Abstract
Chloroquine and hydroxychloroquine are now being widely used for treatment of COVID-19. Both medications prolong the QT interval and accordingly may put patients at increased risk for torsades de pointes and sudden death. Published guidance documents vary in their recommendations for monitoring and managing these potential adverse effects. Accordingly, we set out to conduct a systematic review of the arrhythmogenic effect of short courses of chloroquine or hydroxychloroquine. We searched on MEDLINE and Embase, as well as in the gray literature up to April 17, 2020, for the risk of QT prolongation, torsades, ventricular arrhythmia, and sudden death with short-term chloroquine and hydroxychloroquine usage. This search resulted in 390 unique records, of which 41 were ultimately selected for qualitative synthesis and which included data on 1515 COVID-19 patients. Approximately 10% of COVID-19 patients treated with these drugs developed QT prolongation. We found evidence of ventricular arrhythmia in 2 COVID-19 patients from a group of 28 treated with high-dose chloroquine. Limitations of these results are unclear follow-up and possible publication/reporting bias, but there is compelling evidence that chloroquine and hydroxychloroquine induce significant QT-interval prolongation and potentially increase the risk of arrhythmia. Daily electrocardiographic monitoring and other risk mitigation strategies should be considered in order to prevent possible harms from what is currently an unproven therapy.
Keywords: Arrhythmia, Chloroquine, Coronavirus, COVID-19, Hydroxychloroquine, SARS-CoV-2, Sudden death, Torsades de pointes
Introduction
The aminoquinoline drugs chloroquine and hydroxychloroquine are now in increased use globally as a potential, albeit unproven, treatment option for coronavirus disease 2019 (COVID-19). Regimens vary but are generally equivalent to 400–1200 mg hydroxychloroquine (250–1000 mg chloroquine phosphate) per day for approximately 5 days.1 However, these medications can cause QT prolongation,2 the electrocardiographic (ECG) marker of delayed ventricular repolarization. Delayed repolarization enables early afterdepolarizations, which can trigger a possibly fatal ventricular arrhythmia known as torsades de pointes. Most medications that prolong the QT interval, including aminoquinolines, act by binding to and inhibiting the potassium channel protein product of the gene KCNH2 (also known as hERG), thereby blocking the rapid component of the delayed rectifier potassium current (IKr).3 Repolarization is also maintained by other currents, and it is believed that people with impaired function of these additional currents (such as IKs) are at greater risk for drug-induced QT prolongation and torsades.4 This state, known as one of reduced repolarization reserve, can be brought on by risk factors such as congenital long QT syndrome, hypokalemia, and hypomagnesemia. Bradycardia and heart failure are other risk factors that promote torsades (Table 1 ).5
Table 1.
Risk factors for QT prolongation and torsades de pointes
| General risk factors | Illness-related risk factors |
|---|---|
| Congenital long QT syndrome3 | Hypokalemia5 |
| Use of multiple QT-prolonging medications16 | Hypomagnesemia5 |
| Female sex3 | Sepsis16 |
| Myocardial injury, ischemia, or heart failure16 | |
| Renal impairment16 | |
| Bradycardia (heart rate <60 bpm)5 | |
| Recent conversion from atrial fibrillation3 |
Hence, there is concern about ventricular arrhythmias stemming from the newfound use of these agents. On the one hand, clinical experience with these medications in the Western world is generally with chronic conditions such as lupus. Due to their long half-life (approximately 1 month),6 chronic usage of these drugs will result in more accumulation and greater concentrations than with short-term doses, with theoretical time to steady state of approximately 4 months. Accordingly, the shorter regimens used to treat COVID-19 may be safer. On the other hand, patients with COVID-19 may represent a population at greater arrhythmic risk given the high frequency of myocardial injury, heart failure, and concomitant use of other QT-prolonging medications.7 For example, most protocols suggest combination with azithromycin, another QT-prolonging agent, yet both agents may affect repolarization reserve in ways beyond IKr alone.8 Moreover, interleukin-6 impairs IKr, and hypoxia may also increase the late sodium current (ILATE). As a result, more severely ill COVID-19 patients may be more predisposed to a synergistic torsadogenic effect.9 , 10
Multiple publications with guidance on how to monitor for and manage QT prolongation with chloroquine and hydroxychloroquine in COVID-19 have already appeared. However, their recommendations are not entirely consistent. For example, some authors recommend all patients receive a baseline and repeat ECG,11, 12, 13 whereas others reserve this recommendation for certain higher-risk populations.14
Although ECG monitoring can help prevent torsades,15 possible issues include increased workload, use of personal protective equipment, and exposure to infected patients.11 An adequate knowledge of the potential benefit of ECG monitoring in this setting is essential for informed decision-making. Therefore, we conducted a systematic review of the risk of QT prolongation, torsades, ventricular arrhythmia, and sudden death with short courses of chloroquine or hydroxychloroquine as used in the treatment of COVID-19.
Methods
To complete our systematic review, we searched MEDLINE and Embase with main keywords chloroquine, hydroxychloroquine, QT, torsades, ventricular arrhythmia, cardiac arrest, coronavirus, COVID-19, and sudden death, with associated subject headings (details given in the Supplemental Appendix). Product manufacturers were contacted for relevant studies. To find reports of recent studies, we also searched medRxiv, ClinicalTrials.gov, and the ICTRP (International Clinical Trials Registry Platform) database for COVID-19 studies with keywords chloroquine or hydroxychloroquine. References from eligible full-text studies were searched for further reports.
We excluded preclinical studies, case reports, narrative reviews, and nonconsecutive case series. All other study types were included, provided they gave data allowing estimation of the degree or incidence of QT prolongation, torsades, or sudden death. We excluded studies with single doses of chloroquine or hydroxychloroquine or chronic dosing (≥4 months), intravenous formulations, supratherapeutic doses, studies with primarily pediatric patients, and studies with very small sample sizes (<10 participants). No date or language restrictions were applied. The search was performed up to April 17, 2020.
Studies were screened and reviewed by 1 reviewer, with confirmatory screening and review of a sample performed independently by a second reviewer.
Results
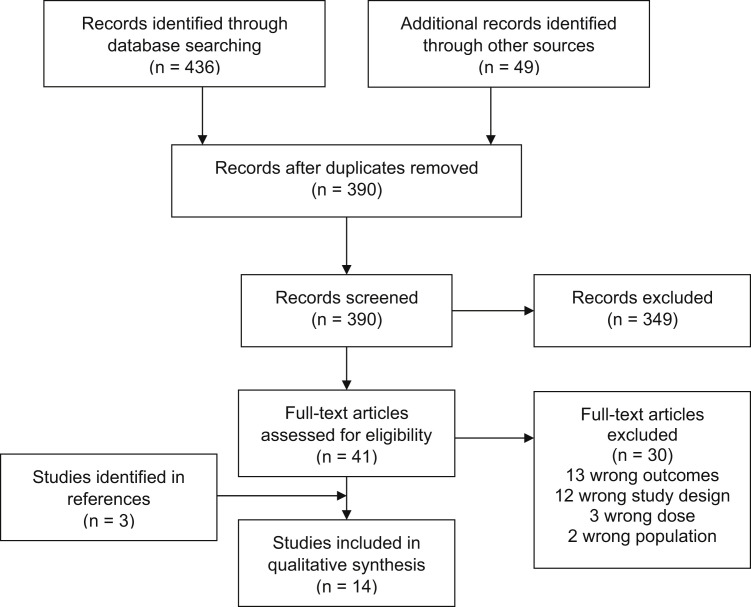
Our search yielded 390 unique records, of which 41 were eligible for full-text review. Of those records, 11 were included in the systematic review, along with an additional 3 identified via reference tracking (Figure 1 ). Study characteristics are listed in Table 2 , and outcomes are summarized in Table 3 .
Figure 1.
Flowchart of study screening and selection.
Table 2.
Characteristics of included studies
| Study first author | Design∗ | Population | Age (y) | Female sex (%) | Baseline comorbidities | Drugs studied | ECG monitoring | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Non–COVID-19 participants | ||||||||
| Haeusler17 | SR of RCTs and cohort studies (n = 1207) | Malaria treatment, prophylaxis, or healthy volunteers | 20.8 | 36.3 | 65% of trials excluded patients with comorbidities | CQ | At least 2 ECGs in all studies | NR |
| Pfizer18 | RCT (n = 119) | Healthy volunteers | 35.5 | 16.4 | NA | CQ phosphate 1000 mg/d, CQ phosphate 1000 mg/d plus azithromycin 500 mg/d, or placebo for 3 days | Baseline and day 3 | Three patients not included in the analysis |
| WHO Evidence Review Group19 | SR of RCTs and cohort studies (n = 23,773) | Malaria treatment | NR | NR | NR | CQ | NR | At least 14 days of follow-up; exact loss to follow-up uncertain |
| COVID-19 participants | ||||||||
| Borba20 | RCT (n = 56) | Patients with ARDS and suspected COVID-19 | 51.1 | 24.7 | Any 67.5%, hypertension 46.3%, DM 25.9%, alcoholism 26%, heart disease 9.3%, asthma 6.2%, CKD 7.5%, liver disease 3.7%, HIV 1.9% | High-dose CQ (CQ diphosphate 1 g twice daily for 6 days) vs low-dose CQ (day 1: CQ diphosphate 750 mg twice daily; days 2–5: 750 mg daily); all patients on IV azithromycin | Baseline and at clinical discretion | No patients reported as lost to follow-up |
| Chen21 | RCT (n = 15) | Moderate COVID-19 patients | 48.6 | 30 | Hypertension 33.3%, DM 6.7% | HCQ sulfate 400 mg/d for 5 days (vs no treatment) | NR | No patients reported as lost to follow-up |
| Chen (unpublished preprint) | RCT (n = 31) | Mild COVID-19 patients | 44.7 | 53.2 | Relevant exclusion criteria: arrhythmias, severe liver disease, or eGFR ≤30 mL/min/1.73 m2 | HCQ sulfate 400 mg/d for 5 days (vs no treatment) | NR | No patients reported as lost to follow-up |
| Chorin22 | Cohort study (n = 84) | Hospitalized COVID-19 patients | 63 | 26 | CAD 11%, CKD 7%, DM 20%, COPD 8%, HF 2%, acute renal failure 6% | HCQ and azithromycin | Baseline and daily | All patients included |
| Gautret23 | Cohort study (n = 80) | Hospitalized COVID-19 patients | 52.5 | 46.2 | Cancer 6.3%, DM 11.2%, hypertension 16.3%, chronic respiratory disease 10.0%, obesity 5.0%, immunosuppression 5.0% | HCQ and azithromycin; other QT-prolonging drugs discontinued | Baseline and day 2 | All patients hospitalized and with 6 days of follow-up included |
| Huang24 | RCT (n = 10) | Moderate and severe COVID-19 patients | 41.5 | 30.0 | 10% hypertension, 10% DM; excluded history of chronic liver or kidney disease, arrhythmia, or other chronic heart disease | CQ phosphate 500 mg twice daily for 10 days | NR | All patients followed for 14 days |
| Mahévas25 | Retrospective cohort study (n = 84) | Hospitalized COVID-19 patients | 59 | 21.7 | Chronic respiratory disease 6%, chronic HF (NYHA III or IV) 1.2%, any cardiovascular condition 45.2%, IDDM 4.8%, CKD 5.0%, liver cirrhosis 1.2%, immunosuppression 9.5% | HCQ sulfate 600 mg/d; 20% also received azithromycin | Baseline, days 3–5 | Follow-up until death, discharge, or day 7 of hospitalization |
| Million (unpublished abstract) | Cohort study (n = 1061) | Hospitalized COVID-19 patients | 43.6 | 53.6 | NR | HCQ and azithromycin for at least 3 days | NR | NR |
| Molina26 | Case series (n = 11) | Hospitalized COVID-19 patients | 58.7 | 36.3 | Obesity 18%, solid cancer 27%, hematologic cancer 18%, HIV 9% | HCQ sulfate 600 mg/d for 10 days and azithromycin 500 mg day 1, then 250 mg/d for 4 days | NR | Follow-up of 10 days |
| Perinel27 | Prospective PK study (n = 13) | COVID-19 patients in critical care | 68 | 15 | 30.7% moderate or severe renal failure, 92% mechanically ventilated | HCQ 200 mg 3 times daily, with dose adjustment to reach trough 1–2 mg/L | NR | Follow-up of ≥5 days |
| Tang28 | Open-label RCT (n = 70) | Mild–moderate (99%) or severe (1%) COVID-19 patients | 48.0 | 44 | DM 16.0%, hypertension 8.0%; liver and renal impairment were exclusion criteria | HCQ sulfate 1200 mg/d for 3 days, then 800 mg/d thereafter (median duration 14 days) | NR | No patients reported as lost to follow-up (median duration 20 days) |
ARDS = acute respiratory distress syndrome; CAD = coronary artery disease; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; CQ = chloroquine; DM = diabetes mellitus; ECG = electrocardiogram; eGFR = estimated glomerular filtration rate; HCQ = hydroxychloroquine; HF = heart failure; HIV = human immunodeficiency virus; IDDM = insulin-dependent diabetes mellitus; IV = intravenous; NA = not applicable; NR = not reported; NYHA = New York Heart Association (functional class); PK = pharmacokinetic; RCT = randomized controlled trial; SR = systematic review; WHO = World Health Organization.
Number of patients treated with chloroquine or hydroxychloroquine (not total number enrolled in the trial). In the Pfizer study, only total number of participants was provided, so this number is shown.
Table 3.
Electrocardiographic and clinical outcomes of included studies
| Study first author | Electrocardiographic outcomes | Clinical outcomes |
|---|---|---|
| Non–COVID-19 participants | ||
| Haeusler17 | NR | No serious cardiac adverse events reported in chloroquine trials with 1702 patients. |
| Pfizer18 | CQ phosphate 1000 mg/d alone: Mean maximum day 3 QTc increase 18.4–35 ms relative to placebo. CQ phosphate 1000 mg/d plus azithromycin 500 mg/d: Increase of 5 ms (upper limit 90% confidence interval: 10 ms) beyond CQ alone. No ECG-related discontinuations reported. |
NR |
| WHO Evidence Review Group19 | NR | No sudden deaths reported in 23,773 courses of CQ for malaria treatment. |
| COVID-19 participants | ||
| Borba20 | QTc >500 ms developed in 7/28 in the high-dose arm and 3/28 in the low-dose CQ arm. | Ventricular arrhythmia developed in 2/28 in the high-dose CQ arm and 0/28 in the low-dose CQ arm. |
| Chen21 | NR | No serious adverse events reported in 15 patients. |
| Chen (preprint) | NR | No serious cardiac events reported in 31 patients. |
| Chorin22 | Increase in QTc from mean baseline of 435 ± 24 ms to mean maximum of 463 ± 32 ms. In 12%, QTc increased by >60 ms, and 11% developed QTc >500 ms. | No arrhythmias reported in 84 patients. |
| Gautret23 | NR | No serious adverse events reported in 80 patients. |
| Huang24 | NR | No adverse cardiac events reported in 10 patients. |
| Mahévas25 | QTc increase >60 ms occurred in 7/84 (1 with QTc >500 ms). | Of 84 patients treated with HCQ, first-degree atrioventricular block developed in 1 patient 2 days after starting HCQ. One patient also developed left bundle branch block on day 8, after being admitted to the ICU and receiving lopinavir-ritonavir. |
| Million (preprint) | NR | No adverse cardiac events reported in 1061 patients. |
| Molina26 | HCQ discontinued in 1/11 due to excessive QT prolongation on day 4 (from 405 to 460–470 ms). | NR |
| Perinel27 | QTc >500 ms occurred in 2/13 patients (from 381 to 510 ms; and from 432 to 550 ms) on days 2 and 3, leading to discontinuation of therapy. | NR |
| Tang28 | No QT prolongation observed in 70 patients. | No arrhythmias reported in 70 patients. |
CQ = chloroquine; ECG = electrocardiogram; HCQ = hydroxychloroquine; ICU = intensive care unit; NR = not reported; WHO = World Health Organization.
Effects on QT interval
A randomized controlled trial of healthy volunteers obtained from Pfizer reported on the ECG effect of chloroquine and its interaction with azithromycin.18 The study found that, compared to placebo, 3 days of chloroquine phosphate 1000 mg/d prolonged QTc by 18.4–35 ms on day 3. The addition of azithromycin 500 mg/d prolonged QTc by a mean of 5 ms beyond chloroquine alone.
For COVID-19 patients, QT data were given in 6 studies (n = 318): 3 preprints (n = 210) and 3 publications (n = 108).22 , 26 , 27 In a cohort of 84 hospitalized patients treated with hydroxychloroquine and azithromycin,22 QTc increased from a mean baseline of 435 ms to a maximum of 463 ms after 3.6 days, with approximately 12% developing QTc >500 ms, a known marker of high arrhythmic risk. The study also noted that baseline QTc poorly predicted this outcome, and that acute renal failure was the strongest predictor of developing acquired long QT syndrome. Other studies generally yielded a similar estimate of the proportion of hospitalized COVID-19 patients developing severe QT prolongation (ie, QTc ≥500 ms or change >60 ms) while taking chloroquine or hydroxychloroquine (approximately 10%).
Effects on ventricular arrhythmias and sudden death
Clinical outcomes were reported across 2 systematic reviews of the cardiotoxicity of quinoline antimalarials. The first review, conducted by the World Health Organization Evidence Review Group in 2016,19 reported no sudden deaths among 23,773 courses of chloroquine for the treatment of malaria, with follow-up ≥14 days. A 2018 systematic review found no events were reported in 1702 subjects given chloroquine for malaria.17 Of note, this review reported an average patient age of 20.8 years and noted that most trials excluded patients with comorbidities.
For COVID-19, 9 studies (a mix of mild, moderate, and severe cases) reported clinical outcomes in a total of 1491 patients treated with chloroquine or hydroxychloroquine. Of these reports, 5 papers (n = 1302) were obtained in prepublication form and 4 papers were published (n = 189).21, 22, 23, 24 Two of 28 patients treated with a high dose of chloroquine (chloroquine diphosphate 1 g twice daily) developed a ventricular arrhythmia. The high-dose arm of the study was subsequently terminated for safety reasons. One study also reported development of first-degree atrioventricular block and left bundle branch block in 2 patients. No other patients studied developed an arrhythmia.
Discussion
Our study found data on short-term chloroquine or hydroxychloroquine use for 2 indications: malaria and COVID-19. Through its use for treatment of malaria, chloroquine may be the drug to which humans have been most exposed, and the resulting real-world experience suggests it is generally safe.17 , 19 Although it did not provide more granular data on arrhythmia or sudden death, a recent unpublished preprint by Lane et al reported an initial 30-day course of hydroxychloroquine for treatment of rheumatoid arthritis, which showed no increase in overall cardiovascular mortality relative to control. This external evidence was consistent with data from studies of malaria included in this review.
However, attempting to apply these data to the current pandemic is limited by the differences in the treated diseases. There is warrant for the concern that COVID-19 patients are a population particularly vulnerable to drug-induced long QT syndrome and arrhythmia. Our included studies indicate that COVID-19 patients treated with these agents are older than participants in malaria studies (mean subject age 46.9 and 20.8 years, respectively) and have more baseline comorbidities. COVID-19 patients have a high frequency of directly arrhythmic risk factors, such as sepsis, multiorgan failure, hypoxia, stress-induced cardiomyopathy, and use of other QT-prolonging agents (eg, azithromycin, selective serotonin reuptake inhibitors, amiodarone). Moreover, COVID-19 may also induce indirect risk factors, such as acute kidney injury, which can cause accumulation of chloroquine and hydroxychloroquine to toxic levels,29 which an included study noted had the best predictive value for QT prolongation.22
The results of this review lend support to the hypothesis that COVID-19 patients may be more susceptible to QT prolongation. A study of chloroquine and azithromycin in 116 healthy volunteers reported no ECG-related drug discontinuations.18 However, among COVID-19 patients, approximately 10% developed QT prolongation to a degree that generally leads to withdrawal of the drug (QTc ≥500 ms or change >60 ms). Although COVID-19 itself may be a confounding factor in this comparison, a preprint of a nonconsecutive case series of COVID-19 patients by Ramireddy et al described greater QTc prolongation with hydroxychloroquine and azithromycin (17 ± 39 ms) compared to azithromycin alone (0.5 ± 40 ms). It also is notable that a randomized trial of lopinavir-ritonavir in severe COVID-19 patients reported only 1 instance of QT prolongation among 95 patients treated with this combination, and no instances among 99 patients who received standard care.30 Although this is an indirect comparison, the considerably smaller proportion with QT prolongation in this population suggests that chloroquine or hydroxychloroquine plays an important role in QT prolongation in COVID-19 patients.
This apparent increased risk of QT prolongation was associated with 2 ventricular arrhythmias in the included studies, both in patients receiving high doses of chloroquine (2 g/d). Although the authors of the study did not report a causality assessment, a causative role of chloroquine is suggested by the lack of events in the lower-dose arm (750 mg twice daily on the first day, then 750 mg/d thereafter) of the randomized trial.20 Nonetheless, the low number of events prevents definitive conclusions about causality. Although there was report of 2 conduction abnormalities with unclear causation, no ventricular arrhythmias were reported at any lower dose, which suggests the relative cardiac safety of these agents when used at typical doses, provided regular ECG monitoring is performed.
Strengths of these findings include our thorough search strategy, which included non-English and industry data. Most included trials seemed to have complete patient follow-up. However, the largest report (n = 1061) had unclear follow-up, and an earlier report from the same institution seems to have included only patients with 6 days of follow-up available, making it possible that some patients may have been lost to follow-up. This requires particular caution in the study of rare toxicities, in which even a single missed event can significantly change estimates of risk. Publication and reporting bias, previously observed in reporting of harms,31 may contribute to underestimation of risk. Moreover, most included data came from preprints that have not gone under peer review, meaning these data are preliminary and possibly more susceptible to such biases.
Published studies of hydroxychloroquine in COVID-19 patients that postdate our search illustrate some of these concerns. One study of 40 patients in intensive care units in France who were treated with hydroxychloroquine 200 mg twice daily for 10 days (with or without azithromycin) noted that 14 (36%) developed QT prolongation, albeit with no arrhythmias reported.32 However, another recent study of 90 hospitalized COVID-19 patients, despite more closely agreeing with our review’s estimate of QT prolongation incidence (20%), also reported an event of torsades de pointes in a patient given hydroxychloroquine and azithromycin.33 This event occurred 3 days after the drugs were discontinued, and other risk factors were present, such as bradycardia, new-onset cardiomyopathy, and use of propofol, a drug that is considered a known risk factor for torsades de pointes.34 Thus, the role played by hydroxychloroquine is uncertain. Nevertheless, given that the long half-lives of these agents still implicate them as possible contributors, this finding serves as a reminder that even a small number of newly reported events could considerably shift risk estimates and, thus, risk–benefit analyses.
Given these limitations and the currently unknown benefit of these agents, it is especially important to take reasonable precautions to minimize possible risk. Because drug-induced QT prolongation was observed early in some included studies, daily ECG monitoring should be considered in all patients regardless of baseline QTc, particularly in patients with other risk factors for torsades (Table 1). Where a lack of resources limits 12-lead ECG placement, alternatives include mobile devices such as KardiaMobile 6L personal 6-lead ECG device or telemetry, or if not available then proactive monitoring and correction (where possible) of risk factors. Although more reliable safety data on COVID-19 from ongoing large randomized trials are expected soon, even ruling out, for example, a 0.1% risk, will still require data on at least 3700 treated patients without any episodes of arrhythmia.35 Our results accordingly lend greater support to the more cautious guidance documents for the near future and are in line with recent guidance from the United States Food and Drug Administration discouraging use of hydroxychloroquine outside of a hospital or trial setting where close supervision is available.36
Conclusion
Our results found evidence of significant QT prolongation in patients with COVID-19 receiving hydroxychloroquine. Arrhythmia was documented during a short course of high-dose chloroquine in critically ill COVID-19 patients. Because of limitations in the current evidence, risk mitigation strategies such as QTc monitoring should be considered in all patients.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrthm.2020.05.008.
Contributor Information
Lior Jankelson, Email: Lior.Jankelson@nyulangone.org.
Giorgio Karam, Email: Giorgio.Karam@dal.ca.
Appendix. Supplementary data
References
- 1.Centers for Disease Control and Prevention Information for Clinicians on Therapeutic Options for Patients with COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html Available at:
- 2.Plaquenil (hydroxychloroquine sulfate). Product monograph. Sanofi-Aventis Canada Inc; Laval, Quebec, Canada: August 2019. [Google Scholar]
- 3.Roden D.M. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 4.Roden D.M. Predicting drug-induced QT prolongation and torsades de pointes. J Physiol. 2016;594:2459–2468. doi: 10.1113/JP270526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kannankeril P., Roden D.M., Darbar D. Drug-induced long QT syndrome. Pharmacol Rev. 2010;62:760–781. doi: 10.1124/pr.110.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White N.J. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis. 2007;7:549–558. doi: 10.1016/S1473-3099(07)70187-1. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:59–60. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roden D.M., Harrington R.A., Poppas A., Russo A.M. Considerations for drug interactions on QTc interval in exploratory COVID-19 treatment. Heart Rhythm. 2020;17:e231–e232. doi: 10.1016/j.hrthm.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazzerini P.E., Boutjdir M., Capecchi P.L. COVID-19, arrhythmic risk and inflammation: mind the gap! Circulation. 2020;142:7–9. doi: 10.1161/CIRCULATIONAHA.120.047293. [DOI] [PubMed] [Google Scholar]
- 10.Plant L.D., Xiong D., Romero J., Dai H., Goldstein S.A.N. Hypoxia produces pro-arrhythmic late sodium current in cardiac myocytes by SUMOylation of NaV1.5 channels. Cell Rep. 2020;30:2225–2236.e4. doi: 10.1016/j.celrep.2020.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giudicessi J.R., Noseworthy P.A., Friedman P.A., Ackerman M.J. Urgent guidance for navigating and circumventing the QTc prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19) Mayo Clin Proc. 2020;95:1213–1221. doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C.-I., Postema P.G., Arbelo E. SARS-CoV-2, COVID-19 and inherited arrhythmia syndromes. Heart Rhythm. 2020;17:1456–1462. doi: 10.1016/j.hrthm.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.COVID-19 Antimicrobial Management Working Group Recommendations for antimicrobial management of adult hospitalized patients with COVID-19. Alberta Health Services; March 22, 2020. https://www.albertahealthservices.ca/assets/info/ppih/if-ppih-covid-19-recommendations.pdf Available from:
- 14.Lakkireddy D.R., Chung M.K., Gopinathannair R. Guidance for cardiac electrophysiology during the coronavirus (COVID-19) pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm. 2020;17:e233–e241. doi: 10.1016/j.hrthm.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drew B.J., Ackerman M.J., Funk M. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55:934–947. doi: 10.1016/j.jacc.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tisdale J.E. Drug-induced QT interval prolongation and torsades de pointes: role of the pharmacist in risk assessment, prevention and management. Can Pharm J (Ott) 2016;149:139–152. doi: 10.1177/1715163516641136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haeusler I.L., Chan X.H.S., Guérin P.J., White N.J. The arrhythmogenic cardiotoxicity of the quinoline and structurally related antimalarial drugs: a systematic review. BMC Med. 2018;16:200. doi: 10.1186/s12916-018-1188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Product monograph. Zithromax (azithromycin dihydrate) Pfizer Canada ULC; Kirkland, Quebec, Canada: May 2019. [Google Scholar]
- 19.WHO Evidence Review Group . World Health Organization; 2017. The cardiotoxicity of antimalarials.https://www.who.int/malaria/mpac/mpac-mar2017-erg-cardiotoxicity-report-session2.pdf Available from. [Google Scholar]
- 20.Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection. JAMA Netw Open. 2020 Apr 1;3(4):e208857. 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed]
- 21.Chen J., Liu D., Liu L. A pilot study of hydroxychloroquine in treatment of patients with moderate coronavirus disease-19 (COVID-19) Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chorin E., Dai M., Shulman E. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nat Med. 2020;26:808–809. doi: 10.1038/s41591-020-0888-2. [DOI] [PubMed] [Google Scholar]
- 23.Gautret P., Lagier J.C., Parola P. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101663. Article 101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang M., Tang T., Pang P. Treating COVID-19 with chloroquine. J Mol Cell Biol. 2020;12:322–325. doi: 10.1093/jmcb/mjaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahévas M., Tran V.T., Roumier M. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369:m1844. doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molina J.M., Delaugerre C., Goff J.L. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perinel S, Launay M, Botelho-Nevers E, et al. Towards optimization of hydroxychloroquine dosing in intensive care unit COVID-19 patients. Published online April 7, 2020. Clin Infect Dis ciaa394 10.1093/cid/ciaa394. [DOI] [PMC free article] [PubMed]
- 28.Tang W., Cao Z., Han M. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smit C., Peeters M.Y.M., van den Anker J.N., Knibbe C.A.J. Chloroquine for SARS-CoV-2: implications of its unique pharmacokinetic and safety properties. Clin Pharmacokinet. 2020;59:659–669. doi: 10.1007/s40262-020-00891-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao B., Wang Y., Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golder S., Loke Y.K., Wright K., Norman G. Reporting of adverse events in published and unpublished studies of health care Interventions: a systematic review. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bessière F, Roccia H, Delinière A, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. Published online May 1, 2020. JAMA Cardiol 10.1001/jamacardio.2020.1787. [DOI] [PMC free article] [PubMed]
- 33.Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). Published online May 1, 2020. JAMA Cardiol 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed]
- 34.CredibleMeds Combined list of drugs that prolong QT and/or cause torsades de pointes (TDP). AZCERT. https://crediblemeds.org/pdftemp/pdf/CombinedList.pdf Available from:
- 35.Newcombe R.N., Altman D.G. Proportions and their differences. In: Altman D.G., Machin D., Bryant T.N., Gardner M.J., editors. Statistics with Confidence. 2nd ed. BMJ Books; London: 2000. pp. 45–56. [Google Scholar]
- 36.U.S. Food and Drug Administration FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. Food and Drug Administration. https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or Available from:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.