Highlights
-
•
The viral load and viral shedding patterns of asymptomatic patients with COVID-19 were described.
-
•
The viral load of asymptomatic patients peaked during the early stage of hospitalization.
-
•
The Ct values of asymptomatic patients (APs) were higher than asymptomatic patients in incubation period (APIs).
-
•
APs still have a duration of viral shedding, suggesting the possibility of transmission during the asymptomatic period.
Keywords: COVID-19, Asymptomatic patients, Viral load, Viral shedding
Abstract
Data are limited on the viral load, viral shedding patterns, and potential infectivity of asymptomatic patients (APs) with coronavirus disease 2019 (COVID-19). This study included 31 adult patients who were virologically confirmed to have COVID-19 but were asymptomatic on admission. Among these 31 patients, 22 presented symptoms after admission and were defined as asymptomatic patients in the incubation period (APIs); the other nine patients remained asymptomatic during hospitalization and were defined as asymptomatic patients (APs). The median cycle threshold (Ct) value of APs (39.0, interquartile range (IQR) 37.5–39.5) was significantly higher than that of APIs (34.5, IQR 32.2–37.0), indicating a lower viral load in APs. However, the duration of viral shedding remained similar in the two groups (7 days, IQR 5–14 days vs. 8 days, IQR 5–16 days). The study findings demonstrated that although APs with COVID-19 have a lower viral load, they still have certain period of viral shedding, which suggests the possibility of transmission during their asymptomatic period. Further longitudinal surveillance of these asymptomatic cases via virus nucleic acid testing are warranted.
In late December 2019, an outbreak of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) struck Wuhan, China, and then rapidly developed into a worldwide pandemic. New evidence has shown that the majority of people with coronavirus infections do not present symptoms, which indicates that a large number of asymptomatic carriers remain undiscovered in the community (Bai et al., 2020, Day, 2020). To understand the natural history and infectiousness of asymptomatic cases of COVID-19, we followed up virologically confirmed COVID-19 patients in Guangzhou Eighth People’s Hospital who had no subjective symptoms on admission, and investigated their viral load and viral shedding patterns.
This study included 31 adult patients who were confirmed to have COVID-19 and were hospitalized in Guangzhou Eighth People’s Hospital from January 23 to March 3, 2020. All of these patients tested positive for SARS-CoV-2 RNA according to real-time RT-PCR assays conducted in the hospital or the local Centers for Disease Control and Prevention (CDC), but were asymptomatic on admission. The diagnosis of COVID-19 was based on the New Coronavirus Pneumonia Prevention and Control Program 6th edition (National Health Commission of China, 2020).
In this study, a commercial real-time PCR kit (Zhongzhi, Guangzhou, China) was used to detect SARS-CoV-2 RNA in nasopharyngeal swab specimens collected according to the World Health Organization (WHO) guidelines (World Health Organization, 2020). The open reading frame 1ab (ORF1ab) and nucleocapsid protein (N) genes were targeted simultaneously, and were amplified and tested. The detailed protocol has been described elsewhere (National Institute for Viral Disease Control and Prevention China, 2020). Clinical samples were quantified and the results expressed in terms of the cycle threshold value (Ct-value), which is defined as the number of cycles required for the fluorescence signal to cross the threshold (i.e., exceed the background level). Samples were considered positive if the Ct-value was ≤40, and only Ct values of positive test results were available. The viral load of patient nasopharyngeal swab samples was estimated by Ct value of the N gene, with lower Ct values indicating a higher viral load.
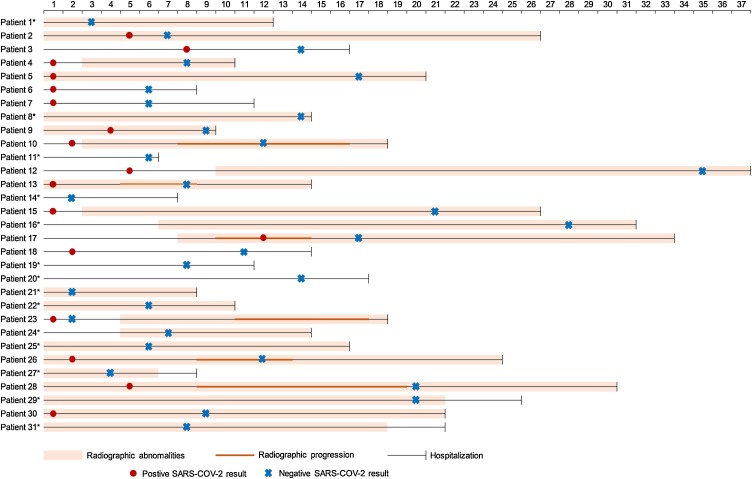
Among 31 participants who did not show any subjective symptoms on admission, 22 presented symptoms after admission; these patients were defined as asymptomatic patients in the incubation period (APIs). The other nine patients remained asymptomatic during hospitalization (APs). The baseline characteristics did not differ significantly between the AP and API groups (Supplementary material Table S1). All patients underwent chest computed tomography (CT) scans on admission and were followed up every 3 days or as necessary. Four (44.44%) APs and nine (41.93%) APIs showed bilateral abnormalities typical of pneumonia. Of the 22 APIs, six (27.27%) showed radiographic progression 8 days (interquartile range (IQR) 7–9 days) after admission, but all exhibited improvement before discharge (Figure 1 ).
Figure 1.
Viral shedding patterns and pulmonary symptoms progression of 31 asymptomatic patients during hospitalization.
*Tested SARS-COV-2 RNA positive before admission. The numbers on the top indicate the number of days since admission.
APs: patient 1–9; APIs: patient 10–31.
Abbreviations: AP, asymptomatic patient; API, asymptomatic patient in incubation period.
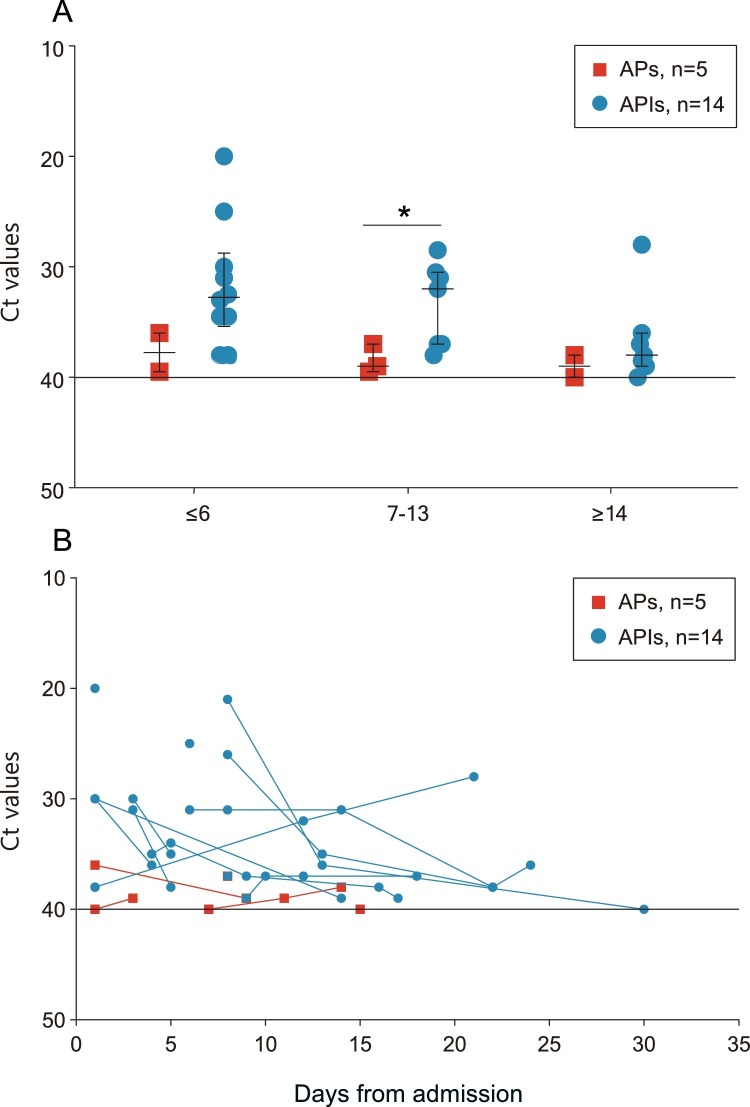
Five APs and 14 APIs who had positive RNA tests during hospitalization had available Ct values. The median Ct values during hospitalization in APs (39.0, IQR 37.5–39.5) was significantly higher than that in APIs (34.5, IQR 32.2–37.0), indicating a significantly lower viral load. To evaluate the viral dynamics of asymptomatic cases of COVID-19, the data were further stratified according to length of hospital stay (LOS) at the time of sampling. The median Ct values of APs were consistently higher during hospitalization than those of APIs (Figure 2 A). The viral load of APs peaked during the first week after admission, while that of APIs peaked during the second week. Serial samples from five APs and 14 APIs were also studied (Figure 2B). All APs tested negative for SARS-CoV-2 RNA within 2 weeks after admission, while APIs tended to have a longer duration of viral shedding, although no significant difference was found.
Figure 2.
Viral dynamics in asymptomatic patients with COVID-19.
The figure shows the viral dynamics of included APs and APIs infected with COVID-19, and each line represents a patient.
Figure 2A shows available Ct values from 5 APs and 14 APIs with COVID-19 at different stages of hospitalization. The solid lines in black show the normal limit of Ct values. Median, quartile 1, and quartile 3 are shown. Figure 2B shows the Ct values of serial samples from 5 APs and 14 APIs with COVID-19. *P values < 0.05.
Abbreviations: Ct, cycle threshold; AP, asymptomatic patient; API, asymptomatic patient in incubation period.
Among the APs, those who never experienced pulmonary symptoms and who tested negative for SARS-CoV-2 RNA later were regarded as having subclinical infections. The other patients may not have shown subjective signs but they developed pulmonary symptoms including ground glass opacities or subsegmental areas of consolidation, and these patients were considered to be in the period of illness. The 22 patients who developed symptoms after admission were considered to be asymptomatic patients who were in the incubation period, which is the time from virus vector exposure to the appearance of disease symptoms and signs.
It was noted that the viral load of APs peaked during the early stage of hospitalization, which was different from the results observed in patients with severe acute respiratory syndrome (SARS) in 2003 (Peiris et al., 2003), while those of APIs peaked during the second week as symptoms appeared. The phenomenon in which APs have a lower viral load than APIs has also been observed for the influenza virus. However, the duration of viral shedding was similar for APs and APIs, which reflected the potential of APs to transmit the virus in the community (Ip et al., 2016).
Overall, this study demonstrated that even though these patients may be asymptomatic, they still have a certain period of viral shedding, which suggests the possibility of transmission during the asymptomatic period. These asymptomatic cases of COVID-19 could be an important source of contagion, and this needs to be controlled by longitudinal surveillance via virus nucleic acid testing. Further studies are needed to investigate and quantify the contribution of persons with asymptomatic COVID-19 infections to COVID-19 transmission.
Ethical approval
The study was approved by the Institutional Ethics Board of Guangzhou Eighth People’s Hospital and the requirement for informed consent was waived by the ethics board.
Funding
This research was supported by the Open Project of Guangdong Provincial Key Laboratory of Tropical Disease Research. The funding source had no role in the design and execution of the study, the data analysis, interpretation of the data, or the decision to submit the results of this study.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge the patients, staff at Guangzhou Eighth People’s Hospital, and staff at the Guangzhou Centers for Disease Control and Prevention (CDC) Department of Infectious Disease Control and Prevention for their input and collaboration in this research.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.05.030.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Bai Y., Yao L., Wei T., Tian F., Jin D.-Y., Chen L. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(February):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M. Covid-19: four fifths of cases are asymptomatic, China figures indicate. BMJ. 2020;369(April) doi: 10.1136/bmj.m1375. m1375. [DOI] [PubMed] [Google Scholar]
- Ip D., Lau L., Leung N., Fang V., Chan K.-H., Chu D. Viral shedding and transmission potential of asymptomatic and paucisymptomatic influenza virus infections in the community. Clin Infect Dis. 2016;(December):64. doi: 10.1093/cid/ciw841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Commission of China . 6th edition. 2020. New coronavirus pneumonia prevention and control program. Available from: http://www.nhc.gov.cn/jkj/s3577/202003/4856d5b0458141fa9f376853224d41d7.shtml [Cited 18 February 2020] [Google Scholar]
- National Institute for Viral Disease Control and Prevention (China) 2020. Specific primers and probes for detection 2019 novel coronavirus. Available from: http://ivdc.chinacdc.cn/kyjz/202001/t20200121_211337.html [Cited 21 January 2020] [Google Scholar]
- Peiris J.S., Chu C.-M., Cheng V., Chan K., Hung I., Poon L. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(June):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. Available from: https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117 [Cited 12 March 2020] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.