Abstract
Study objective
Most coronavirus disease 2019 (COVID-19) reports have focused on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) positive patients. However, at initial presentation, most patients’ viral status is unknown. Determination of factors that predict initial and subsequent need for ICU and invasive mechanical ventilation is critical for resource planning and allocation. We describe our experience with 4,404 persons under investigation and explore predictors of ICU care and invasive mechanical ventilation at a New York COVID-19 epicenter.
Methods
We conducted a retrospective cohort study of all persons under investigation and presenting to a large academic medical center emergency department (ED) in New York State with symptoms suggestive of COVID-19. The association between patient predictor variables and SARS-CoV-2 status, ICU admission, invasive mechanical ventilation, and mortality was explored with univariate and multivariate analyses.
Results
Between March 12 and April 14, 2020, we treated 4,404 persons under investigation for COVID-19 infection, of whom 68% were discharged home, 29% were admitted to a regular floor, and 3% to an ICU. One thousand six hundred fifty-one of 3,369 patients tested have had SARS-CoV-2–positive results to date. Of patients with regular floor admissions, 13% were subsequently upgraded to the ICU after a median of 62 hours (interquartile range 28 to 106 hours). Fifty patients required invasive mechanical ventilation in the ED, 4 required out-of-hospital invasive mechanical ventilation, and another 167 subsequently required invasive mechanical ventilation in a median of 60 hours (interquartile range 26 to 99) hours after admission. Testing positive for SARS-CoV-2 and lower oxygen saturations were associated with need for ICU and invasive mechanical ventilation, and with death. High respiratory rates were associated with the need for ICU care.
Conclusion
Persons under investigation for COVID-19 infection contribute significantly to the health care burden beyond those ruling in for SARS-CoV-2. For every 100 admitted persons under investigation, 9 will require ICU stay, invasive mechanical ventilation, or both on arrival and another 12 within 2 to 3 days of hospital admission, especially persons under investigation with lower oxygen saturations and positive SARS-CoV-2 swab results. This information should help hospitals manage the pandemic efficiently.
Introduction
Background
Coronavirus disease 2019 (COVID-19), which originated in China in December 2019, has now reached pandemic proportions.1 Although most publications have rightfully focused on patients who had a positive polymerase chain reaction (PCR) test result for SARS-CoV-2,2, 3, 4, 5, 6, 7, 8 stress on the health care system has also occurred because of a surge in the number of persons under investigation with symptoms possibly but not exclusively caused by COVID-19. Because of shortages in testing supplies, delays in reporting the results of viral testing, false-negative test results, and daily fluctuations in test results within individual patients,9 , 10 all persons under investigation should be considered to have COVID-19 until proven otherwise.
Editor’s Capsule Summary.
What is already known on this topic
We are in the middle of a coronavirus disease 2019 pandemic. There have been few detailed descriptions of demographics, presentations, and clinical course of undifferentiated emergency department (ED) patients with symptoms suggestive of this illness.
What question this study addressed
What are the characteristics and outcomes of patients presenting to a large New York State adjacent academic ED during early months of the coronavirus disease 2019 pandemic?
What this study adds to our knowledge
Among 4,404 patients, the most common symptoms were cough (72%), fever (63%), dyspnea (43%), myalgias (23%), fatigue (14%), and diarrhea (14%); 57% of those tested were SARS-CoV-2 positive. During the course of this 1-month period, persons under investigation composed the majority of ED visits and admissions. Sixty-eight percent of persons under investigation were discharged home (3% were later readmitted), 29% were admitted to a regular floor, and 3% were admitted to an ICU. Of patients initially admitted to a regular floor, 13% were later transferred to the ICU. By the end of the study period, 2.3% of patients had died.
How this is relevant to clinical practice
Studies of this kind help us understand the disease and anticipate future resource needs.
Importance
Our hospital emergency department (ED) had its first person under investigation on February 7, 2020. Since then, we have observed an increasing number of persons under investigation, and as of April 14, 2020, we have treated approximately 4,600 such patients, with increasing SARS-CoV-2 rule-in rates. Our administration and clinical services have responded by rapid expansion of our capacity, including the ED, and most recently the opening of a field ED tent (with the help of the New York State Department of Health, New York State Department of Environmental Conservation, New York State Department of Homeland Security, and National Guard), in which many of the less ill persons under investigation are treated. Because of nationwide shortages, it is important to be able to predict real-time, future needs for ICU beds and mechanical ventilators according to the number and type of patients arriving at the ED with suspected COVID-19 and number of admissions to regular floors. This would give critical lead time to allocate resources most wisely both now and in future anticipated pandemics, helping to deal efficiently with them.
Goals of This Investigation
In this report, we present a cohort of 4,404 persons under investigation and compare patient clinical characteristics and outcomes based on whether their test results were positive or negative for SARS-CoV-2. We also performed an exploratory analysis regarding patient factors that predicted the need for ICU-level care, need for invasive mechanical ventilation, and death. It is our hope that these data will help other health care institutions in planning for and responding to the COVID-19 pandemic and similar future pandemics.
Materials and Methods
Study Design
We performed a structured, retrospective chart review, consistent with the recommended methodology of Kaji et al,11 in all persons under investigation and presenting to our ED with symptoms suggestive of COVID-19. Our study followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines for cross-sectional studies (http://www.equator-network.org/reporting-guidelines/strobe/). Because of the retrospective design, we received institutional review board approval with waiver of informed consent.
Setting and Selection of Participants
We performed a computerized search of our electronic medical records to identify all patients with a physician order for COVID-19 investigation. In early February, we added to our electronic medical record a specific computerized order for persons under investigation to indicate that the physician suspected that the patient might have COVID-19. Although the definition of persons under investigation for COVID-19 has evolved, we included any patient with signs or symptoms of a flulike illness, including but not limited to fever, cough, shortness of breath, fatigue, myalgia, sore throat, diarrhea, or loss of smell or taste. Patients of all ages were included. Eligible patients presented from March 12, 2020, to April 14, 2020. Our ED is a large, tertiary care, suburban, academic medical center with greater than 100,000 annual ED visits. Our medical center is also a major referral center for the county of Suffolk, NY, on the eastern end of Long Island, with greater than 1.5 million inhabitants.
To expand our capacity to treat persons under investigation, on March 9, 2020, we opened a 16-bed unit in an ambulatory care pavilion that was not being used, in which we treated the less ill patients under investigation. To further expand our capacity, we moved to a field tent on March 24, 2020, on the other side of our university campus. This move was done to create more ED space while the ambulatory care pavilion was being prepared for conversion to an inpatient unit. Use of PCR testing for SARS-CoV-2 varied according to evolving Centers for Disease Control and Prevention recommendations and availability. In general, all patients presenting to the ED tent were tested, whereas all patients presenting to the main ED who required hospital admission and symptomatic health care workers were also tested.
Data Collection and Processing
The source of all data was the electronic medical record. Extraction of data was performed both manually (eg, for items present in the various clinical notes and radiology reports) and automatically (eg, for vital signs and laboratory results). For eligible patients, we extracted demographic information, comorbidities, symptoms, exposure history, vital signs, laboratory results, chest radiograph and chest computed tomographic (CT) imaging results, disposition, (discharge to home, admission to a non–ICU floor, or admission to ICU), and treatments (invasive and noninvasive mechanical ventilation). We defined all study data and variables before initiating the study and trained our data abstractors by using a library of definitions. We periodically monitored data collection and determined the interobserver agreement on the primary outcome on a randomly selected sample of 20 study patients. Interobserver agreement (κ statistic) for invasive mechanical ventilation and ICU admission was excellent (1.0 for both). Agreement for subsequent ICU upgrade was 0.89 (95% confidence interval 0.67 to 0.99).
Outcome Measures
The primary outcomes were ICU admission, invasive mechanical ventilation, and mortality. Secondary outcomes were hospital admission and whether patients had positive test results for SARS-CoV-2 PCR.
Primary Data Analysis
Data are summarized as numbers and frequencies for nominal data and means with SD, or medians with interquartile ranges (IQRs) for continuous data. For all variables and models, we used only the initial findings at ED presentation. Comparisons between groups were performed with χ2 or Fisher’s exact test for categoric data and t tests or Mann-Whitney U tests for continuous data. Exploratory multivariate analysis of the primary and secondary outcomes was performed with potential predictor variables chosen according to biological plausibility and previous reports. Level of significance was defined as P ≤ .05. The rates of ICU admission, invasive mechanical ventilation, and death were calculated with the total number of hospital admissions as the denominator.
Results
Characteristics of Study Subjects
Between March 12, 2020, and April 14, 2020, our ED treated 4,404 persons under investigation, of whom 3,003 (68%) were discharged home, 1,267 (29%) were admitted from the ED to a regular floor, and 122 (3%) were admitted directly from the ED to an ICU; there were 12 deaths in the ED. Of all persons under investigation, 558 were treated in the ambulatory care pavilion and 1,422 in the field tent.
Median age of all persons under investigation was 47 years (IQR 33 to 60 years), 51% were men, 11% were health care workers, and 3.4% were younger than 18 years. Comorbidities included hypertension (25%), diabetes (13%), asthma (9%), coronary artery disease (8%), chronic obstructive pulmonary disease (4%), heart failure (3%), cancer (5%), immunosuppression (4%), chronic kidney disease (4%), previous smoking (2%), and current smoking (6%).
Most common symptoms were cough (72%), fever (63%), shortness of breath (43%, with sputum in 10.4%), myalgias (23%), fatigue (14%), and diarrhea (14%). Sick contacts were reported in 41% of persons under investigation and exposure to a confirmed case of COVID-19 in 28%. Of 2,606 chest radiographs, 1,346 (52%) had an opacity, of which 1,010 (75%) were bilateral. Of 579 chest CTs, 374 (65%) have had an opacity, of which 299 (80%) were bilateral.
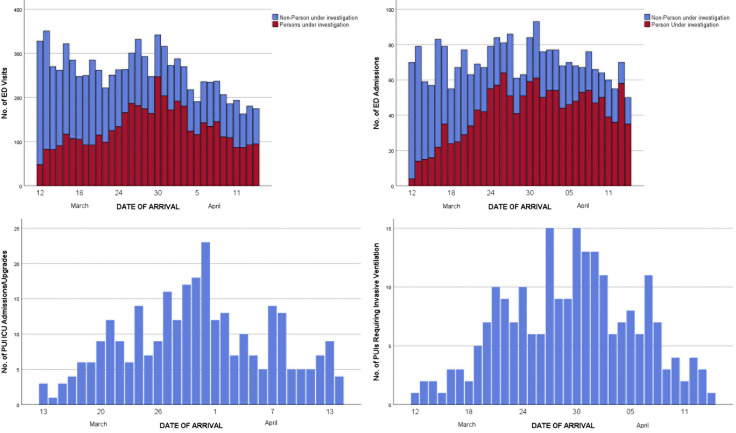
Of 1,267 patients initially admitted to a regular floor, 169 (13%) were upgraded to ICU care within a median of 62 hours (IQR 28 to 106 hours). The number of ED visits of persons under investigation and those not under investigation over time is presented in the Figure . Of all patients treated, 3,369 (76.5%) were tested for SARS-CoV-2. Of 2,897 SARS-CoV-2 tests available to date, 1,651 (57%) were positive and 1,246 (43%) were negative. Invasive mechanical ventilation was required for 221 patients, 4 in the out-of-hospital setting, 50 in the ED, and 167 after admission, within a median 60 hours (IQR 26 to 99 hours). Of patients intubated, 42 have been extubated and discharged, 47 have been extubated and are still in the hospital, and 58 died in the hospital. The remaining patients are still intubated. Median length of intubation for patients already extubated or who died was 6 days (IQR 2 to 10 days). Median time receiving mechanical ventilation was 4.7 days (IQR 2.6 to 8.5 days) for patients who died and 7.1 days (IQR 3.4 to 9.8 days) for the survivors. Between March 12, 2020, and April 14, 2020, 103 patients died, of whom 72 had tested positive for SARS-CoV-2. Overall mortality rate was 103 of 4,404, or 2.3% (95% confidence interval 1.9% to 2.8%).
Figure.
Temporal variation in ED visits (upper left), admissions to a regular floor (upper right), admissions from the ED to the ICU (bottom left), and total number of invasive mechanical ventilations from the ED and previously admitted patients (bottom right).
Of 3,003 visits from persons under investigation that resulted in discharge directly from the ED, 187 patients revisited our ED during the study period; 76 were admitted to a regular floor and 5 directly to the ICU. Thirteen of the admitted patients required invasive mechanical ventilation, and of the 76 floor admissions, 12 were later upgraded to an ICU. Currently, of the 81 admitted patients 42 have been discharged home and 1 died.
Table 1 summarizes patient characteristics and outcomes based on the results of PCR tests. Patients with positive test results for SARS-CoV-2 were more likely to have abnormal findings and bilateral opacities on chest imaging than those with negative results. Table 2 compares patients requiring invasive mechanical ventilation and those not requiring it. Comparison of patients based on whether they were admitted to an ICU is presented in Table E1, available online at http://www.annemergmed.com. The results of the exploratory multivariate analyses are presented in Table E2, available online at http://www.annemergmed.com. Multivariate predictors of positive test results for SARS-CoV-2 were exposure to COVID-19, cough, fever, Hispanic race, smoking history, and hypoxemia. Multivariate predictors of ICU admission or upgrades were positive test results for SARS-CoV-2 and hypoxemia. Multivariate predictors of invasive mechanical ventilation were positive test results for SARS-CoV-2, male sex, hypoxemia, and increased respiratory rate. Multivariate predictors of death were positive test results for SARS-CoV-2, history of chronic obstructive pulmonary disease, age, and hypoxemia.
Table 1.
Comparison of persons under investigation with COVID-19 positive and negative test results (N=2,897 cases with test results completed).
| All PUIs |
Admitted PUIs |
|||||
|---|---|---|---|---|---|---|
| COVID-19 Positive | COVID-19 Negative | Difference (95% CI) | COVID-19 Positive | COVID-19 Negative | Difference (95% CI) | |
| Demographics | ||||||
| No. patients | 1,651 | 1,246 | 737 | 392 | ||
| Age, mean (SD), y | 50 (18) | 47 (20) | 3 (2 to 5) | 60 (18) | 62 (21) | 2 (–4 to 1) |
| Men, No. (%) | 892 (54) | 594 (48) | 6 (3 to 10) | 423 (57) | 209 (53) | 4 (–2 to 10) |
| White, No. (%) | 695 (42) | 797 (64) | –22 (–25 to –18) | 353 (48) | 284 (72) | –25 (–30 to –19) |
| Black, No. (%) | 114 (7) | 97 (8) | –1 (-3 to 1) | 48 (7) | 23 (6) | 1 (–2 to 4) |
| Asian, No. (%) | 55 (3) | 70 (6) | –2 (–4 to –1) | 31 (4) | 15 (4) | 0.4 (–2 to 3) |
| Other, No. (%) | 14 (1) | 9 (1) | 0 (–1 to 1) | 8 (1) | 2 (1) | 0.6 (–1 to 2) |
| Unknown, No. (%) | 773 (47) | 273 (22) | 25 (21 to 28) | 297 (40) | 68 (17) | 23 (17 to 29) |
| Hispanic, No. (%) | 607 (37) | 210 (17) | 20 (17 to 23) | 197 (27) | 50 (13) | 14 (9 to 19) |
| Sick contact, No. (%) | 745 (45) | 544 (44) | 1 (–2 to 5) | 239 (33) | 45 (12) | 21 (16 to 26) |
| COVID-19 contacts, No. (%) | 533 (32) | 387 (31) | 1 (–2 to 5) | 124 (17) | 19 (5) | 12 (8 to 16) |
| HCW, No. (%) | 136 (8) | 259 (21) | –13 (–15 to –10) | 18 (2) | 6 (2) | 0.9 (–0.01 to 3) |
| Nursing home, No. (%) | 113 (7) | 58 (5) | 1 (0.5 to 4) | 107 (15) | 57 (15) | 0 (–4 to 4) |
| Comorbidities, No. (%) | ||||||
| HTN | 461 (28) | 307 (25) | 3 (2 to 7) | 341 (46) | 201 (51) | –5 (–11 to 1) |
| DM | 254 (15) | 123 (10) | 6 (3 to 8) | 196 (27) | 90 (23) | 4 (–2 to 9) |
| Asthma | 106 (6) | 142 (11) | –5 (–7 to –3) | 48 (7) | 39 (10) | –3 (–7 to –0.01) |
| CAD | 122 (7) | 126 (10) | –3 (–5 to –1) | 102 (14) | 97 (25) | –11 (–16 to –6) |
| COPD | 59 (4) | 69 (6) | –2 (–4 to –0.4) | 54 (7) | 57 (15) | –7 (–11 to –4) |
| CHF | 44 (3) | 63 (5) | –2 (–4 to –1) | 39 (5) | 59 (15) | –10 (–13 to –6) |
| Cancer | 66 (4) | 90 (7) | –3 (–5 to –2) | 49 (7) | 66 (17) | –10 (–14 to –7) |
| Immunosuppressed | 66 (4) | 73 (6) | –2 (–4 to –0.02) | 49 (7) | 43 (11) | –4 (–8 to –1) |
| CKD | 75 (5) | 58 (5) | –0.1 (–2 to 2 | 67 (9) | 50 (13) | –4 (–7 to 0) |
| Symptoms, No. (%) | ||||||
| Fever | 1,231 (75) | 635 (51) | 24 (20 to 27) | 540 (73) | 176 (45) | 28 (23 to 34) |
| Cough | 1,290 (78) | 837 (67) | 11 (8 to 14) | 544 (74) | 177 (45) | 29 (23 to 34) |
| Shortness of breath | 748 (45) | 439 (35) | 10 (7 to 14) | 506 (69) | 202 (52) | 17 (11 to 23) |
| Fatigue | 231 (14) | 162 (13) | 1 (–2 to 4) | 163 (22) | 72 (18) | 4 (–1 to 9) |
| Nausea/vomiting | 212 (13) | 116 (9) | 4 (1 to 6) | 139 (19) | 63 (16) | 3 (–2 to 8) |
| Diarrhea | 362 (22) | 153 (12) | 10 (7 to 12) | 168 (23) | 48 (12) | 11 (6 to 15) |
| Vital signs, mean (SD) | ||||||
| Pulse rate, beats/min | 99 (55) | 110 (406) | 11 (–31 to 9) | 101 (54) | 124 (500) | –23 (–60 to 13) |
| Respiratory rate, breaths/min | 20 (6) | 19 (12) | 0.4 (–0.2 to 1) | 22 (7) | 23 (19) | –0.3 (–2 to 1) |
| Systolic blood pressure, mm Hg | 132 (22) | 136 (24) | –4 (–6 to –3) | 128 (25) | 132 (30) | –3 (–7 to –9) |
| Temperature, °C | 37.4 (1.2) | 37.1 (2.1) | 0.4 (0.3 to 0.5) | 37.6 (1.6) | 37.2 (3.2) | 0.4 (0.1 to 0.7) |
| Pulse oximetry (%) | 95.3 (5.5) | 97.2 (2.9) | –1.9 (–2.2 to –1.5) | 93 (7) | 95 (4) | –3 (–4 to –2) |
| Imaging, No. (%) | ||||||
| None ordered | 689 (42) | 594 (48) | –6 (–10 to –2) | 11 (1) | 17 (4) | –3 (–5 to –1) |
| Chest radiograph ordered | 944 (57) | 634 (51) | 6 (3 to 10) | 710 (96) | 360 (92) | 4 (2 to 7) |
| Opacity(ies) on chest radiograph | 701 (74) | 198 (31) | 43 (39 to 48) | 608 (86) | 168 (47) | 39 (34 to 44) |
| Bilateral findings | 569 (81) | 112 (57) | 25 (18 to 31) | 498 (82) | 100 (60) | 23 (16 to 30) |
| Chest CT ordered | 227 (14) | 212 (17) | –3 (–6 to –1) | 211 (29) | 188 (48) | –19 (–25 to –14) |
| Opacity(ies) | 201 (89) | 98 (46) | 42 (35 to 50) | 190 (90) | 94 (50) | 40 (32 to 48) |
| Bilateral findings | 179 (89) | 63 (65) | 24 (15 to 33) | 169 (89) | 62 (67) | 22 (13 to 32) |
| Laboratory values, mean (SD) [n] | ||||||
| Leukocytes (SD), ×103 | 8.0 (5.2) [809] | 11.4 (11.4) [457] | –3.5 (–4.4 to –2.5) | 8.1 (5.3) [728] | 12.0 (12.4) [376] | –3.8 (–4.9 to –2.8) |
| Lymphocytes (SD), % | 15.6 (10.2) [797] | 17.1 (12.5) [436] | –1.5 (–2.8 to –0.1) | 15.0 (9.9) [719] | 15.7 (12.5) [360] | –0.8 (–2.1 to 0.6) |
| ALT, units/L | 44 (51) [785] | 37 (77) [406] | 7 (–0.3 to 14) | 43 (48) [718] | 39 (82) [356] | 5 (–3 to 13) |
| LDH, units/L | 390 (188) [744] | 285 (197) [320] | 105 (80 to 130) | 395 (184) [701] | 288 (203 [299]) | 107 (81 to 133) |
| CRP, mg/dL | 10.5 (9.2) [748] | 7.0 (8.7) [335] | 3.5 (2.4 to 4.7) | 10.9 (9.2) [707] | 7.2 (8.6) [313] | 3.7 (2.5 to 4.9) |
| Ferritin, pg/mL | 1,161 (1,487) [676] | 612 (976) [219] | 549 (338 to 759) | 1,158 (1,470) [659] | 615 (983) [214] | 543 (332 to 754) |
| Procalcitonin, pg/mL | 1.2 (8.8) [747] | 2.3 (11.8) [328] | –1.1 (–2.3 to 0.2) | 1.2 (9.0) [711] | 2.4 (12.1) [309] | –1.2 (–2.5 to 0.2) |
| Troponin, ng/mL | 0.04 (0.14) [666] | 0.05 (0.20) [331] | –0.2 (–0.4 to 0.004) | 0.04 (0.14) [625] | 0.06 (0.22) [291] | –0.02 (–0.05 to 0.002) |
| BNP, pg/mL | 2,608 (10,527) [525] | 7,094 (31,061) [244] | –4,486 (–7,457 to –1,516) | 2,674 (10,715) [502] | 7,582 (32,412) [223] | –4,908 (–8,076 to –1,740) |
| D-dimer, mg/L | 1,021 (3,891) [705] | 1,738 (6,738) [248] | –717 (–1,412 to 173) | 1,025 (3,932) [675] | 1,918 (7,119) [221] | –893 (–1,640 to –146) |
| ED disposition, No. (%) | ||||||
| Discharge | 908 (55) | 852 (68) | –13 (–17 to –10) | — | — | — |
| Regular floor | 678 (41) | 349 (28) | 13 (10 to 17) | 678 (92) | 348 (89) | 3 (–0.3 to 7) |
| ICU admission | 59 (4) | 44 (4) | 0 (–1 to 1) | 59 (8) | 44 (11) | –3 (–7 to 0.3) |
| Died in ED | 6 (0.4) | 2 (0.2) | 0.2 (–0.2 to 0.6) | — | — | — |
| Invasive ventilation, No. (%) | 152 (10) [1,491] | 32 (3) [1,194] | 7 (6 to 9) | 149 (26) [577] | 31 (9) [340] | 17 (12 to 22) |
| Length of ventilation, mean (SD), days | 7.2 (4.4) | 3.7 (3.4) | 3.5 (1.6 to 5.4) | 7.4 (4.3) | 3.9 (3.4) | 3.6 (1.7 to 5.5) |
| Noninvasive ventilation, No. (%) | 41 (2) | 21 (2) | 0.8 (–0.3 to 1.9) | 40 (5) | 20 (5) | 0 (–2 to 3) |
| Mean (SD) hospital LOS, days | 1.4 (2.7) | 1.1 (2.2) | 0.4 (0.2 to 0.6) | 4.7 (3.0) | 3.9 (2.7) | 0.8 (0.3 to 1.2) |
| Mortality (%) | 74 (4.5) | 22 (1.7) | 3 (1 to 4) | 68 (9) | 20 (5) | 4 (1 to 7) |
PUI, Persons under investigation; CI, confidence interval; HCW, health care workers; HTN, hypertension; DM, diabetes mellitus; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; CKD, chronic kidney disease; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; CRP, C-reactive protein; BNP, brain natriuretic peptide; LOS, length of stay.
Table 2.
Comparison of persons under investigation who did or did not receive mechanical ventilation.
| Admitted PUI |
|||
|---|---|---|---|
| Invasive Mechanical Ventilation | No Invasive Mechanical Ventilation | Mean difference (95%CI) | |
| Demographics | |||
| No. patients∗ | 215 | 875 | |
| Age, mean (SD), y | 60 (15) | 59 (20) | 1 (–2 to 3) |
| Men, No. (%) | 145 (67) | 481 (55) | 12 (5 to 20) |
| White, No. (%) | 100 (47) | 633 (61) | –26 (–33 to –19) |
| Black, No. (%) | 12 (6) | 61 (7) | –1 (5 to 2) |
| Asian, No. (%) | 18 (8) | 28 (3) | 5 (2 to 8) |
| Other, No. (%) | 2 (1) | 8 (1) | 0 (–1 to 1) |
| Unknown, No. (%) | 83 (39) | 245 (28) | 4 (–2 to 10) |
| Hispanic, No. (%) | 59 (27) | 179 (21) | 7 (1 to 13) |
| Sick contact, No. (%) | 63 (30) | 203 (23) | 6 (0 to 13) |
| COVID-19 contacts, No. (%) | 38 (18) | 106 (12) | 6 (1 to 11) |
| HCW, No. (%) | 5 (2) | 29 (3) | –1 (–4 to 2) |
| Nursing home, No. (%) | 18 (8) | 126 (14) | –6 (–11 to –1) |
| Comorbidities, No. (%) | |||
| HTN | 100 (47) | 374 (43) | 4 (–4 to 11) |
| DM | 58 (27) | 203 (23) | 4 (–3 to 10) |
| Asthma | 18 (8) | 72 (8) | 0 (–4 to 4) |
| CAD | 32 (15) | 146 (17) | –2 (–7 to 4) |
| COPD | 20 (9) | 87 (10) | –1 (–5 to 4) |
| CHF | 14 (7) | 76 (9) | –2 (–6 to 2) |
| Cancer | 10 (5) | 84 (10) | –5 (–9 to –1) |
| Immunosuppressed | 13 (6) | 69 (8) | –2 (–6 to 2) |
| CKD | 19 (9) | 80 (9) | 0 (–5 to 4) |
| Symptoms, No. (%) | |||
| Fever | 152 (71) | 560 (64) | 7 (0 to 14) |
| Cough | 145 (67) | 572 (65) | 2 (–5 to 9) |
| Shortness of breath | 166 (77) | 551 (63) | 14 (7 to 21) |
| Fatigue | 43 (20) | 189 (22) | –2(–8 to 5) |
| Nausea/vomiting | 33 (15) | 167 (19) | –4 (–10 to 2) |
| Diarrhea | 38 (18) | 162 (19) | –1 (–7 to 5) |
| Vital signs | |||
| Pulse rate, beats/min | 108 (85) | 110 (333) | –2 (–48 to 43) |
| Respiratory rate/min | 25 (9) | 22 (14) | 3 (1 to 5) |
| Systolic blood pressure, mm Hg | 127 (26) | 130 (26) | –3 (–7 to 1) |
| Temperature, °C | 37.7 (1.0) | 37.4 (1.5) | 0.3 (0.1 to 0.5) |
| Pulse oximetry (%) | 90 (9) | 95 (4) | –5 (–6 to –4) |
| Imaging, No. (%) | |||
| None ordered | 1 (0.5) | 29 (3) | –3 (–5 to 0) |
| Chest radiograph ordered | 211 (98) | 811 (93) | 5 (2 to 9) |
| Opacity(ies) on chest radiograph | 184 (87) | 560 (69) | 18 (13 to 24) |
| Bilateral findings | 160 (87) | 414 (74) | 13 (6 to 20) |
| Chest CT ordered | 57 (27) | 333 (38) | –11 (–19 to –4) |
| Opacity(ies) | 48 (84) | 231 (69) | 15 (2 to 28) |
| Bilateral findings | 44 (94) | 181 (78) | 15 (3 to 28) |
| Laboratory values, mean (SD) [n] | |||
| Leukocytes (SD), ×103 | 9.6 (7.6) [211] | 8.9 (6.8) [849] | 0.7 (–0.4 to 1.7) |
| Lymphocytes (SD), % | 12.9 (8.8) [205] | 16.6 (11.7) [832] | –3.7 (–0.4 to –2.0) |
| ALT, units/L | 56 (98) [211] | 38 (41) [819] | 18 (9 to 27) |
| LDH, units/L | 495 (225) [201] | 326 (172) [736] | 169 (140 to 198) |
| CRP, mg/dL | 14.7 (10.4) [206] | 8.1 (7.9) [751] | 6.6 (5.3 to 7.9) |
| Ferritin, pg/mL | 1,459 (1,504) [199] | 841 (1,194) [596] | 618 (412 to 823) |
| Procalcitonin, pg/mL | 2.6 (12.7) [208] | 1.2 (9.2) [751] | 1.4 (–0.2 to 2.9) |
| Troponin ng/mL | 0.05 (0.16) [202] | 0.03 (0.09) [662] | 0.02 (–0.002 to 0.03) |
| BNP, pg/mL | 3,566 (14,783) [172] | 3,603 (19,452) [492] | –37 (–3,230 to 3,157) |
| D dimer, mg/L | 1,475 (3,346) [195] | 1,102 (5,167) [626] | 373 (–400 to 1,145) |
| Noninvasive ventilation, No. (%) | 29 (13) | 19 (2) | 11 (8 to 14) |
| Hospital LOS, mean (SD), days | 6.5 (4.2) | 4.1 (2.7) | 2.4 (1.5 to 3.2) |
| Mortality | 50 (23) | 44 (5) | 18 (14 to 22) |
Based on patients who received mechanical ventilation or were discharged without it.
In general, outcomes were worse in men and older patients (Table 3 ). A comparison of ICU admissions, invasive mechanical ventilation, and mortality is presented in Table 3.
Table 3.
Outcomes for admitted patients, by age and sex.
| ICU Admissions, No. (%) | Invasive Mechanical Ventilation, No. (%)∗ | Deaths, No. (%) | |
|---|---|---|---|
| Age, y | |||
| <25 | 11 (19) | 3 (6) | 0 |
| 25–45 | 45 (20) | 34 (17) | 3 (1) |
| 46–65 | 118 (22) | 94 (22) | 18 (3) |
| 66–80 | 83 (24) | 67 (25) | 33 (9) |
| >80 | 33 (15) | 17 (10) | 41 (19) |
| Sex | |||
| Men | 186 (23) | 145 (23) | 42 (7) |
| Women | 105 (18) | 70 (15) | 53 (7) |
Based on patients who received mechanical ventilation or were discharged without it, n=1,090.
Limitations
Our study has several notable limitations. Although data capture was often contemporaneous or within 24 hours of patient contact, it still is retrospective and therefore subject to all of the limitations of this study design, including selection bias, errors in data entry, and residual confounding. The data regarding the frequency of SARS-CoV-2 testing should be viewed with caution because criteria for testing changed frequently in accordance with Centers for Disease Control and Prevention and Department of Health recommendations and local resources such as test availability. In addition, because of limited sensitivity, many patients with negative test results for SARS-C0V-2 may have had COVID-19. Our predictive models are based on the initial presentation to the ED. Obviously, changes in vital signs, laboratory values, and imaging over time are critical in predicting outcomes. We and others at our center are looking into artificial intelligence and machine learning using time series to further improve our predictions. Our regression models should be considered exploratory and may have resulted in overfitting. Although we report the number of deaths to date, mortality and case fatality rates cannot be directly calculated until all patients are either discharged alive or die while in the hospital. Finally, our results are also limited to a single hospital setting near the epicenter of the COVID-19 pandemic and may not be representative of other settings.
Discussion
Our data show that the burden of COVID-19 goes well beyond just patients with positive test results for SARS-CoV-2. This is especially true because the results of SARS-CoV-2 testing are often known only hours or days after admission and because false-negative results are common. Even though outcomes were generally worse in patients with positive PCR test results, need for ICU-level of care, need for mechanical ventilation, and mortality in persons under investigation who had negative test results was still considerable. We also demonstrated how quickly the percentage of persons under investigation with positive test results for SARS-CoV-2 increased over time. Our most recent data indicate that of all current admissions from the ED, more than two thirds are for persons under investigation.
Although many persons under investigation can be discharged directly from the ED with a relatively small return rate, a significant percentage of patients are admitted directly to an ICU or are later upgraded to an ICU within a median of 62 hours. There are also a significant number of persons under investigation who require invasive mechanical ventilation, with most being intubated a median of 60 hours after hospital arrival. Thus, for every 100 persons under investigation who are admitted to the hospital, 9 will require immediate ICU placement, invasive mechanical ventilation, or both, and another 12 will require ICU placement, invasive mechanical ventilation, or both within approximately 2 to 3 days. When a patient is intubated, the length of mechanical ventilation is considerable, further contributing to the health care burden. As expected, lower oxygen saturations and positive test results for SARS-CoV-2 were associated with need for ICU, need for invasive mechanical ventilation, and mortality. Male sex and respiratory rate were associated with ICU admission, whereas age and a history of chronic obstructive pulmonary disease were associated with mortality. Patients with positive test results for SARS-CoV-2 were more likely to have a known exposure to COVID-19, have cough, have fever, be Hispanic, have a smoking history, and be hypoxemic. However, the results of viral testing were often received after the patient had already been intubated and cannot be relied on to dictate management.
We also showed that extensive findings on chest imaging (bilateral opacities) are common among persons under investigation both with and without viral confirmation, although more common in the former. In accordance with our data, and in an attempt to conserve resources and limit unnecessary contamination of our CT scanner, we have modified our indication for obtaining a CT of the chest. Patients with respiratory distress, hypoxemia, or significant risk with bilateral opacities on a plain chest radiograph do not receive a CT. If the chest radiograph result is negative but clinical suspicion remains high, particularly in the presence of hypoxemia, dyspnea on exertion, or shortness of breath, a CT scan of the chest is performed. Obviously, serial imaging may be required after the patient is admitted, according to their clinical course. Although most patients discharged from the ED do well, a minority will revisit the ED, some even requiring subsequent admission, ICU care, and ventilation. Thus, it is important to emphasize to all discharged patients that they must return immediately to the hospital if their condition worsens.
The risk factors we report appear to be similar to those found in China, Italy, Singapore, and Washington. Increasing age and comorbidities have been associated with a greater need for supplemental oxygen therapy, ICU admission, and mechanical ventilation, and have been associated with mortality.4 , 12, 13, 14, 15, 16, 17, 18, 19, 20 A previous study found that a history of cardiovascular disease, particularly hypertension, was commonly observed in severely ill COVID-19 patients.19 Symptoms of fever and cough were also common in confirmed COVID-19 cases but not predictive of disease severity.17 In our cohort, mortality, when expressed as a percentage of persons under investigation for COVID-19 who died, was similar to that in larger studies in China (1.4% to 4.3%) but lower than that reported from Italy and Washington State, likely because of a younger population and larger cohort.3, 4, 5 , 14 Both Sequential [Sepsis-related] Organ Failure Assessment and Acute Physiology and Chronic Health Evaluation scores have been shown to be higher in nonsurvivors (4.5 and 18, respectively) compared with survivors (1 and 14, respectively), whereas the Confusion Uremia Respiratory Blood Pressure score was not significantly different between these groups.19 , 20 The MuLBSTA score offers an alternate means of risk stratification specific to viral pneumonia, with risk factors of age and comorbidity weighted more heavily, consistent with the COVID-19 pandemic.21
Our study is one of the largest to date and included not only patients with confirmed COVID-19 but also those who were being considered as possibly having COVID-19 and who also contributed to the health care burden. These patients are often treated similarly to COVID-19 patients and must be taken into account to better understand the full scope of the pandemic. It is our sincere hope that this information will help inform other hospitals that are not yet at the epicenter of the pandemic on how best to anticipate and prepare for ICU beds and mechanical ventilators.
In conclusion, the health care burden of the COVID-19 pandemic goes far beyond patients with positive test results for SARS-CoV-2. For every 100 persons under investigation who are admitted to the hospital, 9 will require immediate ICU, invasive mechanical ventilation, or both and another 12 will require ICU or invasive mechanical ventilation within 2 to 3 days, with a median length of mechanical ventilation nearing 1 week. In general, lower oxygen saturations and positive test results for SARS-CoV-2 were associated with worse outcomes. This information should help hospitals anticipate needs for ICU beds and mechanical ventilators and thus be able to deal efficiently with the pandemic.
Acknowledgments
We acknowledge our dedicated health care workers, leadership, and administrative and research staff who helped put together this information.
Footnotes
Please see page 395 for the Editor’s Capsule Summary of this article.
Supervising editor: David A. Talan, MD. Specific detailed information about possible conflict of interest for individual editors is available at https://www.annemergmed.com/editors.
Author contributions: AJS and MCH conceived and designed the study. AJS, KM, and RF supervised the conduct of the trial and data collection. HCT managed the data, including quality control, provided statistical advice on study design, and analyzed the data. AJS drafted the article, and all authors contributed substantially to its revision. AJS takes responsibility for the paper as a whole.
All authors attest to meeting the four ICMJE.org authorship criteria: (1) Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND (2) Drafting the work or revising it critically for important intellectual content; AND (3) Final approval of the version to be published; AND (4) Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding and support: By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
Readers: click on the link to go directly to a survey in which you can provide feedback to Annals on this particular article.
A podcast for this article is available at www.annemergmed.com.
Supplementary Data
Comparison of ICU and Non-ICU Admitted Persons Under Investigation
Table E2. Exploratory Multivariate Predictor Variables.
References
- 1.Zu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [DOI] [PMC free article] [PubMed]
- 2.World Health Organization Novel coronavirus (2019-nCoV): situation report—15. https://www-who-int.proxy.library.stonybrook.edu/docs/default-source/coronaviruse/situation-reports/20200204-sitrep-15-ncov.pdf Available at:
- 3.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorbello M., El-Boghdadly K., Di Giacinto I. Società Italiana di Anestesia Analgesia Rianimazione e Terapia Intensiva (SIAARTI) Airway Research Group, The European Airway Management Society. The Italian COVID-19 outbreak: experiences and recommendations from clinical practice. Anaesthesia. 2020;75:724–732. doi: 10.1111/anae.15049. [DOI] [PubMed] [Google Scholar]
- 8.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.To K.K., Tsang O.T., Leung W.S. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y., Yao L., Li J. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol. 2020; [DOI] [PMC free article] [PubMed]
- 11.Kaji A.H., Schriger D., Green S. Looking through the retroscope: reducing bias in emergency medicine chart review studies. Ann Emerg Med. 2014;64:292–298. doi: 10.1016/j.annemergmed.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 12.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. https://doi.org/10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed]
- 13.Young B.E., Ong S.W.X., Kalimuddin S. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 15.Livingston E., Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020;323:1335. doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- 16.Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395:1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J., Zheng Y., Gou X. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo L., Wei D., Zhang X. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. doi: 10.3389/fmicb.2019.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of ICU and Non-ICU Admitted Persons Under Investigation
Table E2. Exploratory Multivariate Predictor Variables.