Adolescence is a unique period of development that requires special consideration in study design, statistical analyses, and interpretation of results. Biologically, the onset of adolescence is defined by pubertal surges in hormones that can impact neurodevelopment. Phenotypically, adolescence is characterized by a period of increased sensation/novelty-seeking that may manifest in risk-taking behaviors, heightened emotionality, and greater peer influence. Neurobiologically, adolescence is a time when foundational neurocognitive processes are in place and when brain systems are predominantly undergoing specialization to optimally match neurobiological/genetic predisposition with the demands of the particular environment. Conceptually, adolescence has unique features, including the stabilization of neurobehavioral processes into adulthood, such as in cognitive development, as well as processes that show peak expression, such as in models of affective development. Each of these characterizations has critical implications for studying adolescent brain development, both in normative populations and in clinical presentations, many of which first emerge during this period. Below, we contextualize these characterizations of adolescence and discuss key considerations regarding research in adolescent brain development and their implications for understanding psychopathology. While our focus is on study design, statistical analysis, and data interpretation for neuroimaging studies of brain development, many of these considerations also apply to behavioral studies that model developmental change.
A critical first consideration in adolescent research is to operationally define the adolescent period with a clear rationale. Adolescence is now largely considered to span 10 to 24 years of age, although environmental and genetic factors can affect individual timing (1). Puberty is a biological marker of adolescence, but consideration should be given to differences between self-report and hormonal assays (blood-, saliva-, or hair-derived), which can make defining pubertal timing difficult. Optimally, studies should distinguish prepubertal, pubertal, and postpubertal stages to identify adolescent-specific processes. While studies including only adolescent participants can provide important insights, considering all three periods is optimal for determining adolescent-specific processes and to distinguish key developmental transitions (e.g., processes coming online from childhood to adolescence, stabilization from adolescence to adulthood). Relatedly, although group comparisons (e.g., adolescents vs. adults) can provide important information distinguishing processes that are unique to adolescence, the optimal approach, when sample size and outcome measures permit, is to consider age and/or pubertal status as continuous variables. These approaches provide insight into the shape and timing of developmental trajectories and can identify when processes in adolescence are reaching maturity.
Repeated measurements are essential in developmental research. Longitudinal study designs have several advantages, including but not limited to the exploration of temporal precedence among variables and subject-specific developmental trajectories. Single cohort designs, which follow adolescents with the same initial age, have entirely longitudinal developmental effects that can maximize longitudinal path analysis (e.g., mediation), latent change models, and trajectory modeling approaches. However, they also have the potential to confound within-subject developmental effects with other-visit effects [e.g., scanner upgrades, practice effects among tasks, habituation to the scanning environment, and head motion (2)]. This limitation is highlighted by the recent COVID-19 pandemic, which may undermine the ability to disentangle pandemic-related effects (e.g., due to stress, time out of school) from age-related developmental effects. Cohort-sequential/accelerated longitudinal designs, which follow multiple cohorts with various starting ages, may be better able to isolate developmental and visit effects through the inclusion of cross-sectional effects. However, they may be more limited in the ability to leverage within-subject developmental effects, given the potential moderating effects of initial age. Importantly, both designs have limitations in attrition and scan failures, which can introduce bias (e.g., overrepresenting willing participants who may be more mature). We suggest that investigators optimize data collection for their planned analyses and known sources of error. When the aim is to model subject-specific developmental trajectories, as in the neurodevelopment of individual differences, a single cohort design is optimal, but with consideration to confounding nondevelopmental visit effects. Alternatively, group-level normative developmental changes and their physiological underpinnings may be better suited by an accelerated longitudinal design that can control for nondevelopmental visit effects. Simulation studies can be particularly useful in modeling these effects before data collection and can be tailored to study-specific factors (e.g., reliability of included measurements or predicted attrition rate) that inform sample size and requisite power.
A growing number of large-scale big data collection efforts in both the United States (e.g., the Philadelphia Neurodevelopmental Cohort [PNC], Pediatric Imaging, Neurocognition, and Genetics [PING] study, National Consortium on Alcohol and Neurodevelopment in Adolescence [NCANDA], Adolescent Brain Cognitive Development [ABCD] study, and Lifespan Human Connectome Project Development [HCP-D] studies) and Europe (e.g., the NeuroScience in Psychiatry Network [NSPN] Consortium, BrainTime, and Center for Lifespan Changes in Brain and Cognition [LCBC] studies) as well as multisite aggregation of existing data (e.g., the Enhancing Neuro Imaging Genetics Through Meta Analysis [ENIGMA] Consortium) have the potential, for the first time, to provide a rigorous understanding of the replicability and effect sizes of various developmental neuroimaging outcomes. This is critically needed, especially with the multiple comparison thresholding techniques used in neuroimaging and the relatively low reliability of many functional measures. Initial investigations in the ABCD dataset have already delineated important implications in this regard, including the effects of data collection site and scanner manufacturer (2). Importantly, large datasets can also be leveraged to replicate findings regarding normative development and deviations associated with psychopathology (3).
Modeling frameworks that allow for curvilinear longitudinal trajectories and age as a continuous variable, such as multilevel and structural equation models, can capture the uniqueness of the adolescent period. Inverse forms of age (e.g., 1/age) are particularly important for modeling adolescence because they can capture fast growth during childhood, deceleration in adolescence, and stabilization in adulthood. In comparison, quadratic models provide the opportunity to identify peaks during adolescence. Exploratory analyses that test several functional forms should balance model fit with model complexity and use information criteria (e.g., Akaike information criterion/Bayesian information criterion) for model selection. More recently, algorithmic approaches have been adopted for fitting nonlinear developmental trajectories (e.g., general additive models). These approaches have many key advantages, including flexible and quantitatively defined functional forms, and permit the examination of age periods of significant change (4), which can delineate plasticity and growth that can inform predictive models for risk for psychopathology (5) and opportunities for effective interventions. However, these algorithmic nonlinear approaches may require very large sample sizes. In addition, well-powered models of normative development can be used as a template from which to assess impairment given the age of subjects (6).
Potential confounds in developmental neuroimaging studies are well recognized, including head motion, nondevelopmental visit effects, and missing data. Thus, limiting artifacts during imaging acquisition and the use of state-of-the-science approaches to identify and mitigate these effects in the acquired data (e.g., global signal regression, despiking, and template-based artifact removal) are critical. Given that artifact removal techniques can also remove meaningful signal, it becomes important to characterize information loss that varies systematically with age [e.g., global signal regression (7)]. After addressing artifacts in “preprocessing” stages, it is also important to test, report, and potentially control for any remaining associations with age/pubertal status. In addition, missing data need to be integrated in analyses to ensure representative sampling.
The interpretation of findings on adolescent brain development should always be informed by conceptual models of neurodevelopment, by broader theories from the neuroscience literature, and in consideration of limitations of data. This is particularly important given our still-emerging field, which may lead to multiple interpretations that are critical to move toward increased transparency and reproducibility. For example, task-based functional magnetic resonance imaging group differences showing lower blood oxygen level–dependent activation in adolescents or clinical groups compared with adults or healthy populations could be due to limited regional engagement or, alternatively, varied strategies or compensatory processes (8). The consideration of alternative interpretations, including behavioral performance (e.g., task difficulty, distinguishing between correct and error trials, and response speed) and systems-level changes, are thus critical. Within this context, both exploratory and hypothesis-driven designs are needed, but with clear rationale and predictions based on the current status of the literature and informed by open science approaches. Critically, well-validated negative findings are as important as “positive results” in moving the field forward.
A next step in developmental cognitive neuroscience is to characterize the physiological neural mechanisms underlying development. Multimodal imaging approaches that concurrently assess multiple aspects of brain maturation, including those that animal and postmortem studies have shown to undergo unique changes through puberty (myelination, neurotransmitters), can inform underlying developmental mechanisms (9). Acquisitions that go beyond structural, task-based, and resting-state functional magnetic resonance imaging, such as characterizing white matter microstructure (e.g, diffusion tensor imaging and magnetization transfer ratio), tissue iron as a marker for dopamine (e.g., quantitative susceptibility mapping and R2′ mapping), and neurotransmitter systems (e.g., positron emission tomography and magnetic resonance spectroscopy), can move our understanding of normative developmental mechanisms forward and inform the etiology of psychopathology and potential interventions.
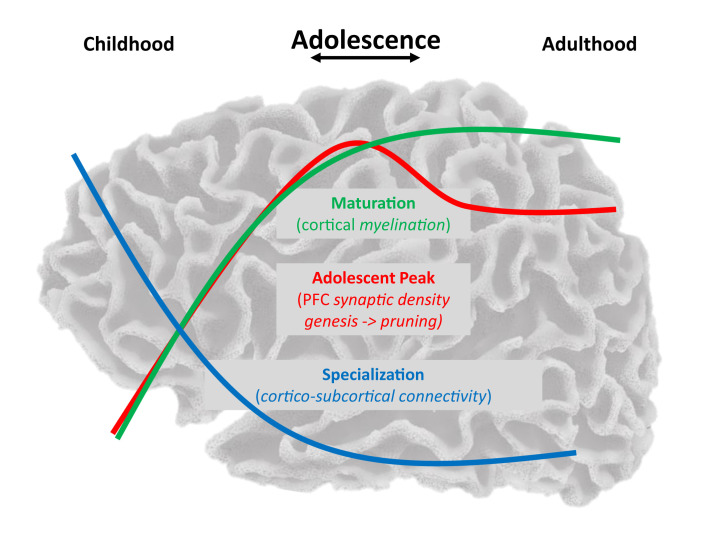
Overall, methodological and analytical approaches that characterize developmental change are critical in characterizing adolescent development and informing normative neurocognitive growth and risk for psychopathology. Notably, development through adolescence must be conceptualized in a nonlinear fashion, characterizing the transition to adult-level trajectories and integrating the multiple and independent brain maturational mechanisms that underlie behavior and determine adult trajectories (Figure 1 ).
Figure 1.
Depiction of curvilinear trajectories from childhood through adolescence and into adulthood, including processes that increase (green) (e.g., myelination, cognitive control, and prefrontal gamma-aminobutyric acid) or decrease (blue) (e.g., synaptic pruning, cortico-subcortical functional connectivity, and prefrontal glutamate) and stabilize into adulthood or show unique peaks in adolescence (red) (e.g., dopamine function and affective processes) (10). PFC, prefrontal cortex.
Acknowledgments and Disclosures
This work was supported by National Institute of Mental Health Grant Nos. R01MH08024, R03MH113090, and R01MH067924 (to BL), and the Staunton Farm Foundation (to BL).
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Sawyer S.M., Azzopardi P.S., Wickremarathne D., Patton G.C. The age of adolescence. Lancet Child Adolesc Health. 2018;2:223–228. doi: 10.1016/S2352-4642(18)30022-1. [DOI] [PubMed] [Google Scholar]
- 2.Marek S., Tervo-Clemmens B., Nielsen A.N., Wheelock M.D., Miller R.L., Laumann T.O. Identifying reproducible individual differences in childhood functional brain networks: An ABCD study. Dev Cogn Neurosci. 2019;40:100706. doi: 10.1016/j.dcn.2019.100706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jalbrzikowski M., Larsen B., Hallquist M.N., Foran W., Calabro F., Luna B. Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: Associations with anxiety and depression. Biol Psychiatry. 2017;82:511–521. doi: 10.1016/j.biopsych.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calabro F.J., Murty V.P., Jalbrzikowski M., Tervo-Clemmens B., Luna B. Development of hippocampal-prefrontal cortex interactions through adolescence. Cereb Cortex. 2020;30:1548–1558. doi: 10.1093/cercor/bhz186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tervo-Clemmens B., Simmonds D., Calabro F., Montez D.F., Lekht J., Day N.L. Early cannabis use and neurocognitive risk: A prospective functional neuroimaging study. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:713–725. doi: 10.1016/j.bpsc.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jalbrzikowski M., Murty V.P., Tervo-Clemmens B., Foran W., Luna B. Age-associated deviations of amygdala functional connectivity in youths with psychosis spectrum disorders: Relevance to psychotic symptoms. Am J Psychiatry. 2019;176:196–207. doi: 10.1176/appi.ajp.2018.18040443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J., Bolt T., Bzdok D., Nomi J.S., Yeo B.T.T., Spreng R.N., Uddin L.Q. Topography and behavioral relevance of the global signal in the human brain. Sci Rep. 2019;9:14286. doi: 10.1038/s41598-019-50750-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luna B., Padmanabhan A., O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsen B., Olafsson V., Calabro F., Laymon C., Tervo-Clemmens B., Campbell E. Maturation of the human striatal dopamine system revealed by PET and quantitative MRI. Nat Commun. 2020;11:846. doi: 10.1038/s41467-020-14693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen B., Luna B. Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci Biobehav Rev. 2018;94:179–195. doi: 10.1016/j.neubiorev.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]