Summary
A 65-year-old man was hospitalized owing to fever (38.6 °C) and dry cough since 4 days. He visited Wuhan 8 days ago. At admission, nasopharyngeal swab samples were taken, and polymerase chain reaction analysis confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA positivity. On day 9, after admission, the chest computed tomography scan showed diffuse ground-glass shadows in the patient's bilateral lungs. On day 11, his respiratory symptoms worsened. Subsequently, type I respiratory failure was diagnosed, coinciding with kidney injury, and subsequently, type II respiratory failure occurred, coupled with multiorgan failure including the heart and liver. However, the patient's constitution worsened although SARS-CoV-2 tests were negative since day 13. He died on day 21. Lung biopsy showed areas of diffuse alveolar damage, characterized by extensive acute alveolitis with numerous intra-alveolar neutrophil, lymphocyte, and macrophage infiltrations. Microthrombi were seen in the dilated pulmonary capillaries. Immunohistochemistry staining for SARS-CoV-2 N protein was negative. Taken together, the patient died of multiorgan failure although the SARS-CoV-2 infection was cleared already, implicating that for disease worsening, no active SARS-CoV-2 infection is required.
Keywords: SARS-CoV-2, Secondary bacterial infection, Multiorgan failure, Coronavirus
1. Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged initially in Wuhan, China, in December 2019 and since then has rapidly spread worldwide. The epidemic has resulted in a total of 82,758 confirmed cases and 4632 death cases in China as of February 24, 2020 (http://www.nhc.gov.cn/xcs/yqfkdt/202004/b504a02486834baf8ed8149701a4175b.shtml). Although about 80% of the confirmed patients are mild cases, mortality of critical cases reaches 49% in some areas [1]. How SARS-CoV-2–infected patients progress to critical disease is a key issue in clinical practice. Only a few case reports of pulmonary pathology about COVID-19 have been published to date [2,3]. Given the lack of more histopathological evidence, pathophysiological alterations underlying COVID-19 progression remain largely unknown. Here, we report about a 65-year-old patient who died of COVID-19. We analyzed his lung tissue and determined the potential correlation between lung pathological alterations and disease progression.
2. Materials and methods
2.1. Case report
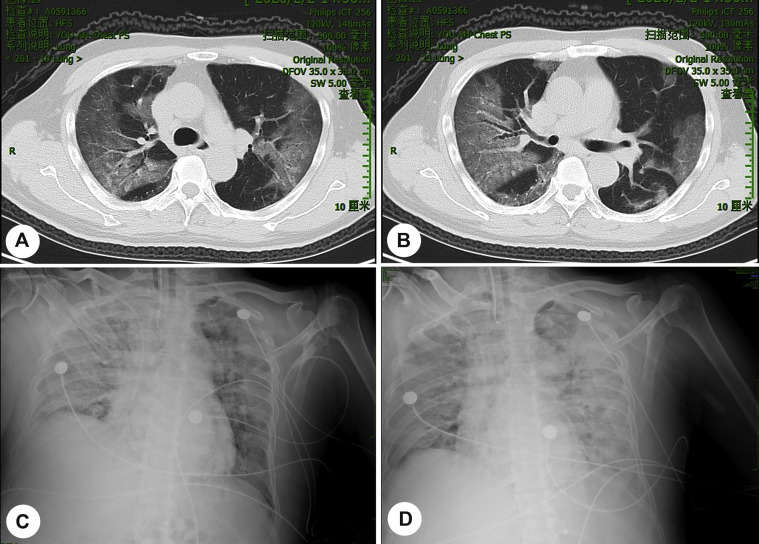
A 65-year-old man was admitted to the hospital owing to fever (38.6 °C) and dry cough for 4 days on January 28, 2020. He returned to Beijing from Wuhan one week ago (January 20, 2020). The patient had no history of any chronic diseases, and he was a nonsmoker. He was confirmed as COVID-19 positive in February 2 by quantitative polymerase chain reaction (PCR) analysis for SARS-CoV-2 RNA of samples collected by nasopharyngeal swabs. On the same day, the chest computed tomography (CT) scan revealed diffuse ground-glass shadows in the bilateral lungs (Fig. 1 A–B). He developed type I respiratory failure (PO2 = 47.6 mmHg and PCO2 = 24.8 mmHg) on February 4. Two days later, the patient received 40 mg of methylprednisolone every 12 h and antibiotic (biapenem) treatment owing to high temperature (39.2 °C), and enlarged pulmonary lesions were visible in chest CT. On February 11, the patient progressed to type II respiratory failure (PO2 = 56 mmHg and PCO2 = 49.4 mmHg). X-ray showed multiple plaque shadows in bilateral lungs, in particular, in the right side (Fig. 1C). To reduce edema of respiratory tracts, the dose of methylprednisolone was increased to 80 mg every 12 h. Unfortunately, pneumonia was further exacerbated (Fig. 1 D).
Fig. 1.
Images of chest CT scans and X-ray. A–B, Chest CT was performed on Feb 2. C, X-ray was performed on Feb 11. D, X-ray was performed on Feb 12. CT, computed tomography.
In the following days, the patient's condition did not improve. He progressed to septic shock; severe metabolic acidosis and respiratory acidosis occurred successively; and then, the patient died on February 14. Table 1 shows the details of disease progression and therapeutic interventions. It is worth noting that since February 6, four subsequent tests of nasopharyngeal swabs were SARS-CoV-2 RNA negative.
Table 1.
Blood biochemistry of the patient during the course of the disease.
| Date | Course | Disease milestones | Management | T (°C) | CoV-2 RNA | Hemoglobin (109/L) | WBC (109/L) | Lymphocytes (109/L) | Lymphocytes (%) | Neutrophils (109/L) | Neutrophils (%) | platelet (109/L) | Procalcitonin (ng/ml) | PO2 (mmHg) (SaO2 = 60–99.6%) |
PCO2 (mmHg) | ALT (IU/L) | TBIL (μmol/L) | Albumin (g/L) | Creatinine (μmol/L) | Troponin (μg/L) | PT (S) | PT-INR | APTT (S) | Fibrinogen (g/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.24 | Day 01 | Fever, dry cough | 38.0 | |||||||||||||||||||||
| 1.28 | Day 04 | Hospitalized | 38.6 | + | ||||||||||||||||||||
| 1.29 | Day 05 | Support care and symptomatic therapy | 38.0 | 136 | 2.95 | 0.81 | 27.4 | 1.85 | 62.7 | 95 | 0.11 | 36 | 8.6 | 41.4 | 71 | 0.016 | 13.5 | 1.20 | 36.3 | 2.87 | ||||
| 2.2 | Day 09 | Confirmed COVID-19 | 38.3 | + | 121 | 2.63 | 0.41 | 15.5 | 2.05 | 78.0 | 60 | 0.14 | 31 | 11.4 | 31.9 | 72 | 0.017 | 13.1 | 1.17 | 34.8 | 3.40 | |||
| 2.4 | Day 11 | Expectoration, chest congestion | 38.3 | 118 | 3.62 | 0.28 | 7.8 | 3.15 | 87.0 | 79 | 0.14 | 47.6 | 24.8 | 31 | 13.8 | 28.1 | 67 | |||||||
| 2.5 | Day 12 | Type I respiratory failure | Intubation and ventilator support | 38.0 | 136 | 5.53 | 0.19 | 3.4 | 5.00 | 90.5 | 53 | 0.35 | 55.7 | 33.8 | 23 | 12.6 | 26.2 | 0.028 | 15.7 | 1.40 | 35.6 | 3.68 | ||
| 2.6 | Day 13 | 40 mg of methylprednisolone every 12 h, 0.3 g of biapenem every 12 h | 39.2 | – | 102 | 4.39 | 0.25 | 5.7 | 3.82 | 86.9 | 81 | 1.90 | 59.7 | 28.1 | 39 | 13.0 | 26.5 | 62 | 17.0 | 1.51 | 34.3 | 2.32 | ||
| 2.8 | Day 15 | Acute renal insufficiency, myocardial injury | 38.2 | – | 96 | 6.11 | 0.24 | 4.0 | 5.38 | 88.0 | 65 | 0.72 | 79.7 | 37.4 | 20 | 8.4 | 27.1 | 167 | 16.9 | 1.50 | 35.6 | 0.46 | ||
| 2.9 | Day 16 | 60 mg of methylprednisolone every 12 h | 38.3 | 98 | 8.24 | 0.26 | 3.2 | 7.41 | 89.9 | 72 | 0.90 | 181.8 | 38.4 | 15 | 11.5 | 26.8 | 172 | 3.432 | 16.4 | 1.45 | 30.0 | 0.43 | ||
| 2.10 | Day 17 | 38.2 | – | 94 | 9.83 | 0.17 | 1.7 | 9.29 | 94.6 | 62 | 0.10 | 51.2 | 44.2 | 22 | 17.0 | 27.3 | 165 | 2.387 | 14.0 | 1.24 | 30.6 | 1.43 | ||
| 2.11 | Day 18 | Type II respiratory failure | 80 mg of methylprednisolone every 12 h, 10 g of immunoglobulin every 12 h | 38.3 | 92 | 10.11 | 0.16 | 1.6 | 9.62 | 95.2 | 46 | 0.42 | 56.0 | 49.4 | 28 | 20.8 | 26.9 | 140 | 2.373 | 14.4 | 1.28 | 32.0 | 2.65 | |
| 2.12 | Day 19 | Decrease of platelet count | 36.0 | 100 | 3.56 | 0.11 | 3.2 | 3.20 | 89.9 | 32 | 2.70 | 89.2 | 50.8 | 19 | 24.8 | 24.0 | 197 | 0.867 | 13.3 | 1.18 | 38.0 | 4.24 | ||
| 2.13 | Day 20 | Acute heart failure, septic shock | Norepinephrine | 37.0 | 78 | 6.63 | 0.42 | 6.3 | 6.15 | 92.7 | 92 | 12.0 | 51.2 | 73.0 | 14 | 36.6 | 24.6 | 305 | 1.463 | 13.2 | 1.17 | 44.0 | 6.09 | |
| 2.14 | Day 21 | Acute hepatic failure | Rescue | 37.0 | – | 66 | 1.70 | 0.21 | 12.4 | 1.40 | 82.3 | 23 | 11.0 | 48.0 | 72.0 | 4837 | 94.6 | 27.5 | 234 | 32.0 | 2.80 | 44.9 | 2.49 |
Abbreviations: WBC, white blood cell; ALT, alanine transaminase; TBIL, total bilirubin; PT, prothrombin time; APTT, activated partial thromboplastin time; INR, internationalization standard value; COVID-19, coronavirus disease 2019.
2.2. RNA test for SARS infection
The Duplex Real-Time PCR Diagnostic Kit for rapid detection of SARS-CoV-2 RNA ORF1ab/N gene was used and purchased from Applied Biological Technologies, Beijing (cat #A7712RC-50T). The kit was approved with 97.6% specificity and 97.1% sensitivity (unpublished data).
2.3. Lung biopsy and specimen examination
With the consent of the patient's family members, we performed percutaneous biopsy in both his lungs with an 18G needle after his death. Three lung tissue specimens were collected (3.1 cm in total length and 0.1 cm in diameter).
The specimens were fixed in neutral formalin for 24 h. Paraffin embedding and sectioning were performed according to routine protocols. Four-micrometer sections were used for hematoxylin and eosin, periodic acid–Schiff (PAS), Grocott-Gomori methenamine silver (GMS), and immunohistochemical (IHC) staining. Primary anti–SARS-CoV-2 N protein antibody was provided by AbMax Biotechnology Beijing Co., Ltd (1:7500, rabbit polyclonal IgG, catalog # 06–0053). TTF-1 (working solution, clone: 8G7G3/1) and cytomegalovirus (CMV, working solution, clone: CCH2+DDG9) were purchased from Zhongshan Golden Bridge Biotechnology, Beijing. Immunohistochemistry was performed using the PV-9000 test system (mouse/rabbit enhanced polymer test system; Zhongshan Golden Bridge Biotechnology, Beijing). Lung tissues without pulmonary infection were used as the negative control. The blank control was performed by replacing the primary antibody with phosphate-buffered saline.
3. Results
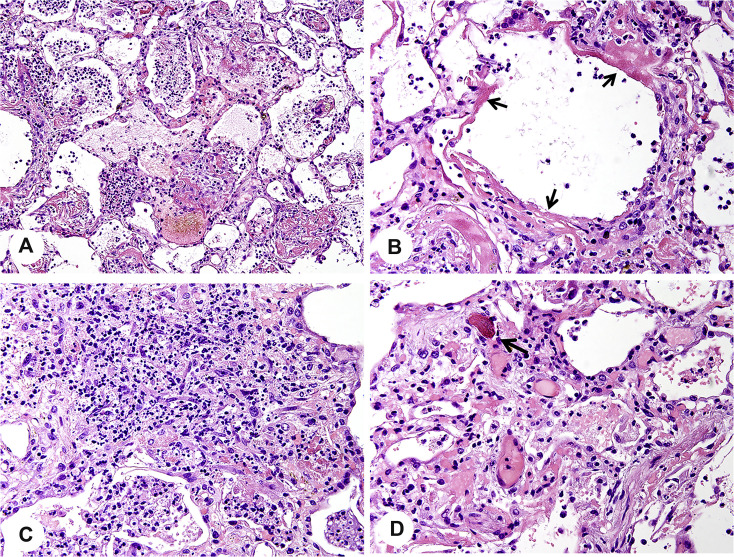
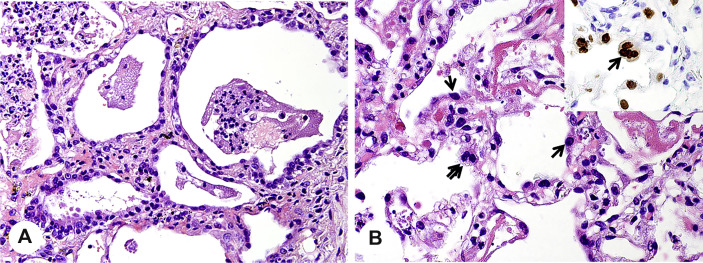
Based on the three biopsied lung tissue specimens, we observed diffuse alveolar damage in the early organizing phase, presenting as focal hyaline membranes, intra-alveolar edema, reactive type II pneumocyte hyperplasia, and focal intra-alveolar fibrosis. Fig. 2 A shows purulent discharge in most of the areas of the alveolar spaces. High-magnification microscopy highlighted extensive areas of acute alveolitis with numerous intra-alveolar neutrophils and some lymphoid cells and alveolar macrophages (Fig. 2B). Intra-alveolar fibrinous exudates with inflammatory cells were present (Fig. 3 A). Hyaline membranes were seen along the surface of the alveolar septa (arrows in Fig. 3B). The alveoli collapsed and fused along with the widened alveolar septum. Proliferating fibroblasts surrounded by neutrophils were observed in the alveolar septum (Fig. 3C). In distinct alveolar septa, pulmonary capillaries were dilated by microthrombi (arrow in Fig. 3D). Alongside with alveolar septa, hyperplasia of alveolar epithelial cells was seen (Fig. 4 A). IHC staining for TTF-1 further showed that these type II pneumocytes were characterized by increase in nuclear size, hyperchromasia, and multinucleated giant cell (Fig. 4B).
Fig. 2.
The alveoli are filled with phlegm. A, Purulent exudates are present in alveolar spaces (H&E, ×200). B, High-magnification microscopy shows a large number of lymphocytes, neutrophils, and macrophages mixed with necrotic cellular debris in a phlegm plug (H&E, ×400). H&E, hematoxylin and eosin.
Fig. 3.
Diffuse alveolar damage with focal organization. A, Intra-alveolar fibrinous exudates with inflammatory cells (H&E, ×200). B, Hyaline membrane formation (H&E, ×600, arrow). C, Proliferating fibroblasts surrounded by neutrophils are seen in widened alveolar septa (H&E, ×400). D, Dilated pulmonary capillaries with microthrombus formation (H&E, ×400, arrow). H&E, hematoxylin and eosin.
Fig. 4.
Type II pneumocyte hyperplasia. A, Hyperplasia of type II pneumocytes (H&E, ×400). B, Type II pneumocyte multinucleation with IHC staining for TTF-1 (H&E, TTF-1, ×600). IHC, immunohistochemical; H&E, hematoxylin and eosin.
No granulomas and virus inclusion were seen in these tissues. IHC staining for anti–SARS-CoV-2 N protein was negative. PAS, GMS, and CMV IHC staining was negative (data not shown), excluding secondary fungi or CMV infection.
4. Discussion
Unfortunately, a 65-year-old male without any preexisting diseases died of COVID-19. At autopsy, severe and diffuse damage was identified in the lung tissue, including type II pneumocyte hyperplasia, intra-alveolar fibrinous exudates with inflammatory cells, hyaline membrane formation, interstitial fibrosis, and microthrombi in capillaries. These pathological phenotypes strongly suggest the occurrence of adult respiratory distress syndrome [4,5] and septic shock (phlegm plugs in alveolar cavities and microthrombi in the capillaries).
It is worthy to note that the patient underwent SARS-CoV-2 RNA tests of nasopharyngeal swabs six times (Table 1). The first two times showed virus positivity (January 28 and February 2). Since February 6, the rest of the four SARS-CoV-2 RNA tests of nasopharyngeal swabs were negative (Table 1). Consistent with the nasopharyngeal swab tests, we did not detect positive results for SARS-CoV-2 N protein by IHC staining in lung tissue after biopsy. These results suggest that SARS-CoV-2 infection might have been cleared at least on February 6, two weeks after presenting to the hospital. Scrutinizing the dynamically altered clinical parameters shown in Table 1, the following time points were critical for clarifying the disease progress: (1) February 2: the CT scan confirmed bilateral pneumonia; (2) February 5: type I respiratory failure; (3) February 8: kidney injury; (4) February 11: type II respiratory failure and cardiac insufficiency; (5) February 13: heart failure and septic shock; and (6) February 14: liver failure and death. In addition to the time points reflecting disease progression, attention should be paid to the time this patient received steroid and antibiotic therapy. As shown in Table 1, the patient received steroid therapy from February 5 to February 14 (death), whereas antibiotic treatment was started on February 6. Based on these analyses, the patient suffered from SARS-CoV-2 viral pneumonia at least on February 2. The virus was inhibited and cleared between February 2 and February 6. Unfortunately, he developed type I respiratory failure on February 5. The deteriorating respiratory disease was not connected to SARS-CoV-2 infection anymore because the virus was undetectable from this time point. Based on his lung histological alterations, such as multiple phlegm plugs in the alveolar spaces, a secondary bacterial infection might have occurred in this patient. Unfortunately, respiratory and blood cultures for bacteria and other organisms were not performed. It has been reported that patients with severe COVID-19 were often superimposed on bacterial or fungal infection [6]. Hence, two treatments might have worsened the disease: steroid therapy [7,8] and a too-late start of the antibiotic therapy [9,10]. Although steroid therapy is useful to prevent a cytokine storm, it inhibits immune responses to pathogen infections (as might have occurred). As shown in Table 1, the lymphocyte number in the patient was impressively less, in particular since initiation of steroid therapy. In addition, high dosage of methylprednisolone treatment led to deleterious pneumonia. Given the missed diagnosis of the potential bacterial infection, antibiotic treatment was started too late in this patient, which might have resulted in multiorgan failure owing to septic shock.
5. Conclusions
Clinical manifestation and lung histological alteration showed that this patient suffered from SARS-CoV-2 viral pneumonia. However, after virus clearance, multiorgan failure, which led to his death, might have been associated with an uncontrolled secondary bacterial infection.
Acknowledgments
The authors are grateful to Dr. Christoph Meyer for discussion and language correction. C.S. and H.L. evaluated the sections and wrote the article. Z.Y. and H.K. collected clinical data. L.M., L.S., and Y.W. performed staining. H.W. edited the manuscript. H.L. analyzed chest X-ray and computed tomography. R.J. and F.L. designed the study.
Footnotes
Competing interestsThe authors do not have any possible conflicts of interest.
References
- 1.The novel coronavirus pneumonia emergency response epidemiology team, Chinese center for disease control and prevention. The epidemiologic characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liuxingbingxue Zazhi.2020, 41:145-151.
- 2.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. June 2020;153(6):725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes K.T., Beasley M.B. Pulmonary manifestations of acute lung injury: more than just diffuse alveolar damage. Arch Pathol Lab Med. 2017;141:916–922. doi: 10.5858/arpa.2016-0342-RA. [DOI] [PubMed] [Google Scholar]
- 5.Libby L.J., Gelbman B.D., Altorki N.K., Christos P.J., Libby D.M. Surgical lung biopsy in adult respiratory distress syndrome: a meta-analysis. Ann Thorac Surg. 2014;98:1254–1260. doi: 10.1016/j.athoracsur.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Annane D., Cavaillon J.M. Orticosteroids in sepsis: from bench to bedside? Shock. 2003;20:197–207. doi: 10.1097/01.shk.0000079423.72656.2f. [DOI] [PubMed] [Google Scholar]
- 8.Salluh J.I., Póvoa P. Corticosteroids in severe sepsis and septic shock: a concise review. Shock. 2017;47(1S Suppl 1):47–51. doi: 10.1097/SHK.0000000000000704. [DOI] [PubMed] [Google Scholar]
- 9.Angus D.C., van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 10.Dendoncker K., Libert C. Glucocorticoid resistance as a major drive in sepsis pathology. Cytokine Growth Factor Rev. 2017;5:85–96. doi: 10.1016/j.cytogfr.2017.04.002. [DOI] [PubMed] [Google Scholar]