Abstract
Angiotensin-converting enzyme (ACE) and its homologue, ACE2, have been mostly associated with hypertensive disorder. However, recent pandemia of SARS-CoV-2 has put these proteins at the center of attention, as this virus has been shown to exploit ACE2 protein to enter cells. Clear difference in the response of affected patients to this virus has urged researchers to find the molecular basis and pathophysiology of the cell response to this virus. Different levels of expression and function of ACE proteins, underlying disorders, consumption of certain medications and the existence of certain genomic variants within ACE genes are possible explanations for the observed difference in the response of individuals to the SARS-CoV-2 infection. In the current review, we discuss the putative mechanisms for this observation.
Keywords: Angiotensin-converting enzyme, ACE, SARS-COV-2
Graphical abstract
1. Introduction
Angiotensin-converting enzyme (ACE) has its homologue, ACE2 discovered in 2000 as a ACE related caroxypeptidase not inhibited by captopril [1,2]. ACE2 was firstly shown to be expressed in the kidneys of both the normotensive and the spontaneously hypertensive rat strains [3]. Subsequent studies demonstrated down-regulation of renal ACE2 in three different models of hypertension [4]. Moreover, circulating and cardiac levels of angiotensin II (AT-II) were shown to increase in the ACE2-null mice. ACE2 is the principal pathway for Ang- [[1], [2], [3], [4], [5], [6], [7]] formation from AT-II (Ang-1-8), protecting against excessive activation of AT1 receptor in the heart tissues, However, newer findings suggested that ACE2 can be an important element in the renin–angiotensin aldosterone system [5]. Following these studies, ACE and ACE2 focused the attention of researchers for their contribution in diverse human disorders. Recently, the new coronavirus (2019-nCoV or SARS-CoV-2) outbreak which has affected people all over the world has further highlighted the role of ACE2. This virus has about 80% sequence identity with the severe acute respiratory syndrome (SARS)-related coronaviruses (SARS-CoVs) and 96% sequence identity to a bat coronavirus. Most remarkably, SARS-COV-2 was shown to utilize the similar cell entry receptor ACE2 as SARS-CoV [6,7]. A recent study has shown that the ACE2-binding pocket for SARS-CoV-2 spike protein receptor-binding domain (RBD) is almost identical to this one of SARS-CoV RBD. Structural protein modeling led to identification of amino acid residues in SARS-CoV-2 RBD that critical in ACE2 binding. Notably, most of these residues are either highly conserved or have comparable side chain chemical properties with the SARS-CoV RBD. This similarity of the structure and amino acid sequence stimulated intensive debate on the convergent evolution of these viruses RBDs under a pressure of enhanced binding to ACE2 [8]. ACE2 has been shown to be expressed as a membrane bound protein in several human tissues such as lung, intestine, heart and kidney. The surface expression of this protein on was demonstrated on ciliated bronchial cells and on the lung alveolar epithelial cells but also in endothelial cells, which was stated a noticeable discovery [9]. Moreover, a recent in silico analysis of RNA-seq profiles verified expression of ACE2 in the mucosa of oral cavity [10].
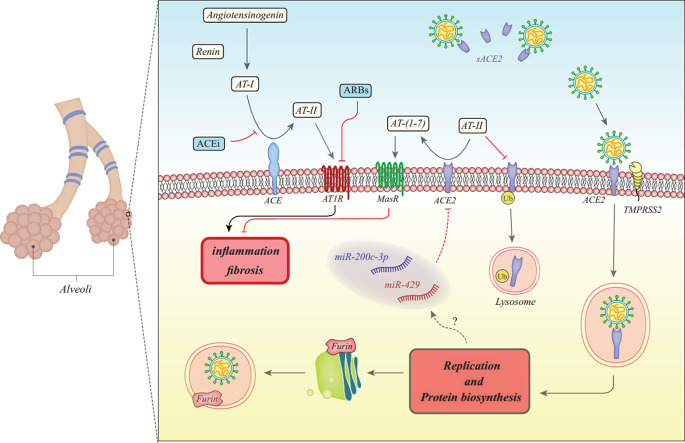
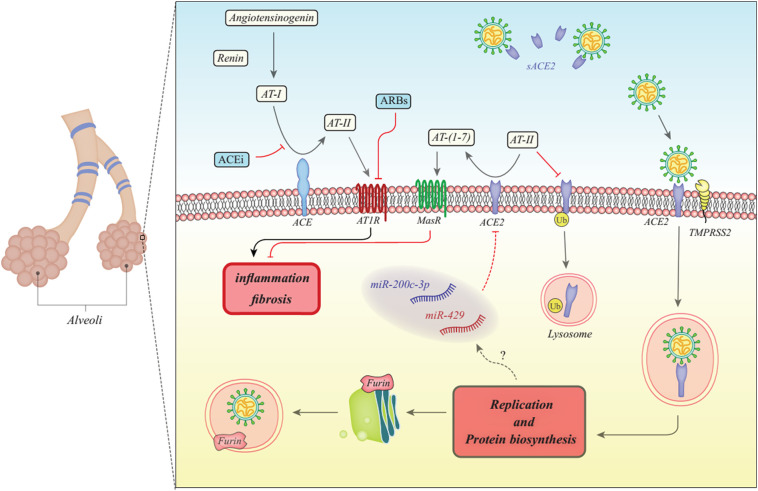
Fig. 1 shows the molecular mechanisms initiated after SARS-CoVs entry into the cells and the significance of ACE and ACE2 in these processes.
Fig. 1.
The first step of conversion of angiotensinogen (AT) to AT-I is catalyzed by renin. Then, ACE converts AT-I to AT-II. Finally, ACE2 cleaves AT-II to produce AT- [[1], [2], [3], [4], [5], [6], [7]]. AT-II can bind with AT1R to initiate inflammation and fibrosis in lung tissue. However, binding of AT- [[1], [2], [3], [4], [5], [6], [7]] with MasR inhibits this process. SARS-CoVs exploits ACE2 for their entrance into the cells. A transmembrane serine protease TMPRSS2 has a crucial role in activation of the fusion of a virus with cell membrane. Moreover, the Furin protease which is proconvertase physiologically required to activate proteins in the Golgi apparatus mediates proteolysis of the spike protein S2 subunit, a unique feature for SARS-CoV-2 [11]. ACE2 levels are decreased in SARS-CoV infected cells leading to increase in AT-II and decrease in AT- [[1], [2], [3], [4], [5], [6], [7]] levels. Based on receptor effects of these proteins mediated by AT1R and MasR, these two alterations have synergic effects on induction of lung fibrosis. Moreover, AT-II has a role in degradation of ACE2 through ubiquitination [12]. SARS-CoVs also enhance expression levels of miR-200c-3p and miR-429 in the infected cells, both of them being regarded as ACE2 targeting miRNAs [13,14] (ACE: angiotensin converting enzyme, ACEi: ACE inhibitor, AT: angiotensinogen, ARB: Angiotensin II receptor blocker).
In the current review, we discuss the expression pattern and function of the both ACE proteins in relation with the underlying disorders, administration of certain medications and the existence of common genomic variants within ACE genes to explain the differences in the response of affected individuals to SARS-COV-2.
2. Expression pattern of ACE and ACE2 in human disorders
In agreement with the role of ACE2 on virus uptake by cells, up-regulation of human ACE2 has increased disease severity in mice infected with SARS-CoV [15]. Moreover, injecting SARS-CoV spike into mice has led to down-regulation of ACE2, thus aggravating the lung injury [16,17]. Consequently, ACE2 functions as the cellular receptor for SARS-CoV entrance but also confers a protective mechanism against lung injury [18]. Based on these investigations, level of expression of ACE2 is an important factor in the SARS-CoV infection. Thus, comorbid conditions that influence expression of this protein might affect severity of disease. Table 1 summarizes the available data on abnormal expression of ACE and ACE2 in human/ animal disorders.
Table 1.
Expression pattern of ACE and ACE2 in human disorders (↑: up-regulation, ↓: down-regulation).
| Disease | Expression/Activity |
Clinical samples | Function | Reference | |
|---|---|---|---|---|---|
| ACE | ACE2 | ||||
| SARS-CoV infection | – | ↑ | Human airway epithelial cells and lung | SARS-CoV preferentially infects well-differentiated ciliated epithelial cells expressing ACE2 | [19] |
| – | ↑ | Human (hu) 293 T kidney cells | Enhanced SARS-CoV S-mediated entry into 293 T cells transiently over-expressing ACE2 | [20] | |
| Diabetes | ↑ | ↑ | STZ induced diabetic rat | p38 MAPK, ERK and JNK hyperphosphorylation with unchanged expression |
[21] |
| ↑ | ↓ | High glucose NRK-52E cells | |||
| ↓ | ↓ | Diabetic Sprague-Dawley rat's kidney | – | [22] | |
| ↑ | ↓ | Kidney tissue from 20 patients with type 2 diabetes | High ACE/ACE2 ratio in type 2 diabetes and overt nephropathy, contributed to renal injury | [23] | |
| Hypertension | ↑ | ↓ | SHR rats | Activated ACE/Ang II/AT1 arm and compromised ACE2/Ang [[1], [2], [3], [4], [5], [6], [7]]/MasR arm in hypertensive brain | [24] |
| ↑ | ↓ | Hypertensive human kidney/heart | Ang II regulates ACE/ACE2 mediated by the AT1-ERK/p38 pathway in mRNA and protein levels |
[25] |
|
| – | ↓ | Hypertensive rat kidney SHR and WKY rats | ACE2 maps to a QTL associated with hypertension in three rat models of high blood pressure | [4] | |
| – | ↑ | Male Sprague-Dawley rats | ACE2 overexpression decreased AT1R and ACE expression and increased AT2R and Mas expression, attenuated proinflammatory cytokines TNF-α, IL-1β and IL-6 in the PVN. | [26] | |
| Kidney disease | ↑ | ↓ | STNx rat kidney | Increased cortical ACE activity and reduced ACE2 activity in the medulla and cortex, increased plasma and urinary ACE2 activity | [27] |
| ↑ | ↑ | 78 renal cortical specimens | Correlation between ACE and ACE2 gene expressions mediated via the local Ang II concentration | [28] | |
| Cardiovascular | – | ↑ | 79 obstructive CAD Patients | Elevated ACE2 activity, an independent predictor of CV mortality and MACE | [29] |
| – | ↑ | Heart and the kidney of GHR−/− mice | Exacerbation of the ACE2/Ang- [[1], [2], [3], [4], [5], [6], [7]]/Mas receptor axis |
[30] |
|
| ↑ | ↑ | Myocardial infarction rat | Elevated expression of both ACE/ACE2 in border/infarct zone and MI-viable myocardium | [31] | |
| – | ↑ | Human heart failure, IDC and ICM | ACE2 is upregulated in human IDC and ICM | [32] | |
| Acute respiratory distress syndrome (ARDS) |
↓ | BALF and lung tissue of LPS-induced ARDS rat | AEC2 attenuates LPS-induced ARDS via the Ang- [[1], [2], [3], [4], [5], [6], [7]]/Mas pathway by inhibiting ERK/NF-κB activation. | [33] | |
| – | – | 31 ARDS patients (51% survivors) | Higher ACE/ACE2 activities in survivors | [34] | |
| Acute lung injury (ALI) |
↓ | LPS-induced ALI rats | The expressions of VDR mRNA and ACE2 mRNA in LPS group was significantly lower than those in normal control group | [35] | |
| ↑ | ↓ | LPS-induced ALI rats/ PMVECS | Up-regulated ACE/Ang II/AT1R axis | [36] | |
| – | ↓ | ACE2 knockout ALI-induced mice | Loss of ACE2 expression resulted in severe ALI phenotypes and rhuACE2 can protect mice from severe acute lung injury | [16] | |
| Neonatal lung injury | – | ↓ | Alveolar epithelial A549 cells | Proteolytic enzymes in meconium effectively degraded ACE-2 in human A549 cells and decreases its protective activity | [37] |
| Smoking | – | ↑ | Human lung tissue | Smokers may be more susceptible to 2019-nCov | [38] |
| Inflammatory bowel disease (IBD) | – | ↑ | CD, UC patients with IBD | ACE2 activity and Ang [[1], [2], [3], [4], [5], [6], [7]] concentrations and the ACE/ACE2 ratio were higher in patients with IBD | [39] |
Streptozotocin (STZ), immunohistochemical staining (IS), Western blot (WB), growth hormone (GH)/insulin-like growth factor-1 (IGF-1) axis, Growth hormone receptor knockout (GHR−/−) mice, major adverse cardiovascular events (MACE), Cardiovascular disease (CV), Idiopathic dilated cardiomyopathy (IDC), ischemic cardiomyopathy (ICM), Pulmonary microvascular endothelial cells (PMVECS), Recombinant human ACE2 protein (rhuACE2), Crohn's disease (CD) and ulcerative colitis (UC).
It is worth mentioning that adult stem cells which have immunomodulatory and pro-reparative activities in the local environment [40] might affect the process of SARS infection and tissue regeneration. The regenerative capacity of these cells [41] can be exploited for avoidance of tissue damage following infection. Yet, clinical evidence in this regard is scarce. Several medications have been shown to alter expression levels of ACE or ACE2. Administration of these medications not only can modify a risk of infection with SARS-CoV, but also can affect the disease course. Table 2 summarizes the results of studies which reported alteration of ACE or ACE2 levels following administration of certain medications.
Table 2.
The effect of different treatments on the expression pattern of ACE and ACE2 (↑: up-regulation, ↓: down-regulation).
| Treatment | Affected protein |
Treated Disease | Samples | Function | Reference | |
|---|---|---|---|---|---|---|
| ACE | ACE2 | |||||
| Calcitriol | – | ↑ | Acute lung injury (ALI) | LPS-induced ALI rats | Calcitriol can increase the expressions of VDR mRNA and ACE2 mRNA and protein levels of VDR and ACE2. | [35] |
| – | ↑ | Hypertensive brain | SHR and WKY rats/BV2 cells | Decreased Ang II, unchanged ACE and increased ACE2 suggested enhanced ACE2/Ang [[1], [2], [3], [4], [5], [6], [7]]/MasR axis in vivo and vitro | [24] | |
| ↓ | ↑ | Diabetic kidney disease | STZ induced diabetic rat /NRK-52E cells | Regulates ACE/ACE2 possibly by p38 MAPK or ERK, but not JNK pathways. | [21] | |
| ↓ | ↑ | Acute lung injury (ALI) | LPS-induced ALI rats/ PMVECS | Iinhibited ACE, AT1R, induced ACE2, suppressed renin and Ang II expression | [36] | |
| ↓ | ↑ | Hypertension | SHR and normotensive WKY rats | Downregulation of Ace in SHR rats and upregulation of Ace2 in normotensive WKY | [42] | |
| ACEI | – | ↑ | hepatic fibrosis | Liver fibrosis/ hepatic stellate cells (HSC) | ACE inhibitors can upregulate ACE2 under conditions of liver injury both in vivo and in vitro. | [43] |
| ↓ | – | myocardial infarction (MI) | Viable myocardium of MI rats | ACE inhibition was associated with inhibited cardiac ACE but ACE2 catalytic activity was unchanged. | [31] | |
| – | ↑ | Acute kidney injury (AKI) | Renal cortex and medulla in STNx-induced AKI | Ramipril had no effect on ACE or ACE2 mRNA expression in either STNx or Control kidneys but increased both cortical and medullary ACE2 activity. | [44] | |
| DIZE | – | ↑ | Hyperoxic lung injury (HLI) | BALF and lung of HLI mice | Inhibited NF-κB pathway, activated Nrf2/HO-1/NQO1 pathway and reduces severity of HLI | [45] |
| – | ↑ | acute kidney injury | Kidney cortex of STNx rat | Increased cortical ACE2 gene expression, increased ACE2 cortex and medulla activity. Reduced cortical ACE activity. | [27] | |
| – | ↑ | myocardial infarction (MI) | AMI rat | Suppressed TNFα, IL-6, reduced COX-2 and iNOS, and activated ACE2/AT1R/MasR pathway. | [46] | |
| ↓ | ↑ | diabetic nephropathy (DN) | Kidney of DN rat | Restored ACE2 levels and further increased of AT2 receptors expression | [47] | |
| Statin | – | ↑ | Diabetes | STZ induced diabetic rat | Combined fluvastatin/insulin treatment more efficiently prevents diabetic cardiomyopathy. | [48] |
| – | ↑ | thickening after vascular balloon injury | Wistar rats | Upregulation of ACE2, an increase in Ang- [[1], [2], [3], [4], [5], [6], [7]], downregulation of AT1, and activation of the P-ERK pathway. | [49] | |
| ↓ | ↑ | diabetic myocardium | STZ induced diabetic rat | Attenuated ACE/ACE2 ratio to normal values | [50] | |
| Fasudil | ↓ | ↑ | Acute pulmonary embolism (APE) | SD rat PAECs | ACE2 activation by ROCK inhibitor for APE treatment | [51] |
| ↓ | ↑ | Myocardial fibrosis | Overload pressure model of SD rats | Fasudil inhibits overload pressure-induced myocardial fibrosis by improving ACE2 and angiotensin [[1], [2], [3], [4], [5], [6], [7]]. | [52] | |
| ↓ | ↑ | Hypertension | Hypertensive DOCA)-salt rat | Increased vascular and plasma ACE2 activity, reduced Ang II and increased Ang- [[1], [2], [3], [4], [5], [6], [7], [8], [9]] plasma levels. | [53] | |
| – | ↑ | Hypoxic pulmonary hypertension (HPH) | Hypoxia-Induced PH rats/PASMC | Up-regulated Ang- [[1], [2], [3], [4], [5], [6], [7]] and ACE2, and lessened HIF-1α attenuate the PVSR and PH. | [54] | |
Acute Lung Injury (ALI), AXCE inhibitor (ACEI), Lipopolysaccharide (LPS), Brain of spontaneously hypertensive rats (SHR), Wistar–Kyoto (WKY), microglial cells (BV2), Streptozotocin (STZ), Rat renal tubular epithelial cells (NRK-52E), Pulmonary microvascular endothelial cells (PMVECS), Hearts of spontaneously hypertensive rats (SHR), Renal tubular epithelial cells cultured in high-glucose medium (MTC), ACE2 agonist diminazene aceturate (DIZE), Bronchoalveolar lavage fluid (BALF), Subtotal nephrectomy (STNx), Acute myocardial infarction (AMI), Sprague-Dawley rats (SD), Fasudil: Rho-associated, coiled-coil containing protein kinase (ROCK) inhibitor, Deoxycorticosterone acetate (DOCA)-salt hypertensive rat, pulmonary vascular structure remodeling (PVSR).
3. Association between ACE/ACE2 polymorphisms and human disorders
Several potentially functional gene polymorphisms have been identified in ACE and ACE2. Associations between these polymorphisms and human disorders have been assessed in different populations. The ACE gene I/D polymorphism, 287-bp sequence insertion or deletion of DNA in intron 16 (rs4340, rs4646994), is perhaps the most studied polymorphism in this regard. Being associated with the onset and course of diabetic nephropathy [55,56], the I/D genotype is regarded as a determinant of ACE expression levels in plasma, cells, and tissues [[57], [58], [59]]. Moreover, the ACE2 rs2074192 and rs2106809 polymorphisms have been associated with lower levels of circulating AT- [[1], [2], [3], [4], [5], [6], [7]] [60]. Table 3 shows the results studies which assessed the association between ACE polymorphisms and human disorders.
Table 3.
Association between ACE/ ACE2 polymorphisms and human disorders in different populations.
| Gene | SNP | Disease | Treatment | Case/Control | Population | Conclusion | Reference |
|---|---|---|---|---|---|---|---|
| ACE | ACE I/D | Kidney Disease and Hypertension | ACE inhibitor ramipril | 347 | African American | II or DD homozygous genotypes and homozygous ACE haplotypes confer faster response to Ramipril. | [61] |
| Hypertension | ACEI Enalapril, Lisinopril or Imidapril | 190 (70 with cough /120 without cough) | Japanese | ACE-inhibitor-induced cough was not related to the ACE polymorphism. | [62] | ||
| ACEI-induced cough | ACE inhibitor | 144/105 | Spanish | The rs4646994 I allele is associated with cough (protective effect in males and risk conferring in females). | [63] | ||
| Erectile dysfunction | Sildenafil | 113/118 | German | ACE II homozygous patients are better responder to sildenafil. | [64] | ||
| hypertrophic cardiomyopathy (HCM) | – | 368 MYPBC3 mutation carriers | Dutch | ACE-DD was significantly associated with the Wigle score. | [65] | ||
| Post exercise CK increase | – | 70 Healthy athletes | Ashkenazi and non-Ashkenazi Caucasian | ACE II/ID was associated with elevated CK activity and higher peak CK levels. | [66] | ||
| Psoriasis | – | 207/ 182 | Austrian Caucasian | ACE II genotype was associated with higher risk of early-onset psoriasis. | [67] | ||
| cardiometabolic disease | Chlorthalidone, calcium channel blocker (amlodipine) or ACEi (lisinopril) | 9309/ 8164 | – | ACE I/D polymorphism was associated with fasting glucose level during antihypertensive treatment. | [68] | ||
| Heart failure (HF) | – | 58 | Canadian Caucasian | AGT (T235)/ACE(D) combined polymorphisms associated with HF predisposition | [69] | ||
| Pneumonia | – | 1239/2400 | Asian Caucasian |
ACE-DD genotype of rs4340 polymorphism is associated with increased risk of pneumonia | [70] | ||
| Multiple sclerosis (MS) | IFN-β1a treatment | 391/ 380 | Persian | Higher prevalence of ACE I allele in MS patients, overrepresentation of the I allele in irresponsive patients to IFN-β. | [71] | ||
| Healthy persons | – | 80 | Caucasians | ACE I allele is associated with higher serum level of ACE. | [72] | ||
| ACE 2 | rs1978124 (A1075G) | Hypertension | Anti-hypertensive (F/M %) 16.05/ 12.59 | 1009/756 | Chinese Han | Significant haplotype: G-T-G-G-A (rs1978124, rs2106809, rs1403543, rs5194, rs56204867) |
[73] |
| Retinopathy T2DM | – | 743 cases DR/DNR F: 237/171 M:182/153 |
Chinese | rs2074192 (TT) and rs714205 (CC) were higher in DR in female (P < .05). | [74] | ||
| rs879922 | T2 Diabetes | – | 275/272 | Uygurs | The rs1978124, rs2048683, rs2074192, rs233575, rs4240157, rs4646156, rs4646188 and rs879922 were associated with T2D. The rs879922 is common maker for T2D and related cardiovascular risks. | [75] | |
| rs2106809 | hypertrophic cardiomyopathy | – | 261/ 609 | Chinese Han | T allele of rs2106809 and C allele of rs6632677 conferred risk for HCM. | [76] | |
| Hypertension | ACEI Benazepril/ Imidapril |
497 hypertensive patients | Chinese Han | Lower BP in CC/CT carrier female | [77] | ||
| – | 246/274 | Odisha, India | ACE (DD) and rs2106809 (TT) were associated with disease in females. | [78] | |||
| Atenolol, Hydrochlorothiazide, Captopril, or Nifedipine | 3408 untreated hypertensive patients | Chinese Han | T allele confers a high risk for hypertension and reduced antihypertensive response to ACE inhibitors. | [79] | |||
| AF | – | 265/289 | Chinese Han | rs2106809 (T) conferred higher risk of AF in males. | [80] | ||
| rs2074192 | Hypertension | – | 647 cases 289 LVH/ 358 |
Chinese Han | ACE2 tag SNPs rs2074192 and rs2106809 as well as major haplotypes CCGC and TCGT are associated with blood pressure and LVH. | [81] | |
| rs4646176 | Blood pressure | High/low-sodium intervention | 1906 cases from 637 families | Chinese Han | rs1514283, rs1514282, and rs4646176 were significantly associated with SBP, DBP, or MAP responses to low and high-sodium intervention. | [82] | |
| rs2285666 (G8790A) | fatal CAD events | – | 1382 CAD/ 453 fatal CAD | Finnish, Swedish, Irish, French | rs2285666 (A) significantly associated with the risk of cardiovascular death in female. | [83] | |
| hypertension T2 Diabetes with stroke |
– | 7251 cases | Han Chinese | G8790A is a risk factor for hypertension in Han-Chinese males, and females from other ethnicities. | [84] |
Wigle's score, a point score system which takes into account the thickness of the ventricular septum, hypertrophic cardiomyopathy (HCM), Blood pressure (BP), Type 2 diabetes mellitus (T2DM), diabetes with retinopathy (DR), without retinopathy (DNR), Hypertrophic cardiomyopathy (HCM), Lone atrial fibrillation (AF), Hypertensive left ventricular hypertrophy (LVH), Systolic/diastolic blood pressure (SBP/DBP).
4. Associations between microRNAs (miRNAs) and ACE-related pathways
MicoRNA (miRNAs) as regulators of gene expression have been involved in several ACE-related pathways and have been shown to alter expression of ACE proteins or being altered by ACE proteins. These small-sized RNAs can bind with the 3′ untranslated region (3′ UTR) of their targets to stimulate degradation of the target mRNA and suppress translation. Moreover, miRNAs can interact with 5′ UTR, coding regions, and promoters, thus regulating gene expression by various mechanisms. Secretion of miRNAs in extracellular components provides them the ability to participate in the cell-cell communication [85]. Table 4 shows the results of studies which assessed association between miRNAs and ACE proteins.
Table 4.
Summary of studies which assessed association between miRNAs and ACE proteins.
| miRNA | Disease | Samples | Function | Reference |
|---|---|---|---|---|
| let-7b | Hypoxic pulmonary hypertension (HPH) | let-7b−/− rat | HIF-1α-dependent hypoxia stimulated let-7b inhibited ACE2 expression via the HIF-1α-let-7b-ACE2 axis and contributed to the HPH | [86] |
| miR-421 | Cardiovascular disease (CVD) | Primary cardiac myofibroblasts | miR-421 down-regulates ACE2 expression. | [14] |
| Chronic Kidney Disease (CKD) | Circulating leukocytes | A significant, inverse correlation between circulating miR-421serum level and the ACE2 expression in leucocytic was shown. Also, ACE2 upregulation following Anti-miR-421 treatment was reported | [87] | |
| miR-1246 | acute lung injury (ALI) | LPS-exposed pulmonary microvascular endothelial cells (PMVECs) | miR-1246 meditates LPS-induced pulmonary endothelial cell apoptosis in vitro and ALI in mouse models, by targeting ACE2. | [88] |
| miR-200c-3p | Acute respiratory distress syndrome (ARDS) | HEK293T cells | Avian influenza virus H5N1 induced the miR-200c-3p upregulation via an NF-κB dependent manner to reduce ACE2 levels and cause lung injury | [13] |
| miR-483-3p | Vascular diseases | human embryonic kidney (HEK-293) | miR-483-3p target 3′-UTRs of AGT, ACE-1, ACE-2 and AT2R | [89] |
| miR-4262 | acute lung injury (ALI) | bleomycin-induced ALI mouse | ACE2-induced suppression of miR-4262, which lead to Bcl-2 protein upregulation, decreased the ALI severity by inhibiting the apoptosis of PECs | [90] |
Hypoxic pulmonary hypertension (HPH), let-7b knockout (let-7b−/−), Doxorubicin-induced heart cardiomyopathy (DHC), ALI-induced apoptosis of pulmonary endothelial cells (PECs).
5. Discussion
In the current study, we reviewed the available literature about the expression pattern of ACE peptidases and the influence of various disorders and medications on the levels of these proteins. Expression level of ACE2 has importance in severity of infection with SARS-COV-2 and the extent of lung injury [18]. Most recently, human recombinant soluble ACE2 (hrsACE2) has been shown to inhibit growth of SARS-CoV-2 and interrupt early stages of infections with this virus [91].
Based on the abundance of genetic modifying factors in determination of ACE2 levels, it is advisable to create a risk predictive panel to determine propensity for severe infection of individual. Whole genome sequencing of the patients' samples is the best method for identification of genetic variants that determine severity of the disorder. If a few genes were recognized that have a significant impact on the variability of COVID-19 course, a genetic test for coronavirus susceptibility could be simple to make, cheap and accurate. However, much more genes could be involved in this process. Perhaps a complex regulatory pattern of genetic expression which is involved in the physiology of the lung and upper respiratory tract shape in addition to ACE2 might contribute in this disorder.
Assessment of association between ACE proteins expression and human disorders has implications in health consequences after recovery from the primary SARS-CoV-2 infection. This would be a next important issue after extinguishing of the pandemia.
Hypertension is reported to be the most common comorbidity in SARS-CoV-2 infection [92], and the ACE protein is a target for ACE-inhibitors which are used in the treatment of hypertension to ultimately decrease the amount of Ang II. Some polymorphisms in ACE gene are reported to influence the efficacy of these inhibitors among them is the homozygous ACE haplotypes which lead to faster response to ramipril [61]. The expression and function of the ACE itself are affected by its polymorphisms which are associated with susceptibility to different diseases such as hypertension and diabetes mellitus [93]. Notably, polymorphisms in both ACE and ACE2 are important in the regulation of the ACE2 expression [94,95].
On the other hand, a meta-analysis has reported association between the administration of ACE inhibitors and reduction in risk of pneumonia. Notably, ACE inhibitors may be more efficient in reducing the risk of pneumonia in Asian patients. Also, treatment with ACE inhibitors was associated with a significant reduction in risk of pneumonia-related mortality compared with controls [96]. This may be also related to the dual effect of ACE2 in viral infection and protection against acute respiratory distress syndrome.
Although the ACE/ACE2 regulation is complicated, it seems that in the absence of ACE the accumulation of angiotensin I may lead to the upregulation of ACE2. Whether this could facilitate the viral infection, is plausible because ACE2 is considered as a specific target for coronavirus treatment [95]. It means that the population-based differences in the ACE2 expression may affect the efficacy of a future antiviral treatment.
In brief, we summarize that coronaviruses, such as SARS-CoV and SARS-CoV-2, utilize ACE2 receptor for cell entry and infection. We know that the most severe consequence of the SARS-CoV-2 is pneumonia, which developes mostly in eldery males and subjects with comorbidities like diabetes, kidney disease, hypertension [97]. Besides, ACE2 has a protective role against acute respiratory distress syndrome. Thus, it can be concluded that decreased ACE2 level contributes to severe consequences of SARS-CoV-2 infection, while ACE2 is essential for the virus-cell fusion. One explanation for this controversy is a viral-induced transitional overexpression of ACE2 at the first stage of the infection [98]. However, a recent in silico analysis of sex bias severity of SARS-CoV-2 infection did not support the association between ACE2 genetic variants and disease severity/sex bias in the Italian population. Yet, TMPRSS2 levels and genetic variants were suggested as potential candidate modulators of the disease course [99].
Accordingly, among the top 38 eQTLs in ACE2, the strongest expression positive eQTL is more prevalent in East Asian females [100]. We also suggest epigenetic regulation by the potential miRNAs targeting on ACE2 transcripts. The results of the Targetscan database (www.targetscan.org) list miR-200c-3p and miR-429 among the most prominent miRNAs that target ACE2. Up-regulation of miR-200c-3p is induced by a viral infection which leads to the downregulation of ACE2 [13]. Also, miR-421 is proved to downregulate ACE2 translation [14].
Interestingly, we have analyzed the well-studied I/D in ACE in Iranian patients with multiple sclerosis and reported association between this polymorphism and response to Interferon-β treatment [71]. Thus, ACE/ ACE2 polymorphisms not only can predispose individuals to diverse diseases, but also they can modulate response of patients to therapeutic options. Both activities have implications on the susceptibility to SARS-CoV-2 infection and the disease course. Another research era might be the identification of the difference between ACE/ACE2 expression levels and their regulating factors, such as the mentioned eQTL and miRNAs, between patients with severe and mild symptoms in different ethnic groups to find the possible effect of ethnicity, gender and the period of the disease on the ACE/ACE2 expression.
In addition to the routine models for investigation of the pathological events during infections, tissue engineering methods particularly “advanced biomaterials” or “functionalized scaffolds” [101] would provide study models to investigate the potential of such approaches in the treatment of the disorder. As an advance in the field of functional studies, the obtained results from “safe” in-vitro models which work without any additive can be applied in human models [102].
Taken together, the data presented above show the diversity of factors that modulate ACE/ ACE2 expression both in physiological conditions and in the course of SARS-CoV-2 infection. Different levels of expression and function of the ACE proteins, underlying disorders such as diabetes and hypertension, administration of certain medications, especially ACE inhibitors and calcitriol, and the existence of certain genomic variants within ACE genes that modulate function or expression of the encoded proteins are possible explanations for the observed difference in the response of individuals to the SARS-CoV-2 infection. Exploration of the role of these factors can lead to design of appropriate therapeutic modalities based on the personalized risks. Such personalized approach is expected to be more effective. Exploitation of the next generation sequencing methods at both genomic and transcriptomic levels would be a practical strategy in this regard.
In conclusion, the observed differences in the course of SARS-CoV-2 infection can be attributed to several genetic factors, comorbidities and administration of medical regimens that modulate expression of ACE proteins.
Acknowledgements
This study was finacially supported by Shahid Beheshti University of Medical Sciences.
Contributor Information
Mohammad Taheri, Email: mohammad_823@yahoo.com.
Marek Sanak, Email: nfsanak@cyf-kr.edu.pl.
References
- 1.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N. A novel angiotensin-converting enzyme–related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87(5):e1–e9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 2.Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000;275(43):33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 3.Tikellis C., Cooper M., Bialkowski K., Johnston C., Burns W., Lew R. Developmental expression of ACE2 in the SHR kidney: a role in hypertension? Kidney Int. 2006;70(1):34–41. doi: 10.1038/sj.ki.5000428. [DOI] [PubMed] [Google Scholar]
- 4.Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417(6891):822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 5.Chappel M., Ferrario C. ACE and ACE2: their role to balance the expression of angiotensin II and angiotensin-(1–7) Kidney Int. 2006;70(1):8–10. doi: 10.1038/sj.ki.5000321. [DOI] [PubMed] [Google Scholar]
- 6.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker M.M., Graham R.L., Donaldson E.F., Rockx B., Sims A.C., Sheahan T. Synthetic recombinant bat SARS-like coronavirus is infectious in cultured cells and in mice. Proc. Natl. Acad. Sci. 2008;105(50):19944–19949. doi: 10.1073/pnas.0808116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020:1–9. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 9.Hamming I., Timens W., Bulthuis M., Lely A., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12(1):1–5. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2014;88(2):1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang K., Gheblawi M., Oudit G.Y. Angiotensin converting enzyme 2: a double-edged sword. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047049. (in press) [DOI] [PubMed] [Google Scholar]
- 13.Liu Q., Du J., Yu X., Xu J., Huang F., Li X. miRNA-200c-3p is crucial in acute respiratory distress syndrome. Cell Discov. 2017;3(1):1–17. doi: 10.1038/celldisc.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambert D.W., Lambert L.A., Clarke N.E., Hooper N.M., Porter K.E., Turner A.J. Angiotensin-converting enzyme 2 is subject to post-transcriptional regulation by miR-421. Clin. Sci. 2014;127(4):243–249. doi: 10.1042/CS20130420. [DOI] [PubMed] [Google Scholar]
- 15.Yang X.-h., Deng W., Tong Z., Y-x Liu, L-f Zhang, Zhu H. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp. Med. 2007;57(5):450–459. [PubMed] [Google Scholar]
- 16.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020:1–5. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia H.P., Look D.C., Shi L., Hickey M., Pewe L., Netland J. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J. Virol. 2005;79(23):14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofmann H., Geier M., Marzi A., Krumbiegel M., Peipp M., Fey G.H. Susceptibility to SARS coronavirus S protein-driven infection correlates with expression of angiotensin converting enzyme 2 and infection can be blocked by soluble receptor. Biochem. Biophys. Res. Commun. 2004;319(4):1216–1221. doi: 10.1016/j.bbrc.2004.05.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin M., Gao P., Zhao T., He L., Li M., Li Y. Calcitriol regulates angiotensin-converting enzyme and angiotensin converting-enzyme 2 in diabetic kidney disease. Mol. Biol. Rep. 2016;43(5):397–406. doi: 10.1007/s11033-016-3971-5. [DOI] [PubMed] [Google Scholar]
- 22.Tikellis C., Johnston C.I., Forbes J.M., Burns W.C., Burrell L.M., Risvanis J. Characterization of renal angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension. 2003;41(3):392–397. doi: 10.1161/01.HYP.0000060689.38912.CB. [DOI] [PubMed] [Google Scholar]
- 23.Mizuiri S., Hemmi H., Arita M., Ohashi Y., Tanaka Y., Miyagi M. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am. J. Kidney Dis. 2008;51(4):613–623. doi: 10.1053/j.ajkd.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Cui C., Xu P., Li G., Qiao Y., Han W., Geng C. Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: role of renin-angiotensin system. Redox Biol. 2019;26:101295. doi: 10.1016/j.redox.2019.101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koka V., Huang X.R., Chung A.C., Wang W., Truong L.D., Lan H.Y. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am. J. Pathol. 2008;172(5):1174–1183. doi: 10.2353/ajpath.2008.070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sriramula S., Cardinale J.P., Lazartigues E., Francis J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc. Res. 2011;92(3):401–408. doi: 10.1093/cvr/cvr242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velkoska E., Patel S.K., Griggs K., Pickering R.J., Tikellis C., Burrell L.M. Short-term treatment with diminazene aceturate ameliorates the reduction in kidney ACE2 activity in rats with subtotal nephrectomy. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0118758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wakahara S., Konoshita T., Mizuno S., Motomura M., Aoyama C., Makino Y. Synergistic expression of angiotensin-converting enzyme (ACE) and ACE2 in human renal tissue and confounding effects of hypertension on the ACE to ACE2 ratio. Endocrinology. 2007;148(5):2453–2457. doi: 10.1210/en.2006-1287. [DOI] [PubMed] [Google Scholar]
- 29.Ramchand J, Patel SK, Srivastava PM, Farouque O, Burrell LM. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PloS one. 2018;13(6). [DOI] [PMC free article] [PubMed]
- 30.Giani J.F., Miquet J.G., Muñoz M.C., Burghi V., Toblli J.E., Masternak M.M. Upregulation of the angiotensin-converting enzyme 2/angiotensin-(1–7)/mas receptor axis in the heart and the kidney of growth hormone receptor knock-out mice. Growth Hormon. IGF Res. 2012;22(6):224–233. doi: 10.1016/j.ghir.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burrell L.M., Risvanis J., Kubota E., Dean R.G., MacDonald P.S., Lu S. Myocardial infarction increases ACE2 expression in rat and humans. Eur. Heart J. 2005;26(4):369–375. doi: 10.1093/eurheartj/ehi114. [DOI] [PubMed] [Google Scholar]
- 32.Goulter A.B., Goddard M.J., Allen J.C., Clark K.L. ACE2 gene expression is up-regulated in the human failing heart. BMC Med. 2004;2(1):19. doi: 10.1186/1741-7015-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y., Zeng Z., Cao Y., Liu Y., Ping F., Liang M. Angiotensin-converting enzyme 2 prevents lipopolysaccharide-induced rat acute lung injury via suppressing the ERK1/2 and NF-κB signaling pathways. Sci. Rep. 2016;6:27911. doi: 10.1038/srep27911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddy R, Asante I, Liu S, Parikh P, Liebler J, Borok Z, et al. Circulating angiotensin peptides levels in acute respiratory distress syndrome correlate with clinical outcomes: a pilot study. PloS one. 2019;14(3). [DOI] [PMC free article] [PubMed]
- 35.Yang J., Jun X., Zhang H. Effect of vitamin D on ACE2 and vitamin D receptor expression in rats with LPS-induced acute lung injury. Chin. J. Emerg. Med. 2016;25(12):1284–1289. [Google Scholar]
- 36.Xu J., Yang J., Chen J., Luo Q., Zhang Q., Zhang H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017;16(5):7432–7438. doi: 10.3892/mmr.2017.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gandhi C.K., Holmes R., Gewolb I.H., Uhal B.D. Degradation of lung protective angiotensin converting enzyme-2 by meconium in human alveolar epithelial cells: a potential pathogenic mechanism in meconium aspiration syndrome. Lung. 2019;197(2):227–233. doi: 10.1007/s00408-019-00201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai G. Bulk and single-cell transcriptomics identify tobacco-use disparity in lung gene expression of ACE2, the receptor of 2019-nCov. medRxiv. 2020 (in press) [Google Scholar]
- 39.Garg M., Burrell L.M., Velkoska E., Griggs K., Angus P.W., Gibson P.R. Upregulation of circulating components of the alternative renin-angiotensin system in inflammatory bowel disease: a pilot study. J. Renin-Angiotensin-Aldosterone Syst. 2015;16(3):559–569. doi: 10.1177/1470320314521086. [DOI] [PubMed] [Google Scholar]
- 40.Tatullo M., Codispoti B., Pacifici A., Palmieri F., Marrelli M., Pacifici L. Potential use of human periapical cyst-mesenchymal Stem Cells (hPCy-MSCs) as a novel stem cell source for regenerative medicine applications. Front Cell Dev Biol. 2017;5:103. doi: 10.3389/fcell.2017.00103. PubMed PMID: 29259970. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ballini A., Scacco S., Coletti D., Pluchino S., Tatullo M. Mesenchymal stem cells as promoters, enhancers, and playmakers of the translational regenerative medicine. Stem Cells Int. 2017;2017 doi: 10.1155/2017/3292810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Machado C.D.S., Ferro Aissa A., Ribeiro D.L., Antunes L.M.G. Vitamin D supplementation alters the expression of genes associated with hypertension and did not induce DNA damage in rats. J. Toxic. Environ. Health A. 2019;82(4):299–313. doi: 10.1080/15287394.2019.1592044. [DOI] [PubMed] [Google Scholar]
- 43.Ml Huang, Li X., Meng Y., Xiao B., Ma Q., Ss Ying. Upregulation of angiotensin-converting enzyme (ACE) 2 in hepatic fibrosis by ACE inhibitors. Clin. Exp. Pharmacol. Physiol. 2010;37(1):e1–e6. doi: 10.1111/j.1440-1681.2009.05302.x. [DOI] [PubMed] [Google Scholar]
- 44.Velkoska E., Dean R.G., Burchill L., Levidiotis V., Burrell L.M. Reduction in renal ACE2 expression in subtotal nephrectomy in rats is ameliorated with ACE inhibition. Clin. Sci. 2010;118(4):269–279. doi: 10.1042/CS20090318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang Y., Gao F., Liu Z. Angiotensin-converting enzyme 2 attenuates inflammatory response and oxidative stress in hyperoxic lung injury by regulating NF-κB and Nrf2 pathways. QJM. 2019;112(12):914–924. doi: 10.1093/qjmed/hcz206. [DOI] [PubMed] [Google Scholar]
- 46.Chen J., Cui L., Yuan J., Zhang S., Ma R., Sang H. Protective effect of diminazene attenuates myocardial infarction in rats via increased inflammation and ACE2 activity. Mol. Med. Rep. 2017;16(4):4791–4796. doi: 10.3892/mmr.2017.7152. [DOI] [PubMed] [Google Scholar]
- 47.Goru S.K., Kadakol A., Malek V., Pandey A., Sharma N., Gaikwad A.B. Diminazene aceturate prevents nephropathy by increasing glomerular ACE2 and AT2 receptor expression in a rat model of type1 diabetes. Br. J. Pharmacol. 2017;174(18):3118–3130. doi: 10.1111/bph.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin Y.H., Min J.J., Lee J.-H., Kim E.-H., Kim G.E., Kim M.H. The effect of fluvastatin on cardiac fibrosis and angiotensin-converting enzyme-2 expression in glucose-controlled diabetic rat hearts. Heart Vessel. 2017;32(5):618–627. doi: 10.1007/s00380-016-0936-5. [DOI] [PubMed] [Google Scholar]
- 49.Li Y.-H., Wang Q.-X., Zhou J.-W., Chu X.-M., Man Y.-L., Liu P. Effects of rosuvastatin on expression of angiotensin-converting enzyme 2 after vascular balloon injury in rats. J. Geriatr. Cardiol. 2013;10(2):151. doi: 10.3969/j.issn.1671-5411.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aguilar C., Ventura F., Rodriguez-Delfin L. Atorvastatin induced increase in homologous angiotensin I converting enzyme (ACE2) mRNA is associated to decreased fibrosis and decreased left ventricular hypertrophy in a rat model of diabetic cardiomyopathy. Rev. Peru. Med. Exp. Salud Publ. 2011;28(2):264–272. doi: 10.1590/s1726-46342011000200013. [DOI] [PubMed] [Google Scholar]
- 51.Xu X., Shi L., Ma X., Su H., Ma G., Wu X. RhoA-rho associated kinase signaling leads to renin-angiotensin system imbalance and angiotensin converting enzyme 2 has a protective role in acute pulmonary embolism. Thromb. Res. 2019;176:85–94. doi: 10.1016/j.thromres.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 52.P-y Z.H.A.N.G., S-y S.H.E.N., CAI H. Effect of fasudil on angiotensin converting enzyme 2 and angiotensin (1-7) in rat myocardium [J] Chin. J. Geriatr. Heart Brain Vessel Dis. 2013;5 [Google Scholar]
- 53.Ocaranza M.P., Rivera P., Novoa U., Pinto M., González L., Chiong M. Rho kinase inhibition activates the homologous angiotensin-converting enzyme-angiotensin-(1–9) axis in experimental hypertension. J. Hypertens. 2011;29(4):706–715. doi: 10.1097/HJH.0b013e3283440665. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y., Liu M., Zhang B., Fu E., Li Z. Fasudil alleviated hypoxia-induced pulmonary hypertension by stabilizing the expression of angiotensin-(1–7) in rats. Eur. Rev. Med. Pharmacol. Sci. 2016;20(15):3304–3312. [PubMed] [Google Scholar]
- 55.Marre M., Bernadet P., Gallois Y., Savagner F., Guyene T.-T., Hallab M. Relationships between angiotensin I converting enzyme gene polymorphism, plasma levels, and diabetic retinal and renal complications. Diabetes. 1994;43(3):384–388. doi: 10.2337/diab.43.3.384. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida H., Kuriyama S., Atsumi Y., Tomonari H., Mitarai T., Hamaguchi A. Angiotensin I converting enzyme gene polymorphism in non-insulin dependent diabetes mellitus. Kidney Int. 1996;50(2):657–664. doi: 10.1038/ki.1996.362. [DOI] [PubMed] [Google Scholar]
- 57.Tiret L., Rigat B., Visvikis S., Breda C., Corvol P., Cambien F. Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am. J. Hum. Genet. 1992;51(1):197. [PMC free article] [PubMed] [Google Scholar]
- 58.Costerousse O., Allegrini J., Lopez M., Alhenc-Gelas F. Angiotensin I-converting enzyme in human circulating mononuclear cells: genetic polymorphism of expression in T-lymphocytes. Biochem. J. 1993;290(1):33–40. doi: 10.1042/bj2900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Danser A.J., Schalekamp M.A., Bax W.A., van den Brink A.M., Saxena P.R., GNA Riegger. Angiotensin-converting enzyme in the human heart: effect of the deletion/insertion polymorphism. Circulation. 1995;92(6):1387–1388. doi: 10.1161/01.cir.92.6.1387. [DOI] [PubMed] [Google Scholar]
- 60.Chen Y., Zhang P., Zhou X., Liu D., Zhong J., Zhang C. Relationship between genetic variants of ACE 2 gene and circulating levels of ACE 2 and its metabolites. J. Clin. Pharm. Ther. 2018;43(2):189–195. doi: 10.1111/jcpt.12625. [DOI] [PubMed] [Google Scholar]
- 61.Bhatnagar V., O’Connor D.T., Schork N.J., Salem R.M., Nievergelt C.M., Rana B.K. Angiotensin-converting enzyme gene polymorphism predicts the time-course of blood pressure response to angiotensin converting enzyme inhibition in the AASK trial. J. Hypertens. 2007;25(10):2082. doi: 10.1097/HJH.0b013e3282b9720e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mukae S., Itoh S., Aoki S., Iwata T., Nishio K., Sato R. Association of polymorphisms of the renin–angiotensin system and bradykinin B2 receptor with ACE-inhibitor-related cough. J. Hum. Hypertens. 2002;16(12):857–863. doi: 10.1038/sj.jhh.1001486. [DOI] [PubMed] [Google Scholar]
- 63.Grilo A., Sáez-Rosas M.P., Santos-Morano J., Sánchez E., Moreno-Rey C., Real L.M. Identification of genetic factors associated with susceptibility to angiotensin-converting enzyme inhibitors-induced cough. Pharmacogenet. Genomics. 2011;21(1):10–17. doi: 10.1097/FPC.0b013e328341041c. [DOI] [PubMed] [Google Scholar]
- 64.Eisenhardt A., Sperling H., Hauck E., Porst H., Stief C., Rübben H. ACE gene I/D and NOS3 G894T polymorphisms and response to sildenafil in men with erectile dysfunction. Urology. 2003;62(1):152–157. doi: 10.1016/s0090-4295(03)00137-7. [DOI] [PubMed] [Google Scholar]
- 65.Kolder I.C., Michels M., Christiaans I., Ten Cate F.J., Majoor-Krakauer D., Danser A.H. The role of renin–angiotensin–aldosterone system polymorphisms in phenotypic expression of MYBPC3-related hypertrophic cardiomyopathy. Eur. J. Hum. Genet. 2012;20(10):1071–1077. doi: 10.1038/ejhg.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamin C., Amir O., Sagiv M., Attias E., Meckel Y., Eynon N. ACE ID genotype affects blood creatine kinase response to eccentric exercise. J. Appl. Physiol. 2007;103(6):2057–2061. doi: 10.1152/japplphysiol.00867.2007. [DOI] [PubMed] [Google Scholar]
- 67.Weger W., Hofer A., Wolf P., El-Shabrawi Y., Renner W., Kerl H. The angiotensin-converting enzyme insertion/deletion and the endothelin-134 3A/4A gene polymorphisms in patients with chronic plaque psoriasis. Exp. Dermatol. 2007;16(12):993–998. doi: 10.1111/j.1600-0625.2007.00620.x. [DOI] [PubMed] [Google Scholar]
- 68.Irvin M.R., Lynch A.I., Kabagambe E.K., Tiwari H.K., Barzilay J.I., Eckfeldt J.H. Pharmacogenetic association of hypertension candidate genes with fasting glucose in the GenHAT study. J. Hypertens. 2010;28(10):2076. doi: 10.1097/HJH.0b013e32833c7a4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zakrzewski-Jakubiak M., De Denus S., Dubé M.P., Bélanger F., White M., Turgeon J. Ten renin-angiotensin system-related gene polymorphisms in maximally treated Canadian Caucasian patients with heart failure. Br. J. Clin. Pharmacol. 2008;65(5):742–751. doi: 10.1111/j.1365-2125.2007.03091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang X., Liu F. Increased risk of pneumonia associated with angiotensin-converting enzyme (CD143) rs4340 polymorphism. Clin. Exp. Med. 2016;16(3):423–428. doi: 10.1007/s10238-015-0356-3. [DOI] [PubMed] [Google Scholar]
- 71.Yaeghmaie R., Ghafouri-Fard S., Noroozi R., Tavakoli F., Taheri M., Ayatollahi S.A. Polymorphisms in the angiotensin I converting enzyme (ACE) gene are associated with multiple sclerosis risk and response to interferon-β treatment. Int. Immunopharmacol. 2018;64:275–279. doi: 10.1016/j.intimp.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 72.Rigat B., Hubert C., Alhenc-Gelas F., Cambien F., Corvol P., Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J. Clin. Invest. 1990;86(4):1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li J., Feng M., Wang Y., Li Y., Zhang Y., Li L. The relationship between three X-linked genes and the risk for hypertension among northeastern Han Chinese. J. Renin-Angiotensin-Aldosterone Syst. 2015;16(4):1321–1328. doi: 10.1177/1470320314534510. [DOI] [PubMed] [Google Scholar]
- 74.Meng N., Zhang Y., Ma J., Li H., Zhou F., Qu Y. Association of polymorphisms of angiotensin I converting enzyme 2 with retinopathy in type 2 diabetes mellitus among Chinese individuals. Eye. 2015;29(2):266–271. doi: 10.1038/eye.2014.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu C., Li Y., Guan T., Lai Y., Shen Y., Zeyaweiding A. ACE2 polymorphisms associated with cardiovascular risk in Uygurs with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2018;17(1):127. doi: 10.1186/s12933-018-0771-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang S.X., Fu C.Y., Zou Y.B., Wang H., Shi Y., Xu X.Q., Chen J.Z., Song X.D., Huan T.J., Hui R.T. Polymorphisms of angiotensin-converting enzyme 2 gene associated with magnitude of left ventricular hypertrophy in male patients with hypertrophic cardiomyopathy. Chin. Med. J. 2008;121(1):27–31. [PubMed] [Google Scholar]
- 77.Chen Y., Liu D., Zhang P., Zhong J., Zhang C., Wu S. Impact of ACE2 gene polymorphism on antihypertensive efficacy of ACE inhibitors. J. Hum. Hypertens. 2016;30(12):766–771. doi: 10.1038/jhh.2016.24. [DOI] [PubMed] [Google Scholar]
- 78.Patnaik M., Pati P., Swain S.N., Mohapatra M.K., Dwibedi B., Kar S.K. Association of angiotensin-converting enzyme and angiotensin-converting enzyme-2 gene polymorphisms with essential hypertension in the population of Odisha, India. Ann. Hum. Biol. 2014;41(2):145–152. doi: 10.3109/03014460.2013.837195. [DOI] [PubMed] [Google Scholar]
- 79.Fan X., Wang Y., Sun K.F., Zhang W., Yang X., Wang S. Polymorphisms of ACE2 gene are associated with essential hypertension and antihypertensive effects of captopril in women. Clin. Pharmacol. Ther. 2007;82(2):187–196. doi: 10.1038/sj.clpt.6100214. [DOI] [PubMed] [Google Scholar]
- 80.Wang S.-X., Tao T., Fu Z.-Q., Xie X.-Z., Hao W., Wang Y.-T. Polymorphisms of angiotensin-converting enzyme 2 gene confer a risk to lone atrial fibrillation in Chinese male patients. Chin. Med. J. 2013;126(24):4608–4611. [PubMed] [Google Scholar]
- 81.Fan Z., Wu G., Yue M., Ye J., Chen Y., Xu B. Hypertension and hypertensive left ventricular hypertrophy are associated with ACE2 genetic polymorphism. Life Sci. 2019;225:39–45. doi: 10.1016/j.lfs.2019.03.059. [DOI] [PubMed] [Google Scholar]
- 82.Zhao Q., Hixson J.E., Rao D.C., Gu D., Jaquish C.E., Rice T. Genetic variants in the apelin system and blood pressure responses to dietary sodium interventions: a family-based association study. J. Hypertens. 2010;28(4):756. doi: 10.1097/HJH.0b013e3283370d32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vangjeli C., Dicker P., Tregouet D.-A., Shields D.C., Evans A., Stanton A.V. A polymorphism in ACE2 is associated with a lower risk for fatal cardiovascular events in females: the MORGAM project. J. Renin-Angiotensin-Aldosterone Syst. 2011;12(4):504–509. doi: 10.1177/1470320311405557. [DOI] [PubMed] [Google Scholar]
- 84.Lu N., Yang Y., Wang Y., Liu Y., Fu G., Chen D. ACE2 gene polymorphism and essential hypertension: an updated meta-analysis involving 11,051 subjects. Mol. Biol. Rep. 2012;39(6):6581–6589. doi: 10.1007/s11033-012-1487-1. [DOI] [PubMed] [Google Scholar]
- 85.O’Brien J., Hayder H., Zayed Y., Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang R., Su H., Ma X., Xu X., Liang L., Ma G. MiRNA let-7b promotes the development of hypoxic pulmonary hypertension by targeting ACE2. Am. J. Phys. Lung Cell. Mol. Phys. 2019;316(3) doi: 10.1152/ajplung.00387.2018. L547-L57. [DOI] [PubMed] [Google Scholar]
- 87.Trojanowicz B., Imdahl T., Ulrich C., Fiedler R., Girndt M. Circulating miR-421 targeting leucocytic angiotensin converting enzyme 2 is elevated in patients with chronic kidney disease. Nephron. 2019;141(1):61–74. doi: 10.1159/000493805. [DOI] [PubMed] [Google Scholar]
- 88.Fang Y., Gao F., Hao J., Liu Z. microRNA-1246 mediates lipopolysaccharide-induced pulmonary endothelial cell apoptosis and acute lung injury by targeting angiotensin-converting enzyme 2. Am. J. Transl. Res. 2017;9(3):1287. [PMC free article] [PubMed] [Google Scholar]
- 89.Kemp J.R., Unal H., Desnoyer R., Yue H., Bhatnagar A., Karnik S.S. Angiotensin II-regulated microRNA 483-3p directly targets multiple components of the renin–angiotensin system. J. Mol. Cell. Cardiol. 2014;75:25–39. doi: 10.1016/j.yjmcc.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bao H., Gao F., Xie G., Liu Z. Angiotensin-converting enzyme 2 inhibits apoptosis of pulmonary endothelial cells during acute lung injury through suppressing MiR-4262. Cell. Physiol. Biochem. 2015;37(2):759–767. doi: 10.1159/000430393. [DOI] [PubMed] [Google Scholar]
- 91.Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., del Pozo C.H., Prosper F., Romero J.P. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020 Apr 24 doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M., Liu X.Q., Chen R.C., Tang C.L., Wang T., Ou C.Q. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur. Respir. J. 2020 Jan 1 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mafra F.F., Gattai P.P., Macedo M.M., Mori M.A., Araujo R.C. The angiotensin-I-converting enzyme insertion/deletion in polymorphic element codes for an AluYa5 RNA that downregulates gene expression. Pharmacogenomics J. 2018;18(4):517–527. doi: 10.1038/s41397-018-0020-x. [DOI] [PubMed] [Google Scholar]
- 94.Yi L., Gu Y., Wang X., An L., Xie X., Shao W. Association of ACE, ACE2 and UTS2 polymorphisms with essential hypertension in Han and Dongxiang populations from North-Western China. J. Int. Med. Res. 2006;34(3):272–283. doi: 10.1177/147323000603400306. [DOI] [PubMed] [Google Scholar]
- 95.Cao Y., Li L., Feng Z., Wan S., Huang P., Sun X. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6(1):1–4. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Caldeira D., Alarcão J., Vaz-Carneiro A., Costa J. Risk of pneumonia associated with use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers: systematic review and meta-analysis. Bmj. 2012;345 doi: 10.1136/bmj.e4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang P.-H. Increasing host cellular receptor—angiotensin-converting enzyme 2 (ACE2) expression by coronavirus may facilitate 2019-nCoV infection. bioRxiv. 2020 doi: 10.1002/jmv.26139. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Asselta R., Paraboschi E.M., Mantovani A., Duga S. 2020. ACE2and TMPRSS2 Variants and Expression as Candidates to Sex and Country Differences in COVID-19 Severity in Italy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen J., Jiang Q., Xia X., Liu K., Yu Z., Tao W. 2020. Individual Variation of the SARS-CoV2 Receptor ACE2 Gene Expression and Regulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barry M., Pearce H., Cross L., Tatullo M., Gaharwar A.K. Advances in nanotechnology for the treatment of osteoporosis. Curr. Osteoporos. Rep. 2016;14(3):87–94. doi: 10.1007/s11914-016-0306-3. [DOI] [PubMed] [Google Scholar]
- 102.Marrazzo P., Paduano F., Palmieri F., Marrelli M., Tatullo M. Highly efficient in vitro reparative behaviour of dental pulp stem cells cultured with standardised platelet lysate supplementation. Stem Cells Int. 2016;2016 doi: 10.1155/2016/7230987. [DOI] [PMC free article] [PubMed] [Google Scholar]