Highlights
-
•
The management of COVID-19 patients who suffer from stroke poses a challenge to healthcare professionals.
-
•
There are no specific clinical guidelines established in handling COVID-19 positive patients who are eligible for thrombolysis.
-
•
There is a probable relationship of COVID-19 and Stroke and effective monitoring is warranted.
Keywords: Coronavirus, COVID-19, SARS-CoV-2, Stroke, IV-RTPA
Abstract
The 2019 Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) which was first reported in Wuhan, China last December 2019, has been declared an emergency by the World Health Organization but eventually progressed to become a Pandemic. To date, Coronavirus Disease 2019 (COVID-19) has affected at least 100,000 individuals worldwide, reaching thousands of mortalities (Zhou et al., 2020; World Health Organization, 2020). In the Philippines, the number of COVID-19 confirmed positive cases is over 636 and is expected to rise (Department of Health, 2020). Respiratory infections alongside their comorbidities can induce acute myocardial infarction and acute ischemic stroke (Warren-Gash et al., 2018) [3]. These may further bring challenges in the management and administration of Intravenous (IV) Alteplase in eligible patients. Currently, there are no case reports in the administration IV Altepase in ischemic stroke patients who are COVID-19 positive. We present a case of a 62-year old female who was admitted due to cough, colds and shortness of breath of 2 weeks duration and was tested to be COVD-19 positive. She suffered from an ischemic stroke while in the Medical Intensive Care Unit and was given Intravenous thrombolysis.
1. Introduction
IV Alteplase is administered in ischemic stroke patients who are eligible for thrombolysis [4]. There is no contraindication in the administration of IV Alteplase in patients with communicable diseases [4]. COVID-19 infected more than 100,000 individuals worldwide and its numbers are still increasing [1], [8]. Administering IV Alteplase in COVID-19 positive patients poses a risk and challenge to the healthcare professionals. Respiratory infections can trigger acute coronary events and strokes along with the patient’s comorbidities [3], [5]. A link between COVID-19 and stroke and its outcome when given Intravenous Alteplase has yet to be reported.
2. Case report
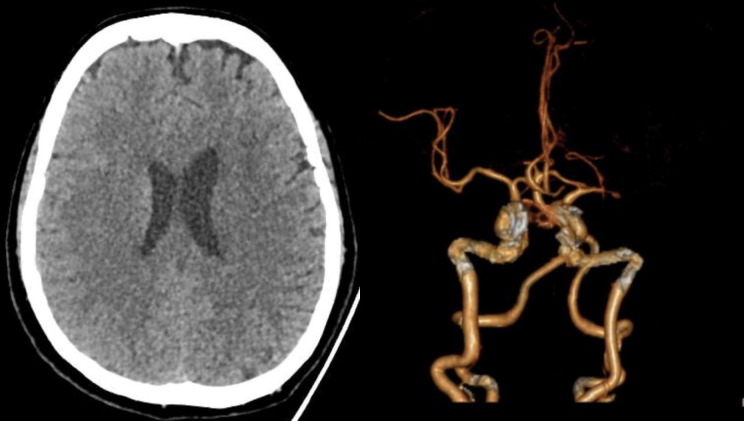
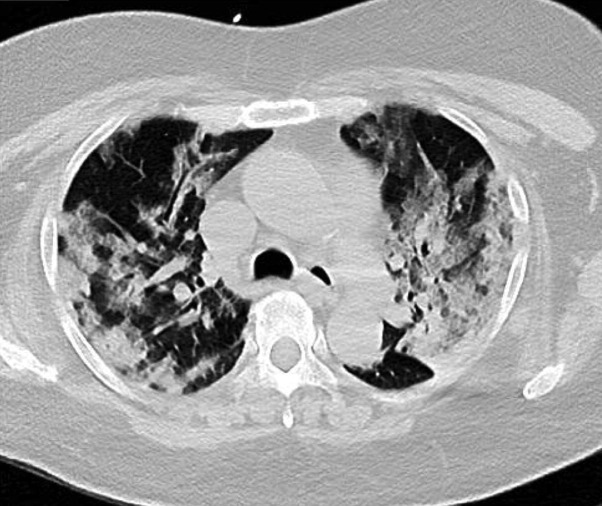
A 62-year old female, known hypertensive, prediabetic, dyslipidemic with a history of transient ischemic attack last 2019 presented to the emergency department with cough and colds of 2 weeks duration associated with shortness of breath. She was initially managed as a case of Community Acquired Pneumonia, but was tagged as a Person Under Investigation (PUI) due to her occupation as a health provider. She was admitted in the Medical Intensive Care Unit, (converted to the hospital’s COVID Critical Unit) with necessary precautions undertaken. During the first hospital day, she developed sudden onset of severe dysarthria and right upper and lower extremity weakness, giving a National Institutes of Health Stroke Scale (NIHSS) of 4. Plain cranial CT scan showed a subtle hypodensity in the left centrum semiovale and corona radiata (Fig. 1 a). CT-angiography showed significant stenosis in the left M1 segment of the Middle Cerebral Artery (Fig. 1b). Intravenous thrombolysis (IV rTPA) was initiated at a dose of 0.9 mg/kg body weight 3.4 h post ictus with 10% bolus of the total dose given initially, followed by the remaining dose as an infusion over 1 h. Immediately after IV rTPA, the patient’s right leg weakness resolved with an NIHSS of 3. Repeat plain cranial CT scan 24 h post-IV rTPA showed absence of hemorrhage. Cautious hydration was made and Aspirin 100 mg was started. However, on day 3 post ictus, his right leg was seen to be moving side to side with a cumulative NIHSS of 6. There were fluctuations in motor strength thereafter for which adjustment in IV hydration was made. She was confirmed COVID-19 positive by reverse transcription polymerase chain reaction (rRT-PCR) along with the classic findings on High-resolution CT (HRCT) of the chest showing areas of ground glass densities with focal areas of consolidation predominantly in both perihilar and peripheral regions of the lung (Fig. 2 ). The patient is being managed for the COVID-19 infection and is still admitted with mild dysarthria, right upper extremity falls before 10 s and right lower extremity drift (NIHSS of 4). Laboratory tests are as follows (please refer to Table 1 .)
Fig. 1.
(A) Plain cranial CT scan shows subtle hypodensity on the Left Corona radiata and Centrum Semiovale denoting acute infarction. (B) CT angiography shows atherosclerotic intracranial arteries with significant stenosis at the M1 segment of the MCA.
Fig. 2.
Areas of ground glass densities with focal areas of consolidation predominantly in both perihilar and peripheral regions.
Table 1.
Laboratory and Imaging Results of the patient.
| TEST | SCORE | Reference range |
|---|---|---|
| WBC | 13,200 | 4800–10,800 mm3 |
| Lymphocyte count | 11% | 19–48% |
| Platelet count | 409,000 | 150,000–400,000/mm3 |
| Creatinine | 0.69 | 0.55–1.02 mg/dL |
| Lactate Dehydrogenase | 406 | 85–227 U/L |
| Highly-sensitive cardiac troponin I | 14.5 | <11 ng/dL |
| Prothrombin time | 12.9 | 11.9–14.2 s |
| CRP | 192 | <6 mg/dL |
| ESR | 86 | 0–30 mm/hr |
| Procalcitonin | 0.80 | <0.5 ng/ml – low risk for sepsis >2.0 ng/ml – High risk for severe sepsis or septic shock |
| D-Dimer | 1160 | 0–246 ng/ml |
| Serum ferritin | 4609.33 | 4.63–204 ng/ml |
| Albumin | 2.3 | g/dL |
| ALT | 34 | 14–59 U/L |
| AST | 25 | 15–37 U/L |
| Imaging features | ||
| Consolidation | Present | |
| Ground-glass opacity | Present | |
| Bilateral pulmonary infiltration | Present | |
3. Discussion
On January 30, 2020, the WHO declared COVID-19 as a global emergency [2]. The most common symptoms are fever, cough and body malaise [6], [7]. Sars-CoV-2 infects the respiratory tract by inducing release of inflammatory cytokines such as interleukin (IL)1β and IL-6 by binding to the Toll Like Receptor (TLR), triggering an inflammatory cascade and resulting in Acute Respiratory Distress Syndrome. Suppression of the inflammatory mediators have been shown to limit injury [10]. Increased inflammatory biomarkers (D-dimer, C-Reactive Protein and Fibrinogen) have been shown to increase stroke severity and disability within 30 days [11], while erythrocyte sedimentation rate has not been shown to predict the outcome of stroke [12]. Chest imaging of COVID-19 patients show consolidation, ground glass opacities and bilateral lung involvement, which were consistent with findings in our patient [1], [6], [7]. Diagnostic parameters of COVID-19 patients show elevated D-dimer, fibrinogen, erythrocyte sedimentation rate, lactate dehydrogenase and C-reactive protein which are seen in our patient (Table 1). These levels are directly proportional to the clinical outcome and mortality. Procalcitonin levels are normal unless there is a concomitant bacterial infection [1], [6], [7], [11].
Currently, there are no reports elucidating the direct relationship of COVID-19 and its influence on stroke outcome. Possible mechanisms that may explain acute ischemic events in COVID-19 patients include cardiovascular compromise in the setting of infection, reduced oxygenation in the setting of acute respiratory distress syndrome, and systemic inflammation causing thrombosis or plaque disruption. This patient had a coexisting cerebral vascular disease that may have an impact on the outcome of immediate treatment of stroke. Together with antithrombotics, the balance in hemodynamic status is paramount in treating such patients pre- and post-thrombolysis. Fluid management therefore remains a challenge among stroke patients who are COVID-19 positive as acute respiratory distress syndrome is a known potential sequelae, especially in those with multiple comorbidities. This reported case had severe stenosis in the left M1 segment of the middle cerebral artery which would make thrombolysis a better option as we anticipate the multisystemic problems that COVID patients are faced with. To mention, myocarditis, the second common mentioned cause of fatality next to severe lung infection may exacerbate heart failure. Vice versa, acute heart failure should also be evaluated for myocarditis as a primary complication of COVID-19 [9]. Whether this is secondary to viral infiltration, or hypoxia and cytokine storm mounting in response to systemic infection [13], the aggressiveness in stroke management remains a priority because stroke is reversible.
Given the current setting however, management of stroke among COVID-19 positive patients is a struggle in most countries due to hindrance from rapid response of the stroke team. This is likely from extrinsic factors such as inadequate Personal Protective Equipment (PPE) as well as lack of imaging modalities solely dedicated for COVID-19 patients to avoid rapid viral transmission. These occur on top of the already existing problem of shortage of healthcare professionals as they are inadvertently exposed and hence, quarantined. Multiple reports of cases will help explore other possibilities of a causal relationship or none, and define guidelines in the management of stroke among COVID-19 positive cases.
4. Conclusion
The management of COVID-19 patients who suffer from stroke poses a challenge to healthcare professionals. There are no specific clinical guidelines established in handling COVID-19 positive patients who are eligible for thrombolysis. Management of hemodynamic status must always balance the risk and benefits of such treatment in COVID positive cases. There is no direct causal relationship of stroke with COVID-19 as of yet but given the fact that COVID-19 patients are predisposed to developing neurologic complications such as acute vascular events, monitoring these complications in the setting of COVID-19 infection is highly warranted. We therefore propose the following: 1) Development of an Acute Stroke Unit capable of managing COVID-19 patients and 2) Establishment of a clinical pathway and guidelines for the management of these complications in COVID-19 patients.
Acknowledgments
Acknowledgements
This material would not be possible without the support of the Brain Attack Team in St. Lukes Medical Center – Global City. We would also like to thank and commend our stroke nurses: Alyssa Mae C. Domingo, Evita C. Trias, Joana Lyn M. Racpan-Cauntay, Karen Czarina S. Illano and Maria Joanne D. Francisco for their contributions in this case.
Contributor Information
Christian Oliver C. Co, Email: cochristianoliver@gmail.com.
Jeryl Ritzi T. Yu, Email: jerylyu@gmail.com.
Lina C. Laxamana, Email: lclaxamana@stlukes.com.ph.
Deborah Ignacia A. David-Ona, Email: didona@stlukes.com.ph.
References
- 1.Zhou Fei, Yu Ting, Du Ronghui, Fan Guohui, Liu Ying, Liu Zhibo. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2020. A report about coronavirus disease 2019 (COVID-19)https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200313-sitrep-53-covid-19.pdf?sfvrsn=adb3f72_2 Retrieved from. [Google Scholar]
- 3.Warren-Gash C., Blackburn R., Whitaker H. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J. 2018;51:1701794. doi: 10.1183/13993003.01794-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Keyser Jacques, Gdovinová Zuzana, Uyttenboogaart Maarten, Vroomen Patrick C., Luijckx Gert Jan. Intravenous alteplase for stroke beyond the guidelines and in particular clinical situations. Stroke AHA. 2007;38:2612–2618. doi: 10.1161/STROKEAHA.106.480566. [DOI] [PubMed] [Google Scholar]
- 5.Elkind Mitchell SV, Harrington Robert A, Benjamin Ivor J. Role of the American Heart Association in the global COVID-19 pandemic. DOI: 10.1161/CIRCULATIONAHA.120.046749. [DOI] [PMC free article] [PubMed]
- 6.Huang Yihui, Tu Mengqi, Wang Shipei, Chen Sichao, Zhou Wei, Chen Danyang, et al. Travel medicine and infectious disease. https://doi.org/10.1016/j.tmaid.2020.101606. [DOI] [PMC free article] [PubMed]
- 7.Rodriguez-Morales Alfonso J, Cardona-Ospina Jaime A, Gutiérrez-Ocampo Estefanía, Villamizar-Peña Rhuvi, Holguin-Rivera Yeimer, Escalera-Antezana Juan Pablo, et al. On behalf of the Latin American network of coronavirus disease 2019-COVID-19 research (LANCOVID-19). Travel Med Infect Dis https://doi.org/10.1016/j.tmaid.2020.101623.
- 8.Department of Health, Updates on novel coronavirus disease (COVID-19). Retrieved from https://www.doh.gov.ph/2019-nCov; 2020 Manila, Philippines.
- 9.Zeng J.H., Liu Y., Yuan J., Wang F., Wu W., Li J. First case of COVID-19 infection with fulminant myocarditis complication: case report and insights. Preprints. 2020:2020030180. doi: 10.20944/preprints202003.0180.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conti P., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Frydas I. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2) doi: 10.23812/CONTI-E. pii: 1. doi: 10.23812. [DOI] [PubMed] [Google Scholar]
- 11.Melake M., El-Kabany R., Al-Emam A., El-Shereef A., Okda M. J Neurol Res North Am. 2016 The role of D-dimer, fibrinogen and C-reactive protein as plasma biomarkers in acute ischemic stroke. Available at: https://www.neurores.org/index.php/neurores/article/view/362/346 [Date accessed: 23 Mar. 2020] [Google Scholar]
- 12.Çomoğlu Selim Selçuk, Çİlli̇ler Aslı Ece, Güven Hayat. Erythrocyte sedimentation rate: can be a prognostic marker in acute ischemic stroke? Turk J Cerebrovasc Dis. 2013;19(1):18–22. doi: 10.5505/tbdhd.2013.32042. [DOI] [Google Scholar]
- 13.Chen L., Deng H., Cui H. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017;9(6):7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]