflammatory factors may be an important indicator of complications. To our knowledge, this study is the first comprehensive and large-scale assessment report on the immune status of this population. The results from this study may further help us understand the immune function of newborns delivered by mothers infected with SARS-CoV-2 and improve treatment and prevention strategies.6 , 7 , 20
Although no evidence of vertical transmission of SARS-CoV-2 from mother to child has been found,3 , 4 , 21 the potential effects of the maternal inflammatory response on development and immunologic function in exposed neonates are unknown.20 , 22 As the epidemic situation in China has gradually been controlled, a spreading trend was focused in Europe and the Middle East.23 Elderly individuals and those with underlying diseases are at a high risk of developing critical illness or even dying.2 Newborns and children who are immunocompromised or immune immature suffer relatively few infections, accounting for approximately 2.0% of the total cases confirmed, and have a low rate of severe and critical illness.5 , 24 The incidence of COVID-19 in pregnant women or the proportion of the entire infected population has not yet been reported, but according to epidemiological data of SARS and Middle East respiratory syndrome, maternal mortality rates are as high as 25% and 23%.6 , 7 Coronavirus infection in pregnant women can cause severe complications and multiple organ damage in newborns.25 , 26
One patient developed abdominal distension and bloody stools and rapidly developed NEC in the third week after birth. Her blood and stool cultures and rotavirus and enterovirus detection test results were all negative, which is very similar to the 2 cases of neonatal intestinal perforation reported during the SARS epidemic.25 Five cases of SARS-CoV-2 nucleic acid tests were judged to be “false positive” the first or second time; and none of the 5 newborns developed clinical manifestations, such as fever, respiratory abnormalities, and gastrointestinal symptoms. We speculate that this “false positive” is not caused by vertical transmission but may be related to the detection sensitivity of reagents or the presence of trace virus or sample contamination in the environment.
Because of the role of the placental barrier, the newborn is in a relatively closed environment in the uterus.27 CD4+CD25+Foxp3 regulatory T cells play a dominant role in the formation of immune tolerance with the mother.15 , 27 , 28 After the birth of the newborn, immune cells are stimulated by external antigens, thus affecting their later directional differentiation. This feature may lead to atypical symptoms when neonatal infections occur, but pathogenic microorganisms tend to spread easily.29 , 30 The absolute value of each lymphocyte subgroup in these neonates was in the normal range, and T cells, CD19 cells, and CD16-CD56 cells were positively correlated with lymphocyte counts. This result indicates that COVID-19 mothers did not affect the differentiation of fetal lymphocytes. Serum IgM levels in most of the cases were not increased; only in 10 (19.61%) cases did IgM levels increase slightly, suggesting that the fetus may not be at risk of virus exposure or infection in utero.15 , 31
In this group of data, with the exception of IL-6 and IL-10, there was no abnormal increase in other cytokines in newborns. In the first week after birth, a large amount of IL-6 can be produced after infection in neonates, increasing the resistance to infection and aiding healing of damaged tissues at birth.32 Studies have shown that regulatory T cells expressing IL-10 exert immune tolerance and control the intensity of the inflammatory response. IL-6 promotes TH2 and TH17 cell differentiation, participating in systemic inflammation and autoimmune responses.31 IL-10 and IL-6 maintain the balance of a moderate inflammatory response and prevent the IL-6–mediated excessive amplified TH17 inflammatory response from becoming uncontrolled.30
In our study, the reason for a nondecreased level of IL-10 in newborns may be the continued existence of fetal immune tolerance after birth. The elevated level of IL-6 was positively correlated with that of IL-10, suggesting that fetal immune tolerance was not affected by maternal SARS-CoV-2 infection. In the case of the newborn with NEC, IL-6 content was as high as 109.26 pg/mL (38-fold increase), and the IL-6/IL-10 ratio was 28.8, which was 9.5 times higher than the average level of 3.0. This result implied that the abnormal increase in IL-6 levels without IL-10 regulation might have been an essential indicator of complications. Because there was only 1 case in the study, a further study is needed to clarify the clinical implication of IL-6/IL-10.
There are limitations to the study. First, a healthy control group could not be set for ethical reasons. Second, the follow-up time was limited. Although the newborns were monitored for immune function at birth, dynamic changes in the indexes were not detected afterward. Moreover, the types of immune cell subtypes and cytokines were limited, and further interpretation of lymphocyte function has not yet been made.
Conclusions
Our results showed that the mother’s infection had no significant effect on the cellular and humoral immunologic status of the newborn and that lymphocyte differentiation was not seriously unbalanced. However, we should pay attention to the relationship between the abnormal elevation of IL-6 levels and serious complications.
Clinical implications.
COVID-19 in the third trimester does not affect the cellular or humoral immunity of the fetus; abnormally elevated IL-6 levels may be related to complications seen in patients with COVID-19.
Acknowledgments
The opinions expressed reflect the collective views of the coauthors. We thanks Dr Nitin Saksena, Head of Epigenes, Melbourne, Australia, for editing the manuscript.
Footnotes
This work was supported by the Science and Technology Department of Hubei Province (grant no. 2019-nCoV. No. 2020FCA011).
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Appendix
References
- 1.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang P., Liu P., Li D., Zhao D. Corona virus disease 2019, a growing threat to children? J Infect. 2020;80:671–693. doi: 10.1016/j.jinf.2020.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA [published online ahead of print February 24, 2020]. https://doi.org/10.1001/jama.2020.2648. [DOI] [PubMed]
- 6.Schwartz D.A., Graham A.L. Potential maternal and infant outcomes from coronavirus 2019-nCoV (SARS-CoV-2) infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12:194. doi: 10.3390/v12020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen S.A., Smulian J.C., Lednicky J.A., Wen T.S., Jamieson D.J. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstetr Gynecol. 2020;222:415–426. doi: 10.1016/j.ajog.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong S.F., Chow K.M., Leung T.N., Ng W.F., Ng T.K., Shek C.C. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang P., Wang X., Liu P., Wei C., He B., Zheng J. Clinical characteristics and risk assessment of newborns born to mothers with COVID-19. J Clin Virol. 2020;127:104356. doi: 10.1016/j.jcv.2020.104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poon L.L.M., Peiris M. Emergence of a novel human coronavirus threatening human health. Nat Med. 2020;26:317–319. doi: 10.1038/s41591-020-0796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz AT, Zeichner SL. COVID-19 in children: initial characterization of the pediatric disease [published online ahead of print March 16, 2020]. Pediatrics. https://doi.org/10.1542/peds.2020-0834. [DOI] [PubMed]
- 13.Kollmann T.R., Kampmann B., Mazmanian S.K., Marchant A., Levy O. Protecting the newborn and young infant from infectious diseases: lessons from immune ontogeny. Immunity. 2017;46:350–363. doi: 10.1016/j.immuni.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olin A., Henckel E., Chen Y., Lakshmikanth T., Pou C., Mikes J. Stereotypic immune system development in newborn children. Cell. 2018;174:1277–1292.e14. doi: 10.1016/j.cell.2018.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.China NHCotPsRo, ed. National Health Commission of the People's Republic of China; China: 2020. China NHCO. Diagnosis and treatment of COVID-19 (5th Edition) [In Chinese] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases [published online ahead of print February 26, 2020]. Radiology. https://doi.org/10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed]
- 18.Xiao AT, Tong YX, Zhang S. False-negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: rather than recurrence [published online ahead of print April 9, 2020]. J Med Virol. https://doi.org/10.1002/jmv.25855. [DOI] [PMC free article] [PubMed]
- 19.Amatuni G.S., Sciortino S., Currier R.J., Naides S.J., Church J.A., Puck J.M. Reference intervals for lymphocyte subsets in preterm and term neonates without immune defects. J Allergy Clin Immunol. 2019;144:1674–1683. doi: 10.1016/j.jaci.2019.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chua MSQ, Lee JCS, Sulaiman S, Tan HK. From the frontlines of COVID-19—how prepared are we as obstetricians: a commentary [published online ahead of print May 5, 2020]. BJOG. https://doi.org/10.1111/1471-0528.16257. [DOI] [PubMed]
- 21.Liu Y, Chen H, Tang K, Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy [March 4, 2020]. J Infect. https://doi.org/10.1016/j.jinf.2020.02.028. [DOI] [PMC free article] [PubMed]
- 22.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P. Coronavirus infections and immune responses. J Med Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bordi L., Nicastri E., Scorzolini L., Di Caro A., Capobianchi M.R., Castilletti C. Differential diagnosis of illness in patients under investigation for the novel coronavirus (SARS-CoV-2), Italy, February 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.8.2000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Q., Chen Y.-C., Chen C.-L., Chiu C.-H. SARS-CoV-2 infection in children: transmission dynamics and clinical characteristics. J Formosan Med Assoc. 2020;119:670–673. doi: 10.1016/j.jfma.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shek C.C., Ng P.C., Fung G.P., Cheng F.W., Chan P.K., Peiris M.J. Infants born to mothers with severe acute respiratory syndrome. Pediatrics. 2003;112:e254. doi: 10.1542/peds.112.4.e254. [DOI] [PubMed] [Google Scholar]
- 26.Kam KQ, Yung CF, Cui L, Lin Tzer Pin R, Mak TM, Maiwald M, et al. A well infant with coronavirus disease 2019 (COVID-19) with high viral load [published online ahead of print February 28, 2020]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa201. [DOI] [PMC free article] [PubMed]
- 27.Vishnu Bhat B., Manoj Kumar Kingsley S. New York: Elsevier Inc; 2018. Innate immunity at birth; pp. 15–35. [Google Scholar]
- 28.Weitkamp J.H., Lewis D.B., Levy O. 10th Ed. Elsevier, Inc; New York: 2018. Immunology of the fetus and newborn: Avery’s diseases of the newborn. pp453-481.e7. [Google Scholar]
- 29.Jansen J.M., Gerlach T., Elbahesh H., Rimmelzwaan G.F., Saletti G. Influenza virus-specific CD4+ and CD8+ T cell-mediated immunity induced by infection and vaccination. J Clin Virol. 2019;119:44–52. doi: 10.1016/j.jcv.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Heinonen S., Rodriguez-Fernandez R., Diaz A., Oliva Rodriguez-Pastor S., Ramilo O., Mejias A. Infant immune response to respiratory viral infections. Immunol Allergy Clin North Am. 2019;39:361–376. doi: 10.1016/j.iac.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Debock I., Flamand V. Unbalanced neonatal CD4(+) T-cell immunity. Front Immunol. 2014;5:393. doi: 10.3389/fimmu.2014.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]