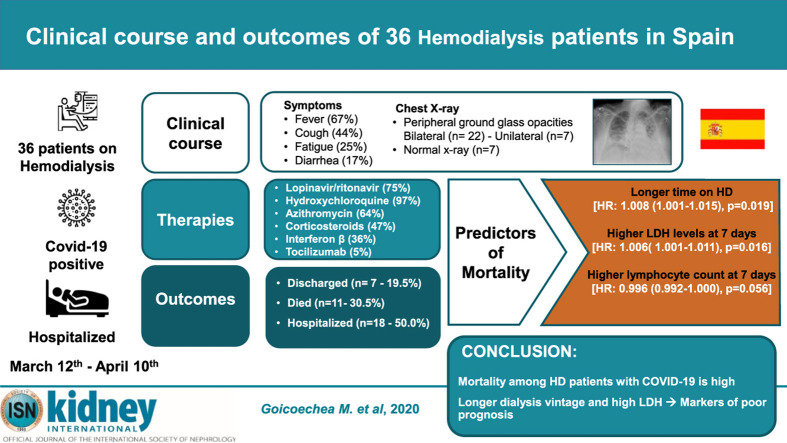
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia emerged in Wuhan, China in December 2019. Unfortunately, there is a lack of evidence about the optimal management of novel coronavirus disease 2019 (COVID-19), and even less is available in patients on maintenance hemodialysis therapy than in the general population. In this retrospective, observational, single-center study, we analyzed the clinical course and outcomes of all maintenance hemodialysis patients hospitalized with COVID-19 from March 12th to April 10th, 2020 as confirmed by real-time polymerase chain reaction. Baseline features, clinical course, laboratory data, and different therapies were compared between survivors and nonsurvivors to identify risk factors associated with mortality. Among the 36 patients, 11 (30.5%) died, and 7 were able to be discharged within the observation period. Clinical and radiological evolution during the first week of admission were predictive of mortality. Among the 36 patients, 18 had worsening of their clinical status, as defined by severe hypoxia with oxygen therapy requirements greater than 4 L/min and radiological worsening. Significantly, 11 of those 18 patients (61.1%) died. None of the classical cardiovascular risk factors in the general population were associated with higher mortality. Compared to survivors, nonsurvivors had significantly longer dialysis vintage, increased lactate dehydrogenase (490 U/l ± 120 U/l vs. 281 U/l ± 151 U/l, P = 0.008) and C-reactive protein levels (18.3 mg/dl ± 13.7 mg/dl vs. 8.1 mg/dl ± 8.1 mg/dl, P = 0.021), and a lower lymphocyte count (0.38 ×103/µl ± 0.14 ×103/µl vs. 0.76 ×103/µl ± 0.48 ×103/µl, P = 0.04) 1 week after clinical onset. Thus, the mortality among hospitalized hemodialysis patients diagnosed with COVID-19 is high. Certain laboratory tests can be used to predict a worsening clinical course.
Keywords: coronavirus, COVID-19, hemodialysis, mortality, SARS-CoV-2
Graphical abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection emerged in Wuhan, China in December 2019 and has spread rapidly worldwide.1 , 2 SARS-CoV-2 is a single-strain RNA virus that typically causes respiratory damage in humans and animals. Severe infections can lead to multisystem disorders. Clinical presentation is highly variable, from an asymptomatic or very mild course (up to 80%), to severe involvement with unilateral or bilateral pneumonia (approximately 15%), to a very serious course with bilateral pneumonia and respiratory distress that requires ventilatory support in the intensive care unit (ICU; 3%–5%).1, 2, 3 In very severe cases, immune response can trigger a strong inflammatory reaction accompanied by a cytokine storm that may worsen respiratory symptoms, even leading to death.2 , 3
Mortality estimates in the general population range from 1.4% to 8%.1, 2, 3 Mortality risk significantly increases if the patient requires admission to the ICU, standing between 16% and 78%.3 These rates vary according to the criteria for ICU admission; in Italy, ICU mortality was around 26%.1 Factors associated with a poor prognosis include advanced age, male sex, and previous comorbidity, particularly cardiovascular events, diabetes mellitus, chronic obstructive pulmonary disease, or a history of cancer.4 , 5
The impact of this virus on patients with chronic kidney disease is poorly understood.6, 7, 8 Given the advanced age and comorbidity of these patients, mortality could be higher than that in the general population, especially in patients on dialysis therapy. There is good-quality data related to preventive and isolation measures that must be carried out in hemodialysis units to prevent the spread of the virus,6, 7, 8 but we still do not know the specific characteristics of the disease in this population. To date, only isolated observations or small case series on prevalence and mortality rate have been reported.9, 10, 11, 12, 13
The objectives of this observational study were to describe the clinical manifestations of SARS-CoV-2 infection in maintenance hemodialysis (MHD) patients, identify prognostic factors, and analyze the impact of different treatment schemes on mortality.
RESULTS
Patients and clinical characteristics
Thirty-six MHD patients out of 282 followed in 2 reference hemodialysis units were hospitalized with confirmed coronavirus disease 2019 (COVID-19) starting on March 12, 2020. The age of the patients was 71 ± 12 years (range, 29–90 years), and 64% were male. Coexisting conditions are shown in Table 1 . Most patients had hypertension (97%), diabetes mellitus (64%), and dyslipidemia (67%). The most common symptoms at admission were fever (67%) and cough (44%), followed by fatigue (25%) and diarrhea (17%). Poor oxygen saturation (<95%) breathing room air was observed in 22 of 36 patients (61%).
Table 1.
Demographic, clinical, and radiological features
| Variables | Total (n = 36) | Survivors (n = 25) | Nonsurvivors (n = 11) | P |
|---|---|---|---|---|
| Age, yr | 71 ± 12 | 69 ± 14 | 75 ± 6 | 0.082 |
| Male | 23 (64) | 17 (68) | 6 (54) | 0.439 |
| Body mass Index | 26.5 ± 4.3 | 27.2 ± 4.5 | 25.1 ± 3.6 | 0.531 |
| On-line hemodiafiltration | 12 (33.3) | 12 (48) | 0 (0) | 0.014 |
| Comorbidity | ||||
| Hypertension | 35 (97) | 25 (100) | 10 (91) | 0.306 |
| Diabetes | 23 (64) | 17 (68) | 6 (54) | 0.475 |
| Coronary heart disease | 8 (22) | 7 (28) | 1 (9) | 0.388 |
| Dyslipidemia | 24 (67) | 18 (72) | 6 (54) | 0.446 |
| Chronic obstructive lung disease | 7 (19) | 6 (24) | 1 (9) | 0.400 |
| Atrial fibrillation | 11 (31) | 7 (28) | 4 (36) | 0.703 |
| Charlson comorbidity index | 5.56 ± 1.78 | 5.84 ± 1.91 | 4.90 ± 1.30 | 0.100 |
| Symptoms | ||||
| Fever | 24 (67) | 9 (82) | 15 (60) | 0.268 |
| Cough | 16 (44) | 10 (40) | 6 (54) | 0.483 |
| Fatigue | 9 (25) | 5 (20) | 4 (36) | 0.409 |
| Diarrhea, nausea or vomiting | 6 (17) | 4 (16) | 2 (18) | 0.609 |
| Time from illness onset to hospital admission, d | 2.9 (0–15) | 3.2 (0–15) | 2.1 (0–7) | 0.332 |
| Oxygen saturation | 93.6 ± 4.4 | 94.7 ± 2.9 | 91.3 ± 6.2 | 0.030 |
| Admission chest X-ray | ||||
| Bilateral peripheral ground-glass opacity | 22 (61) | 16 (64) | 6 (55) | 0.215 |
| Unilateral opacity | 7 (19) | 4 (16) | 3 (27) | |
| Normal X-ray | 7 (19) | 6 (24) | 1 (9) | |
Data are n (%), mean ± SD, or median (range).
All patients that had a positive real-time reverse transcriptase polymerase chain reaction (rRT-PCR) test were hospitalized. Three cases did not have typical symptoms at the time of diagnosis of SARS-CoV-2 infection: one had an episode of intradialytic hemodynamic instability; another had an isolated fever peak during a hemodialysis session; and the third patient had a nosocomial transmission. There were 3 documented nosocomial transmissions.
No differences in pre-existing comorbidities or clinical presentation were observed between survivors and nonsurvivors, except oxygen saturation on presentation was statistically significantly lower in nonsurvivors (94.7% ± 2.9% vs. 91.3% ± 6.2%, P = 0.03). Lung abnormalities on initial chest X-ray were observed in 29 patients (80%). Peripheral ground-glass opacities, the typical radiologic pattern, were bilateral in 22 patients and unilateral in 7 patients. Seven of 36 patients had a normal X-ray at admission.
Treatment scheme
Twenty-seven patients (75%) received lopinavir/ritonavir for antiviral therapy. Hydroxychloroquine was administered in all patients but one. Azithromycin was administered in 23 patients (64%), corticosteroids in 17 (47%), interferon β in 13 (36%), and tocilizumab in 2 (5%). Drugs, dosages, and treatment schemes are described in Supplementary Table S1.
Dialysis scheme
All patients were included in MHD programs with a median time on dialysis therapy of 29 months (1 week–285 months). Nineteen patients had an arteriovenous fistula, and 17 patients had a permanent central venous catheter. During admission, convective volume ranged from 22 to 32 L per session.
Clinical evolution and outcomes
By April 10th, 7 patients were discharged, and 11 (30.5%) patients died during hospitalization, all due to respiratory failure. The median time until discharge was 13 days after symptom onset and 11.4 days after admission, and the median time of death was 9.3 days after symptom onset and 7.2 days after admission.
Changes of laboratory parameters
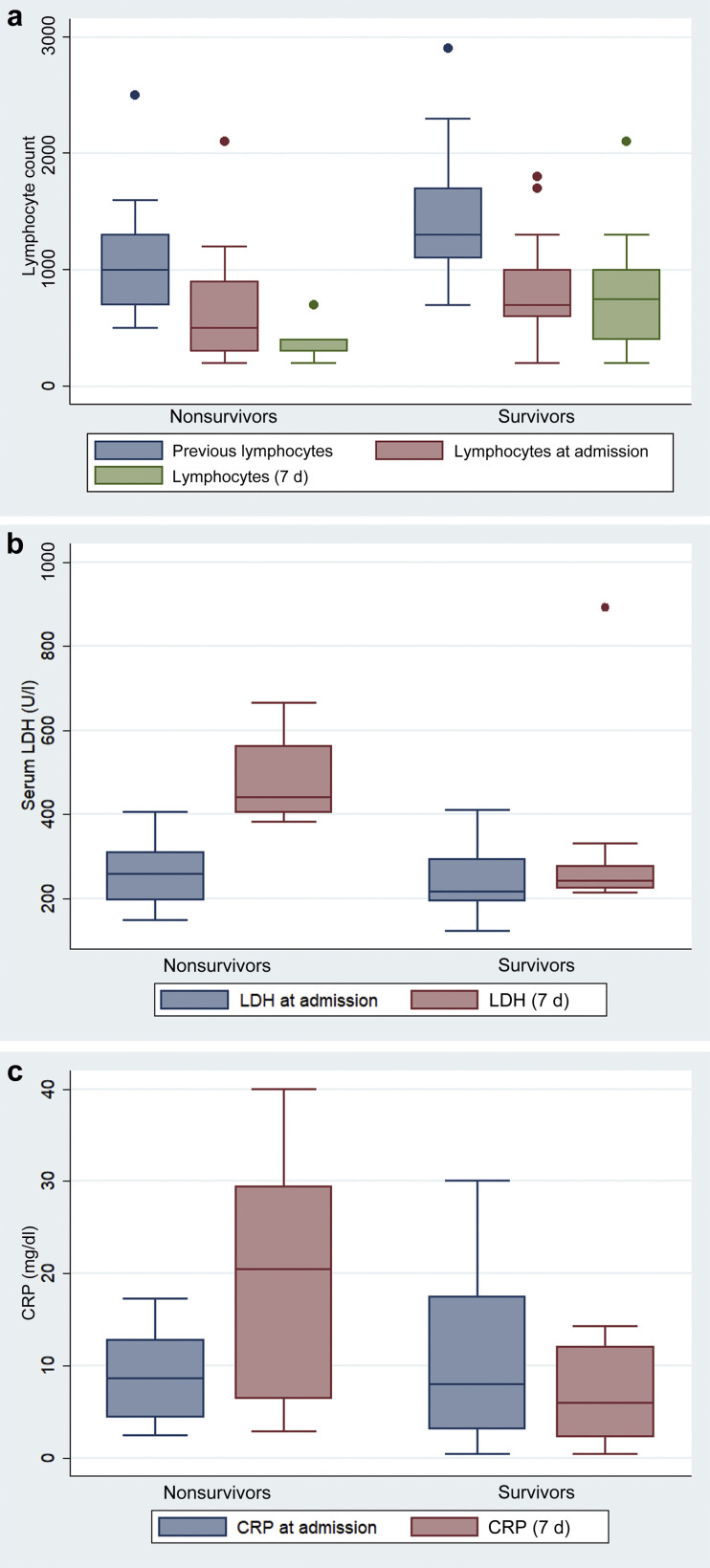
One week after admission, we observed mild worsening of anemia, increased serum lactate dehydrogenase (LDH) levels, and a reduction in serum total protein and albumin levels (Table 2 ). Lymphocyte count was the lowest on day 7 after illness onset in nonsurvivors (0.38 ± 0.14 vs. 0.76 ± 0.48 [×109/μl]; P = 0.040). In addition, on day 7, in comparison with patients who survived, nonsurvivors had higher LDH (490 ± 120 vs. 281 ± 151 U/l; P = 0.008) and higher C-reactive protein serum levels (18.3 ± 13.7 vs. 8.1 ± 8.1 mg/dl; P = 0.021; Figure 1 a–c).
Table 2.
Laboratory findings at admission and 1 week after clinical onset
| Laboratory variables | Total | Survivors | Nonsurvivors | P valuea |
|---|---|---|---|---|
| Lymphocyte count, ×109 | ||||
| Baseline | 0.79 ± 0.47 | 0.83 ± 0.41 | 0.67 ± 0.62 | 0.418 |
| Day 7 | 0.66 ± 0.45 | 0.76 ± 0.48 | 0.38 ± 0.14 | 0.040 |
| Hemoglobin, g/l | ||||
| Baseline | 10.6 ± 1.4 | 10.6 ± 1.5 | 10.6 ± 1.1 | 0.129 |
| Day 7 | 9.8 ± 1.9 | 9.6 ± 1.6 | 10.7 ± 2.7 | 0.172 |
| P valueb | 0.001 | 0.001 | ||
| Platelet count, ×109 | ||||
| Baseline | 1.64 ± 0.66 | 1.74 ± 0.58 | 1.37 ± 0.83 | 0.133 |
| Day 7 | 1.78 ± 0.73 | 1.89 ± 0.76 | 1.48 ± 0.55 | 0.177 |
| Serum LDH, U/l | ||||
| Baseline | 235 ± 58 | 225 ± 59 | 274 ± 32 | 0.269 |
| Day 7 | 329 ± 166 | 281 ± 151 | 490 ± 120 | 0.008 |
| P valueb | 0.016 | 0.029 | ||
| Serum ALT, U/l | ||||
| Baseline | 29.2 ± 51.3 | 29.5 ± 54.2 | 19.2 ± 12.6 | 0.722 |
| Day 7 | 32.9 ± 43.8 | 37.3 ± 48.6 | 17.0 ± 7.7 | 0.323 |
| Serum GGT, U/l | ||||
| Baseline | 44.6 ± 43.7 | 45.2 ± 47.3 | 41.0 ± 4.5 | 0.242 |
| Day 7 | 84.7 ± 118 | 91.8 ± 127 | 42.0 ± 5.2 | 0.515 |
| Serum total alkaline phosphatases, U/l | ||||
| Baseline | 147 ± 135 | 121 ± 117 | 167 ± 158 | 0.481 |
| Day 7 | 128 ± 101 | 119 ± 99 | 146 ± 108 | 0.577 |
| Serum total bilirubin, mg/dl | ||||
| Baseline | 0.56 ± 0.87 | 0.53 ± 0.93 | 0.70 ± 0.53 | 0.431 |
| Day 7 | 0.43 ± 0.26 | 0.36 ± 0.18 | 0.80 ± 0.34 | 0.005 |
| Creatine phosphate kinase, U/l | ||||
| Baseline | 80.2 ± 64.8 | 85.0 ± 69.0 | 57.0 ± 36.8 | 0.817 |
| Day 7 | 81.0 ± 106 | 76.9 ± 112.0 | 100.5 ± 84.7 | 0.697 |
| Serum D-dimer, ng/ml | ||||
| Baseline | 375 (212–853) | 381 (234–879) | 794 (124–1615) | 0.881 |
| Day 7 | 572 (409–1031) | 572 (409–1711) | 674 (281–10135) | 0.510 |
| P valueb | 0.039 | |||
| Serum procalcitonin, μg/l | ||||
| Baseline | 0.38 (0.21–0.85) | 0.40 (0.21–0.86) | 0.52 (0.19–2.49) | 0.798 |
| Day 7 | 0.50 (0.26–1.31) | 0.37 (0.24–1.03) | 1.78 (0.36–5.6) | 0.079 |
| Serum C-reactive protein, mg/dl | ||||
| Baseline | 9.6 ± 7.5 | 10.3 ± 8.3 | 7.4 ± 4.3 | 0.568 |
| Day 7 | 10.6 ± 10.4 | 8.1 ± 8.1 | 18.3 ± 13.7 | 0.021 |
| P valueb | 0.039 | |||
| Serum albumin, g/dl | ||||
| Baseline | 3.7 ± 0.4 | 3.7 ± 0.4 | 4.0 ± 0.9 | 0.181 |
| Day 7 | 3.2 ± 0.3 | 3.2 ± 0.3 | 3.2 ± 0.3 | 0.955 |
| P valueb | 0.001 | 0.001 | 0.002 | |
| Total protein, g/dl | ||||
| Baseline | 6.7 ± 0.7 | 6.7 ± 0.7 | 6.9 ± 0.8 | 0.196 |
| Day 7 | 5.6 ± 0.6 | 5.6 ± 0.6 | 5.7 ± 0.7 | 0.493 |
| P valueb | 0.001 | 0.001 | 0.002 |
ALT, alanine aminotransferase; GGT, gamma-glutamyl transferase; LDH, lactate dehydrogenase.
Data are n (%), mean ± SD, or median (range).
Differences between survivors and non-survivors.
Differences between baseline and day 7.
Figure 1.
(a) Lymphocyte count during the illness for survivors compared with nonsurvivors. Previous lymphocyte count (1 month before admission), lymphocyte count at admission, and lymphocyte count 7 days after clinical onset. (b) Serum lactate dehydrogenase (LDH) during the illness for survivors compared with nonsurvivors. Shown is LDH at admission and LDH 7 days after clinical onset. (c) Serum C-reactive protein (CRP) during the illness for survivors compared with nonsurvivors. Shown is CRP at admission and CRP 7 days after clinical onset.
Radiological evolution
All patients developed radiological uni or bilateral pulmonary consolidation or infiltrates on X-ray examination during hospitalization, including the 7 patients that had normal chest X-ray features at admission.
Radiological improvement during the first week was observed in only 2 patients of 36, 16 remained stable, and 18 had radiological worsening. The worst X-ray scores were seen 7 days after initial onset of symptoms, with 85.7% prevalence of bilateral pneumonia. Lung abnormalities were still present in 6 of 7 patients at the time of discharge.
Clinical evolution (respiratory failure/oxygen therapy requirement)
Peripheral oxygen saturation at room air was 94% ± 4% at admission. During hospitalization, all patients required oxygen supplement therapy: 24 (67%) through nasal cannula (up to 4 l/min) and 12 (33%) through face mask with oxygen reservoir bag (5–0 l/min). A total of 12 patients (33%) required assisted mechanical ventilation, but only one patient was admitted to the ICU, due to severe comorbidities in the other 11 patients that limited invasive measures. Clinical and radiological worsening during the first week of admission was associated with mortality such that 11 of 18 patients (61.1%) that worsened eventually died (logRank, 10.918; P = 0.001).
Prognostic factors related to hospital death
Established risk factors in the general population, such as age, diabetes, obesity, coronary heart disease, or chronic obstructive lung disease were not associated with higher mortality in this cohort (Supplementary Table S2). Those patients with a longer dialysis vintage had higher mortality (1.008 [1.001–1.015] per month on hemodialysis; P = 0.019). Lower oxygen saturation at admission was associated with higher hospital mortality in the univariate analysis, but not when it was adjusted by age. Regarding laboratory parameters, increased LDH levels (1.006 [1.001–1.011]; P = 0.016) were associated with increased hospital death. Higher lymphocyte count at 7 days (hazard ratio, 0.996 [0.992–1.000]; P = 0.056) was associated with better survival, but it did not reach a level of statistical significance after adjustment for age (Supplementary Table S2). Other biochemical data previously related to worse prognosis in COVID-19 and/or in MHD patients (D dimer, C-reactive protein, procalcitonin, or albumin levels) were not associated with mortality in age-adjusted analyses. Baseline characteristics, clinical features, radiological features, and mortality comparing patients who received either azithromycin or corticosteroids with patients who did not receive these treatments are shown in Supplementary Table S3.
DISCUSSION
The primary aim of the report is to provide descriptive information including clinical features, laboratory data, treatment schemes, and prognostic factors in a case series of hospitalized patients on MHD therapy with COVID-19. Data on literature in this population are scarce. Yiqiong et al. compared blood test results in 37 MHD patients with those from unaffected MHD patients and healthy subjects. They also reported on mortality (6 of them died, 31 were hospitalized), but there were no data on treatment schedules.11
Clinical and radiological presentation
In our cohort, early clinical symptoms at admission were milder than those in the general population, with lower rates of fever (67% vs. 88%), fatigue (25% vs. 45.6%), and cough (44% vs. 57.6%).14 This result is consistent with similar findings in the MHD population reported by Wang et al. and Yiqiong et al.,10 , 11 which could be related to the immune system dysfunction of MHD patients.11 Although most patients had no evidence of more marked fatigue, a high proportion presented with basal peripheral oxygen saturation under 95% on admission (61%). This finding could be due to the well-known rightward shift of the hemoglobin-oxygen dissociation curve in MHD patients.15 Most patients (85.7%) presented with or developed bilateral pneumonia with the typical radiological pattern of peripheral ground-glass bilateral opacities, consistent with other published data.16 , 17
Treatment scheme
To date, no randomized controlled trials have been published comparing different therapies for COVID-19 in patients on MHD therapy. Our study population received a number of different treatment protocols that are commonly reported. In the general population, Gautret et al. 18 reported a virological cure with the combination of hydroxychloroquine and azithromycin in a select cohort of patients, but there is very low certainty of the efficacy results with this treatment, mainly due to a very high-risk selection bias, making any claims of effectiveness highly uncertain. No studies were found specifically examining the role of steroids for the treatment of the acute COVID-19. Corticosteroids were widely used in China to prevent the development of acute respiratory distress syndrome in patients with COVID-19 pneumonia.19, 20, 21 Limited studies suggest that early treatment of corticosteroids could decrease the need for mechanical ventilation in these patients, and therefore decrease mortality.21 , 22 The probable beneficial effects of azithromycin or corticosteroid treatment in patients on MHD must be confirmed in clinical trials with an adequate sample size.
Mortality
The mortality rate (30.5%) was much higher than that observed in the general population (1.4%–8%),1, 2, 3 and even higher than the 26% ICU mortality rate reported by Grasselli et al. 1 in Italy. This difference may be explained by the older age of the patients and the presence of multiple comorbid conditions, especially the high cardiovascular comorbidity observed in patients with end-stage kidney disease. However, classical cardiovascular risk factors in the general population were not associated with higher mortality in this cohort, probably limited by the small sample size. Also, a mild clinical presentation at diagnosis did not guarantee a benign course, as all patients developed radiological abnormalities. The first week of hospitalization was crucial to assess the prognosis.
The mortality rate in our cohort was higher than that reported by Yiqiong et al. 11—30.5% vs. 16.2%—but the patients in the Chinese cohort were also younger than those in our series, albeit only minimally (66 vs. 71 years).11 Mortality reported in 2 other series from Italy are much closer to our findings: Scarpioni et al. 12 found a mortality rate of 41% in Piacenza, Italy (n = 41), and Alberici et al. 13 reported a mortality rate of 25% in Brescia, Italy (n = 21).
In this cohort, the main parameters that were associated with mortality were dialysis vintage and several laboratory findings, namely low lymphocyte count and high LDH, and total bilirubin and CRP levels 7 days after clinical onset, in accord with previous reports, although only LDH remained statistically significant after adjustment for age.1, 2, 3, 4, 5 , 9, 10, 11, 12, 13 LDH has been shown to have prognostic value in Pneumocystis jirovecii pneumonia,23 which could also be applicable to COVID-19. Interestingly, no laboratory findings at baseline were predictors of mortality. D-dimer levels, which are shown to be associated with outcomes in the general population are probably not good predictors of mortality in this population, as elevated levels have been described in MHD patients in stable conditions, and there is evidence of D-dimer clearance with dialysis therapy affecting its concentration independent of disease states. The cause of death in all our patients was respiratory distress syndrome due to COVID-19, as it was in the Italian cohorts.12 , 13 These data contrast with the Wuhan cohort, in which the main cause of death was a cardiovascular event.11
Our study has some limitations. First, due to the fact that it is retrospective, laboratory tests such as interleukin-6 and serum ferritin measures were not done in all patients. Therefore, their role could not be evaluated in predicting in-hospital death. Second, we do not know the incidence of SARS-CoV-2 infection in our dialysis facility because a real-time reverse transcriptase polymerase chain reaction test could only be performed in symptomatic patients. Third, at the time of publication of these results, several patients are still hospitalized, which could create a bias in the interpretation of our findings. Fourth, the elevated mortality rates observed with the first “steroid-free” treatment schemes might be related to other confounding factors not related to treatment modifications (e.g., better clinical experience, improved logistical conditions or, perhaps, a virulence reduction over time). Lastly, interpretation of our findings might be limited by the small sample size.
In conclusion, mortality among hospitalized MHD patients diagnosed with COVID-19 infection is strikingly high. Several laboratory parameters at day 7 after hospital admission could be used to assess the clinical outcome of these patients.
PATIENTS AND METHODS
Study design
The study was an observational, analytical, retrospective, single-center study.
Included were all patients on MHD therapy admitted to the hospital with positive rRT-PCR testing for SARS-CoV-2 from March 12, 2020 to April 10, 2020. For data collection, demographic and clinical features, laboratory and radiological data, treatment schemes, and mortality rates were registered.
Laboratory procedures
Methods for laboratory confirmation of SARS-CoV-2 infection have been described elsewhere.24 Routine blood examinations included complete blood count, coagulation profile, and serum biochemistry (including liver function tests, creatine kinase, LDH, total proteins, and albumin). Laboratory parameters were measured at admission, at 1 week after clinical onset, and either at discharge or before death.
Clinical and radiological evolution
Clinical improvement, stability, or worsening were defined based on the need for oxygen therapy at admission, at 1 week after clinical onset, and either at discharge or before death. Radiological improvement, stability, or worsening were evaluated by successive chest X-ray results collected at admission, at 1 week after clinical onset, and either at discharge or before death. Unfavorable evolution was defined as severe hypoxia with oxygen therapy requirements greater than 4 l/min and worsening or appearance of X-ray pulmonary infiltrates.
Treatment scheme
Throughout the outbreak, the treatment scheme was modified according to a decision of our hospital commission. Patients were initially treated with lopinavir/ritonavir plus hydroxychloroquine and interferon beta. In a second phase, starting March 21st, patients were treated with lopinavir/ritonavir plus hydroxychloroquine or azithromycin with hydroxychloroquine, and methylprednisolone was administered in cases of radiological worsening. Tocilizumab was indicated for cases with radiological and clinical worsening and for patients with interleukin-6 levels >40 pg/ml who met criteria for ICU admission. The study was approved by the local research ethics committee.
Dialysis scheme
During admission, all patients received three, 4-hour dialysis sessions per week with a similar dialysis prescription: post-dilution on-line hemodiafiltration with an auto-substitution control system (AutoSub Plus, FMC, Bad Homburg, Germany) in 5008 dialysis monitors, dialyzer surface 1.8 m2 (helixone or triacetate membranes, depending on history of hypersensitivity). The dialysis prescription was individualized according to previous patient regimes and evolution during admission.
Statistical analysis
Qualitative variables are presented with their frequency distribution. Quantitative variables are summarized with their mean ± SD or median and interquartile range. Baseline variables have been compared following the CONSORT regulations according to clinical relevance. If any confusion variable was detected, the appropriate models have been adjusted. The association between qualitative variables has been evaluated with the χ2 test or Fisher's exact test. The quantitative variables have been analyzed using Student's t test (for comparisons of one variable with 2 categories) and/or an analysis of variance. Using this technique, mean differences due to the individual or main effect of each factor and/or the effect of their interactions have been evaluated. In all cases, the distribution of the variables has been checked against the theoretical models, and the hypothesis of variance homogeneity has been tested. Univariable and multivariable logistic regression methods were used to explore the risk factors associated with in-hospital death. Cox proportional hazard models were used to determine the influence of different treatments adjusted for age. All statistical analyses were performed with SPSS 21.0 software (Chicago, IL). Statistical significance was considered a 2-sided P value <0.05.
Disclosure
All the authors declared no competing interests.
Footnotes
Table S1. Treatment scheme modified according to decision by local hospital commission.
Table S2. Risk factors associated with in-hospital death.
Table S3. Baseline characteristics and clinical and radiological features comparing patients who received either azithromycin or corticosteroids with those who did not receive these treatments.
Supplementary Material
References
- 1.Grasselli G., Zangrillo A., Zanella A. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yi Y., Lagniton P.N.P., Ye S. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16:1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madjid M, Safavi-Naeini P, Solomon SD, et al. Potential effects of coronaviruses on the cardiovascular system: a review [e-pub ahead of print]. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.1286. Accessed March 27, 2020. [DOI] [PubMed]
- 4.Wynants L., Van Calster B., Bonten M.M.J. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) [e-pub ahead of print]. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.1017. Accessed March 27, 2020. [DOI] [PMC free article] [PubMed]
- 6.Basile C, Combe C, Pizzarelli F, et al. Recommendations for the prevention, mitigation and containment of the emerging SARS-CoV-2 (COVID-19) pandemic in haemodialysis centres. Nephrol Dial Transplant. 2020;35:737–741. [DOI] [PMC free article] [PubMed]
- 7.de Sequera Ortiz P, Quiroga Gili B, de Arriba de la Fuente G, et al.; representing the Spanish Society of Nephrology. Protocol against coronavirus diseases in patients on renal replacement therapy: dialysis and kidney transplant [English, Spanish; e-pub ahead of print]. Nefrologia. https://doi.org/10.1016/j.nefro.2020.03.001. Accessed April 8, 2020. [DOI] [PMC free article] [PubMed]
- 8.Li J, Xu G. Lessons from the experience in Wuhan to reduce risk of COVID-19 infection in patients undergoing long-term hemodialysis. Clin J Am Soc Nephrol. 2020;15:717–719. [DOI] [PMC free article] [PubMed]
- 9.Ferrey AJ, Choi G, Hanna RM, et al. A case of novel Coronavirus Disease 19 in a chronic hemodialysis patient presenting with gastroenteritis and developing severe pulmonary disease. Am J Nephrol. 2020;51:337–342. [DOI] [PMC free article] [PubMed]
- 10.Wang R, Liao C, He H, et al. COVID-19 in hemodialysis patients: a report of 5 cases [e-pub ahead of print]. Am J Kidney Dis. https://doi.org/10.1053/j.ajkd.2020.03.009. Accessed May 14, 2020. [DOI] [PMC free article] [PubMed]
- 11.Yiqiong Ma, Bo Diao, Xifeng Lv, et al. 2019 novel coronavirus disease in hemodialysis (HD) patients: report from one HD center in Wuhan, China. medRxiv preprint. Available at: 10.1101/2020.02.24.20027201. Accessed February 27, 2020. [DOI]
- 12.Scarpioni R., Manini A., Valsania T. Covid-19 and its impact on nephropathic patients: the experience at Ospedale "Guglielmo da Saliceto" in Piacenza. G Ital Nefrol. 2020;37(2) [PubMed] [Google Scholar]
- 13.Alberici F., Delbarba E., Manenti C. Management of patients on dialysis and with kidney transplantation during the SARS-CoV-2 (COVID-19) pandemic in Brescia, Italy. Kidney Int Rep. 2020;5:580–585. doi: 10.1016/j.ekir.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E. American network of Coronavirus Disease 2019-COVID-19 research (LANCOVID-19). Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metivier F., Marchais S.J., Guerin A.P. Pathophysiology of anaemia: focus on the heart and blood vessels. Nephrol Dial Transplant. 2000;15(suppl 3):14–18. doi: 10.1093/oxfordjournals.ndt.a027970. [DOI] [PubMed] [Google Scholar]
- 16.Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel Coronavirus (COVID-19) pneumonia [e-pub ahead of print]. Radiology. https://doi.org/10.1148/radiol.2020200370. Accessed February 13, 2020.
- 17.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in Coronavirus Disease 2019 (COVID-19) in China: a report of 1014 cases [e-pub ahead of print]. Radiology. https://doi.org/10.1148/radiol.2020200642. Accessed February 26, 2020. [DOI] [PMC free article] [PubMed]
- 18.Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial [e-pub ahead of print]. Int J Antimicrob Agents. https://doi.org/10.1016/j.ijantimicag.2020.105949. Accessed March 20, 2020. [DOI] [PMC free article] [PubMed] [Retracted]
- 19.Zhai P., Ding Y., Wu X. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020;55:105955. doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China [e-pub ahead of print]. JAMA Intern Med. https://doi.org/10.1001/jamainternmed.2020.0994. Accessed March 13, 2020. [DOI] [PMC free article] [PubMed]
- 22.Li T. Diagnosis and clinical management of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection: an operational recommendation of Peking Union Medical College Hospital (V2.0) Emerg Microbes Infect. 2020;9:582–585. doi: 10.1080/22221751.2020.1735265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt J.J., Lueck C., Ziesing S. Clinical course, treatment and outcome of Pneumocystis pneumonia in immunocompromised adults: a retrospective analysis over 17 years. Crit Care. 2018;22:307. doi: 10.1186/s13054-018-2221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.