Abstract
COVID-19 is a global public health emergency with more than one million positive cases across the globe. COVID-19 has a multifaceted presentation. We are herein to report two cases of SARS-CoV-2 induced rhabdomyolysis with an initial presentation of weakness and elevated creatinine kinase (CK). Both patients had no respiratory symptoms, they only complained of generalized weakness and were found to have elevated CK. Routine chest X-ray showed bilateral infiltrates in both cases and subsequently reverse-transcription polymerase chain reaction (RT-PCR) for SARS-CoV-2 was positive. To the best of our knowledge, there was only one literature to date documented SARS-CoV-2 induced rhabdomyolysis as a late complication of COVID-19 patient. Our cases showed that elevated CK and rhabdomyolysis can be the sole initial presentation of patients with COVID-19 and total CK should be ordered in every patient on admission.
Keywords: SARS-CoV-2, COVID-19, Rhabdomyolysis, Elevated creatinine kinase
1. Introduction
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a public health emergency with more than one million cases worldwide and close to 60,000 deaths as of April 3rd, 2020 [1]. SARS-CoV-2 has been identified as a novel enveloped RNA beta-coronavirus [2]. Patients with SARS-CoV-2 infection mainly present with respiratory tract symptoms, with complications related to cytokine storm syndrome and acute respiratory distress syndrome (ARDS) [3]. Numerous case reports have been published to date that described the clinical characteristics of COVID-19. To our knowledge, these are the first two cases presenting initially as an isolated muscle weakness with increased CK, without fever or respiratory symptoms.
2. Case presentation
2.1. Case 1
A 75-year-old female with past medical history of coronary artery disease status post percutaneous coronary intervention, hypertension and gastroesophageal eflux disease (GERD) presented to our emergency room complaining of generalized weakness and decrease in appetite for 4 days duration. Patient denies muscle pain, respiratory symptoms, fever, chills and rigors. She had no recent travel or known sick exposure. Initial vital signs were stable and initial physical examination was only notable for decrease air entry to the lungs bilaterally with rhonchi and crackles bilaterally. Laboratory findings noted to have elevated troponin of 0.663 ng/ml (0.00–0.45 ng/ml) with electrocardiogram (EKG) not showing any ischemic changes. Total CK was elevated to 2767 U/l (38–176 U/l), hypernatremia of 152 mmol/l (136–145 mmol/l), elevated AST and ALT 198 U/l (10–36 U/l) and 63 (6–29 U/l) respectively as well as elevated blood urea nitrogen and creatinine to 31 mg/dl (6–24 mg/dl) and 1.2 mg/dl (05–1.0 mg/dl) respectively. Complete blood count otherwise within normal limits. Urinalysis was positive for blood with no red blood cells seen microscopically. Chest X-ray performed at baseline showed left lower patchy opacities (Fig. 1 ). Based on the troponin elevation and non-specific symptoms in elderly, patient was loaded with aspirin 324 mg, atorvastatin 80 mg, clopidogrel 600 mg and heparin drip for possible acute coronary symptoms. The next day, patient underwent echocardiogram which showed a left ventricular ejection fraction of 35–40% with hypokinesis of the basal to mid anterior and anteroseptal wall, in addition to a linear density in the descending aorta that can represent an aortic dissection. A CT angiogram was done to clarify the echocardiogram findings, no evidence of dissection or aneurysmal changes in the descending aorta but it showed multiple bilateral ground-glass patchy opacities (Fig. 2 ). Subsequently, nasopharyngeal swab for SARS-CoV-2 RT-PCR was sent and result came back positive. Inflammatory markers including LDH, CRP, DDimer and Ferritin were markedly elevated to 497 U/l(122–222 U/l), 3.7 mg/dl (0.0–0.8 mg/dl), 573 ng/ml (0.0–500 ng/ml) and 2134 ng/ml (11–307 ng/ml) respectively. Patient was then treated with azithromycin, hydroxychloroquine, vancomycin and intravenous cefepime. Also, chest physiotherapy and prone position were encouraged. On day three, patient became more lethargic and noted to have acute encephalopathy with increasing oxygen requirement, she was upgraded to the intensive care unit for close hemodynamically monitoring, supplemental oxygen and continue on supportive care. She also received fluids intermittently with close monitoring of kidney functions and CK level. Patient's oxygenation was improving, CK trending down. She was clinically improved, inflammatory markers were down-trending with normalized CK on discharge.
Fig. 1.
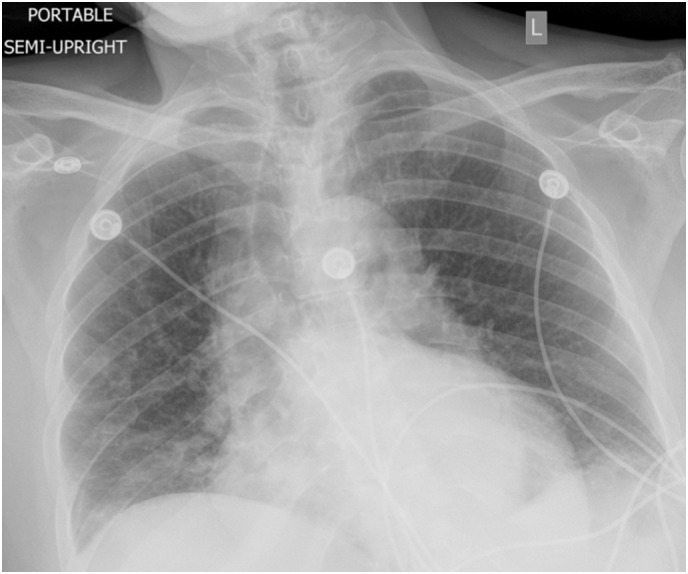
Chest X-ray portable view showing left lower lobe infiltrates.
Fig. 2.
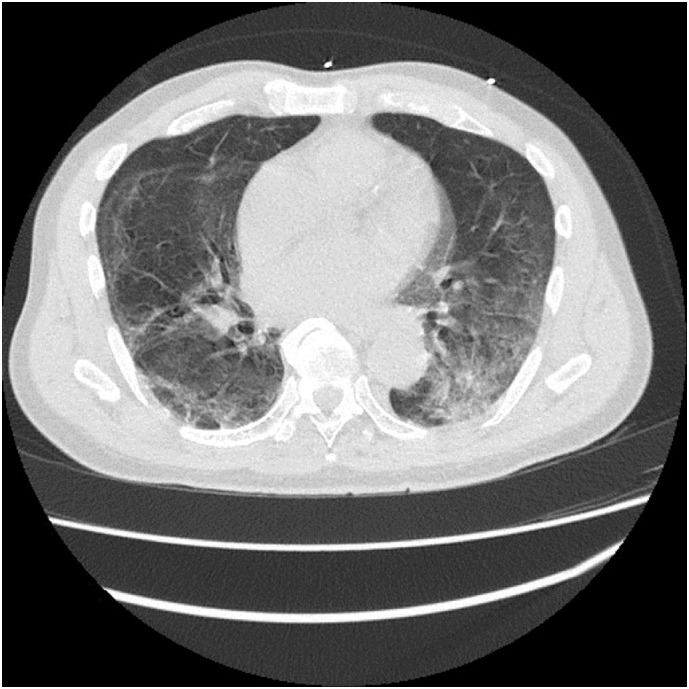
Chest CT showing bilateral ground glass opacities (axial section).
2.2. Case 2
A 71-year-old gentleman with past medical history of hypertension, seizure, chronic kidney disease and overactive bladder presented to the emergency room for repetitive leg twitching and generalized weakness. Patient complained of intermittent leg twitching associated with tingling and numbness sensation at the lateral portion of the upper thigh radiating down to the posterior mid-calf. Otherwise, he denied any hematuria, respiratory symptoms, fever, chill, and rigors. Patient also denied tobacco, alcohol and illicit drug use. Initial vital signs were unremarkable, he was afebrile, hemodynamically stable and saturating well on room air. Physical examination including neurological examination was unremarkable. Nonetheless, he had decrease air entry to the lungs bilaterally with crackles heard diffusely. Chemistry profile showed an increase CK level to 1859, with elevated BUN and creatinine to 78 mg/dl and 3.6 mg/dl from the baseline of 27 mg/dl and 1.9 mg/dl. Troponin was mildly elevated to 0.249 ng/ml. Urinalysis was positive for blood with few red blood cells. Complete blood counts were within normal limits except for the neutrophil to lymphocyte ratio of 7.3. EKG showed new onset atrial fibrillation with controlled heart rate. CT head showed old right lacunar infarct with no evidence of acute event. Chest x-ray showed signs of multifocal pneumonia with suspicion of COVID-19 (Fig. 3 ). Subsequently nasopharyngeal swab for SARS-CoV-2 RT-PCR was sent, which came back positive. Inflammatory markers including LDH, DDimer, ferritin and CRP were markedly elevated to 538 U/l, 989 ng/ml, 1003 ng/ml and 18.8 mg/dl, Procalcitonin was elevated to 9.65 ng/ml (0.0–0.05 ng/ml). Patient was started on doxycycline, ceftriaxone and Hydroxychloroquine. He received 1.5 l bolus of normal saline on admission for rhabdomyolysis. Patient had a spike of fever the next day with progressive worsening kidney function. Creatinine was elevated to 5.6 but total CK was down-trending. Patient has a CHA₂DS₂-VASc Score for atrial fibrillation stroke risk of 4 and was then started on metoprolol tartrate 25 mg BID and therapeutic dose of heparin. He was deteriorated on Day 3 of admission (tachypneic at rate of 40, tachycardic, atrial fibrillation with rapid ventricular rate at of 160–170 beats per minute and oxygen saturation was 89% on non-rebreather mask 100%, which ultimately lead to mechanical ventilation. Kidney functions continued to deteriorate, and the patient was started on hemodialysis. Inflammatory markers peaked at 4 to 6 days after his admission and then started to trend down. Patient was still mechanical ventilated with FiO2 50%, PEEP 8 and tidal volume of 450 at the time of writing this case.
Fig. 3.
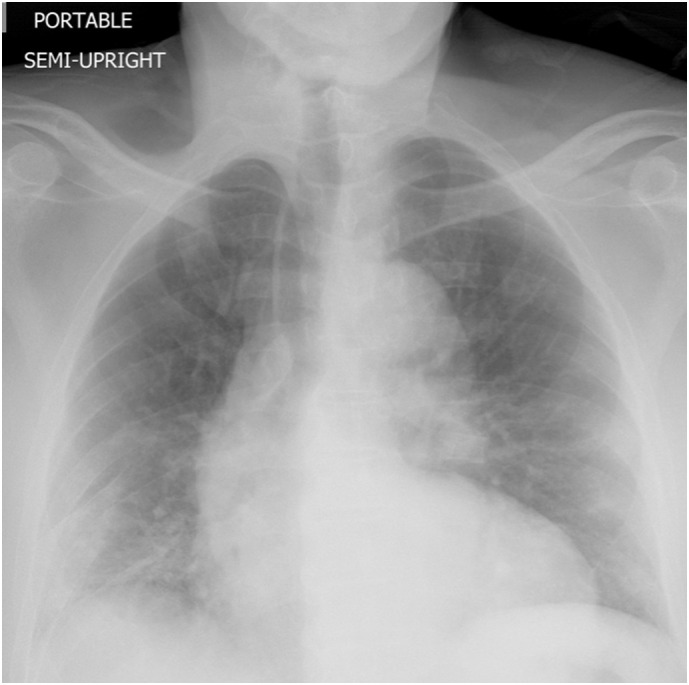
Chest X-ray showing bilateral multifocal patchy opacities more on the peripheral and basal regions.
3. Discussion
COVID-19 has emerged as a public health emergency. World Health Organization (WHO) has declared COVID-19 as a pandemic in March 2020 after the disease had spread to 110 countries and territories [4]. The pathogen that causes the disease - SARS-CoV-2 (previously referred as 2019-nCoV) is a novel enveloped RNA beta-coronavirus. Since the first case reported in Wuhan, China in December 2019, more than one million of cases have been reported in 205 countries and territories as of April 2020 [1].
Patients with SARS-CoV-2 infection mainly present with respiratory tract symptoms. They tend to present as a viral pneumonia picture with fever, cough, dyspnea, fatigue, muscle and joint aches. It has a wide spectrum of illness ranging from mild to critical with around 80% has mild symptoms (asymptomatic or mild pneumonia) as reported by Chinese Center for Disease Control and Prevention that included approximately 44,500 confirmed infections [5].
Rhabdomyolysis, a syndrome with a triad of muscle weakness, pain and red colored urine is characterized by skeletal muscle damage and necrosis, resulting in release of intracellular chemicals into the blood circulation [6]. CK, an enzyme present in the muscle will be markedly elevated. Rhabdomyolysis has been associated with a variety of viral and bacterial infections. The common reported acute viral infection associated with rhabdomyolysis include influenza, parainfluenza, Epstein-Barr virus, Cytomegalic virus, Herpes Simplex virus, and Human Immunodeficiency Virus as well as other respiratory tract pathogens [[7], [8], [9], [10], [11], [12]].
To our knowledge, SARS-CoV-2 is rarely associated with rhabdomyolysis and the incidence of SARS-CoV-2 induced rhabdomyolysis was not well documented and reported. To our best effort, we are only able to find a literature describing this rare phenomenon to date [13]. In that case report, Min and Tong reported that rhabdomyolysis was a late manifestation of COVID-19 [13]. Nonetheless, in both of our cases, elevated CK and rhabdomyolysis seem to be the presenting symptom.
Laboratory abnormalities associated with COVID-19 include lymphopenia, prolonged prothrombin time, elevated D-dimer, fibrinogen and lactate dehydrogenase as well as elevated inflammatory markers such as ferritin and CRP [[14], [15], [16]]. Patient rarely have elevated CK. Bilateral infiltrates and ground-glass opacities were the common findings on chest X-ray and computed tomography (CT) scan of the chest [17].
Both of our patient presented with typical symptoms of rhabdomyolysis with generalized weakness and muscle pain, although they did not complain of any dark urine. Surprisingly, both patients had no fever or respiratory symptoms at their initial presentation that raise the suspicions of COVID-19 infections on initial admission. Laboratory investigations only notable for elevated CK and troponin. Nonetheless, imaging showed bilateral infiltrates at the peripheral and basal regions consistent of COVID-19 disease.
Treatment for rhabdomyolysis involved vigorous fluid resuscitation and management of electrolyte abnormalities, in order to prevent acute kidney injury and severe metabolic disturbances. Although there is no consensus guideline, nonetheless, extensive fluids resuscitation is not recommended in the early stage of COVID-19 management. However, in both our patient, they were given fluids while maintaining adequate urine output and avoid positive fluid balance.
4. Conclusion
In conclusion, we report two unusual presentation of SARS-CoV-2 induced rhabdomyolysis. To our knowledge, this atypical presentation of elevated CK and rhabdomyolysis in elderly patients as the initial manifestations of COVID-19 was not reported in the literature yet. Our cases emphasize the need to keep a high suspicion for COVID 19 in elderly patients presenting with non-specific findings, always obtain chest imaging and consider checking inflammatory markers, when in doubt obtain serology or RT-PCR for SARS-CoV-2.
Funding
None.
Statement of ethics
Patient/Health proxy has given the written and oral informed consent to publish the cases including publication of images.
Declaration of competing interest
All authors including Kok Hoe Chan, Iyad Farouji, Amany Abu Hanoud and Jihad Slim declare no competing conflict of interest.
References
- 1.World Health Organization . Vol. 74. World Health Organization; 2020. Novel coronavirus (2019-nCoV): situation report. [Google Scholar]
- 2.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Director-general's remarks at the media briefing on 2019-nCoV on 11 February 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Giannoglou G.D., Chatzizisis Y.S., Misirli G. The syndrome of rhabdomyolysis: pathophysiology and diagnosis. Eur J Intern Med. 2007;18:90. doi: 10.1016/j.ejim.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Pesik N.T., Otten E.J. Severe rhabdomyolysis following a viral illness: a case report and review of the literature. J Emerg Med. 1996;14:425. doi: 10.1016/0736-4679(96)00078-9. [DOI] [PubMed] [Google Scholar]
- 8.O’Connor J.V., Iyer S.K. Myoglobinuria associated with parainfluenza type 2 infection. N Y State J Med. 1982;82:1469. [PubMed] [Google Scholar]
- 9.Ueda K, Robbins DA, Iitaka K, Linnemann CC Jr. Fatal rhabdomyolysis associated with parainfluenza type 3 infection. Hiroshima J Med Sci 1978; 27:99. [PubMed]
- 10.Sertogullarindan B., Ozbay M.B., Ertem F.U. Rhabdomyolysis associated with mycoplasma pneumoniae infection. Pol Arch Med Wewn. 2013;123:66. [PubMed] [Google Scholar]
- 11.Posner M.R., Caudill M.A., Brass R., Ellis E. Legionnaires’ disease associated with rhabdomyolysis and myoglobinuria. Arch Intern Med. 1980;140:848. [PubMed] [Google Scholar]
- 12.Naschitz J.E., Yeshurun D., Shagrawi I. Rhabdomyolysis in pneumococcal sepsis. Am J Med. 1989;87:479. doi: 10.1016/s0002-9343(89)80842-3. [DOI] [PubMed] [Google Scholar]
- 13.Jin M., Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19 [published online ahead of print, 2020 Mar 20] Emerg Infect Dis. 2020;26(7) doi: 10.3201/eid2607.200445. [doi:10.3201/eid2607.200445] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


